The anaphase-promoting complex/cyclosome (APC/C) inhibitor Emi2 contains a destruction box (D-box) found in APC/C substrates, but does not appear to inhibit the APC/C by a “pseudosubstrate” mechanism. Rather, it inhibits transfer of ubiquitin from the E2 to substrates. The D-box promotes Emi2-APC/C association, but the zinc-binding region plays the critical role in APC/C inhibition.
Abstract
Vertebrate eggs are arrested at Metaphase II by Emi2, the meiotic anaphase-promoting complex/cyclosome (APC/C) inhibitor. Although the importance of Emi2 during oocyte maturation has been widely recognized and its regulation extensively studied, its mechanism of action remained elusive. Many APC/C inhibitors have been reported to act as pseudosubstrates, inhibiting the APC/C by preventing substrate binding. Here we show that a previously identified zinc-binding region is critical for the function of Emi2, whereas the D-box is largely dispensable. We further demonstrate that instead of acting through a “pseudosubstrate” mechanism as previously hypothesized, Emi2 can inhibit Cdc20-dependent activation of the APC/C substoichiometrically, blocking ubiquitin transfer from the ubiquitin-charged E2 to the substrate. These findings provide a novel mechanism of APC/C inhibition wherein the final step of ubiquitin transfer is targeted and raise the interesting possibility that APC/C is inhibited by Emi2 in a catalytic manner.
INTRODUCTION
Anaphase is initiated by the activation of a large multi-subunit protein complex known as the anaphase-promoting complex/cyclosome (APC/C). The APC/C is a 12-subunit E3 ubiquitin ligase that mediates polyubiquitylation of numerous proteins, targeting them for proteasomal degradation. Many APC/C substrates are critical cell cycle regulators, including Cyclin B and Securin, whose degradation is essential for the metaphase–anaphase transition. Although the functions of all 12 subunits are not fully understood, it is known that the APC/C recruits an E2 (UbcH5 or UbcH10, charged by an E1) to the core subunit APC11, recruits substrates destined for polyubiquitylation (facilitated by an activator), and transfers ubiquitin from the charged E2 to the substrate (Castro et al., 2005; Peters, 2006; Sullivan and Morgan, 2007). During the transfer step, the APC/C promotes the release of ubiquitin from the charged E2 before linking ubiquitin to the substrate (Ozkan et al., 2005).
Two E2 enzymes are known to support APC/C activity, UbcH5 and UbcH10 (UbcX in Xenopus; Peters, 2006). The N-terminus of UbcH10 has also been implicated in the regulation of APC/C ubiquitylation activity (Summers et al., 2008). Although APC/C core subunits confer a basal level of substrate interaction, the APC/C activators Cdc20 and Cdh1 can significantly enhance substrate binding to the APC/C by recognizing specific sequence motifs within substrates, such as the Destruction box (D-box [DB]) and KEN box (Peters, 2006; Yu, 2007; Matyskiela and Morgan, 2009). A recent study of APC/CCdc20 identified an additional important activator function; although the C-terminal WD40 domain of Cdc20 was required for substrate binding, the C-box within the N-terminus was required for activation of intrinsic APC/C ligase activity, a previously unrecognized step in APC/C-mediated substrate ubiquitylation (Kimata et al., 2008a).
APC/C activity is tightly regulated at several levels. Association of the APC/C with its activators is regulated throughout the cell cycle by phosphorylation (both of the APC/C and the activators), and levels of the E2, UbcH10, also fluctuate during the cell cycle (Castro et al., 2005; Peters, 2006). In addition, APC/C activity is restrained by a variety of inhibitors. At metaphase, the spindle assembly checkpoint (SAC) holds the APC/C in check until all kinetochores are properly attached to the microtubules. Several protein factors implicated in SAC function, such as Mad2 and BubR1 (Mad3 in yeast), can independently inhibit the APC/C in vitro (Musacchio and Salmon, 2007). During S and G2 phases, the inhibitor Emi1 prevents premature activation of the APC/C, allowing the accumulation of Cyclin A as well as Geminin, essential for preventing rereplication of DNA (Di Fiore and Pines, 2007; Machida and Dutta, 2007). Finally, Emi2, the meiotic APC/C inhibitor, maintains a prolonged M phase arrest in eggs before fertilization (known as cytostatic factor, or CSF, arrest; Wu and Kornbluth, 2008). The actions of these APC/C inhibitors are also governed by a variety of regulatory mechanisms, to ensure appropriately timed APC/C activation.
Recently, a number of APC/C inhibitors have been shown to function as APC/C pseudosubstrates. Emi1 was the first APC/C inhibitor shown to compete with substrates for APC/C binding in a D-box–dependent manner, though this mechanism could not fully explain the essential role played by the zinc-binding region (ZBR) found at the C-terminus of the protein (Miller et al., 2006). Later, Mad3 (Burton and Solomon, 2007), Acm1 (Choi et al., 2008), Securin (Marangos and Carroll, 2008), Mes1 (Kimata et al., 2008b), and the N-terminus of BubR1 (Malureanu et al., 2009) were all found capable of competing with substrates for APC/C binding. These findings, taken together with the observation that Emi2 also contains an apparent D-box, have led to the strong suggestion that Emi2 might also act as a pseudosubstrate inhibitor. However, we show here that the D-box of Emi2 is dispensable for its APC/C inhibitory activity. Examination of each step in substrate ubiquitylation by the APC/C has revealed that Emi2 exerts its APC/C inhibitory effect by precluding the transfer of ubiquitin from the charged E2 to its substrate and that it is this step in ubiquitylation, rather than substrate binding, that is inhibited by Emi2.
