Adenomatous polyposis coli is a cytoskeletal organizer and a scaffold for mediating degradation of the Wnt effector β-catenin. We uncouple these different APC functions and show that GSK3β/CKI phosphorylation regulates APC clusters and cell migration independently of cell–cell adhesion and β-catenin transcriptional activity.
Abstract
Adenomatous polyposis coli (APC), a tumor suppressor commonly mutated in cancer, is a cytoskeletal organizer for cell migration and a scaffold for GSK3β/CKI-mediated phosphorylation and degradation of the Wnt effector β-catenin. It remains unclear whether these different APC functions are coupled, or independently regulated and localized. In primary endothelial cells, we show that GSK3β/CKI-phosphorylated APC localizes to microtubule-dependent clusters at the tips of membrane extensions. Loss of GSK3β/CKI-phosphorylated APC from these clusters correlates with a decrease in cell migration. GSK3β/CKI-phosphorylated APC and β-catenin at clusters is degraded rapidly by the proteasome, but inhibition of GSK3β/CKI does not increase β-catenin–mediated transcription. GSK3β/CKI-phosphorylated and -nonphosphorylated APC also localize along adherens junctions, which requires actin and cell–cell adhesion. Significantly, inhibition of cell–cell adhesion results in loss of lateral membrane APC and a concomitant increase in GSK3β/CKI-phosphorylated APC in clusters. These results uncouple different APC functions and show that GSK3β/CKI phosphorylation regulates APC clusters and cell migration independently of cell–cell adhesion and β-catenin transcriptional activity.
INTRODUCTION
Adenomatous polyposis coli (APC) protein is the product of a tumor suppressor gene mutated in colorectal (Groden et al., 1991) and other cancers (reviewed in Polakis, 1997). APC is a large protein, with multiple binding domains for a variety of proteins that are involved in different signaling and cytoskeletal functions of APC (reviewed in McCartney and Nathke, 2008).
APC plays an integral role in canonical Wnt signaling by controlling levels of β-catenin, a key effector in the Wnt pathway (reviewed in Nelson and Nusse, 2004). In the absence of Wnt signaling, β-catenin is down-regulated through its association with a multiprotein complex of APC, axin, glycogen synthase kinase 3β (GSK3β), and casein kinase I (CKI; the destruction complex). Sequential phosphorylation of β-catenin by CKI and GSK3β leads to ubiquitination and degradation of β-catenin by the proteasome. Activation of Wnt signaling inhibits GSK3β, leading to accumulation of nonphosphorylated β-catenin, which binds Tcf/Lef family of transcription factors and induces target gene expression. Deletion of axin and β-catenin–binding sites on APC prevents β-catenin degradation and leads to unchecked Wnt signaling and cancer progression (Munemitsu et al., 1995; Korinek et al., 1997; Morin et al., 1997). CKI and GSK3β also phosphorylate APC (Ikeda et al., 2000; Rubinfeld et al., 2001), but consequences of this phosphorylation on APC function(s) are not well understood.
The subcellular location of the APC/β-catenin destruction complex is unclear, as it has been suggested to be on the apical membrane (Reinacher-Schick and Gumbiner, 2001) and at cell–cell contacts (Maher et al., 2009). Complicating interpretation of these results is the fact that many of these experiments were performed in colon carcinoma cell lines harboring mutations in either APC or β-catenin, which can lead to changes in activity of the destruction complex and distribution of APC. APC has been detected at lateral plasma membrane in some cells (Miyashiro et al., 1995; McCartney et al., 1999; Yu et al., 1999; Rosin-Arbesfeld et al., 2001), and loss of APC correlates with defective intercellular adhesion (Hamada and Bienz, 2002; Faux et al., 2004), although this notion has been challenged in other studies in Drosophila (McCartney et al., 2006). β-Catenin also plays an essential role in cell–cell adhesion through its association with cadherins and α-catenin (reviewed in Nelson, 2008). It is unclear whether APC regulates β-catenin in the cadherin complex.
APC also binds directly to actin (Moseley et al., 2007) and microtubules (Munemitsu et al., 1994; Smith et al., 1994), as well as many actin and microtubule regulatory proteins (reviewed in Barth et al., 2008). Little is known about the role of APC in regulating the actin cytoskeleton, but APC stabilizes microtubules and stimulates microtubule assembly and bundling in vitro (Munemitsu et al., 1994; Smith et al., 1994). In several cell types APC predominantly localizes in punctate clusters at cell extensions where microtubule plus ends are focused (Nathke et al., 1996; Mimori-Kiyosue et al., 2000; Etienne-Manneville and Hall, 2003; Votin et al., 2005). It is unknown whether there is a functional relationship between APC at cell–cell contacts and in clusters at membrane extensions. APC clusters are thought to promote cell extension and cell migration by affecting microtubule stability (Wen et al., 2004; Kita et al., 2006; Kroboth et al., 2007; Purro et al., 2008). Although phosphorylation of APC is thought to be important in regulating APC functions in cell migration (Etienne-Manneville and Hall, 2003), the phosphorylation state of APC during cell migration (or other processes) has not been directly shown. Furthermore, it remains unknown if APC clusters are also associated with proteasomal degradation of β-catenin.
Together, these studies have identified different functions and subcellular distributions of APC in signaling and cytoskeleton regulation. However, it is unclear whether these functions of APC are coupled or are independently regulated and localized. Here we have used a combination of morphological and biochemical approaches in primary human umbilical vein endothelial cells (HUVECs) to probe the relationship between different functions and distributions of endogenous, wild-type APC. We have identified distinct APC complexes that localize along cell–cell contacts and in punctate clusters at cell extensions. Our results indicate that these complexes have different cytoskeleton requirements for localization and different GSK3β/CKI phosphorylation states, turnover kinetics, and functions.
MATERIALS AND METHODS
Cell Culture, Antibodies, and Reagents
HUVECs (Lonza CC-2519; Basel, Switzerland) were cultured in EBM plus Supplement and Growth Factor Kit (Lonza, CC-3121 + CC-4133) and maintained for up to 12 passages. Madin-Darby canine kidney (MDCK) II cells were cultured in DMEM (Sigma Aldrich, St. Louis, MO) plus 10% fetal bovine serum and antibiotics. HL-60 cells (a gift from Sean Collins, Stanford University, Stanford, CA) were cultured in RPMI with HEPES (Invitrogen, Carlsbad, CA) plus 17% fetal bovine serum and antibiotics and were differentiated by addition of 1.3% DMSO. All cells were grown at 37°C, 5% CO2. Antibodies for immunofluorescence (IF), immunoblotting (IB), and immunoprecipitation (IP) were purchased from BD Transduction Laboratories (Lexington, KY): VE-cadherin (IF), β-catenin (IF, IB, IP), and EB1 (IF); from Santa Cruz Biotechnology (Santa Cruz, CA): VE-cadherin C19 (IB, IP), APC H290 (IB; Davies et al., 2007); Chemicon International (Temecula, CA): α-catenin (IB, IP); Enzo Life Sciences (New York, NY): α-catenin (IF, IB); Cell Signaling (Beverly, MA: phospho-β-catenin Ser45 (IF, IB) and phospho-β-catenin Ser33/Ser31/Thr41 (IF, IB); Sigma Aldrich: α-tubulin (IF); Zymed (South San Francisco, CA) ZO-1 (IF, IB); and ECM Biosciences (Versailles, KY): β-catenin (IB). We also used a polyclonal APC (anti-APC3) antibody raised and affinity-purified against an epitope between amino acids 2130–2884 for IF and IP (baculovirus vector containing APC3 fragment was a gift from Paul Polakis, Genentech; see also Rubinfeld et al., 1993, 1995); a polyclonal β-catenin antibody raised against the 12 C-terminal amino acids (IF, IB, IP), which recognizes phosphorylated and nonphosphorylated forms; a monoclonal E-cadherin antibody raised against the extracellular domain (IF, IB; cells were a gift from Barry Gumbiner, University of Virginia, Charlottesville, VA); and a monoclonal AMER1 antibody raised against amino acids 2–285 of human AMER1 (a gift from Juergen Behrens, University of Erlangen-Nuremberg, Erlangen, Germany). Fluorescent phalloidin was purchased from Molecular Probes (Eugene, OR). Secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA) and Molecular Probes. Chemical reagents were purchased from Sigma Aldrich: GSK3β inhibitor (SB216763), MG132 (C2211), cycloheximide (C7698), nocodazole (M1404), and cytochalasin D (C8273); Calbiochem (La Jolla, CA): CKI inhibitor D4476 (218705); and New England Biolabs (Beverly, MA): lambda protein phosphatase (P0753). If incubation times for various chemical reagents were different, addition of reagents was staggered so total time course of the experiment was the same for all conditions. Lipofectin (Invitrogen) and OmniFector (VennNova, Pompano Beach, FL) were used according to manufacturer's instructions. Human β-catenin small interfering RNA (siRNA) was purchased from Dharmacon (Boulder, CO): CTNNB1 siGENOME SMARTpool (M-003482–00).
