Increased CGRP expression in sensory neurons is associated with inflammatory pain. We examined the molecular basis of CGRP expression and found that acid-sensing nociceptor Trpv1 is activated under inflammatory acidic environments and up-regulates the CGRP expression through CaMK-CREB cascade.
Abstract
Increased production of calcitonin gene-related peptide (CGRP) in sensory neurons is implicated in inflammatory pain. The inflammatory site is acidic due to proton release from infiltrating inflammatory cells. Acid activation of peripheral nociceptors relays pain signals to the CNS. Here, we examined whether acid activated the transient receptor potential vanilloid subtype 1 (Trpv1), a widely recognized acid-sensing nociceptor and subsequently increased CGRP expression. Chemically induced inflammation was associated with thermal hyperalgesia and increased CGRP expression in dorsal root ganglion (DRG) in rats. In organ cultures of DRG, acid (pH 5.5) elevated CGRP expression and the selective Trpv1 antagonist 5′-Iodoresiniferatoxin decreased it. Trpv1-deficient DRG showed reduced CGRP increase by acid. Of note, many of CGRP/Trpv1-positive DRG neurons exhibited the phosphorylation of cAMP response element-binding protein (CREB), a nociceptive transcription factor. Knockdown of CREB by small interfering RNA or a dominant-negative form of CREB diminished acid-elevated CGRP expression. Acid elevated the transcriptional activity of CREB, which in turn stimulated CGRP gene promoter activity. These effects were inhibited by a Ca2+/calmodulin-dependent protein kinase (CaMK) inhibitor KN-93. In conclusion, our results suggest that inflammatory acidic environments activate Trpv1, leading to an up-regulation of CGRP expression via CaMK-CREB cascade, a series of events that may be associated with inflammatory pain.
INTRODUCTION
Calcitonin gene-related peptide (CGRP) is a neuropeptide that is widely distributed in the peripheral and central nervous systems. It has been proposed that the release of CGRP from perivascular nerves causes vasodilatation, resulting in neurogenic inflammation. CGRP release into the dorsal spinal cord is associated with nociceptive transmission (Benemei et al., 2009). Clinically, CGRP levels in the circulation have been found to be elevated during migraines and cluster headache attacks (Goadsby et al., 1990; Fanciullacci et al., 1995), as well as in myocardial infarction (Preibisz, 1993). Interestingly, recent studies have demonstrated that CGRP knockout mice show an attenuated response to chemical pain and inflammation (Salmon et al., 2001). These reports indicate the importance of CGRP regulation for therapeutic approaches to control pain.
The vanilloid receptor transient receptor potential vanilloid subtype 1 (Trpv1) is a ligand-gated nonselective cation channel that is activated by capsaicin, noxious heat (temperatures >43°C), and protons (Caterina et al., 1997; Tominaga et al., 1998; Premkumar et al., 2002). Trpv1 is abundantly expressed in nociceptive neurons in the dorsal root ganglia (DRG) and is involved in various physiological functions (Caterina et al., 1997; Immke and Gavva, 2006). Recent studies have demonstrated that Trpv1 is a critical regulator of inflammatory pain. Trpv1-deficient mice fail to develop thermal hyperalgesia in an experimental inflammation model (Caterina et al., 2000). Activation of Trpv1 causes the release of neurotransmitters such as CGRP and substance P from peripheral and central nerve terminals, resulting in pain and neurogenic inflammation (Szallasi and Blumberg, 1999). Moreover, several Trpv1 antagonists have been reported to alleviate thermal hyperalgesia associated with inflammation (Szallasi et al., 2007).
It is well documented that capsaicin significantly and dose-dependently increases the release of CGRP from skin (Kilo et al., 1997) and that activation of Trpv1 by capsaicin induces the exocytosis of CGRP from sensory neurons (Meng et al., 2007; Meng et al., 2009). Furthermore, capsaicin and low pH induce a significant increase in CGRP release in mouse heart but not in mice lacking the Trpv1 receptor (Strecker et al., 2005). Despite its clear involvement in inflammatory pain, the molecular mechanism underlying CGRP expression through Trpv1 activation remains to be elucidated.
A variety of inflammatory mediators, such as prostaglandin E2, bradykinin, and protons, play crucial roles in the induction of pain. Among these, only protons can directly activate Trpv1 in sensory neurons (Tominaga et al., 1998). Indeed, sensory neurons from DRGs isolated from Trpv1-deficient mice show reduced responses to acid (Caterina et al., 2000). Because local tissue acidosis is a predominant feature of inflammation (Bevan and Geppetti, 1994), proton-activated Trpv1 probably participates in the induction of inflammatory pain. Elucidation of the intracellular molecular events triggered by protons via Trpv1 will contribute to increased understanding of the mechanism underlying inflammatory pain.
Trpv1 activation induces Ca2+ influx into neurons, leading to activation of Ca2+-mediated signal transduction, including activation of Ca2+/calmodulin-dependent protein kinase (CaMK) (Means, 2000) and protein kinase C isoenzymes (Mellstrom et al., 2008). Of note, many studies have reported that CaMK signaling is critical for a variety of neuronal functions (Ribar et al., 2000; Kang et al., 2001; Griffith, 2004). CaMK activates the transcription factor cAMP response element-binding protein (CREB) via phosphorylation of Ser-133 (Mayr and Montminy, 2001). Activated CREB induces target gene expression and regulates various neuronal functions (Lonze and Ginty, 2002). Importantly, the inflammation caused by complete Freund's adjuvant (CFA) increases phosphorylation of CREB in DRG neurons (Tamura et al., 2005). Collectively, these studies raise the possibility that protons contribute to inflammatory pain by modulating CaMK-CREB signaling cascades via Trpv1.
In this study, we investigated the intracellular signaling pathways initiated by Trpv1 activation by protons in DRG neurons. Using an experimental inflammation model in Trpv1-deficient mice, we found that proton activation of Trpv1 is responsible for the induction of CGRP expression. We also found that protons activate the CaMK-CREB pathway through Trpv1 activation and that this signaling cascade is involved in the regulation of CGRP expression. Our findings will increase the understanding of the molecular mechanism of inflammatory pain.
MATERIALS AND METHODS
Reagents
5′-Iodoresiniferatoxin (I-RTX) and capsazepin were purchased from Tocris Cookson (Bristol, United Kingdom) and Sigma-Aldrich (St. Louis, MO), respectively. KN-93 was obtained from BIOMOL Research Laboratories (Plymouth Meeting, PA).
Animals
Trpv1-deficient mice were kindly provided by Dr. Makoto Tominaga (National Institutes of Natural Sciences, Okazaki, Japan; Caterina et al., 2000). Male C57BL/6J mice aged 7 wk and male Sprague Dawley (SD) rats aged 5–7 wk were obtained from SLC Japan (Hamamatsu, Japan). Animals were maintained in an animal room with free access to drinking water and basal diet, under controlled conditions and a 12:12-h light/dark cycle. All animal experiments were approved by the Institutional Animal Care and Use Committee of Osaka University Graduate School of Dentistry.
Animal Model of Inflammation
Male SD rats (6 wk old) received a subcutaneous injection into the right hind paw of 100 μl of CFA (Wako Pure Chemical Industries, Osaka, Japan), prepared in an oil/saline (1:1) emulsion. The same volume of saline was injected into the left hind paw as a control. Wild-type and Trpv1-deficient mice were given an injection of 50 μl of solution. Signs of acute inflammation, including redness and swelling, were observed in the right hind paw 24 h after CFA injection.