MATERIALS AND METHODS
Plasmids and Proteins Preparation
Recombinant glutathione S-transferase (GST) or maltose binding protein (MBP) fusion Emi2 fragments (aa 489-651) were prepared as previously described (Wu et al., 2007a,b). Emi2 mutants (C583A, R529A, and L532A) were prepared using the QuickChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). hCdc20 was cloned into BamHI and XhoI sites in pFastbac vector and transfected into Sf9 cells for recombinant protein production. hCdc20 (aa 1-151) was cloned into the BamHI and NotI sites of pEnter3C vector and recombined into pDest15 vector (Invitrogen, Carlsbad, CA) for the bacterial production of recombinant protein. Emi2 fragment (aa 489-651) and hCyclin B1 (aa 1-106) was cloned separately into the NotI sites of pEnter3C vector containing hCdc20 (aa 1-151) to generate C-Emi2 and Cdc20-Cyclin B chimeric proteins. The DB of hCyclin B1 (aa 1-106) on the chimera protein was mutated (R42A, L45A) with the same kit described above. The constructs were recombined into pDest15 vector for recombinant protein production and also into the pCDNA3 vector for production of in vitro–translated (IVT) proteins, which were generated using the TNT Quick Coupled Transcription/Translation System (Promega, Madison, WI) in the presence of 35S-labeled methionine and cysteine (MP Biomedicals, Solon, OH).
The plasmids used to make recombinant GST-Cdc27 and IVT Nek2A were gifts from Dr. Hiro Yamano (UCL Cancer Institute, London, United Kingdom). The plasmids to make wild-type (WT) and DB mutant GST-Cyclin B N-terminus (aa 1-70, 2×) were gifts from Dr. Tim Hunt (London Research Institute, London, United Kingdom). The plasmids used to make IVT APC/C subunits were gifts from Dr. Peter Jackson (Genentech, CA).
Extracts and Cyclin B Degradation Assay
CSF extracts and interphase extracts (also referred as crude S extract) were prepared as previously described (Murray, 1991; Smythe and Newport, 1991). M phase extract were prepared by incubating the interphase extract with purified His-hCyclin B1 lacking the first 13 amino acids. This protein was made as described previously in baculovirus-infected Sf9 cells (Wu et al., 2007b).
For the Cyclin B degradation assay, extracts were incubated with various Emi2 mutants at room temperature for 10 min before Ca2+ addition; 0.8 mM Ca2+ was added to extracts to induce release from CSF arrest.
Antibodies, Immunoprecipitation, and Immunodepletion
Antibodies used in this study were as follows: anti-cyclin B1 as previously described (Hochegger et al., 2001); anti-Cdc27 (Santa Cruz Biotechnology, Santa Cruz, CA) for immunoprecipitation; anti-Cdc27 (Transduction Laboratories, Lexington, KY) for immunoblotting; anti-flag (Sigma, St. Louis, MO); anti-MBP (Cell Signaling, Beverly, MA); anti-GST (Santa Cruz); and anti UbcH10 (Boston Biochem, Boston, MA).
For Cdc27 immunoprecipitation, 4 μg of antibodies was coupled to protein A Sepharose beads and incubated in 100 μl of Xenopus egg extracts for 2 h at 4°C.
The antibody used for Cdc20 immunodepletion from Xenopus egg extracts was a gift from Dr. Hiroyuki Yamano, and the depletion procedure was performed as described previously (Hayes et al., 2006).
APC/C Assay
Unless otherwise specified, immunoprecipitated APC/C was incubated with 4 μl of IVT human Cdc20 or Cdh1 at 22°C for 30 min; then 50 ng of hE1, 0.5 μg UbcH10, 10 μg of ubiquitin, and an energy regenerating system were added together with 2 μl of IVT 35S-labeled human Cyclin B1 fragment (aa 1-106) as a substrate. Reactions were incubated at 22°C for 45 min with constant shaking, quenched with SDS sample buffer, analyzed by SDS-PAGE and autoradiography, and quantified by phosphorimager.
RESULTS
Characterization of DB and ZBR Regions within the Emi2 C-terminus
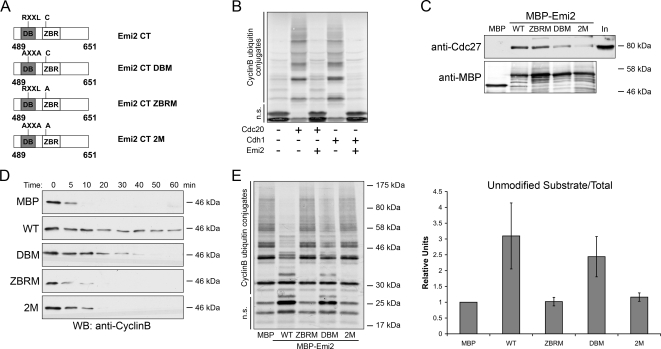
The C-terminus (aa 489-651) of Emi2 was previously shown to be essential for its APC/C inhibitory activity (Wu et al., 2007b; see diagram, Figure 1A). As confirmed in Figure 1B, the recombinant Emi2 C-terminus could inhibit either Cdc20 or Cdh1-dependent APC/C activity in vitro (Figure 1B). Analysis of the Emi2 C-terminal fragment revealed two potential functional domains: a putative DB, found in a number of APC/C substrates (aa 529-536), and a previously reported ZBR (aa 583-624). To evaluate the relative importance of these two domains for Emi2 function, we mutated them and assayed the mutants for both APC/C binding and APC/C inhibition. As shown in Figure 1C, mutations in either the DB (DBM; R529A, L532A) or ZBR (ZBRM; C583A) compromised APC/C binding, with the DB mutant having a more profound effect. Combination of both mutants (2M: the double mutant) further decreased binding, indicating that the DB and ZBR domains cooperated to achieve optimal APC/C association. CSF extracts supplemented with either recombinant Emi2 ZBRM or the double mutant exhibited very similar kinetics of Cyclin B degradation to the buffer control, consistent with a complete loss of APC/C inhibitory activity for these mutants (Figure 1D). In contrast, the DB mutant was considerably less impaired in its ability to inhibit Cyclin B degradation (Figure 1D). Similar results were obtained in an in vitro APC/C assay; using Emi2 variants at similar concentrations, the ZBRM was more impaired than the DBM in its ability to prevent Cyclin B ubiquitylation (Figure 1E). These data strongly suggest that although the DB may contribute to APC/C inhibition, there is a particularly critical role for the intact ZBR in APC/C inhibition by Emi2.