IF Microscopy
Cells were plated on collagen-coated coverslips for minimum 12 h before further treatments or fixation. Cells were fixed for 20 min (3% paraformaldehyde [EM Sciences], 0.1% saponin) blocked for 1 h (2% bovine serum albumin [BSA], 1% goat serum, 0.1% saponin), and stained with primary and secondary antibodies for 1 h. Methanol fixation (100% for 10 min) was used only for EB1 localization. Coverslips were mounted using Vectashield with DAPI (Vector Laboratories, Burlingame, CA) and imaged using Zeiss Axiovert 200 with 100× and 63× 1.4 NA objectives (Carl Zeiss MicroImaging, Thornwood, NY). Images were acquired using AxioCam mRM camera and AxioVision Rel. 4.6 software (Carl Zeiss MicroImaging). Quantification of proteins in clusters was performed in context of wound-healing assay because membrane extensions uniformly oriented to wound edge. For quantification of proteins to lateral plasma membrane, cell–cell contacts were traced using free-hand tool in ImageJ (http://rsb.info.nih.gov/ij/). Integrated density was corrected for cell background. For analysis of APC and phosphorylated β-catenin at the lateral plasma membrane, cell–cell contacts were first traced using a bona fide cell–cell contact marker (β-catenin, VE-cadherin), and then the corresponding region of APC or phosphorylated β-catenin on the second channel was measured.
Live Cell Imaging and Tracking
Live cell imaging and tracking was performed with help of Feng-Chiao Tsai (Stanford University, Stanford, CA) as described previously (Vitorino and Meyer, 2008). HUVECs were plated to confluency on collagen-coated 96-well clear-bottom tissue culture plates (Corning, Lowell, MA) for 24 h and then stained with 100 ng/ml Hoechst 33342 (Invitrogen) for 20 min. Monolayers were wounded using a high-throughput scratching tool, washed, and incubated with various chemical reagents. Cells were imaged every 15 min in a 37°C, 5% CO2 chamber with a 4× objective on an automated fluorescent microscope (ImageXpress 5000A, Molecular Devices, Sunnyvale, CA). Cells were tracked using Hoechst-stained nuclei with a MatLab particle tracking algorithm (MathWorks, Natick, MA) as described previously to determine coordination and directionality parameters (Vitorino and Meyer, 2008). Rate of wound closure was determined by measuring the cell-free area at 0 and 6 h using ImageJ.
Extractions and IPs
Unless otherwise stated, extractions were performed on cells grown at least 12 h on tissue culture plates and were 60–80% confluent. Cells were washed, extracted for 10 min (50 mM Tris-HCl, pH 7.4, 100 mM NaCl, 2 mM MgCl2, 0.5% NP40 (Nonidet P-40; Sigma Aldrich), 1 mM Pefabloc, 5 μg/ml each leupeptin, pepstatin, and aprotinin, 1 U α2-macroglobulin, 20 μg/ml TPCK), scraped, and centrifuged at 14,000 × g for 10 min. Postnuclear supernatants were used for subsequent IPs or diluted in SDS-sample buffer for analysis on SDS-PAGE. For IPs, 400 μl of lysate was incubated with 5 μl of antibody for 1 h, followed by 1 h with protein A- or G-Sepharose (GE Healthcare, Waukesha, WI), at 4°C with end-over-end rotation. Beads were washed four times before resuspension in SDS-sample buffer. Extractions and IPs were run on 3–8% Criterion XT Tris-acetate gels (Bio-Rad, Richmond, CA) and transferred to polyvinylidene fluoride (Immobilon-FL, Millipore, Bedford, MA). Membranes were imaged on Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, NW). All Western blot quantification was performed in ImageJ.
Tcf/Lef Transcription
Tcf/Lef-mediated transcription was measured using Dual-Light reporter system (Applied Biosystems, Bedford, MA). Cells were cotransfected with β-galactosidase and either TOPFLASH (luciferase reporter with Tcf/Lef binding sites) or FOPFLASH (luciferase reporter with mutated Tcf/Lef-binding sites; a gift from Marc van de Wetering and Hans Clevers, Hubrecht Institute, Utrecht, The Netherlands). A subset were also transfected with stabilized β-catenin construct (GFP-ΔGSK-βcat) in which four GSK3β phosphorylation sites (Ser33, Ser37, Thr41, and Ser35) are mutated to alanine (Barth et al., 1999) or green fluorescent protein (GFP) empty vector. Twenty-fours after transfection cells were treated with chemical reagents before preparation of lysates. Lysates and other solutions were prepared as outlined in Dual-Light protocol (Applied Biosystems). For each sample, luciferase and β-galactosidase activity were measured in triplicate. β-Galactosidase activity was used to normalize luciferase activity for transfection efficiency.
Cell Barrier Function
For paracellular diffusion assays, HUVECs were plated to confluency on collagen-coated 0.4-μm-pore polycarbonate Transwell filters (Corning) 24 h before treatment with various chemical reagents. Alexa-647 Dextran (Molecular Probes, 10,000 MW) at 50 μg/ml was added to the apical compartment, cells were incubated at 37°C for 1 h; then equal volume aliquots of apical and basal compartment media were collected, and amount of A647-Dextran was determined using the Odyssey Infrared Imaging System (Li-Cor Biosciences). For transmigration assays, HUVECs were plated to confluency on collagen-coated 5-μm-pore polycarbonate Transwell filters (Corning) for 3 d before treatment with various chemical reagents. To start migration, HUVEC medium was removed, and 1 × 106 differentiated HL-60 cells were added to apical chamber. HL-60 media containing 20 nM N-formyl-MET-LEU-PHE (fMLP; Sigma Aldrich; 59880-97-6) was added to lower chamber. Cells were incubated at 37°C for 1 h. Migration was stopped by removing filter inserts, and the number of HL-60 cells in the basal compartment was determined using a hemocytometer.
RESULTS
Simultaneous Localization of APC in Clusters and at the Lateral Membrane
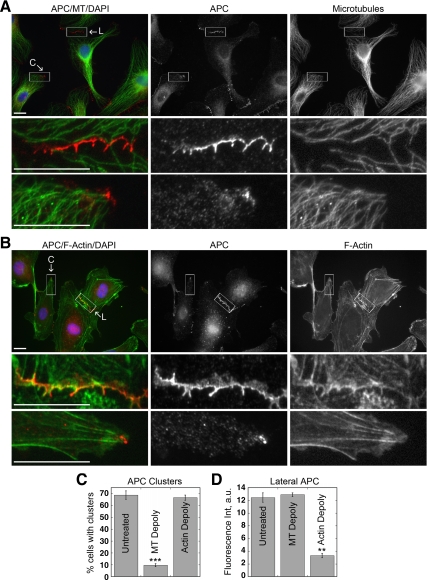
Endogenous APC is localized in two distinct structures in HUVECs: 1) in clusters at free plasma membrane extensions of ∼70% of cells in subconfluent conditions or wound-healing assays (Figure 1, A and C); and 2) along the lateral plasma membrane at cell–cell contacts in tear-drop-like protrusions (Figure 1B), which are not associated with either caveolin or clathrin (E.S.H., unpublished results). Additionally, we detected punctate APC staining at tricellular corners of ∼20% of cells in confluent monolayers (Supplemental Figure S1A; Figure 2D). These spots of APC staining appeared locally distinct from neighboring lateral membrane APC staining and exhibited characteristics similar to APC clusters at cell extensions, although we cannot exclude that they are simply enrichments of lateral membrane APC. Microtubules (Figure 1A; Supplemental Figure S1A) and EB1-deocrated microtubule plus-tips (Supplemental Figure S1, B and C) were densely focused at APC clusters, as observed previously in other cell types, but relatively few microtubules projected into regions along the lateral membrane containing APC. In contrast, actin filaments colocalized with lateral membrane APC, but relatively few actin filaments projected into APC clusters (Figure 1B).
Figure 1.
APC is localized to different subcellular sites by actin and microtubule cytoskeletons. (A and B) Subconfluent HUVECs fixed and stained for APC (red), α-tubulin or phalloidin (green), and DAPI (blue). Boxed regions of clusters (C) or lateral membrane (L) correspond to enlarged images. Scale bar, 20 μm. (C and D) Quantification of APC localization at wound edge (C) or cell–cell contacts (D) in HUVECs treated with 33 μM nocodazole or 200 nM cytochalasin D for 1 h. (D) More than 150 contacts were measured for each condition. Mean values ± SEM from three independent experiments. ***p < 0.0001, **p = 0.0004 by Student's t test.
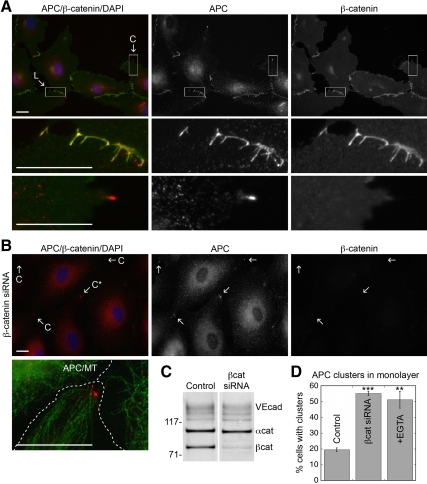
Figure 2.
APC at lateral membranes, but not in clusters, colocalizes with β-catenin and requires cell–cell adhesion for localization. (A) HUVECs fixed and stained for APC (red), β-catenin (green), and DAPI (blue). Boxed regions of clusters (C) or lateral membrane (L) correspond to enlarged images. Scale bar, 20 μm. (B) HUVECs treated with 0.5 μM β-catenin siRNA for 72 h, fixed, and stained for APC (red), β-catenin (green), and DAPI (blue). APC clusters are indicated with (C). C* marks cluster in enlarged image showing APC (red) and α-tubulin (green) staining. Cell borders are indicated with dashed line. Scale bar, 20 μm. (C) Western blot of 0.5% NP40 extracts from control or β-catenin siRNA-treated HUVECs with antibodies to VE-cadherin, α-catenin, and β-catenin. In siRNA treated cells β-catenin, α-catenin, and VE-cadherin levels were decreased ∼70, 15, and 25%, respectively (average of two experiments). (D) Quantification of % cells within confluent monolayers with APC clusters. Cells were treated with β-catenin siRNA or 2 mM EGTA for 1 h. Mean values ± SEM from three independent experiments. ***p < 0.0001, **p = 0.0005 by Student's t test.