Plantar Test
Thermal hyperalgesia induced by CFA injection was measured by the plantar test (Hargreaves et al., 1988). In brief, animals were placed in an acrylic box with a glass pane floor and the plantar surface of their hind paws was exposed to a beam of infrared radiant heat (Plantar test 7370; Ugo Basile, Comerio, Italy). The paw withdrawal latencies were measured three times per session, separated by a minimum interval of 5 min. Paw withdrawals due to locomotion or weight shifting were not counted, and the trials were repeated.
Isolation and Culture of Primary DRG Neurons
DRGs isolated from SD rats, wild-type mice, and Trpv1−/− mice were cultured in poly-d-lysine–coated 35-mm dish (BD Biosciences Discovery Labware, Bedford, MA) with Ham's F-12 medium (Invitrogen, Carlsbad, CA) containing 2% B-27 supplement (Invitrogen) for 24 h.
Primary culture of DRG neurons was performed as described previously using male SD rats aged 6–8 wk (Sango et al., 1991). Thirty to 35 ganglia (from the thoracolumbar level) were dissected from the spinal column of each animal and were dissociated with 0.2% collagenase (Worthington Biochemicals, Freehold, NJ) at 37°C for 90 min and 0.25% trypsin (Sigma-Aldrich) at 37°C for 15 min. Then, ganglia were subjected to density gradient centrifugation (1500 rpm for 5 min) with 30% Percoll (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). The cells were seeded onto poly-d-lysine–coated eight-well culture slides (BD Biosciences Discovery Labware) or 35-mm culture dishes (BD Biosciences Discovery Labware) in Ham's F-12 medium containing 2% B-27 supplement (Invitrogen). The cells were grown in culture for 48 h before experiments.
Immunohistochemistry
Rats were anesthetized with ether and fixed by perfusion with 4% paraformaldehyde in 0.1 M phosphate buffer through the left cardiac ventricle. The spinal column of rats was removed and postfixed in the same fixative for 24 h. L4 and L5 DRG were excised and embedded in paraffin. The sections were microwaved for 5 min in 0.01 M citrate buffer and blocked with 1% bovine serum albumin for 30 min. For double immunofluorescence, sections were incubated overnight at 4°C with appropriate combinations of guinea pig anti-TRPV1 polyclonal antibody (pAb, 1:500; Neuromics, Edina, MN) and rabbit anti-CGRP pAb (1:10,000; Sigma-Aldrich), guinea pig anti-CGRP pAb (1:1000; Progen Biotechnik, Heidelberg, Germany), or guinea pig anti-TRPV1 pAb and rabbit anti-phospho-CREB pAb (1:500; Millipore, Billerica, MA), and rabbit anti-CGRP pAb or rabbit anti-TRPV1 (1:500; Calbiochem, San Diego, CA) and mouse anti-CaMK II monoclonal antibody (mAb, 1:300; BIOMOL Research Laboratories) or mouse anti-CaMK IV mAb (1:200; Abnova, Taipei, Taiwan). As secondary antibodies, Alexa Fluor 555-conjugated anti-mouse immunoglobulin (Ig)G (1:500; Invitrogen), Alexa Fluor 488-conjugated anti-guinea pig IgG (1:500; Invitrogen), Alexa Fluor 488 anti-rabbit IgG (1:500; Invitrogen), and Alexa Fluor 555-conjugated anti-rabbit IgG (1:500; Invitrogen) were incubated for 1 h at room temperature. The sections were coverslipped with VECTASHIELD mounting medium (Vector Laboratories, Burlingame, CA).
RNA Preparation and Real-Time Polymerase Chain Reaction (PCR)
DRGs were isolated from the spinal column of each animal and grown in an organ culture system. The medium was changed into pH 5.5 medium and treated with or without I-RTX. Total RNA was isolated using RNAeasy Mini kit (QIAGEN, Valencia, CA), and cDNA was synthesized using PrimeScript First Strand cDNA Synthesis kit (Takara Bio, Shiga, Japan). Real-time PCR was performed using the TaqMan PCR protocol and a 7300 real-time PCR system (Applied Biosystems, Tokyo, Japan). TaqMan primers and probes used for the amplification were as follows: mouse CGRP, sense 5′-CCAGTGGGTGAGGAGAAAGTC-3′, antisense 5′-AAGCAAGACTAGAAGCTCTACTAGG-3′, and probe 5′-ACTGCCCTTGCTCTCTGCCATCTTCC-3′; mouse β-actin, sense 5′-TTAATTTCTGAATGGCCCAGGTCT-3′, antisense 5′-ATTGGTCTCAAGTCAGTGTACAGG-3′, and probe 5′-CCTGGCTGCCTCAACACCTCAACCC-3′); rat CGRP, sense 5′-ATCTAAGCGGTGTGGGAATCTG-3′, antisense 5′-TTCTTGCCAGGTGCTCCAAC-3′, and probe 5′-TGCTGGGCACGTACACACAAGACCTC-3′; rat Trpv1, sense 5′-CTGACGGCAAGGATGACTACC-3′, antisense 5′-ACCTCAGGGAGAAGCTCAGG-3′, and probe 5′-CGCTTGACGCCCTCACAGTTGCCT-3′; and rat β-actin, sense 5′-ACAACCTTCTTGCAGCTCCTC-3′, antisense 5′-CGACGAGCGCAGCGATATC-3′ and probe 5′-CCACACCCGCCACCAGTTCGCC-3′. mRNA expression levels were normalized to that of β-actin.
Western Blotting
Western blotting was performed as described previously (Hata et al., 2005). In brief, cells were rinsed twice with phosphate-buffered saline and solubilized in lysis buffer (20 mM HEPES, pH 7.4, 150 mM NaCl, 1 mM EGTA, 1.5 mM MgCl2, 10% glycerol, 1% Triton X-100, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM AEBSF, and 0.2 mM sodium orthovanadate). The lysates were centrifuged for 10 min at 4°C at 15000 × g and boiled in SDS sample buffer containing 5% β-mercaptoethanol for 5 min. The supernatants were separated by SDS-polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, immunoblotted with primary antibodies, and visualized with horseradish peroxidase-coupled anti-mouse or anti-rabbit IgG antibody using an ECL detection kit (Lumigen, Southfield, MI). Antibodies were purchased from Cell Signaling Technology (Danvers, MA).
Cell Culture
The rat DRG cell line F11 was a generous gift from Dr. Guichao Zeng (Medical College of Georgia, Augusta, GA). Trpv1-F11 cells, which were stably transfected with rat Trpv1cDNA, were generated as described previously (Nagae et al., 2006). F11 and F11-Trpv1 cells were maintained in DMEM, pH 7.4 (Sigma-Aldrich) supplemented with 10% fetal bovine serum and 100 μg/ml kanamycin sulfate (Meiji Seika, Tokyo, Japan) in a humidified atmosphere of 5% CO2 in air.
Intracellular Ca2+ Measurement
Intracellular Ca2+ responses of F11 and F11-Trpv1 cells were assessed using the fluorescent Ca2+ probe Fluo-3 (Calcium Kit-Fluo 3; Dojin Chemical, Kumamoto, Japan). Cells were cultured on 96-well plates at a density of 15,000/well for 24 h, and then they were loaded with Fluo-3 acetoxymethyl ester (4.4 μM) in loading buffer containing 1.25 mM probenecid and 0.04% Pluronic F-127 for 1 h at 37°C. Wells were then incubated with recording buffer, and Fluo-3–loaded cells were excited at 458 nm and emission was recorded at 538 nm using a Fluoroskan ascent microplate reader (Thermo Fischer Scientific, Yokohama, Japan). Fluorescence was recorded every 1 s, and data are expressed as increasing ratio (ΔF/F0).