Figure 1.
Characterization of DB and ZBR regions within the Emi2 C-terminus. (A) Emi2 C terminal (CT) mutants. DBM, D-box mutant; ZBRM, zinc-binding region mutant; 2M, double mutant. All relevant experiments in this article were performed with Emi2 CT. (B) In vitro APC/C assay was performed in the presence or absence of 600 nM GST-Emi2 with either Cdc20 or Cdh1 as the activator. Conversion of radiolabeled Cyclin B to ubiquitylated forms was monitored by autoradiography. n.s., nonmodified substrates. (C) recombinant MBP-Emi2, 50 nM (WT or mutants), was conjugated to amylose beads and incubated in Crude S extract for 20 min at 22°C. Beads were washed five times with PBS (supplemented with 300 mM NaCl and 0.1% Triton). The amount of associated Cdc27 was detected by Western blotting. In, Input. (D) recombinant MBP or MPB-Emi2, 50 nM (WT or mutants), was added into CSF extracts supplemented with Ca2+. Aliquots removed at the indicated times were analyzed by SDS-PAGE and immunoblotted for Cyclin B. (E) recombinant MBP or MPB-Emi2, 800 nM (WT or mutants), was added into in vitro APC/C assay with IVT Cdc20 as the activator. The immunoprecipitated APC/C was preincubated with Emi2 and Cdc20 together with E1, E2, and Ubiquitin for 1 h before substrates were added. Conversion of radiolabeled Cyclin B to ubiquitylated forms was monitored by autoradiography and phosphorimager. Four independent experiments were further quantified with ImageQuant5.0 (Molecular Dynamics, Sunnyvale, CA). n.s., nonmodified substrates.
DB Promotes Binding of Emi2 to APC/C, Whereas ZBR Is Critical for Its APC/C Inhibitory Activity
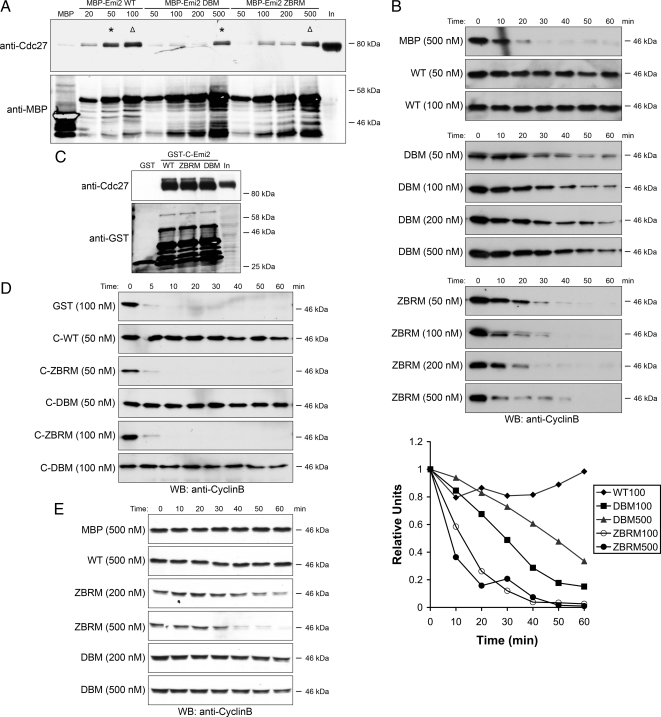
Although binding of both the ZBR and DB Emi2 mutants was impaired, the amount of APC/C associated with Emi2 in the CSF extract could be restored to endogenous levels by simply elevating the levels of mutant protein (Figure 2A). Interestingly, even at concentrations that restored APC/C binding, the ZBR mutant failed to delay Cyclin B degradation (Figure 2B, bottom panel). In contrast, the inhibitory activity of DB mutant was largely restored when binding was restored, increasing in a dose-dependent manner as more protein was added to the extract (Figure 2B, middle panel). These data indicate that although both domains contributed to APC/C binding, the APC/C inhibitory activity resided mainly in the ZBR of Emi2, whereas the function of the D-box was simply to promote the binding required for inhibition by another function/domain of Emi2. To further test this hypothesis, we constructed a chimeric protein that has the N-terminus of Cdc20 (aa 1-151) and C-terminus of Emi2 (aa 489-651) linked together (referred to as C-Emi2). Because the C-Box in the N-terminus of Cdc20 interacts strongly with APC/C, the presence of the D-box on C-Emi2 is not required for APC/C binding. Indeed, as shown in Figure 2C, both the DBM and ZBRM GST-C-Emi2 bound to APC/C as strongly as WT. If the sole function of the D-box is to promote APC/C binding, we expected that the DBM C-Emi2 would be as potent as the WT in APC/C inhibition. As shown in Figure 2D, 50 nM DBM GST-C-Emi2 completely blocked Cyclin B degradation, demonstrating its robust ability to inhibit the APC/C. We also included the ZBRM GST-C-Emi2 as a negative control to demonstrate that, even in the context of the fusion protein, the ZBR is required for the APC inhibitory activity of Emi2. Finally, to demonstrate that the ZBR is the critical domain for APC/C inhibition, we directly added recombinant mutant proteins into CSF extract and observed that the ZBR mutant, which contained an intact D-box, worked in a dominant negative manner, causing Cyclin B degradation without addition of Ca2+. At the same time, the DB mutant Emi2 did not have such an effect (Figure 2E). Taken together, these findings argue strongly against a D-box–dependent pseudosubstrate mechanism of APC/C inhibition by Emi2 and point to a model in which the ZBR plays a critical role.