The microtubule and the actin cytoskeletons were selectively depolymerized with nocodazole and cytochalasin D, respectively, to examine effects on APC localization. Microtubule depolymerization caused a ∼85% decrease in cells with APC clusters (Figure 1C), but did not affect APC on lateral membranes (Figure 1D). In contrast, actin depolymerization did not affect APC clusters (Figure 1C), but caused a ∼75% decrease in the amount of APC at lateral membranes (Figure 1D). These results show that APC complexes in clusters and at lateral membranes have distinct cytoskeleton requirements for localization. Our results agree with previous studies that showed that microtubules were required for APC clusters in MDCK epithelial cells (Nathke et al., 1996), whereas actin was required for lateral plasma membrane localization of APC in MDCK epithelial cells (Rosin-Arbesfeld et al., 2001) and Drosophila (APC2; Townsley and Bienz, 2000).
Lateral Membrane Localization of APC Requires Cell–Cell Adhesion
β-Catenin, a component of adherens junctions and a known APC-binding partner in the Wnt signaling pathway, colocalized with APC at lateral membranes (Figure 2A) but was found in <10% of APC clusters in membrane extensions (n = 133). VE-cadherin and α-catenin (E.S.H., unpublished results) also colocalized with APC on lateral membranes, but in <8% of APC clusters (n = 157), irrespective of cell confluency (Supplemental Figure S2, A and B).
To test if β-catenin was required for APC localization, β-catenin was depleted by siRNA (∼70% reduction; Figure 2, B and C). This resulted in a loss of VE-cadherin from lateral membranes (E.S.H., unpublished results) and disrupted cell–cell adhesion. Significantly, APC localization to lateral membranes was also completely abolished (Figure 2B). Concomitantly, there was a ∼70% increase in cells with APC clusters in confluent monolayers (Figure 2, B and D). To distinguish effects of β-catenin depletion or loss of cell–cell adhesion on APC localization, we disrupted VE-cadherin–based cell–cell adhesion by extracellular Ca2+ chelation. This resulted in a loss of lateral membrane APC and a concomitant increase in the percent of cells with APC clusters in the monolayer (Figure 2D; Supplemental Figure S2C). Neither condition caused the relocalization of adherens junction proteins to APC clusters (Figure 2B; Supplemental Figure S2C).
APC and the tight junction marker protein ZO-1 showed partial colocalization at the lateral membrane of HUVECs (Supplemental Figure S3A). In contrast to adherens junction proteins (see Figure 3), we did not detect any ZO-1 binding to APC in IPs from HUVEC extracts (Supplemental Figure S3B). In endothelial cells tight junctions and adherens junction are more intermingled, which may explain why we detected colocalization of these two proteins despite the lack of binding between APC and ZO-1. In addition, β-catenin depletion, although removing APC from lateral membrane (Figure 2B), did not affect tight junction localization (Supplemental Figure S3C), indicating that tight junctions are not sufficient to retain APC to the lateral membrane. Together, these data suggest a functional link between APC and ZO-1 does not exist. In contrast, APC requires components of the adherens junction complex for localization to the lateral plasma membrane.
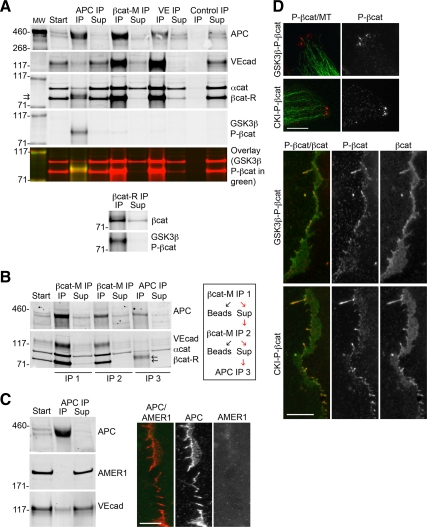
Figure 3.
APC is in a complex with phosphorylated β-catenin, which is distinct from the VE-cadherin complex. (A–C) Western blot of IPs from 0.5% NP40 extracts of HUVECs. (A) APC, β-catenin (mouse and rabbit antibodies), VE-cadherin, and control IPs blotted for APC, VE-cadherin, β-catenin (mouse and rabbit), α-catenin, and GSK3β-P-βcat; representative of four independent experiments. An overlay image of the GSK3β-P-βcat blot (green) and β-catenin/α-catenin blot (red) shows the phosphorylated form of β-catenin migrates more slowly than the nonphosphorylated form. (B) β-Catenin mouse antibody was used for two sequential IPs (IP1 and IP2) to deplete unphosphorylated β-catenin from extracts. APC was immunoprecipitated from IP2 supernatant (IP3), and blotted for APC, VE-cadherin, β-catenin, and α-catenin; representative of two independent experiments. (C) APC immunoprecipitate blotted for APC, AMER1, and VE-cadherin. IF image of subconfluent HUVECs fixed and stained for APC (red) and AMER1 (green). Only enlarged image of lateral membrane is shown. Scale bar, 10 μm. (D) HUVECs fixed and stained for GSK3β-P-βcat or CKI-P-βcat (red) and α-tubulin (green) to show clusters at sites of membrane extensions or GSK3β-P-βcat or CKI-P-βcat (red) and mouse β-catenin (green) to show lateral plasma membrane. Only enlarged images of clusters or lateral membrane are shown. Scale bar, 10 μm.
APC Is in a Complex with Phosphorylated β-Catenin, Which Is Distinct from the Adherens Junction Complex
To determine if APC associates with adherens junction proteins, proteins were immunoprecipitated and Western-blotted for APC, β-catenin, and VE-cadherin. We quantified the amount of each protein remaining in the supernatant after IP and compared this with the amount in the starting material. Although APC, VE-cadherin, β-catenin, and α-catenin coimmunoprecipitated in all cases (Figure 3A), only ∼10% of total VE-cadherin coimmunoprecipitated with APC and ∼10% of total APC coimmunoprecipitated with VE-cadherin (n = 4). Thus, ∼90% of VE-cadherin and APC are in different protein complexes. Therefore, it seems unlikely that VE-cadherin is directly localizing the majority of APC to cell–cell contacts. AMER1, which has been reported to bind and recruit APC to the plasma membrane (Grohmann et al., 2007), did not coimmunoprecipitate with APC, and we did not observe colocalization of endogenous AMER1 with APC in HUVECs (Figure 3C).
Approximately 45% of APC coimmunoprecipitated with its known binding partner β-catenin (n = 4; Figure 3A). β-Catenin comprised two closely migrating bands in APC immunoprecipitates (Figure 3A; arrows). To determine if either of these two bands represented the phosphorylated form of β-catenin, we used antibodies specific for the GSK3β- or CKI-phosphorylation sites on β-catenin (referred to as GSK3β-P-βcat and CKI-P-βcat, respectively). The GSK3β-P-βcat and CKI-P-βcat antibodies only recognize β-catenin when it is phosphorylated at either Ser33/Ser37/Thr41 or Ser45, respectively (Wu et al., 2009). Indeed, the slower migrating β-catenin band was recognized by the GSK3β-P-βcat and CKI-P-βcat antibodies (Figure 3A; CKI-P-βcat Western blot not shown); note that we also observed GSK3β-P-βcat and CK1-P-βcat in β-catenin IPs (Figure 3A), but only if we used a polyclonal rabbit β-catenin antibody (βcat-R), as our mouse mAb (βcat-M) did not recognize the phosphorylated forms of β-catenin. The amount of GSK3β/CKI-P-βcat was low in whole cell extracts (see Figure 4), but was concentrated in APC immunoprecipitates. Significantly, neither GSK3β-P-βcat nor CKI-P-βcat was detected in VE-cadherin immunoprecipitates (Figure 3A). Only ∼5% of total β-catenin was not bound to either APC or VE-cadherin (n = 4), suggesting there is very little free β-catenin in HUVECs, similar to previous results in MDCK epithelial cells (Stewart and Nelson, 1997). In addition, we detected very little β-catenin staining in the nucleus or cytoplasm (Figure 2A), further indicating the majority of β-catenin is in a complex with either APC or VE-cadherin at the lateral membrane.
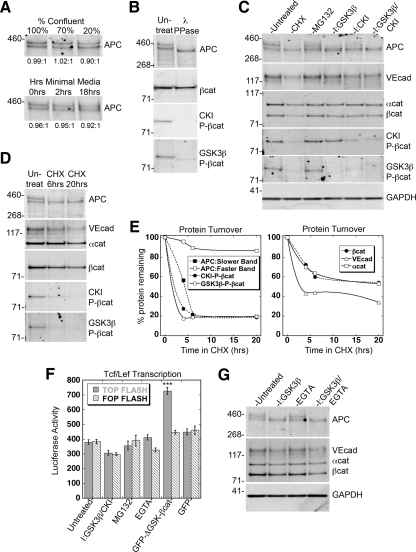
Figure 4.