Knockdown of CREB
CREB small interfering RNA (siRNA; siCREB-1, 5′-AUCAGUUACACUAUCCACAGACUCC-3′; siCREB-2, 5′-UACCAUUGUUAGCCAGCUGUAUUGC-3′) and control siRNA were designed and purchased from Invitrogen. siRNA was transfected into F11-Trpv1 cells using Lipofectamine RNAiMAX reagents (Invitrogen) according to the manufacturer's protocol. Knockdown of endogenous CREB and its effect on CGRP mRNA expression were examined after 48 h.
Constructs for Reporter Assay
The 3×CRE-Luc plasmid, which contains three tandem CRE sequences upstream of a minimal promoter (minP), and the luciferase gene were purchased from Promega (Madison, WI). To construct luciferase fusion plasmids containing the CGRP promoter, promoter regions were amplified by PCR using rat genomic DNA as the template and cloned into the pGL4 Basic Vector (Promega). The sequence of the CGRP gene promoter was downloaded from UCSC Genome Bioinformatics (Santa Cruz, CA). Mutation of cAMP response element (CRE) was generated using the site directed mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's protocol. The sequence of the mutated CRE was confirmed by DNA sequence analysis.
Reporter Assay
F11 and F11-Trpv1 cells were plated at a density of 10,000 cells/well into 24-well plates. Cells were transfected with plasmids using FuGENE 6 (Roche Applied Science, Indianapolis, IN) according to the manufacturer's protocol. Twenty-four hours after transfection, medium was changed to pH 5.5 medium, and cells were cultured for 1 d with or without I-RTX or KN-93. Then, cells were lysed with lysis buffer and luciferase activity was measured by GloMax 96 microplate luminometer (Promega). Transfection efficiency was normalized by determining the activity of Renilla luciferase.
Generation of Adenovirus
The recombinant adenovirus carrying FLAG-tagged A-CREB cDNA was constructed by homologous recombination between the expression cosmid cassette and the parental virus genome in human embryonic kidney (HEK)293 cells using the adenovirus construction kit (Takara Bio) as described previously (Hata et al., 2008). Flag-tagged A-CREB cDNA was kindly provided by Dr. Charles Vinson (National Cancer Institute, Bethesda, MD). The viruses were confirmed to retain no proliferative activity in the cells other than HEK293 cells, because they lack both E1A and E1B domain of adenovirus. Titers of the viruses were analyzed by modified point assay (Hata et al., 2008).
Chromatin Immunoprecipitation (ChIP) Assays
ChIP assays were performed using ChIP-IT Express kit (Active Motif, Carlsbad, CA). In brief, F11-Trpv1 cells were treated with pH 5.5 or pH 7.4 medium for 24 h and then fixed with 1% formaldehyde for 20 min. The sheared chromatin was prepared using sonication shearing. Chromatin immunoprecipitation was performed with antibodies against CREB (Millipore), acetyl-histone H3 (Millipore), and normal rabbit IgG (Active motif) as a control. Immunoprecipitated DNA was amplified by PCR using the following primers, which are specific for the rat CGRP promoter: sense (−384 to −365), 5′-GGGGCACGATTAGAATCAGA-3′ and antisense (−30 to −11), 5′-TCCTGCAGCTGCTCTTATTCC-3′.
Statistical Analysis
Data are presented as the mean ± SD. The data from the behavioral test were analyzed using one-way analysis of variance followed by Fisher's protected least significant difference post hoc test (StatView; SAS Institute, Cary, NC) for comparisons between groups. Student's t test was conducted when two groups were compared. P values of <0.05 were considered significant.
RESULTS
Involvement of Proton-induced Trpv1 Activation in the Up-Regulation of CGRP Expression
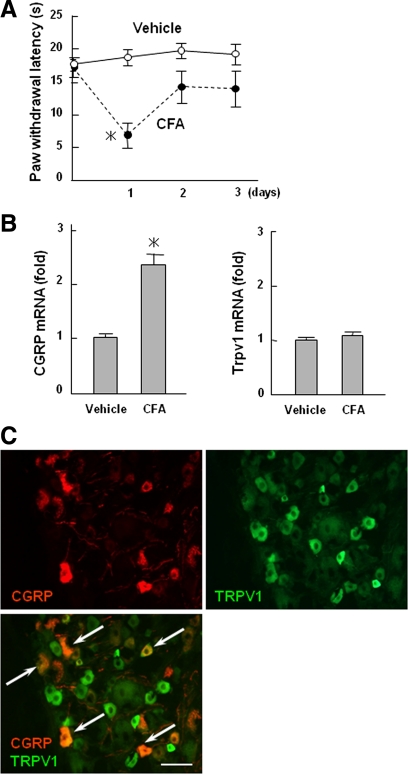
To clarify the relationship between CGRP expression and Trpv1, we first studied a CFA-induced inflammation model in rats. As shown in Figure 1A, animals exhibited thermal hyperalgesia in the CFA-injected paw, a sign of inflammation and the hyperalgesia peaked at day 1. Using this model, we found that the expression of CGRP mRNA was increased in the ipsilateral DRG of CFA-injected animals (Figure 1B). Immunohistochemical analysis demonstrated that CGRP-positive neurons were partially overlapped with Trpv1 in DRG neurons (Figure 1C). Because the expression of Trpv1 mRNA was not changed by inflammation (Figure 1B), we focused on the functional activation of Trpv1 and not quantitative changes in Trpv1.
Figure 1.
Relationship between CGRP and Trpv1 expression in DRG neurons in CFA-induced inflammation. (A) Thermal hyperalgesia in an inflammation rat model induced by CFA. Withdrawal latency for thermal stimulation in vehicle- or CFA-injected paw is shown as the mean ± SD. *p < 0.005 versus vehicle. (B) Total RNA was isolated from contralateral and ipsilateral L3–6 DRGs at 1 d after CFA injection. The expression of CGRP and Trpv1 mRNA was analyzed with real-time PCR. The data are expressed as fold activation normalized to control (mean ± SD). *p < 0.05 versus vehicle. (C) Immunohistochemical analyses of CGRP and Trpv1 in DRGs. Sections were double-stained with antibodies as indicated in the panels. Arrows indicated the colocalization of Trpv1 and CGRP in DRG neurons (arrows). Bar, 50 μm.