Figure 2.
DB promotes binding of Emi2 to APC/C, whereas ZBR, is critical for its APC/C inhibitory activity. (A) Recombinant MBP or MBP-Emi2 (WT or mutant) at indicated concentrations was incubated in Crude S extract for 20 min at 22°C at the concentration as indicated and then pulled out with amylose beads. Samples were analyzed as in Figure 1C. Asterisks indicate that the amount of APC/C associated with 500 nM DBM was equal to that with 50 nM WT; triangles indicate that the amount of APC/C associated with 500 nM ZBRM was equal to that with 100 nM WT. In, Input. (B) Recombinant MBP or MBP-Emi2 (WT or mutant) at indicated concentrations were added into CSF extracts supplemented with Ca2+. Samples were analyzed as in Figure 1D; five samples as indicated were quantified with ImageQuant5.0. (C) recombinant GST-C-Emi2, 100 nM (WT or mutants), was conjugated to Glutathione beads and incubated in CSF extract supplemented with 50 nM MBP-Emi2 for 30 min at 22°C. Beads were washed five times with PBS (supplemented with 300 mM NaCl and 0.1% Triton). The amount of associated Cdc27 was detected by Western blotting. In, Input. (D) Recombinant GST or GST-C-Emi2, 50 nM or 100 nM (WT or mutants), was added into CSF extracts supplemented with Ca2+. Aliquots removed at the indicated times were analyzed by SDS-PAGE and immunoblotted for Cyclin B. (E) Recombinant MBP or MPB-Emi2 proteins (WT or mutants) at indicated concentrations were added into CSF extracts. Aliquots removed at the indicated times were analyzed by SDS-PAGE and immunoblotted for Cyclin B.
Emi2 Inhibits Cdc20-dependent Activation of APC/C Activity But Not Substrate Binding
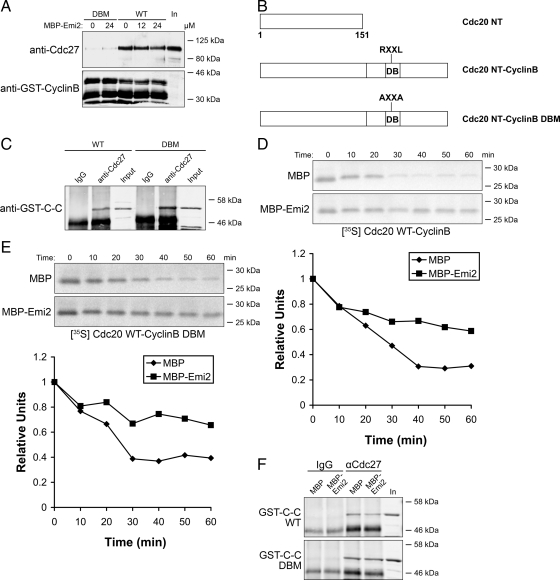
Consistent with Emi2 not being a pseudosubstrate inhibitor, addition of a large excess of WT recombinant Emi2 (24 μM; note that the endogenous concentration of Emi2 was estimated to be 15 nM) to M phase extract did not reduce the Cyclin B-APC/C binding (Figure 3A), reinforcing the conclusion that substrate competition could not account for APC/C inhibition by Emi2. A recent study showed that Cdc20 could not only promote substrate binding to the APC/C but could also activate the APC/C's E3 ubiquitin ligase activity (Kimata et al., 2008a). Although Emi2 did not prevent the substrate-binding step, we considered it possible that it might inhibit the Cdc20-dependent APC/C activation step. To address this, we produced a chimeric protein consisting of the hCdc20 N-terminus (aa 1-151) directly linked to Cyclin B (aa 1-106; Figure 3B). Consistent with a previous report (Kimata et al., 2008a), when physically linked together, this chimeric protein (also referred as C-C) could bind to the APC/C in a D-box-independent manner (Figure 3C). An assay to monitor degradation of the chimeric protein was then performed in a Cdc20-depleted CSF extract. Although endogenous Cyclin B remained stable after addition of Ca2+ (Supplementary Figure S1), the chimeric protein was quickly degraded. More importantly, the degradation could be blocked by addition of exogenous Emi2 into the extract, suggesting that Emi2 was indeed capable of inhibiting the Cdc20-dependent activation of APC/C ligase activity (Figure 3D). The experiment was repeated with the D-box mutant chimeric protein, which, as predicted, could also be degraded upon Ca2+ addition to the CSF extract, and the degradation of the DB mutant chimeric protein was also prevented by exogenous Emi2 (Figure 3E). To exclude the possibility that Emi2 is promoting the dissociation of the chimeric protein from APC/C, we performed a binding experiment and found that the amount of GST-C-C (both WT and DB mutant) associated with the APC/C was not affected by the presence of Emi2 (Figure 3F). These data confirmed the conclusion that Emi2 could not work by blocking substrate recruitment and suggested that either some aspect of Cdc20-mediated APC/C activation or a fundamental feature of APC/C E3 ligase activity was inhibited by Emi2.