Phosphorylation by GSK3β/CKI, but not cell–cell adhesion, regulates stability and turnover of APC and β-catenin. (A–E and G) Western blots of 0.5% NP40 extracts of HUVECs for APC, VE-cadherin, β-catenin, α-catenin, GSK3β- or CKI-P-βcat, and GAPDH; a representative example is shown in which all relevant conditions were run on the same gel. Quantification of Western blots was performed with ImageJ and presented in the accompanying text. (A) HUVECs grown at different % confluency or in the absence of serum and growth factors for indicated time. Ratio of the two APC bands does not change significantly under all conditions. (B) Extracts treated with 1000 U λ protein phosphatase for 30 min at 30°C. (C) Extracts prepared from HUVECs treated with either 10 μM cyclohexamide (CHX) for 6 h, 10 μM MG132 for 4 h, 20 μM GSK3β inhibitor for 1 h, 50 μM CKI inhibitor for 4 h, or 20 μM GSK3β+50 μM CKI inhibitors for 4 h. No difference was found when HUVEC lysates were prepared by 0.5% NP40 extraction or SDS-whole cell lysis (data not shown). (D) Extracts prepared from HUVECs treated with 10 μM CHX for indicated times. (E) Quantification of % protein remaining after 0, 4, 6, or 20 h in the presence of CHX. Depending on time point and protein, n = 2–18. Half-lives were calculated from linear portions of the graph. (F) Reporter luciferase assay using extracts from HUVECs transfected with either TOPFLASH Tcf/Lef-driven luciferase reporter (gray bars) or FOPFLASH (dashed bars, negative control with mutated Tcf/Lef-binding sites). Cells were either treated with indicated chemical reagents or cotransfected with GFP-ΔGSK-βcat or GFP empty vector. For treatment with chemical reagents, at 24 h after transfection HUVECs were treated with either 20 μM GSK3β+50 μM CKI inhibitors for 4 h, 10 μM MG132 for 4 h, or 5 mM EGTA for 1 h before preparation of lysates. Luciferase activity was normalized by activity of cotransfected β-galactosidase. Mean values ± SEM from either two (cotransfection) or three (chemical reagents) independent experiments performed in triplicate. ***p < 0.0001 by Student's t test. (G) Extracts prepared from HUVECs treated with 20 μM GSK3β inhibitor, 5 mM EGTA, or both for 1 h.
This striking difference in the forms of β-catenin bound to APC and VE-cadherin was confirmed by immunodepleting nonphosphorylated β-catenin from HUVEC extracts and then examining β-catenin in APC and VE-cadherin complexes. As GSK3β-P-βcat and CKI-P-βcat immunoprecipitated with the rabbit polyclonal antibody (βcat-R), but not the mouse monoclonal β-catenin antibody (βcat-M; Figure 3A), we used the βcat-M antibody to selectively remove the nonphosphorylated form of β-catenin from HUVEC extracts. After two sequential IPs with βcat-M, nonphosphorylated β-catenin was no longer detected in the supernatant (Figure 3B; IP2 supernatant) but APC still coimmunoprecipitated GSK3β/CKI-P-βcat (Figure 3B; IP3). Together, these data show that β-catenin is in a complex with both APC and VE-cadherin, but GSK3β/CKI-P-βcat is only in a complex with APC.
IF microscopy showed that GSK3β-P-βcat and CKI-P-βcat localized in punctate clusters at membrane extensions and along lateral plasma membranes, similar to APC (Figure 3D). Also similar to APC, disruption of cadherin-based cell–cell adhesion with Ca2+ chelation increased the percent of cells with GSK3β-P-βcat and CKI-P-βcat clusters, but did not significantly change total amount of these proteins on Western blot (E.S.H., unpublished results).
Axin associates with APC, β-catenin, GSK3β, and CKI and thus is a central component of the destruction complex that mediates the phosphorylation and down-regulation of β-catenin (Hart et al., 1998; Nakamura et al., 1998). In an effort to determine if axin colocalized with APC clusters and/or APC at the lateral membrane, we tested eight different axin antibodies from both commercial and noncommercial sources. However, we were unable to localize axin and detected only nonspecific, space-filling staining (E.S.H., unpublished results).
GSK3β and CKI Phosphorylate APC
We consistently detected two closely migrating APC bands in Western blots of HUVEC extracts; the average ratio of the slower and faster migrating bands was 0.92:1 (n = 15) and was unaffected by changes in cell confluency or the presence of serum or growth factors (Figure 4A). Treatment of cell extracts with λ phosphatase collapsed these two APC bands into a third faster migrating band (Figure 4B).
Because there are GSK3β and CKI phosphorylation sites on APC (Ikeda et al., 2000; Rubinfeld et al., 2001), we tested whether GSK3β or CKI activity was responsible for the electrophoretic mobility shift of APC. GSK3β and CKI inhibitors each caused a significant decrease in the level of the slower migrating APC band (74%, n = 12; 58%, n = 7, respectively) and an increase in the level of the faster migrating band (35%, n = 12; 13%, n = 7, respectively; Figure 4C). Significantly, we find that even in the presence of both inhibitors, the degree to which the faster migrating APC band was increased (36%, n = 8) was much less than the amount by which the slower migrating band was decreased (92%, n = 8), indicating that dephosphorylation of APC alone cannot explain the disappearance of the slower migrating band and a portion of APC may be targeted for degradation (see below).
Note that the GSK3β inhibitor selectively removed GSK3β-P-βcat, and not CKI-P-βcat, whereas the CKI inhibitor removed both CKI-P-βcat and GSK3β-P-βcat (Figure 4C) because GSK3β phosphorylation requires priming by CKI (Liu et al., 2002), demonstrating the specificity of these inhibitors. Removal of inhibitors resulted in the reappearance of the slower migrating APC band and the phosphorylated forms of β-catenin within 30–60 min (E.S.H., unpublished results). These results indicate that only the slower migrating band of APC (referred to as GSK3β/CKI-P-APC) is phosphorylated by GSK3β and CKI. However, because λ phosphatase caused both APC bands to collapse into a third faster migrating species (Figure 4B) and only the slowest migrating APC band is phosphorylated by GSK3β and CKI (Figure 4C), another, as yet unidentified kinase may phosphorylate APC.
GSK3β/CKI-Phosphorylation Regulates the Stability of APC and β-Catenin
We tested whether the phosphorylation state of APC and β-catenin affected their stability by measuring protein turnover by cycloheximide chase and Western blotting. The GSK3β/CKI-phosphorylated forms of APC and β-catenin decreased rapidly (t1/2 ∼ 3.5 h), whereas non-GSK3β/CKI-P-APC was stable (t1/2 > 30 h; Figure 4, D and E). Total β-catenin, α-catenin, and VE-cadherin had similar biphasic decreases: ∼40–60% decreased quickly (t1/2 ∼ 7.5 h for β-catenin and α-catenin; ∼4.5 h for VE-cadherin), and the remaining protein was stable (t1/2 > 30 h; Figure 4, D and E).
To test whether the decrease in the GSK3β/CKI-phosphorylated forms of APC and β-catenin in the presence of cycloheximide was due to ubiquitin-mediated degradation, we treated HUVECs with MG132, which is a potent, cell-permeable inhibitor of the proteasome and reduces the degradation of ubiquitin-conjugated proteins. After 4 h, GSK3β/CKI-P-APC increased 22% and non-GSK3β/CKI-P-APC increased 31% (n = 13); GSK3β-P-βcat and CKI-P-βcat increased 25 and 12%, respectively (n = 6). The levels of total β-catenin, α-catenin, and VE-cadherin did not increase under these conditions (Figure 4C; see also Allport et al., 1997). Taken together with the results from coIP experiments (Figure 3, A and B), these data indicate that GSK3β/CKI-P-APC and GSK3β/CKI-P-βcat is distinct from the adherens junction complex, and is degraded by the proteasome. In addition, we suggest that an active destruction complex is present in HUVECs, as phosphorylation by GSK3β/CKI and subsequent ubiquitination and degradation through the proteasome are key steps in the regulation of β-catenin by this complex.
Inhibition of GSK3β/CKI-Phosphorylation of APC and β-Catenin Does Not Increase Tcf/Lef-mediated Transcription
Changes in the level of cytosolic β-catenin effect its nuclear signaling activity (reviewed in Nelson and Nusse, 2004). Basal levels of Tcf/Lef-mediated transcription were low in HUVECs (Figure 4F; Untreated and GFP control), probably due to the very low level of free β-catenin (Figure 3A). Significantly, treatment with both GSK3β and CKI inhibitors, or MG132, did not increase Tcf/Lef-mediated transcription (Figure 4F).
This result was surprising because inhibition of GSK3β is downstream of Wnt activation and results in β-catenin accumulation and activation of Wnt-target genes (reviewed in Nelson and Nusse, 2004). However, consistent with the lack of Tcf/Lef activation in the presence of GSK3β and CKI inhibitors, we found the level of β-catenin had not increased—in fact the level decreased 22% (n = 25) in the presence of inhibitors and was unchanged by MG132 treatment (Figure 4C). This result was confirmed with three different β-catenin antibodies and by extracting HUVECs with either 0.5% NP40 or 2% SDS. We confirmed that canonical Wnt-responsive signaling pathway is active in these cells (Wright et al., 1999; Goodwin et al., 2006) by expressing high levels of a stabilized β-catenin construct (GFP-ΔGSK-βcat) lacking GSK3β/CKI phosphorylation sites (Figure 4F). However, even in the presence of GFP-ΔGSK-βcat, Tcf/Lef activation was low compared with similar experiments performed in MDCK cells (see Supplemental Figure S5D), suggesting that HUVECs may have additional endogenous mechanisms in place to control levels of β-catenin available for transcription.