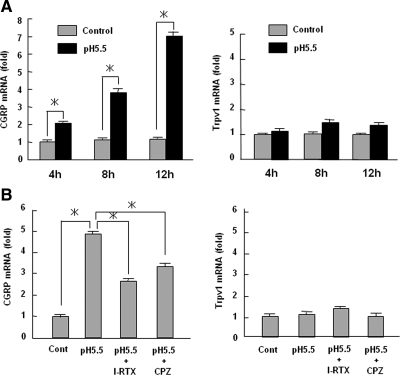
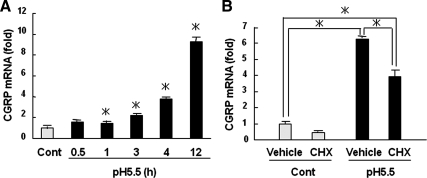
To examine the effects of Trpv1 activation on CGRP production, we treated the DRGs with acidic medium (pH 5.5) in organ culture and investigated CGRP mRNA expression. Treatment with acidic medium had no effects on apoptosis and cell viability (data not shown). As shown in Figure 2A, low pH medium increased CGRP mRNA expression in a time-dependent manner. The selective Trpv1 antagonist I-RTX and nonselective antagonist capsazepine significantly inhibited the increase in CGRP mRNA expression caused by acid stimulation (Figure 2B). In contrast, the expression of Trpv1 mRNA was not changed by acid stimulation (Figure 2, A and B). CGRP mRNA expression was weakly but significantly increased at 1 h after acidic treatment (Figure 3A). However, the increase of CGRP mRNA was partially sensitive to the inhibition of protein synthesis by cycloheximide (Figure 3B). These data suggest that CREB directly promoted CGRP expression but that yet unknown events related to protein synthesis are also involved in CGRP mRNA stimulation by acid.
Figure 2.
Up-regulation of CGRP expression via proton-mediated Trpv1 activation. (A) DRGs were treated with pH 5.5 or pH 7.4 medium for the indicated hours. Total RNA isolated from the DRGs was used for real-time PCR analyses of CGRP and Trpv1. The data were expressed as fold activation normalized to control (mean ± SD). *p < 0.05 versus control. (B) Rat DRGs were treated with pH 5.5 medium for 12 h with or without Trpv1 antagonists (I-RTX or capsazepine [CPZ]). The data are expressed as fold activation normalized to control (mean ± SD). *p < 0.05 versus control.
Figure 3.
Time-dependent induction of CGRP by Trpv1 activation. (A) Rat DRGs were treated with pH 5.5 medium for the indicated hours, and CGRP expression was detected by real-time PCR. (B) Rat DRGs were pretreated with 1 μM cycloheximide (CHX) for 1 h and stimulated with pH 5.5 medium for an additional 12 h. Total RNA was used for real-time PCR analysis of CGRP. The data are expressed as fold activation normalized by control (mean ± SD). *p < 0.05.
Role of Trpv1 in the Induction of Inflammatory Pain and Elevation of CGRP Expression in DRGs
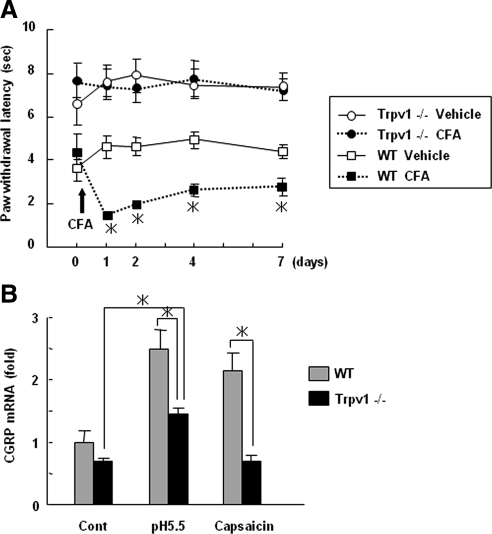
We next verified the significance of Trpv1 to inflammatory pain and CGRP expression by analyzing Trpv1-deficient mice. As shown in Figure 4A, Trpv1-deficient mice did not exhibit thermal hyperalgesia associated with CFA-induced inflammation. Of note, acidic conditions and capsaicin attenuated to induce up-regulation of CGRP mRNA in DRGs isolated from Trpv1-deficient mice, as was seen in wild-type DRGs (Figure 4B). These results suggest that Trpv1 activation by protons is critical for the up-regulation of CGRP mRNA expression.
Figure 4.
Role of Trpv1 in the induction of inflammatory pain and elevation of CGRP expression in DRGs. (A) Behavioral consequences of inflammation induced by CFA in wild-type (WT) and Trpv1−/− mice. The data were expressed as paw withdrawal latency for thermal stimulation and are shown as the mean ± SEM. *p < 0.05 versus vehicle-injected paw in WT mice. (B) DRGs isolated from WT and Trpv1−/− mice were treated with pH 5.5 medium or with 1 μM capsaicin for 12 h. Total RNA isolated from the DRGs was used for real-time PCR analysis of CGRP. The data are expressed as fold activation normalized to control (mean ± SD). *p < 0.05 versus WT.
Involvement of Trpv1 Activation by Acidic Conditions in the Phosphorylation of CREB
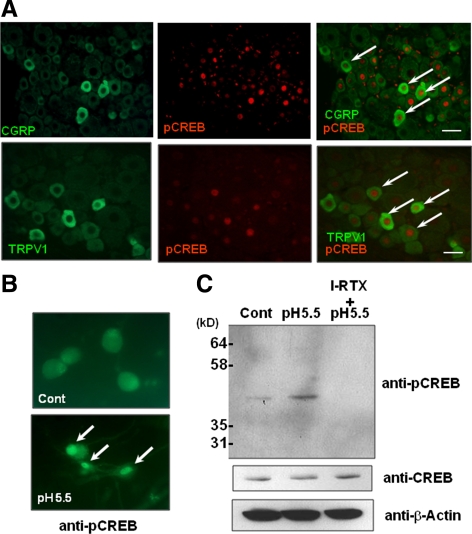
Although we found that protons and activation of Trpv1 up-regulated CGRP expression, the precise molecular mechanism is unclear. Thus, we next examined the intracellular signaling cascades by which protons and Trpv1 control CGRP expression. Because inflammation is known to activate the transcription factor CREB in DRG neurons (Tamura et al., 2005), and because CREB plays essential roles in neural functions (Lonze and Ginty, 2002), we hypothesized that CREB is involved in Trpv1-mediated regulation of CGRP expression. Of note, immunohistochemical analysis demonstrated that both Trpv1- and CGRP-positive neurons were mildly colocalized for phosphorylated CREB (Figure 5A). We next examined whether protons control the phosphorylation and activation of CREB through Trpv1 using primary neuronal cells isolated from rat DRGs. Low pH, 5.5, induced the phosphorylation of CREB in the nucleus (Figure 5B). Western blotting analysis also demonstrated that low pH medium induced phosphorylation of CREB, which was inhibited by I-RTX, suggesting that protons up-regulated the transcriptional activity of CREB through Trpv1 in DRG neurons (Figure 5C). Together, these results suggest that biological interactions between Trpv1 and CREB are involved in the regulation of CGRP expression in DRG neurons.
Figure 5.
Role of Trpv1 activation in the phosphorylation of CREB in DRG neurons. (A) Immunohistochemical analyses of CGRP, Trpv1, and pCREB in rat DRGs. CGRP/Trpv1 and pCREB were partially colocalized in DRG neurons (arrows). Bar, 50 μm. (B) Primary cultured DRG neurons were treated with pH 7.4 medium (cont) and pH 5.5 medium for 2 min and immunostained with pCREB antibody. Arrows indicate the nuclear accumulation of pCREB. (C) Primary cultured DRG neurons were treated with medium at pH 5.5 with or without I-RTX, and the cell lysates were immunoblotted with anti-pCREB, anti-CREB, and anti-β-actin antibodies.