Figure 3.
Emi2 inhibits Cdc20 dependent activation of APC/C activity but not substrate binding. (A) WT or DBM GST-Cyclin B (aa 1-70, 2×; 2.5 μM) conjugated to glutathione beads was incubated in M phase extract in the absence or presence of recombinant MBP-Emi2 at indicated concentrations for 20 min at 22°C. GST-Cyclin B was retrieved from the extract by centrifugation, washed, and immunoblotted for Cdc27 or GST. In, Input. (B) Diagram of the chimeric protein. hCdc20 aa 1-151 was linked with hCyclin B1 1-106 (Cdc20 NT-Cyclin B). Cdc20 NT-Cyclin B DBM: Cdc20 NT-Cyclin B with R42A, L45A mutation on Cyclin B. (C) APC/C immunoprecipitated from M phase extracts was incubated in XB buffer with 250 nM recombinant GST-Cdc20 NT-Cyclin B (GST-C-C; WT or DBM) for 20 min at 22°C. APC/C beads were retrieved and washed. The amount of associated GST-C-C was detected by Western blotting. In, Input. (D) IVT 35S labeled Cdc20 NT-Cyclin B was added to Cdc20-depleted CSF extract supplemented with 60 nM recombinant MBP or MBP-Emi2. After Ca2+ addition, aliquots removed at the indicated times were subjected to autoradiography for IVT Cdc20 NT-Cyclin B as well as to Western blotting for endogenous Cyclin B (see Supplementary Figure S1). Results were quantified with ImageQuant5.0. (E) Same as in Figure 2C except that Cdc20 NT-Cyclin B DBM was analyzed. (F) APC/C immunoprecipitated from M phase extracts was incubated in XB buffer with 250 nM recombinant GST-Cdc20 NT-Cyclin B (GST-C-C) in the presence of 500 nM MBP or MBP-Emi2 for 20 min at 22°C. APC/C beads were retrieved and washed. The amount of associated GST-C-C was detected by Western blotting. In, Input.
Emi2 Inhibits APC/C by Blocking the Transfer of Ubiquitin from Activated E2 to Substrates
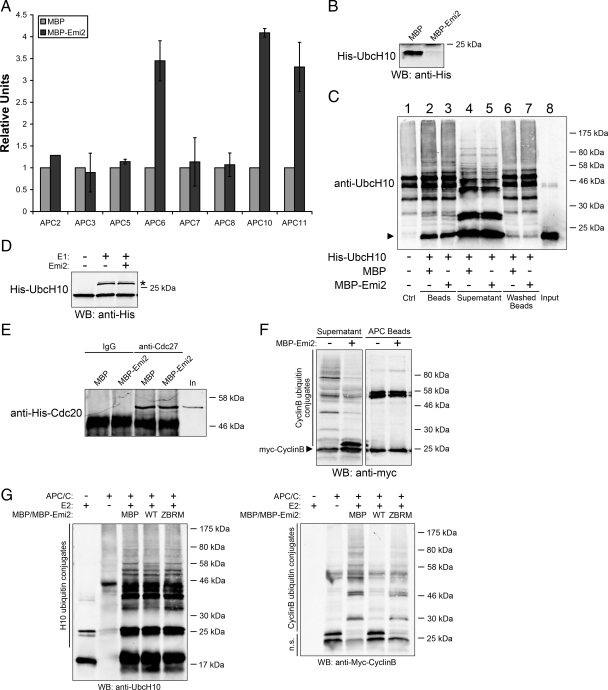
In an attempt to identify the binding site(s) of Emi2 on APC/C, we tested binding between recombinant Emi2 and IVT-radiolabeled individual APC/C subunits and found that Emi2 could interact directly with APC6, APC10, and APC11 in the absence of other APC/C components. (Quantitation of binding of the radiolabeled APC subunits to MBP/MBP-Emi2 is shown in Figure 4A.) As the APC/C recruits E2s through subunit APC11, the observed binding to this APC subunit prompted us to hypothesize that Emi2 might inhibit the APC/C by preventing E2 binding. In this regard, we were interested to find that UbcH10 and Emi2 competed for APC/C binding in vitro as preincubation of APC/C with Emi2 prevented UbcH10 binding (Figure 4B); however, this effect was not observed under ubiquitylation conditions (in the presence of E1, E2, ubiquitin, and an energy-regenerating system). UbcH10, in either its unmodified or ubiquitylated form, associated with APC/C equally well in the presence or absence of Emi2 (Figure 4C). Furthermore, we tested and confirmed that Emi2 did not affect the charging of E2 (Figure 4D) or the binding of Cdc20 to APC/C (Figure 4E). Indeed, Emi2 could inhibit polyubiquitin chain formation on Cyclin B in an in vitro APC/C assay lacking E1 and ubiquitin if E2 precharged with ubiquitin was added to the reaction (Figure 4F), strongly suggesting that it is ubiquitin transfer from charged E2 to the substrate that is targeted by Emi2. (Note again that the amount of Cyclin B bound to the APC/C was not affected by Emi2.)
Figure 4.