Cell–Cell Adhesion Does Not Affect β-Catenin Turnover or Tcf/Lef-mediated Transcription
We considered additional mechanisms that might lead to an increase in β-catenin levels and hence Tcf/Lef-mediated transcription. A relationship between cadherin function and β-catenin–mediated transcription has been previously proposed in Drosophila (Cox et al., 2000). More recently, it has been reported that E-cadherin–based cell–cell adhesion may regulate Wnt signaling by limiting accumulation of unphosphorylated β-catenin in the cytosol (Maher et al., 2009). However, disruption of VE-cadherin–based cell–cell adhesion in HUVECs did not result in increased Tcf/Lef-mediated transcription (Figure 4F). In fact, rather than increasing the level of β-catenin, the amount decreased 10% (n = 3), and levels of both APC bands were unchanged (Figure 4G). Combining GSK3β inhibition with disruption of cell–cell adhesion also did not result in an increase in β-catenin levels (Figure 4G). Together, these data show that β-catenin levels are strictly regulated and that cadherin-based cell–cell adhesion does not play a role in regulating β-catenin transcriptional activity in HUVECs.
GSK3β/CKI-phosphorylated APC Localizes in Clusters at Membrane Extensions and May Promote Directed Cell Migration
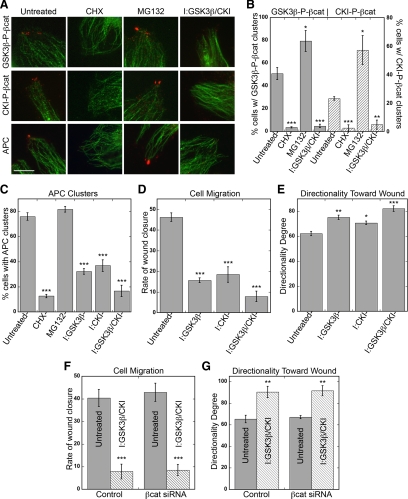
Treatment with GSK3β and CKI inhibitors resulted in a ∼90 and 78% reduction in cells with GSK3β-P-βcat and CKI-P-βcat clusters, respectively (Figure 5, A and B). Cycloheximide treatment also reduced the percent of cells with GSK3β-P-βcat and CKI-P-βcat in clusters (Figure 5, A and B), consistent with the reduced level of phosphorylated β-catenin (Figure 4, D and E). In contrast, MG132 treatment significantly increased the number and size of GSK3β-P-βcat and CKI-P-βcat clusters (Figure 5, A and B), consistent with the increased total amount of phosphorylated β-catenin (Figure 4C). These data confirm that phosphorylated β-catenin is in clusters, but the activity of the proteasome limits the amount of phosphorylated β-catenin that can accumulate at these sites.
Figure 5.
Pharmacological inhibition of GSK3β/CKI removes APC and phosphorylated β-catenin from clusters and decreases directed cell migration. (A–C) HUVECs in wound-healing assay incubated with either 10 μM CHX for 6 h, 10 μM MG132 for 4 h, 20 μM GSK3β inhibitor for 4 h, 50 μM CKI inhibitor for 4 h, or 20 μM GSK3β+50 μM CKI inhibitors for 4 h. Cells were fixed and stained for GSK3β-P-βcat, CKI-P-βcat, or APC (red) and α-tubulin (green). (A) Representative images from wound edge are shown. Scale bar, 10 μm. (B and C) Quantification of % cells at wound edge with phosphorylated β-catenin clusters (GSK3β-P-βcat; gray bars, CKI-P-βcat; dashed bars; B) or APC (C). Mean values ± SEM from three independent experiments. ***p < 0.0001, **p = 0.0008, *p < 0.009 by Student's t test. (D–G) Confluent HUVECs scratch-wounded and immediately incubated with 20 μM GSK3β inhibitor, 50 μM CKI inhibitor, or 20 μM GSK3β+50 μM CKI inhibitors. Cells were imaged every 15 min in a 37°C, 5% CO2 chamber. (D and F) Quantification of rate of wound closure from 0 to 6 h. Mean values ± SEM from two independent experiments performed in triplicate. ***p < 0.0001 by Student's t test. (E and G) Directional migration toward scratch wound edge. Directionality is defined as average angle toward scratch wound edge, with 0 indicating complete orientation toward wound and 180 indicating complete orientation away from wound. Mean values ± SEM from two independent experiments performed in triplicate. ***p < 0.0001, **p < 0.0006, *p = 0.003 by Student's t test.
We observed similar trends for APC in clusters. Treatment with GSK3β or CKI inhibitor caused a ∼55% reduction in APC clusters, which was additive in the presence of both inhibitors (Figure 5, A and C; see also Purro et al., 2008). Because, GSK3β and CKI inhibition decreased the level of the slower migrating GSK3β/CKI-P-APC band but not the faster migrating band (Figure 4C), we suggest that GSK3β/CKI-P-APC is the predominant form of APC present in clusters. This conclusion is supported by 1) decreased levels of GSK3β/CKI-P-APC (Figure 4, D and E) and loss of APC in clusters at cell extensions with cycloheximide (Figure 5, A and C); and 2) increased levels of both bands of APC with MG132 (Figure 4C), which caused larger clusters with ∼ 35% increase in the amount of APC (n = 140), even though the percent of cells with APC clusters was only slightly increased (Figure 5, A and C).
APC clusters are thought to coordinate microtubules at cell extensions and loss of APC correlates with a decrease in cell migration (Sansom et al., 2004; Kroboth et al., 2007) and microtubule stability (Kroboth et al., 2007). Therefore, we tested whether GSK3β/CKI-P-APC, the main component of clusters at HUVEC membrane extensions, plays a role in HUVEC migration using a wound-healing assay. Treatment with GSK3β or CKI inhibitor resulted in a ∼67 and 60% decrease in the rate of wound closure, respectively, and the effect was additive in the presence of both inhibitors (Figure 5D). The average angle toward the scratch-wound edge was increased in the presence of GSK3β and CKI inhibitors, indicating that cells were not only migrating slower but also with less directionality (Figure 5E), probably due to loss of centrosome polarity (Etienne-Manneville and Hall, 2003).
Because GSK3β and CKI inhibition reduced the level of both phosphorylated β-catenin and APC in clusters, we could not exclude the possibility that the observed effect on cell migration was also due to the inhibition of GSK3β/CKI-P-βcat, rather than APC. To distinguish roles of phosphorylated β-catenin and APC, we treated HUVECs with β-catenin siRNA and performed the wound-healing assay in the presence or absence of GSK3β and CKI inhibitors. Inhibition of GSK3β and CKI in β-catenin siRNA cells reduced the rate of wound closure (Figure 5F) and decreased directionality (Figure 5G) to a level similar to that of control cells treated with inhibitors. These results implicate a positive role for GSK3β/CKI-P-APC clusters, but not GSK3β/CKI-P-βcat clusters, in promoting directed cell migration in HUVECs. Thus, our studies suggest that GSK3β/CKI-P-APC has a role in regulating cell behavior that is independent of previously known roles of APC in regulating β-catenin–mediated transcription.
A Complex of APC and β-Catenin at the Lateral Membrane Is Phosphorylated by GSK3β/CKI
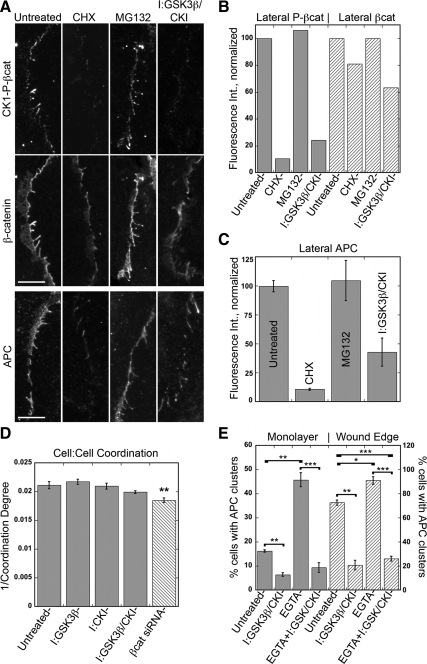
APC and phosphorylated β-catenin are localized to lateral plasma membrane in HUVEC at sites of cell–cell contacts (Figures 1–3). Treatment with GSK3β and CKI inhibitors reduced by ∼ 75% the amount of GSK3β/CKI-P-βcat localized to the lateral membrane (Figure 6, A and B). Inhibition of protein synthesis with cycloheximide also depleted GSK3β/CKI-P-βcat from the lateral membrane (Figure 6, A and B). Surprisingly, treatment with MG132 had little effect on the amount of GSK3β/CKI-P-βcat localized along the lateral membrane (Figure 6, A and B). We also observed a ∼35% reduction in total β-catenin (Figure 6, A and B) and VE-cadherin (E.S.H., unpublished results) in the presence of GSK3β and CKI inhibitors, indicating an overall decrease in the level of the adherens junction complex.
Figure 6.