Acidic Conditions Increased CGRP Expression through CREB
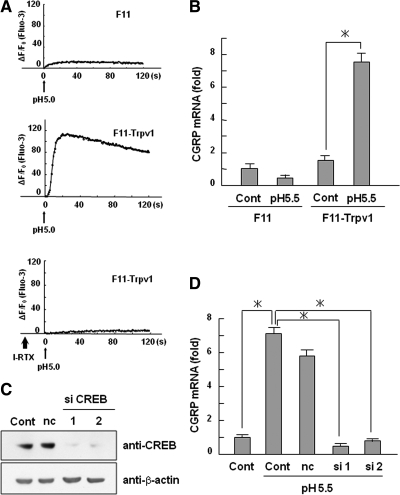
We next investigated the effects of protons on the transcriptional activity of CREB using the rat DRG neuronal cell line F11. Because F11 cells have been reported to express very low levels of Trpv1, we used F11 cells that had been stably transfected with Trpv1 cDNA (Nagae et al., 2006). We observed that acidic conditions induced a strong increase in intracellular [Ca2+] in F11-Trpv1 cells as assessed by Fluo-3, a selective intracellular fluorescent probe for Ca2+, whereas F11 cells did not show this strong Ca2+ influx (Figure 6A). Importantly, the induction of Ca2+ influx in F11-Trpv1 cells was attenuated by treatment with I-RTX, suggesting that exogenous Trpv1 functions properly. Using these cells, we found that low pH medium increased CGRP mRNA in F11-Trpv1 cells, whereas no changes were detected in parental F11 cells (Figure 6B). Moreover, knockdown of CREB inhibited the CGRP mRNA expression induced by low pH medium in F11-Trpv1 cells (Figure 6, C and D).
Figure 6.
Involvement of CREB in the up-regulation of CGRP mRNA after Trpv1 activation. (A) Intracellular Ca2+ responses of Fluo-3–loaded F11 and F11-Trpv1 cells treated with acidic conditions were measured using a fluorescent microplate reader. Plots show the fluorescent increase rate (ΔF/F0). In the bottom graph, F11-Trpv1 cells were preincubated with 5 μM I-RTX for 10 min. (B) F11 and F11-Trpv1 cells were treated with pH 5.5 medium for 8 h. Total RNA isolated from these cells was used for real-time PCR analysis of CGRP. The data are expressed as fold activation normalized to control (mean ± SD). *p < 0.05. (C) Knockdown of CREB by siRNA. Cells were transfected with CREB siRNA or control siRNA, and the level of CREB protein was analyzed with Western blotting. (D) F11-Trpv1 cells transfected with CREB siRNA or control siRNA (nc) were treated with pH 5.5 medium for 8 h. The data are expressed as fold activation normalized to control (mean ± SD). *p < 0.05.
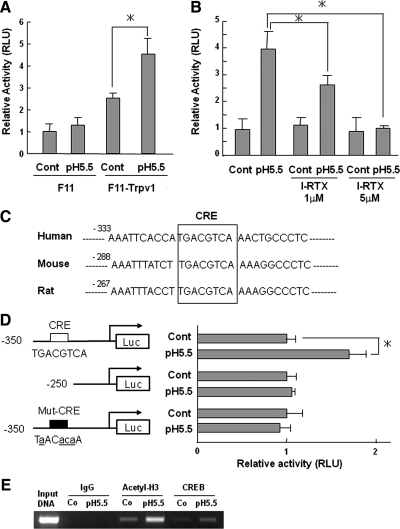
We next examined whether CREB was involved in the regulation of CGRP expression induced by Trpv1 activation at the transcriptional level using F11 and F11-Trpv1 cells. Low pH medium significantly increased CREB transcriptional activity in F11-Trpv1 cells as determined by a reporter assay using 3×CRE-Luc, whereas no changes were detected in parental F11 cells (Figure 7A). Consistent with the results in Figure 5C, I-RTX reduced CREB transcriptional activity in a dose-dependent manner (Figure 7B). Comparison of the human, mouse, and rat CGRP promoter sequences revealed that CRE, the putative binding element of CREB, is highly conserved in the promoter region of the CGRP gene (Figure 7C). Thus, we assessed the effects of protons on CGRP promoter activity using a reporter assay. Low pH, 5.5, increased CGRP gene promoter activity by approximately twofold over control (Figure 7D, top two bars). Elevated promoter activity was decreased by the deletion or mutation of CRE (Figure 7D, middle two bars and bottom two bars). ChIP assays further demonstrated that CREB directly bound to CRE in the CGRP promoter (Figure 7E).
Figure 7.
Up-regulation of CREB transcriptional activity through proton-activated Trpv1. (A) Effect of acid stimulation on transcriptional activity of CREB. F11 and F11-Trpv1 cells were transfected with 3×CRE-Luc plasmids, and cells were treated with pH 5.5 medium for 24 h. The data are expressed as fold activation normalized to control (mean ± SD). *p < 0.05 versus control. (B) Effect of I-RTX on transcriptional activity of CREB. F11-Trpv1 cells were transfected with 3×CRE-Luc plasmids, and cells were treated with pH 5.5 medium for 24 h with indicated concentrations of I-RTX. The data are expressed as fold activation normalized to control (mean ± SD). *p < 0.05 versus pH 5.5. (C) Sequence comparison of human, mouse, and rat CGRP proximal promoters. Sequence information was downloaded from UCSC Genome Bioinformatics and analyzed by visual scanning. (D) Deletion and mutation analyses of CGRP gene promoter activity. The reporter plasmids indicated were transfected into F11-Trpv1 cells, which were treated with pH 5.5 medium. The data are shown as fold activation normalized to control (mean ± SD). *p < 0.05 versus control. (E) F11-Trpv1 cells were treated with pH 5.5 medium for 24 h. The binding ability of CREB to the CGRP promoter was analyzed with a chromatin immunoprecipitation assay using the indicated antibodies and a primer set for the rat CGRP promoter region. Input indicates the PCR products from genomic DNA without immunoprecipitation. Co, control.
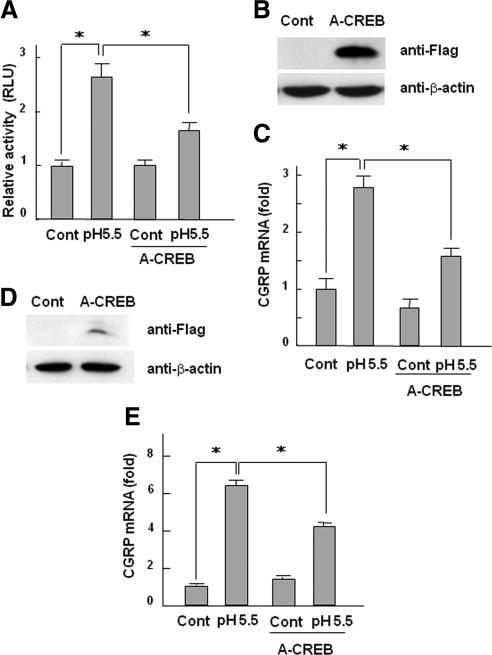
To further examine whether CREB regulates CGRP expression, we next investigated the effects of A-CREB, a dominant-negative form of CREB. We found that A-CREB decreased CGRP promoter activity induced by low pH medium (Figure 8A). Of note, overexpression of A-CREB using the adenovirus system reduced the expression of CGRP induced by acidic conditions in both F11-Trpv1 cells and DRG organ culture (Figure 8, B–E). These data collectively suggest that protons activate CREB transcriptional activity through Trpv1 and directly regulate CGRP gene promoter activity.
Figure 8.