Emi2 inhibits APC/C by blocking the transfer of ubiquitin from activated E2 to substrates. (A) MBP or MBP-Emi2 conjugated to amylose beads were incubated with 35S-labeled IVT APC subunits for 1 h at 22°C. Beads were washed and bound IVT APC subunits were examined by SDS-PAGE and autoradiography. Results from at least three independent experiments for each subunit were quantified with ImageQuant5.0. (B) APC/C immunoprecipitated from mitotic extracts was preincubated with MBP or MBP-Emi2 (600 nM) for 15 min at 22°C. Purified His-UbcH10 was added to both samples and incubated for 1 h at 22°C. APC/C beads were washed and the bound UbcH10 was analyzed by Western blotting. (C) APC/C was immunoprecipitated from mitotic extracts and incubated with purified His-UbcH10 in the presence of MBP or MBP-Emi2 (600 nM) for 1 h at 22°C. E1, ubiquitin, and an energy regeneration system were also added to the reactions. Beads (washed or not with PBS supplemented with 300 mM NaCl and 0.1% Triton-100) and supernatant were separated and analyzed by SDS-PAGE and immunoblotted for UbcH10. Lane 1 was APC/C immunoprecipitant without UbcH10. The arrow indicates unmodified His-UbcH10. (D) His-UbcH10 was incubated with E1, ubiquitin and an energy regenerating system in the presence or absence of 600 nM Emi2 for 30 min at 22°C. Reactions were stopped with addition of sample buffer and charging of UbcH10 was analyzed by His immunoblotting. The asterisk indicates charged/activated E2. (E) APC/C immunoprecipitated from M phase extracts was incubated in XB buffer with recombinant His-Cdc20 in the presence of 500 nM MBP or MBP-Emi2 for 20 min at 22°C. APC/C beads were retrieved and washed. The amount of associated His-Cdc20 was detected by Western blotting. (F) APC/C immunoprecipitated from mitotic extracts were incubated with precharged UbcH10, Cyclin B, and an energy-regenerating system in the presence or absence of MBP-Emi2 (600 nM) for 1 h at 22°C. Sample buffer was added to supernatant and APC/C beads separately. The formation of ubiquitin conjugates on Cyclin B was analyzed by Myc Western blotting. (G) In vitro APC/C assay was performed in the presence of 500 nM MBP or MBP-Emi2 (WT or ZBRM) and the formation of ubiquitin conjugates on both UbcH10 and Cyclin B were analyzed by Western blotting for UbcH10 or Myc. n.s., nonmodified substrates.
As reported previously, ubiquitin transfer by APC/C from a charged E2 to its substrate first requires the release of ubiquitin from the activating site on E2 (Ozkan et al., 2005). On the basis of the observation that APC/C dependent polyubiquitylation of E2 itself, which would also require ubiquitin release was not affected by Emi2, we conclude that it is the latter step in which ubiquitin is transferred to the substrate that is inhibited by Emi2 (Figure 4G).
Emi2 Inhibits APC/C in a Catalytic Manner
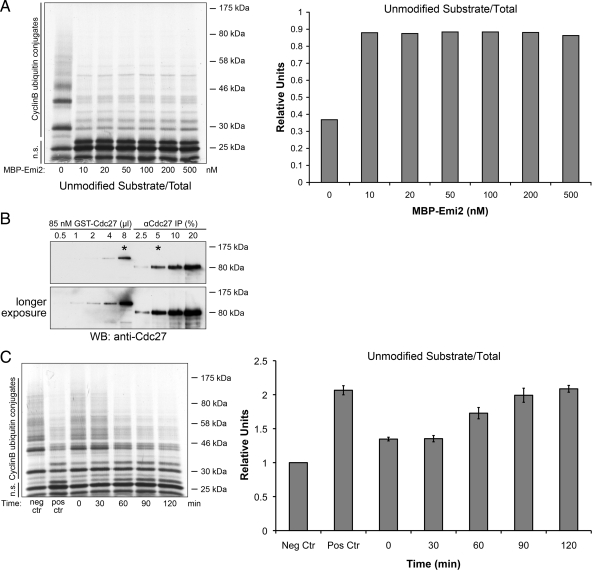
In the CSF extract, APC/C was estimated to be 160 nM (data not shown), and it could be fully inhibited by 15 nM Emi2. This fact that Emi2 inhibits APC/C in a substoichiometrical manner strongly suggests that it functions catalytically. However, it remains possible that only a small pool of APC/C is active in the extract and the rest is kept inactive by other mechanism. As a stronger piece of evidence, in an in vitro APC/C assay with Emi2 substoichiometric to APC/C, we found that 10 and 500 nM Emi2 achieved similar degrees of inhibition on 340 nM APC/C (Figure 5A; the quantification of APC/C in the assay was shown in Figure 5B), which again renders the substrate competition model very unlikely. To test the hypothesis that Emi2 might act catalytically to inhibit the APC/C in a ZBR-dependent manner, we preincubated immunoprecipitated APC/C with minimal Emi2 (5 nM) before substrate addition. Interestingly, we found that preincubation strengthened the inhibition in a time-dependent manner (Figure 5C), strongly suggesting that Emi2 inhibits the APC in a catalytic manner, potentially modifying either APC/C components or tightly associated factors to achieve the inhibition.
Figure 5.
Emi2 inhibits APC/C in a catalytic manner. (A) APC/C were immunoprecipitated from M phase extract and incubated with 1 μM recombinant His-Cdc20 and MBP-Emi2 at indicated concentrations for 45 min before substrate was added. Conversion of radiolabeled Cyclin B to ubiquitylated forms was monitored by autoradiography and phosphorimager. Results were further quantified with ImageQuant5.0. n.s., nonmodified substrates. (B) APC/C immunoprecipitated from same egg extract used in Figure 5A (Note that in experiments shown in Figure 5A, extract was driven into M phase as described in Materials and Methods) were quantified with recombinant GST-Cdc27 by Western blotting. Asterisks indicate that 5% of the amount of APC/C from one sample of the APC/C assay in A is equal to the amount of 8 μl recombinant GST-Cdc27 (85 nM). As the total volume of each sample in the APC/C assay was 40 μl, the concentration of APC/C in A was calculated to be 340 nM. (C) APC/C were immunoprecipitated from M phase extract and incubated with 1 μM recombinant His-Cdc20 and 5 nM MBP-Emi2 for indicated length of time before substrate was added. Conversion of radiolabeled Cyclin B to ubiquitylated forms was monitored by autoradiography and phosphorimager. Results were further quantified with ImageQuant5.0. Neg Ctr, 5 nM MBP was added instead of MBP-Emi2; Pos Ctr, 500 nM MBP-Emi2 was added; n.s., nonmodified substrates.