Pharmacological inhibition of GSK3β/ CKI reduces APC and phosphorylated β-catenin at lateral membrane but does not significantly affect cell coordination. (A–C) HUVECs incubated with either 10 μM CHX for 6 h, 10 μM MG132 for 4 h, or 20 μM GSK3β+50 μM CKI inhibitors for 4 h. Cells were fixed and stained for β-catenin and either GSK3β-P-βcat, CKI-P-βcat, or APC. (A) Representative images of CKI-P-βcat, β-catenin, and APC; CKI-P-βcat and β-catenin are from same image. Only enlarged images of lateral plasma membrane are shown. Scale bar, 10 μm. (B) Quantification of β-catenin, GSK3β-P-βcat, and CKI-P-βcat at lateral plasma membrane; 50 contacts from two independent experiments were measured. Fluorescence intensity at the lateral membrane was normalized so untreated samples are labeled as 100%. Because GSK3β-P-βcat and CKI-P-βcat showed similar changes in fluorescence intensity at lateral plasma membrane, these results were combined and represented as phosphorylated β-catenin (gray bars). (C) Quantification of APC at lateral plasma membrane; 100 contacts from two independent experiments were measured. (D) Tracking of cells in wound monolayer using Hoechst-stained nuclei over 6 h. Coordination degree is defined as average difference of angle between target cell and its neighbors in degrees. Value corresponding to 1/coordination degree is plotted, so a smaller number indicates less coordination between cells. Mean values ± SEM from two independent experiments performed in triplicate. **p = 0.002 by Student's t test. (E) HUVECs were treated with 2 mM EGTA, 20 μM GSK3β+50 μM CKI inhibitors, or both for 2 h and fixed and stained for APC, β-catenin, and α-tubulin. Quantification of % cells within confluent monolayer (gray bars) or from wound edge (dashed bars) with APC clusters. Mean values ± SEM from three independent experiments. ***p < 0.0001, **p < 0.0006, *p = 0.009 by Student's t test.
These inhibitors also affected APC localization along the lateral membrane (Figure 6, A and C). Cycloheximide and GSK3β and CKI inhibitors caused a decrease in APC along the lateral membrane (∼88 and 57% reduction, respectively; Figure 6, A and C). Because cycloheximide and GSK3β and CKI inhibition effectively depleted the amount of GSK3β/CKI-P-APC in cell extracts, it is likely the remaining APC at the lateral membrane is not phosphorylated by these kinases and corresponds to the faster migrating APC band on Western blots (Figure 4C). A Drosophila GSK3β (Zw3) mutant showed reduced cortical localization of APC2 (McCartney et al., 2001), suggesting that in both flies and humans GSK3β-mediated phosphorylation of APC regulates its localization to the lateral membrane. Similar to phosphorylated β-catenin, MG132 treatment had little or no effect on the amount of APC localized along the lateral membrane (Figure 6, A and C), despite an increase in both APC bands on Western blot (Figure 4C), suggesting these proteins at the lateral membrane may be degraded by a nonproteasomal pathway. Taken together, these results indicate that under basal conditions GSK3β/CKI-P-APC is along the lateral membrane and at clusters, whereas the complex of non-GSK3β/CK1-P-APC is along the lateral membrane but not at clusters.
Disruption of cell–cell contacts removed all APC from the lateral membrane and resulted in a significant increase in APC in clusters (Figure 2, C and D; Supplemental Figure S2C), but did not alter the amounts of either APC band on Western blots (Figure 4G). To test whether both complexes of APC could localize to clusters, we disrupted cell–cell adhesion and inhibited GSK3β and CKI. As before, loss of cell–cell adhesion increased the percent of HUVECs with APC clusters, whereas inhibition of GSK3β and CKI decreased APC clusters (Figure 6E). In combination, however, GSK3β and CKI inhibition blocked the effect of loss of cell–cell adhesion and significantly reduced the percent of cells with APC clusters (Figure 6E). These results indicate that although disruption of cell–cell adhesion releases both GSK3β/CKI-P-APC and non-GSK3β/CKI-P-APC from the lateral membrane, only GSK3β/CKI-P-APC can relocalize to clusters.
GSK3β/CKI Inhibition Does Not Affect Cell–Cell Adhesion
The reduction in APC at the lateral membrane caused by inhibition of GSK3β and CKI could be due to direct or indirect effects of these inhibitors on cell–cell adhesion. We performed several classic adhesion assays including hanging drop and spinning aggregation assays (Kim et al., 2000; Shimoyama et al., 2000; Ehrlich et al., 2002), but HUVECs did not form strong cell–cell contacts because endothelial cell–cell adhesion is weaker and more dynamic than that of epithelial cells (reviewed in Schnoor and Parkos, 2008).
As an alternative approach, we used live cell tracking to examine cell coordination in HUVEC monolayers. This is an indirect measure of the overall cell–cell adhesive properties of the monolayer, which is obtained by tracking individual cells over time and calculating the average difference of angle (coordination degree) between a target cell and its neighbors (Vitorino and Meyer, 2008). There was no significant change in cell coordination in the presence of GSK3β and CKI inhibitors (Figure 6D), even though the levels of β-catenin and VE-cadherin were reduced overall (Figure 4C) and along lateral membranes (Figure 6, A and B). Note also GSK3β or CKI inhibition did not significantly affect HUVEC monolayer permeability to small molecules or neutrophils (Supplemental Figure S4, A and B). Because efficient knockdown of VE-cadherin reduced cell coordination in HUVECs (Vitorino and Meyer, 2008), we assume the levels of adherens junction proteins that remained at the lateral membrane in the presence of GSK3β and CKI inhibitors are sufficient to maintain cell–cell adhesion in HUVECs.
These results indicate that GSK3β- and CKI-phosphorylated forms of APC and β-catenin do not play a major role in regulating HUVEC cell–cell adhesion. However, we cannot rule out that the non-GSK3β/CKI-phosphorylated complex of APC is involved in cell–cell adhesion, because we cannot selectively remove this complex. We failed to deplete APC from HUVECs using a variety of strategies due to the fact that ∼50% of APC has a t1/2 >30 h (Figure 4, D and E). Depletion of β-catenin resulted in complete loss of lateral membrane APC localization (Figure 2B) and a decrease in cell coordination (Figure 6D; dashed bar), but VE-cadherin is also depleted from lateral membranes. Thus, these effects on cell–cell adhesion cannot be attributed to APC alone.
In MDCK Cells APC Localizes to Clusters, But Not to the Lateral Plasma Membrane, and Is Phosphorylated by GSK3β/CKI
To examine whether results from HUVECs could be generalized to other cell types, we repeated several key experiments in MDCK epithelial cells, a cell line that is typically used to study APC function. APC localized in punctate clusters at cell extensions where microtubules were focused, as reported previously (Nathke et al., 1996; Barth et al., 2002) and similar to APC localization in clusters in HUVECs. In contrast to HUVECs, however, we did not detect significant amounts of APC at cell–cell contacts (Supplemental Figure S5A). Consistent with this finding, APC did not coimmunoprecipitate with E-cadherin or α-catenin from MDCK extracts, although the phosphorylated form of β-catenin did coimmunoprecipitate with APC (Supplemental Figure S5B).
Two APC bands are detected in Western blots of MDCK cell extracts, similar to HUVECs. Inhibition of GSK3β and CKI collapsed the slower migrating band (Supplemental Figure S5C) and reduced cells with APC clusters ∼50% (Supplemental Figure S5, E and F), indicating that APC clusters in MDCK cells are also phosphorylated by these kinases. Inhibition of GSK3β and CKI efficiently removed phosphorylated β-catenin from cells, but the levels of neither total β-catenin (Supplemental Figure S5C) nor Tcf/Lef-mediated transcription (Supplemental Figure S5D) were affected, similar to results in HUVECs. MG132 treatment and disruption of cell–cell adhesion also did not increase total β-catenin levels or Tcf/Lef-mediated transcription (Supplemental Figure S5, C and D). As in HUVECs, only high transient expression of GFP-ΔGSK-βcat significantly increased Tcf/Lef-mediated transcription (Supplemental Figure S5D). Thus, although we find differences in the localization of APC between HUVECs and MDCK cells, both cell types have clusters containing GSK3β/CKI-P-APC, which may have functions independent of roles in β-catenin–mediated transcription.
DISCUSSION
In primary HUVECs, endogenous APC localizes in two distinct structures that are associated with different cytoskeletons (Figure 7): 1) in microtubule-enriched, punctate clusters that are restricted to membrane extensions at the free edge of cells, and at tricellular corners of cells in confluent monolayers; and 2) in teardrop-like protrusions along cell–cell contacts in association with actin filaments, and a few microtubules. Currently we do not know how APC localizes to these sites, but the different requirements for actin and microtubule cytoskeletons suggest distinct targeting mechanisms. APC has been described previously in punctate clusters in multiple cell types (Nathke et al., 1996; Mimori-Kiyosue et al., 2000; Etienne-Manneville and Hall, 2003; Votin et al., 2005) and at the lateral plasma membrane of other cells (Miyashiro et al., 1995; Yu et al., 1999; Rosin-Arbesfeld et al., 2001), but this is the first observation of robust, endogenous APC staining at both sites in untreated cells. The simultaneous localization of full-length endogenous APC to both of these sites in primary HUVECs enabled us to examine their functional and regulatory relationship.
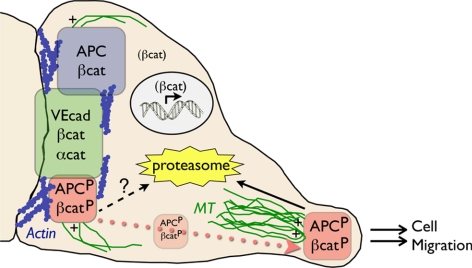
Figure 7.