Effects of A-CREB on the up-regulation of CGRP mRNA expression by protons. (A) F11-Trpv1 cells were transfected with CGRP(350)-Luc constructs together with empty vector or the A-CREB expression vector as indicated. Cells were treated with pH 5.5 medium for 24 h. The data are shown as fold activation normalized to control (mean ± SD). *p < 0.05 versus pH 5.5. (B) F11-Trpv1 cells were infected with adenovirus carrying A-CREB and cultured for 2 d. Cell lysates were used for immunoblotting with anti-FLAG and anti-β-actin antibodies. (C) F11-Trpv1 cells infected with or without adenovirus carrying A-CREB were treated with pH 5.5 medium for 8 h. The expression of CGRP mRNA was determined with real-time PCR. The data are shown as fold activation normalized to control (mean ± SD). *p < 0.05 versus pH 5.5. (D) DRGs isolated from rats were infected with adenovirus carrying A-CREB and cultured for 2 d. DRG lysates were analyzed using immunoblotting with anti-FLAG and anti-β-actin antibodies. (E) DRGs infected with or without adenovirus carrying A-CREB were treated with pH 5.5 medium for 12 h. The expression of CGRP mRNA was determined with real-time PCR. The data are shown as fold activation normalized to control (mean ± SD). *p < 0.05 versus pH 5.5.
Involvement of CaMK in the Activation of CREB by Acidic Conditions
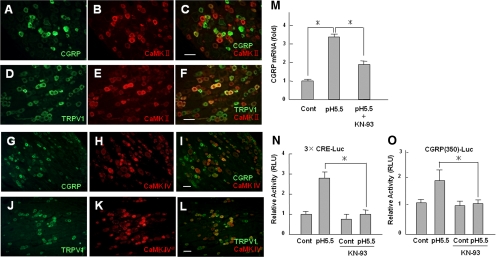
To further explore the intracellular signaling cascade triggered by Trpv1 activation, we investigated the involvement of CaMK. Immunohistochemical analysis revealed that CaMK II and CaMK IV were moderately colocalized with CGRP- and Trpv1-positive neurons in DRGs (Figure 9, A–L). Interestingly, the increased expression of CGRP mRNA induced by protons was significantly reduced by treatment with the CaMK inhibitor KN-93 (Figure 9M). Similarly, as determined by the reporter assay, proton-mediated up-regulation of CREB transcriptional activity was decreased by KN-93 (Figure 9, N and O). These data suggest that the CaMK-CREB signaling pathway participates in the up-regulation of CGRP expression through activation of Trpv1.
Figure 9.
Involvement of CaMK in CREB activity following Trpv1 activation in DRG neurons. Immunohistochemical analyses of CGRP and CaMKII (A–C), Trpv1 and CaMKII (D–F), CGRP and CaMKIV (G–I), and Trpv1 and CaMKIV (J–L) in DRG. DRG sections were double-stained with antibodies as indicated in the figures. Bar, 50 μm (M) DRGs isolated from rats were treated with pH 5.5 medium with or without the CaMK inhibitor KN-93, which was added 1 h before acid stimulation. The expression of CGRP mRNA was determined with real-time PCR. The data are shown as fold activation normalized to control (mean ± SD). *p < 0.05 versus pH 5.5. (N and O) Effects of KN-93 on the transcriptional activity of CREB. F11-Trpv1 cells were transfected with 3×CRE-Luc (N) or CGRP (350)-Luc, and cells were treated with pH 5.5 medium for 24 h with or without KN-93. Cells were preincubated with KN-93 for 1 h before treatment with pH 5.5 medium. The data are expressed as fold activation normalized to the untreated control (mean ± SD). *p < 0.05 versus pH 5.5.
DISCUSSION
Tissue acidosis caused by protons is a characteristic feature of inflammation. Protons have long been thought to contribute to the induction of pain (Reeh and Kress, 2001). However, the underlying molecular basis by which protons induce inflammatory pain is largely unknown. In this study, we show that the activation of Trpv1 by protons up-regulates CGRP expression in DRG neurons. We also show that the activation of the transcription factor CREB is involved in the promotion of CGRP production.
Accumulating evidence has shown that Trpv1 is a critical mediator of inflammatory pain (Caterina et al., 1997; Julius and Basbaum, 2001). Direct activation and sensitization of Trpv1 has been reported to cause inflammatory pain, which is mediated by complex mechanisms (Numazaki et al., 2003; Jin et al., 2004; Zhuang et al., 2004; Mandadi et al., 2006; De Petrocellis et al., 2007). For example, inflammatory mediators sensitize Trpv1, which consequently causes hypersensitivity to thermal and chemical stimuli (Mandadi et al., 2006). Although the hypersensitivity to protons was not well documented in that study, it is possible that severe inflammatory pain is associated with a decrease in the threshold for proton-mediated Trpv1 activation.
In addition to Trpv1, protons directly activate acid-sensing ion channel (ASIC) family members, which are also expressed in nociceptive sensory neurons (Lingueglia, 2007). Indeed, many studies suggest that ASICs play important roles in pain from tissue acidosis associated with inflammation (Voilley, 2004; Wemmie et al., 2006). However, evidence suggests that proton-gated ASICs are not involved in the activation of the CaMK-CREB signaling pathway described in this study. ASICs are proton gated and primarily related to amiloride-sensitive Na+ channels but not Ca2+ channels. Moreover, low pH did not increase the transcriptional activity of CREB in parental F11 cells (Figure 6A), which express normal levels of ASIC1a, -1b, and -3 (Nagae et al., 2006). However, because CGRP- and ASIC3-immunoreactive neurons are colocalized (Ichikawa and Sugimoto, 2002), consistent with the notion that ASICs may be involved in CGRP up-regulation, signaling cascades other than CaMK-CREB may play a role in proton-activated ASICs.
Although we focused on CREB as a transcriptional regulator of CGRP expression in this study, several groups have reported the involvement of other transcription factors in the regulation of CGRP expression. Viney et al. (2004) reported that the forkhead protein Foxa2 and the bHLH-Zip proteins USF-1 and -2 bind to an 18-base pairs enhancer region in the CGRP promoter. In addition, retinoic acid has been reported to suppress CGRP expression through nuclear retinoic acid receptors (Lanigan et al., 1993).
In addition to these factors, several groups reported that transcriptional activity of CREB was modulated by various signaling cascades in the activation of CGRP promoter. Watson and Latchman (1995) reported that nerve growth factor phosphorylated the CREB and increased the CGRP gene promoter activity through CRE. It has also been reported that p42/p44 mitogen-activated protein kinase and protein kinase A (PKA) are involved in the CGRP gene promoter activation (Freeland et al., 2000). Moreover, Bidwell et al. (2010) revealed that prostaglandin E2 activates CREB transcriptional activity via a signaling pathway of PKA. These reports collectively indicate that various stimuli and signaling pathways other than CREB are involved in the regulation of CGRP expression. Thus, it is not unexpected that immunohistochemical analysis revealed that CGRP-positive cells were not 100% overlapped with pCREB-, CaMK-, and Trpv1-positive cells (Figures 1C, 5A, and 9, A–L).
An acidic microenvironment is also generated in malignant tumors (Fukumura and Jain, 2007). Increased production and extrusion of protons are associated with features of tumor malignancy such as invasion, metastasis, and angiogenesis (Kato et al., 2005; Gatenby et al., 2006; Zhou et al., 2006). Cancer-induced pain is an important clinical problem. However, little is known about the biological relationship between cancer pain and acidosis. Of note, Ghilardi et al., (2005) have reported that selective blockade of TRPV1 attenuates bone cancer pain. It also has been reported that endogenous CGRP released by neurons facilitates tumor-associated angiogenesis and tumor growth (Toda et al., 2008). Therefore, our findings can be applied toward better understanding of the mechanisms of cancer pain and the possible future development of therapeutic interventions for the treatment of cancer pain.