DISCUSSION
Mechanism of APC/C Inhibition by Emi2
The importance of APC/C inhibition by Emi2 during oocyte maturation and CSF maintenance is well recognized (Wu and Kornbluth, 2008). However, the mechanism of Emi2 action has not been elucidated. In this study, we show that although the ZBR is critical for the function of Emi2, its DB is dispensable. Unlike many other APC/C inhibitors, including a closely related protein, Emi1, Emi2 does not act as a pseudosubstrate inhibitor. Instead, Emi2 targets the last step of substrate ubiquitylation by APC/C, where the ubiquitin is transferred from charged E2 to the substrate. In particular, it blocks the reception of ubiquitin by the substrate rather than the release of ubiquitin from charged E2. Indeed, when linked to the Cdc20 N-terminus, Cyclin B degradation became D-box independent, yet still inhibitable by Emi2. Coupled with the observation that Emi2 can act substoichiometrically in vitro, these data rule out a model wherein Emi2 prevents the D-box dependent binding of substrates to prohibit their ubiquitylation and raise the interesting possibility that Emi2 either acts via a hit and run mechanism or has some intrinsic catalytic activity.
The Substrate Specificity of APC/C
As previously shown, Emi2 mediated APC/C inhibition selectively targets Cyclin B but not Cyclin A (Wu et al., 2007a). In the course of this study, we observed that, similar to Cyclin A, the APC/C dependent degradation of Nek2A is also not inhibitable by Emi2 (Supplementary Figure S2). The substrate specificity of APC/C is a fascinating topic and recent studies have yielded significant insights into this phenomenon. APC/CCdc20 and APC/CCdh1 are known to have different, albeit overlapping, sets of substrates and the two activators are differently regulated (Peters, 2006). In M phase, APC/C activity is mainly controlled by the SAC although some proteins, like Cyclin A, can be degraded in a checkpoint-independent manner. Recent studies have also revealed that Cyclin A could be degraded in the presence of an active SAC due to Cks binding (Wolthuis et al., 2008). Our observations on Emi2 have raised the interesting possibility that there maybe some similarity between the APC/C inhibition mechanism under CSF and SAC conditions. In addition, these data also suggest that some APC/C inhibitors may have independent means to modulate APC/C substrate specificity, which has not yet been explored. For instance, in theory, Emi2 could alter the APC/C such that it only allows ubiquitylation of a specific subset of substrates.
Roles of the ZBR on Emi2
Another known APC/C inhibitor, Xnf7 (Casaletto et al., 2005), has been identified as a RING domain E3 ligase and its ligase activity appears to be essential for their function. There are similarities between the ZBR and the Xnf7 RING, suggesting that Emi2 could potentially act as an E3 ligase to promote APC/C inhibition in a ZBR-dependent manner. Indeed, we have observed ZBR-dependent autoubiqtuitylation of Emi2 in vitro (W.T. and S.K., unpublished observations). However, whether this activity mediates APC/C inhibition remains to be determined. Xnf7 and Emi2 may share similar mechanisms of action in response to SAC and CSF signals, respectively. Consistent with the idea that Emi2 inhibits the APC/C via a catalytic activity rather than through physical association, it is worth noting that majority of Emi2 is not present in the same fractions as APC/C by gel filtration (Wu et al., 2007b). It seems reasonable to postulate that Emi2 must bind the APC/C, regardless of its mechanism of action, as close juxtaposition of Emi2 and that the APC/C is a likely prerequisite for any APC/C modification to occur. This would provide a potential explanation for the partial defect in action of the D-box mutant observed in Figure 1, D and E. Alternatively, because Emi2 appears capable of binding APC11, another intriguing possibility is that a RING–RING interaction between Emi2 and APC11 renders it incapable of promoting ubiquitylation on a subset of APC/C substrates. It is clear that understanding the roles of Emi2's ZBR is critical for fully elucidating its mechanism of APC/C inhibition.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Hongtao Yu, Hiro Yamano, Peter Jackson and Tim Hunt for their generous sharing of reagents. This work was supported by RO1 GM067225 and GM088175 to SK.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-08-0708) on June 9, 2010.