APC forms three distinct complexes in endothelial cells. APC localizes to in punctate clusters at membrane extensions, where microtubule (MT) plus ends are focused, and at the lateral plasma membrane with actin and cell–cell adhesion proteins. These two populations of APC comprise three distinct molecular complexes. Clusters, which promote directed cell migration, are comprised of GSK3β/CKI-P-APC (APCP) and GSK3β/CKI-P-βcat (βcatP) and are turned over rapidly through the proteasome. At the lateral membrane, GSK3β/CKI-P-APC and GSK3β/CKI-P-βcat associate separately from adherens junction complex and may be degraded in part by a nonproteasomal pathway, whereas non-GSK3β/CKI-phosphorylated complexes of APC and β-catenin are relatively stable. GSK3β/CKI-P-APC and GSK3β/CKI-P-βcat can relocalize from the lateral membrane into clusters upon disruption of cell–cell adhesion.
Complexes of APC located along lateral membranes and in clusters were not only spatially distinct, but also biochemically different based on phosphorylation status, protein turnover rates, and associated binding partners. Clusters were enriched in GSK3β/CKI-P-APC and GSK3β/CKI-P-βcat, which were rapidly degraded by the proteasome. Lateral membranes contained a complex of GSK3β/CKI-P-APC and GSK3β/CKI-P-βcat that was separate from adherens junction complex and might be partially degraded by a nonproteasomal pathway, and a complex of non-GSK3β/CKI-phosphorylated APC and β-catenin that was relatively stable (Figure 7). The requirement for GSK3β/CKI phosphorylation to retain APC in clusters corresponds with results from Purro et al. (2008) who found that pharmacological inhibition of GSK3β resulted in loss of APC from the growth cone periphery in DRG neurons. However, to our knowledge, our work is the first to directly analyze the phosphorylation state of APC at both clusters and the lateral membrane.
It was previously shown that phosphorylation of APC by GSK3β inhibited interaction of APC with microtubules in vitro. This effect required priming by protein kinase A (PKA; Zumbrunn et al., 2001). However, CKI is the priming kinase for GSK3β-mediated phosphorylation of APC (Liu et al., 2002). Thus, the functional significance of this PKA/GSK3β-mediated phosphorylation of APC in vivo is unknown.
APC in clusters is required for directional cell migration in a variety of cells (Sansom et al., 2004; Kroboth et al., 2007), and our results suggest a similar role for APC in HUVECs. Importantly, our results implicate a role for GSK3β/CKI-P-APC, but not GSK3β/CKI-P-βcat, in regulating cell migration that is independent of roles in regulating β-catenin–mediated transcription. We hypothesize GSK3β/CKI-P-APC may regulate cell migration by directly affecting microtubule dynamics, similar to the proposed role of APC in other cell types (Wen et al., 2004; Kita et al., 2006; Kroboth et al., 2007; Purro et al., 2008).
The role for APC along the lateral membrane is less clear. Previous studies implicated APC in cell–cell adhesion because expression of full-length APC in colon carcinoma cells restored cell–cell adhesion (Faux et al., 2004), and a loss-of-function allele of Drosophila APC2 resulted in cell–cell adhesion defects (Hamada and Bienz, 2002). It was reported in another study, however, that loss of both APC1 and APC2 did not impair cadherin-based cell–cell adhesion in Drosophila (McCartney et al., 2006). From our results, it seems unlikely that APC regulates cell–cell adhesion directly, as the interaction of APC with VE-cadherin is minimal. Also, inhibition of GSK3β and CKI did not significantly effect cell coordination or cell barrier functions, indicating that GSK3β/CKI-phosphorylated forms of APC and β-catenin were not required to maintain cell–cell adhesion in HUVECs. However, APC could control cell–cell adhesion indirectly by regulating the availability of β-catenin for engagement into adherens junctions or by regulating vesicle trafficking to and from the plasma membrane by tethering microtubules to cell–cell contacts (reviewed in Kamal and Goldstein, 2000). Alternatively, lateral membrane GSK3β/CKI-P-APC could be a sensor for balancing cell–cell adhesion and cell migration. We found that dispersal of lateral membrane complexes of APC upon loss of cell–cell adhesion resulted in a concomitant increase in the amount of GSK3β/CKI-P-APC in clusters. We suggest that rapid recruitment of GSK3β/CKI-P-APC from cell–cell contacts into clusters in cell extensions might signal increased microtubule dynamics necessary for cell migration.
Although our data suggest that GSK3β/CKI-P-APC clusters may regulate cell migration independent of β-catenin transcriptional activity, our results showed that these clusters, rather than APC at lateral membranes, were associated with proteasomal degradation of APC and β-catenin. This is in contrast to reports that the APC/β-catenin destruction complex localizes to cell–cell contacts (Maher et al., 2009). However, Maher et al. examined APC distributions in SW480 cells, a colon carcinoma cell line expressing a C-terminal–truncated mutant of APC that lacks binding sites for microtubules, EB1, β-catenin, and axin. Significantly, this mutant APC cannot target β-catenin for ubiquitination and degradation (Yang et al., 2006) nor form clusters. Hence, functions and regulation of the APC complex in clusters that we identified in primary HUVECs could not be identified in SW480 cells.
Maher et al. (2009) also reported that cadherin-based cell adhesion in SW480 and MDCK cells increased the activity of the destruction complex, thus limiting Wnt signaling. We did not observe cell–cell adhesion–dependent changes in APC or β-catenin phosphorylation or Tcf/Lef-mediated transcription in either HUVECs or MDCK cells. Instead, we found that disruption of cell–cell adhesion relocalized GSK3β/CKI-P-APC and GSK3β/CKI-P-βcat to clusters, indicating that clusters can be sites where the destruction complex is active, thus suppressing β-catenin–mediated transcription even in the absence of cell–cell adhesion. It is unclear why these studies came to different conclusions.
It was surprising that β-catenin–mediated transcription was not increased in either HUVECs or MDCK cells upon direct inhibition of GSK3β/CKI. However, the results from our transcriptional assays are consistent with our Western blot analysis showing β-catenin levels are not enhanced upon treatment with these specific inhibitors. We currently do not know the molecular basis of this result, but these data suggest that cells may contain multiple endogenous mechanisms for maintaining tight control over levels of free β-catenin. However, this feature has allowed us to begin to define a function for endogenous GSK3β/CKI-P-APC in cell migration in the absence of compounding effects from APC's role in transcriptional signaling.
In summary, we have defined three APC complexes in primary HUVECs using a combination of morphological and biochemical approaches. Two of these complexes associate with cell–cell contacts and contain either GSK3β/CKI- or non-GSK3β/CKI-P-APC. The other complex localizes in clusters at cell extensions and contains only GSK3β/CKI-P-APC (Figure 7). GSK3β/CKI-P-APC at cell–cell contacts may comprise, at least in part, of a source of APC in clusters upon loss of cell–cell adhesion. Significantly, none of these complexes appear to be involved directly in regulating cell–cell adhesion. Independent of β-catenin transcriptional activity, the complex of APC in clusters may function as a regulator of directional cell migration. Thus we have uncoupled different APC functions and have shown that they are independently regulated and localized.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Angela Barth and other members of the Nelson laboratory for technical help and advice; Feng-Chiao Tsai for help with live cell imaging and tracking; and Drs. Sean Collins, Juergen Behrens, Barry Gumbiner, Paul Polakis, Marc van de Wetering, and Hans Clevers for reagents. This work was supported by NIH Grant GM035527 to WJN, and a postdoctoral fellowship from Jane Coffin Childs Fund for Medical Research to E.S.H.
Abbreviations used:
- APC
adenomatous polyposis coli
- αcat
α-catenin
- βcat
β-catenin
- CKI
casein kinase 1
- CKI-P-βcat
casein kinase I–phosphorylated β-catenin
- EB1
end-binding protein-1
- Ecad
epithelial cadherin
- GSK3β
glycogen synthase kinase 3 beta
- GSK3β/CKI-P-APC
glycogen synthase kinase 3 beta/casein kinase 1–phosphorylated APC
- GSK3β-P-βcat
glycogen synthase kinase 3 β–phosphorylated β-catenin
- HUVEC
human umbilical vein endothelial cells
- Lef
lymphoid-enhancing factor
- MDCK
Madin-Darby canine kidney cells
- Tcf
T-cell factor
- TOP
Tcf optimal promoter
- VEcad
vascular endothelial cadherin.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-03-0235) on June 2, 2010.