There was a concern that exposing cells to pH 5.5 for 24 h would be detrimental, causing cell death. Accordingly, we investigated the effects of pH 5.5 medium on the apoptosis and cell viability. We observed that F11-Trpv1 neuronal cells treated with1 μM staurosporine as positive control showed prominent cell death under a microscopy, whereas the cells were not affected by the treatment with pH 5.5 (data not shown). Consistent with these results, pH 5.5 medium had no effects on caspase-3/7 activity and cell viability compared with control (data not shown). From these results, it is unlikely that pH 5.5 medium is toxic, causing cell death.
In summary, we show that proton-activated Trpv1 up-regulates CGRP expression through the activation of CREB, leading to the induction of pain. These findings provide insight into the molecular mechanisms underlying proton-mediated inflammatory pain.
ACKNOWLEDGMENTS
We are grateful to Dr. Makoto Tominaga for providing Trpv1-deficient mice. We thank Dr. Riko Nishimura (Osaka University Graduate School of Dentistry, Osaka, Japan) and Dr. Ritsuko Masuyama (Nagasaki University Graduate School of Biomedical Science, Nagasaki, Japan) for discussion. This work was supported by Grant-in-Aid for Scientific Research on Priority Areas (to T. Y.) and Grant-in-Aid for Young Scientists B (to M. N.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and the 21st Century COE Program (to T. Y.). This study is partially supported by the Research Grant of the Princess Takamatsu Cancer Research Fund grant 08-24020.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-01-0049) on June 9, 2010.
REFERENCES
- Benemei S., Nicoletti P., Capone J. G., Geppetti P. CGRP receptors in the control of pain and inflammation. Curr. Opin. Pharmacol. 2009;9:9–14. doi: 10.1016/j.coph.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Bevan S., Geppetti P. Protons: small stimulants of capsaicin-sensitive sensory nerves. Trends Neurosci. 1994;17:509–512. doi: 10.1016/0166-2236(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Bidwell P., Joh K., Leaver H. A., Rizzo M. T. Prostaglandin E2 activates cAMP response element-binding protein in glioma cells via a signaling pathway involving PKA-dependent inhibition of ERK. Prostaglandins Other Lipid Mediat. 2010;91:18–29. doi: 10.1016/j.prostaglandins.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Caterina M. J., Leffler A., Malmberg A. B., Martin W. J., Trafton J., Petersen-Zeitz K. R., Koltzenburg M., Basbaum A. I., Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Caterina M. J., Schumacher M. A., Tominaga M., Rosen T. A., Levine J. D., Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L., Starowicz K., Moriello A. S., Vivese M., Orlando P., Di Marzo V. Regulation of transient receptor potential channels of melastatin type 8 (TRPM8): effect of cAMP, cannabinoid CB(1) receptors and endovanilloids. Exp. Cell Res. 2007;313:1911–1920. doi: 10.1016/j.yexcr.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Fanciullacci M., Alessandri M., Figini M., Geppetti P., Michelacci S. Increase in plasma calcitonin gene-related peptide from the extracerebral circulation during nitroglycerin-induced cluster headache attack. Pain. 1995;60:119–123. doi: 10.1016/0304-3959(94)00097-X. [DOI] [PubMed] [Google Scholar]
- Freeland K., Liu Y. Z., Latchman D. S. Distinct signalling pathways mediate the cAMP response element (CRE)-dependent activation of the calcitonin gene-related peptide gene promoter by cAMP and nerve growth factor. Biochem. J. 2000;345:233–238. [PMC free article] [PubMed] [Google Scholar]
- Fukumura D., Jain R. K. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc. Res. 2007;74:72–84. doi: 10.1016/j.mvr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatenby R. A., Gawlinski E. T., Gmitro A. F., Kaylor B., Gillies R. J. Acid-mediated tumor invasion: a multidisciplinary study. Cancer Res. 2006;66:5216–5223. doi: 10.1158/0008-5472.CAN-05-4193. [DOI] [PubMed] [Google Scholar]
- Ghilardi J. R., et al. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J. Neurosci. 2005;25:3126–3131. doi: 10.1523/JNEUROSCI.3815-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goadsby P. J., Edvinsson L., Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann. Neurol. 1990;28:183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- Griffith L. C. Regulation of calcium/calmodulin-dependent protein kinase II activation by intramolecular and intermolecular interactions. J. Neurosci. 2004;24:8394–8398. doi: 10.1523/JNEUROSCI.3604-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K., Dubner R., Brown F., Flores C., Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hata K., Nishimura R., Muramatsu S., Matsuda A., Matsubara T., Amano K., Ikeda F., Harley V. R., Yoneda T. Paraspeckle protein p54nrb links Sox9-mediated transcription with RNA processing during chondrogenesis in mice. J. Clin. Invest. 2008;118:3098–3108. doi: 10.1172/JCI31373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata K., Nishimura R., Ueda M., Ikeda F., Matsubara T., Ichida F., Hisada K., Nokubi T., Yamaguchi A., Yoneda T. A CCAAT/enhancer binding protein beta isoform, liver-enriched inhibitory protein, regulates commitment of osteoblasts and adipocytes. Mol. Cell. Biol. 2005;25:1971–1979. doi: 10.1128/MCB.25.5.1971-1979.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa H., Sugimoto T. The co-expression of ASIC3 with calcitonin gene-related peptide and parvalbumin in the rat trigeminal ganglion. Brain Res. 2002;943:287–291. doi: 10.1016/s0006-8993(02)02831-7. [DOI] [PubMed] [Google Scholar]
- Immke D. C., Gavva N. R. The TRPV1 receptor and nociception. Semin. Cell Dev. Biol. 2006;17:582–591. doi: 10.1016/j.semcdb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Jin X., Morsy N., Winston J., Pasricha P. J., Garrett K., Akbarali H. I. Modulation of TRPV1 by nonreceptor tyrosine kinase, c-Src kinase. Am. J. Physiol. Cell Physiol. 2004;287:C558–C563. doi: 10.1152/ajpcell.00113.2004. [DOI] [PubMed] [Google Scholar]
- Julius D., Basbaum A. I. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Kang H., Sun L. D., Atkins C. M., Soderling T. R., Wilson M. A., Tonegawa S. An important role of neural activity-dependent CaMKIV signaling in the consolidation of long-term memory. Cell. 2001;106:771–783. doi: 10.1016/s0092-8674(01)00497-4. [DOI] [PubMed] [Google Scholar]
- Kato Y., Lambert C. A., Colige A. C., Mineur P., Noel A., Frankenne F., Foidart J. M., Baba M., Hata R., Miyazaki K., Tsukuda M. Acidic extracellular pH induces matrix metalloproteinase-9 expression in mouse metastatic melanoma cells through the phospholipase D-mitogen-activated protein kinase signaling. J. Biol. Chem. 2005;280:10938–10944. doi: 10.1074/jbc.M411313200. [DOI] [PubMed] [Google Scholar]