REFERENCES
- Burton J. L., Solomon M. J. Mad3p, a pseudosubstrate inhibitor of APCCdc20 in the spindle assembly checkpoint. Genes Dev. 2007;21:655–667. doi: 10.1101/gad.1511107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaletto J. B., Nutt L. K., Wu Q., Moore J. D., Etkin L. D., Jackson P. K., Hunt T., Kornbluth S. Inhibition of the anaphase-promoting complex by the Xnf7 ubiquitin ligase. J. Cell Biol. 2005;169:61–71. doi: 10.1083/jcb.200411056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro A., Bernis C., Vigneron S., Labbe J. C., Lorca T. The anaphase-promoting complex: a key factor in the regulation of cell cycle. Oncogene. 2005;24:314–325. doi: 10.1038/sj.onc.1207973. [DOI] [PubMed] [Google Scholar]
- Choi E., Dial J. M., Jeong D. E., Hall M. C. Unique D box and KEN box sequences limit ubiquitination of Acm1 and promote pseudosubstrate inhibition of the anaphase-promoting complex. J. Biol. Chem. 2008;283:23701–23710. doi: 10.1074/jbc.M803695200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fiore B., Pines J. Emi1 is needed to couple DNA replication with mitosis but does not regulate activation of the mitotic APC/C. J. Cell Biol. 2007;177:425–437. doi: 10.1083/jcb.200611166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes M. J., Kimata Y., Wattam S. L., Lindon C., Mao G., Yamano H., Fry A. M. Early mitotic degradation of Nek2A depends on Cdc20-independent interaction with the APC/C. Nat. Cell Biol. 2006;8:607–614. doi: 10.1038/ncb1410. [DOI] [PubMed] [Google Scholar]
- Hochegger H., Klotzbucher A., Kirk J., Howell M., le Guellec K., Fletcher K., Duncan T., Sohail M., Hunt T. New B-type cyclin synthesis is required between meiosis I and II during Xenopus oocyte maturation. Development. 2001;128:3795–3807. doi: 10.1242/dev.128.19.3795. [DOI] [PubMed] [Google Scholar]
- Kimata Y., Baxter J. E., Fry A. M., Yamano H. A role for the Fizzy/Cdc20 family of proteins in activation of the APC/C distinct from substrate recruitment. Mol. Cell. 2008a;32:576–583. doi: 10.1016/j.molcel.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Kimata Y., Trickey M., Izawa D., Gannon J., Yamamoto M., Yamano H. A mutual inhibition between APC/C and its substrate Mes1 required for meiotic progression in fission yeast. Dev. Cell. 2008b;14:446–454. doi: 10.1016/j.devcel.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Machida Y. J., Dutta A. The APC/C inhibitor, Emi1, is essential for prevention of rereplication. Genes Dev. 2007;21:184–194. doi: 10.1101/gad.1495007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malureanu L. A., Jeganathan K. B., Hamada M., Wasilewski L., Davenport J., van Deursen J. M. BubR1 N terminus acts as a soluble inhibitor of cyclin B degradation by APC/C(Cdc20) in interphase. Dev. Cell. 2009;16:118–131. doi: 10.1016/j.devcel.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangos P., Carroll J. Securin regulates entry into M-phase by modulating the stability of cyclin B. Nat. Cell Biol. 2008;10:445–451. doi: 10.1038/ncb1707. [DOI] [PubMed] [Google Scholar]
- Matyskiela M. E., Morgan D. O. Analysis of activator-binding sites on the APC/C supports a cooperative substrate-binding mechanism. Mol. Cell. 2009;34:68–80. doi: 10.1016/j.molcel.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. J., Summers M. K., Hansen D. V., Nachury M. V., Lehman N. L., Loktev A., Jackson P. K. Emi1 stably binds and inhibits the anaphase-promoting complex/cyclosome as a pseudosubstrate inhibitor. Genes Dev. 2006;20:2410–2420. doi: 10.1101/gad.1454006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. W. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- Musacchio A., Salmon E. D. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- Ozkan E., Yu H., Deisenhofer J. Mechanistic insight into the allosteric activation of a ubiquitin-conjugating enzyme by RING-type ubiquitin ligases. Proc. Natl. Acad. Sci. USA. 2005;102:18890–18895. doi: 10.1073/pnas.0509418102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J. M. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat. Rev. Mol. Cell Biol. 2006;7:644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- Smythe C., Newport J. W. Systems for the study of nuclear assembly, DNA replication, and nuclear breakdown in Xenopus laevis egg extracts. Methods Cell Biol. 1991;35:449–468. doi: 10.1016/s0091-679x(08)60583-x. [DOI] [PubMed] [Google Scholar]
- Sullivan M., Morgan D. O. Finishing mitosis, one step at a time. Nat. Rev. Mol. Cell Biol. 2007;8:894–903. doi: 10.1038/nrm2276. [DOI] [PubMed] [Google Scholar]
- Summers M. K., Pan B., Mukhyala K., Jackson P. K. The unique N terminus of the UbcH10 E2 enzyme controls the threshold for APC activation and enhances checkpoint regulation of the APC. Mol. Cell. 2008;31:544–556. doi: 10.1016/j.molcel.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolthuis R., Clay-Farrace L., van Zon W., Yekezare M., Koop L., Ogink J., Medema R., Pines J. Cdc20 and Cks direct the spindle checkpoint-independent destruction of cyclin A. Mol. Cell. 2008;30:290–302. doi: 10.1016/j.molcel.2008.02.027. [DOI] [PubMed] [Google Scholar]
- Wu J. Q., Hansen D. V., Guo Y., Wang M. Z., Tang W., Freel C. D., Tung J. J., Jackson P. K., Kornbluth S. Control of Emi2 activity and stability through Mos-mediated recruitment of PP2A. Proc. Natl. Acad. Sci. USA. 2007a;104:16564–16569. doi: 10.1073/pnas.0707537104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. Q., Kornbluth S. Across the meiotic divide—CSF activity in the post-Emi2/XErp1 era. J. Cell Sci. 2008;121:3509–3514. doi: 10.1242/jcs.036855. [DOI] [PubMed] [Google Scholar]
- Wu Q., et al. A role for Cdc2- and PP2A-mediated regulation of Emi2 in the maintenance of CSF arrest. Curr. Biol. 2007b;17:213–224. doi: 10.1016/j.cub.2006.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H. Cdc 20, a WD40 activator for a cell cycle degradation machine. Mol. Cell. 2007;27:3–16. doi: 10.1016/j.molcel.2007.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.