- Kilo S., Harding-Rose C., Hargreaves K. M., Flores C. M. Peripheral CGRP release as a marker for neurogenic inflammation: a model system for the study of neuropeptide secretion in rat paw skin. Pain. 1997;73:201–207. doi: 10.1016/S0304-3959(97)00108-5. [DOI] [PubMed] [Google Scholar]
- Lanigan T. M., Tverberg L. A., Russo A. F. Retinoic acid repression of cell-specific helix-loop-helix-octamer activation of the calcitonin/calcitonin gene-related peptide enhancer. Mol. Cell. Biol. 1993;13:6079–6088. doi: 10.1128/mcb.13.10.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingueglia E. Acid-sensing ion channels in sensory perception. J. Biol. Chem. 2007;282:17325–17329. doi: 10.1074/jbc.R700011200. [DOI] [PubMed] [Google Scholar]
- Lonze B. E., Ginty D. D. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Mandadi S., Tominaga T., Numazaki M., Murayama N., Saito N., Armati P. J., Roufogalis B. D., Tominaga M. Increased sensitivity of desensitized TRPV1 by PMA occurs through PKCepsilon-mediated phosphorylation at S800. Pain. 2006;123:106–116. doi: 10.1016/j.pain.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Mayr B., Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- Means A. R. Regulatory cascades involving calmodulin-dependent protein kinases. Mol. Endocrinol. 2000;14:4–13. doi: 10.1210/mend.14.1.0414. [DOI] [PubMed] [Google Scholar]
- Mellstrom B., Savignac M., Gomez-Villafuertes R., Naranjo J. R. Ca2+-operated transcriptional networks: molecular mechanisms and in vivo models. Physiol. Rev. 2008;88:421–449. doi: 10.1152/physrev.00041.2005. [DOI] [PubMed] [Google Scholar]
- Meng J., Ovsepian S. V., Wang J., Pickering M., Sasse A., Aoki K. R., Lawrence G. W., Dolly J. O. Activation of TRPV1 mediates calcitonin gene-related peptide release, which excites trigeminal sensory neurons and is attenuated by a retargeted botulinum toxin with anti-nociceptive potential. J. Neurosci. 2009;29:4981–4992. doi: 10.1523/JNEUROSCI.5490-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J., Wang J., Lawrence G., Dolly J. O. Synaptobrevin I mediates exocytosis of CGRP from sensory neurons and inhibition by botulinum toxins reflects their anti-nociceptive potential. J. Cell Sci. 2007;120:2864–2874. doi: 10.1242/jcs.012211. [DOI] [PubMed] [Google Scholar]
- Nagae M., Hiraga T., Wakabayashi H., Wang L., Iwata K., Yoneda T. Osteoclasts play a part in pain due to the inflammation adjacent to bone. Bone. 2006;39:1107–1115. doi: 10.1016/j.bone.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Numazaki M., Tominaga T., Takeuchi K., Murayama N., Toyooka H., Tominaga M. Structural determinant of TRPV1 desensitization interacts with calmodulin. Proc. Natl. Acad. Sci. USA. 2003;100:8002–8006. doi: 10.1073/pnas.1337252100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preibisz J. J. Calcitonin gene-related peptide and regulation of human cardiovascular homeostasis. Am. J. Hypertens. 1993;6:434–450. doi: 10.1093/ajh/6.5.434. [DOI] [PubMed] [Google Scholar]
- Premkumar L. S., Agarwal S., Steffen D. Single-channel properties of native and cloned rat vanilloid receptors. J. Physiol. 2002;545:107–117. doi: 10.1113/jphysiol.2002.016352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeh P. W., Kress M. Molecular physiology of proton transduction in nociceptors. Curr. Opin. Pharmacol. 2001;1:45–51. doi: 10.1016/s1471-4892(01)00014-5. [DOI] [PubMed] [Google Scholar]
- Ribar T. J., Rodriguiz R. M., Khiroug L., Wetsel W. C., Augustine G. J., Means A. R. Cerebellar defects in Ca2+/calmodulin kinase IV-deficient mice. J. Neurosci. 2000;20:RC107. doi: 10.1523/JNEUROSCI.20-22-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon A. M., Damaj M. I., Marubio L. M., Epping-Jordan M. P., Merlo-Pich E., Changeux J. P. Altered neuroadaptation in opiate dependence and neurogenic inflammatory nociception in alpha CGRP-deficient mice. Nat. Neurosci. 2001;4:357–358. doi: 10.1038/86001. [DOI] [PubMed] [Google Scholar]
- Sango K., Horie H., Sotelo J. R., Takenaka T. A high glucose environment improves survival of diabetic neurons in culture. Neurosci. Lett. 1991;129:277–280. doi: 10.1016/0304-3940(91)90480-h. [DOI] [PubMed] [Google Scholar]
- Strecker T., Messlinger K., Weyand M., Reeh P. W. Role of different proton-sensitive channels in releasing calcitonin gene-related peptide from isolated hearts of mutant mice. Cardiovasc. Res. 2005;65:405–410. doi: 10.1016/j.cardiores.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Szallasi A., Blumberg P. M. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- Szallasi A., Cortright D. N., Blum C. A., Eid S. R. The vanilloid receptor TRPV 1, 10 years from channel cloning to antagonist proof-of-concept. Nat. Rev. Drug Discov. 2007;6:357–372. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- Tamura S., Morikawa Y., Senba E. Up-regulated phosphorylation of signal transducer and activator of transcription 3 and cyclic AMP-responsive element binding protein by peripheral inflammation in primary afferent neurons possibly through oncostatin M receptor. Neuroscience. 2005;133:797–806. doi: 10.1016/j.neuroscience.2005.02.046. [DOI] [PubMed] [Google Scholar]
- Toda M., et al. Neuronal system-dependent facilitation of tumor angiogenesis and tumor growth by calcitonin gene-related peptide. Proc. Natl. Acad. Sci. USA. 2008;105:13550–13555. doi: 10.1073/pnas.0800767105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M., Caterina M. J., Malmberg A. B., Rosen T. A., Gilbert H., Skinner K., Raumann B. E., Basbaum A. I., Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Viney T. J., Schmidt T. W., Gierasch W., Sattar A. W., Yaggie R. E., Kuburas A., Quinn J. P., Coulson J. M., Russo A. F. Regulation of the cell-specific calcitonin/calcitonin gene-related peptide enhancer by USF and the Foxa2 forkhead protein. J. Biol. Chem. 2004;279:49948–49955. doi: 10.1074/jbc.M406659200. [DOI] [PubMed] [Google Scholar]
- Voilley N. Acid-sensing ion channels (ASICs): new targets for the analgesic effects of non-steroid anti-inflammatory drugs (NSAIDs) Curr. Drug Targets Inflamm. Allergy. 2004;3:71–79. doi: 10.2174/1568010043483980. [DOI] [PubMed] [Google Scholar]
- Watson A., Latchman D. The cyclic AMP response element in the calcitonin/calcitonin gene-related peptide gene promoter is necessary but not sufficient for its activation by nerve growth factor. J. Biol. Chem. 1995;270:9655–9660. doi: 10.1074/jbc.270.16.9655. [DOI] [PubMed] [Google Scholar]
- Wemmie J. A., Price M. P., Welsh M. J. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29:578–586. doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Zhou J., Schmid T., Schnitzer S., Brune B. Tumor hypoxia and cancer progression. Cancer Lett. 2006;237:10–21. doi: 10.1016/j.canlet.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Zhuang Z. Y., Xu H., Clapham D. E., Ji R. R. Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J. Neurosci. 2004;24:8300–8309. doi: 10.1523/JNEUROSCI.2893-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]