REFERENCES
- Allport J. R., Ding H., Collins T., Gerritsen M. E., Luscinskas F. W. Endothelial-dependent mechanisms regulate leukocyte transmigration: a process involving the proteasome and disruption of the vascular endothelial-cadherin complex at endothelial cell-to-cell junctions. J. Exp. Med. 1997;186:517–527. doi: 10.1084/jem.186.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth A. I., Caro-Gonzalez H. Y., Nelson W. J. Role of adenomatous polyposis coli (APC) and microtubules in directional cell migration and neuronal polarization. Semin. Cell Dev. Biol. 2008;19:245–251. doi: 10.1016/j.semcdb.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth A. I., Siemers K. A., Nelson W. J. Dissecting interactions between EB1, microtubules and APC in cortical clusters at the plasma membrane. J. Cell Sci. 2002;115:1583–1590. doi: 10.1242/jcs.115.8.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth A. I., Stewart D. B., Nelson W. J. T cell factor-activated transcription is not sufficient to induce anchorage-independent growth of epithelial cells expressing mutant beta-catenin. Proc. Natl. Acad. Sci. USA. 1999;96:4947–4952. doi: 10.1073/pnas.96.9.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. T., McEwen D. G., Myster D. L., Duronio R. J., Loureiro J., Peifer M. A screen for mutations that suppress the phenotype of Drosophila armadillo, the beta-catenin homolog. Genetics. 2000;155:1725–1740. doi: 10.1093/genetics/155.4.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. L., Roberts G. T., Stuart N., Wakeman J. A. Analysis of a panel of antibodies to APC reveals consistent activity towards an unidentified protein. Br. J. Cancer. 2007;97:384–390. doi: 10.1038/sj.bjc.6603873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich J. S., Hansen M. D., Nelson W. J. Spatio-temporal regulation of Rac1 localization and lamellipodia dynamics during epithelial cell–cell adhesion. Dev. Cell. 2002;3:259–270. doi: 10.1016/s1534-5807(02)00216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S., Hall A. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature. 2003;421:753–756. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]
- Faux M. C., Ross J. L., Meeker C., Johns T., Ji H., Simpson R. J., Layton M. J., Burgess A. W. Restoration of full-length adenomatous polyposis coli (APC) protein in a colon cancer cell line enhances cell adhesion. J. Cell Sci. 2004;117:427–439. doi: 10.1242/jcs.00862. [DOI] [PubMed] [Google Scholar]
- Goodwin A. M., Sullivan K. M., D'Amore P. A. Cultured endothelial cells display endogenous activation of the canonical Wnt signaling pathway and express multiple ligands, receptors, and secreted modulators of Wnt signaling. Dev. Dyn. 2006;235:3110–3120. doi: 10.1002/dvdy.20939. [DOI] [PubMed] [Google Scholar]
- Groden J., et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- Grohmann A., Tanneberger K., Alzner A., Schneikert J., Behrens J. AMER1 regulates the distribution of the tumor suppressor APC between microtubules and the plasma membrane. J. Cell Sci. 2007;120:3738–3747. doi: 10.1242/jcs.011320. [DOI] [PubMed] [Google Scholar]
- Hamada F., Bienz M. A Drosophila APC tumour suppressor homologue functions in cellular adhesion. Nat. Cell Biol. 2002;4:208–213. doi: 10.1038/ncb755. [DOI] [PubMed] [Google Scholar]
- Hart M. J., de los Santos R., Albert I. N., Rubinfeld B., Polakis P. Downregulation of beta-catenin by human Axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Curr. Biol. 1998;8:573–581. doi: 10.1016/s0960-9822(98)70226-x. [DOI] [PubMed] [Google Scholar]
- Ikeda S., Kishida M., Matsuura Y., Usui H., Kikuchi A. GSK-3beta-dependent phosphorylation of adenomatous polyposis coli gene product can be modulated by beta-catenin and protein phosphatase 2A complexed with Axin. Oncogene. 2000;19:537–545. doi: 10.1038/sj.onc.1203359. [DOI] [PubMed] [Google Scholar]
- Kamal A., Goldstein L. S. Connecting vesicle transport to the cytoskeleton. Curr. Opin. Cell Biol. 2000;12:503–508. doi: 10.1016/s0955-0674(00)00123-x. [DOI] [PubMed] [Google Scholar]
- Kim J. B., Islam S., Kim Y. J., Prudoff R. S., Sass K. M., Wheelock M. J., Johnson K. R. N-Cadherin extracellular repeat 4 mediates epithelial to mesenchymal transition and increased motility. J. Cell Biol. 2000;151:1193–1206. doi: 10.1083/jcb.151.6.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita K., Wittmann T., Nathke I. S., Waterman-Storer C. M. Adenomatous polyposis coli on microtubule plus ends in cell extensions can promote microtubule net growth with or without EB1. Mol. Biol. Cell. 2006;17:2331–2345. doi: 10.1091/mbc.E05-06-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinek V., Barker N., Morin P. J., van Wichen D., de Weger R., Kinzler K. W., Vogelstein B., Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Kroboth K., Newton I. P., Kita K., Dikovskaya D., Zumbrunn J., Waterman-Storer C. M., Nathke I. S. Lack of adenomatous polyposis coli protein correlates with a decrease in cell migration and overall changes in microtubule stability. Mol. Biol. Cell. 2007;18:910–918. doi: 10.1091/mbc.E06-03-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Li Y., Semenov M., Han C., Baeg G. H., Tan Y., Zhang Z., Lin X., He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- Maher M. T., Flozak A. S., Stocker A. M., Chenn A., Gottardi C. J. Activity of the beta-catenin phosphodestruction complex at cell-cell contacts is enhanced by cadherin-based adhesion. J. Cell Biol. 2009;186:219–228. doi: 10.1083/jcb.200811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney B. M., Dierick H. A., Kirkpatrick C., Moline M. M., Baas A., Peifer M., Bejsovec A. Drosophila APC2 is a cytoskeletally-associated protein that regulates wingless signaling in the embryonic epidermis. J. Cell Biol. 1999;146:1303–1318. doi: 10.1083/jcb.146.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney B. M., McEwen D. G., Grevengoed E., Maddox P., Bejsovec A., Peifer M. Drosophila APC2 and Armadillo participate in tethering mitotic spindles to cortical actin. Nat. Cell Biol. 2001;3:933–938. doi: 10.1038/ncb1001-933. [DOI] [PubMed] [Google Scholar]
- McCartney B. M., Nathke I. S. Cell regulation by the Apc protein Apc as master regulator of epithelia. Curr. Opin. Cell Biol. 2008;20:186–193. doi: 10.1016/j.ceb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- McCartney B. M., Price M. H., Webb R. L., Hayden M. A., Holot L. M., Zhou M., Bejsovec A., Peifer M. Testing hypotheses for the functions of APC family proteins using null and truncation alleles in Drosophila. Development. 2006;133:2407–2418. doi: 10.1242/dev.02398. [DOI] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y., Shiina N., Tsukita S. Adenomatous polyposis coli (APC) protein moves along microtubules and concentrates at their growing ends in epithelial cells. J. Cell Biol. 2000;148:505–518. doi: 10.1083/jcb.148.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashiro I., Senda T., Matsumine A., Baeg G. H., Kuroda T., Shimano T., Miura S., Noda T., Kobayashi S., Monden M. Subcellular localization of the APC protein: immunoelectron microscopic study of the association of the APC protein with catenin. Oncogene. 1995;11:89–96. [PubMed] [Google Scholar]
- Morin P. J., Sparks A. B., Korinek V., Barker N., Clevers H., Vogelstein B., Kinzler K. W. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Moseley J. B., Bartolini F., Okada K., Wen Y., Gundersen G. G., Goode B. L. Regulated binding of adenomatous polyposis coli protein to actin. J. Biol. Chem. 2007;282:12661–12668. doi: 10.1074/jbc.M610615200. [DOI] [PubMed] [Google Scholar]
- Munemitsu S., Albert I., Souza B., Rubinfeld B., Polakis P. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc. Natl. Acad. Sci. USA. 1995;92:3046–3050. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemitsu S., Souza B., Muller O., Albert I., Rubinfeld B., Polakis P. The APC gene product associates with microtubules in vivo and promotes their assembly in vitro. Cancer Res. 1994;54:3676–3681. [PubMed] [Google Scholar]
- Nakamura T., Hamada F., Ishidate T., Anai K., Kawahara K., Toyoshima K., Akiyama T. Axin, an inhibitor of the Wnt signalling pathway, interacts with beta-catenin, GSK-3beta and APC and reduces the beta-catenin level. Genes Cells. 1998;3:395–403. doi: 10.1046/j.1365-2443.1998.00198.x. [DOI] [PubMed] [Google Scholar]
- Nathke I. S., Adams C. L., Polakis P., Sellin J. H., Nelson W. J. The adenomatous polyposis coli tumor suppressor protein localizes to plasma membrane sites involved in active cell migration. J. Cell Biol. 1996;134:165–179. doi: 10.1083/jcb.134.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J. Regulation of cell–cell adhesion by the cadherin-catenin complex. Biochem. Soc. Trans. 2008;36:149–155. doi: 10.1042/BST0360149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J., Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P. The adenomatous polyposis coli (APC) tumor suppressor. Biochim. Biophys. Acta. 1997;1332:F127–147. doi: 10.1016/s0304-419x(97)00008-5. [DOI] [PubMed] [Google Scholar]
- Purro S. A., Ciani L., Hoyos-Flight M., Stamatakou E., Siomou E., Salinas P. C. Wnt regulates axon behavior through changes in microtubule growth directionality: a new role for adenomatous polyposis coli. J. Neurosci. 2008;28:8644–8654. doi: 10.1523/JNEUROSCI.2320-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinacher-Schick A., Gumbiner B. M. Apical membrane localization of the adenomatous polyposis coli tumor suppressor protein and subcellular distribution of the beta-catenin destruction complex in polarized epithelial cells. J. Cell Biol. 2001;152:491–502. doi: 10.1083/jcb.152.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin-Arbesfeld R., Ihrke G., Bienz M. Actin-dependent membrane association of the APC tumour suppressor in polarized mammalian epithelial cells. EMBO J. 2001;20:5929–5939. doi: 10.1093/emboj/20.21.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld B., Souza B., Albert I., Muller O., Chamberlain S. H., Masiarz F. R., Munemitsu S., Polakis P. Association of the APC gene product with beta-catenin. Science. 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B., Souza B., Albert I., Munemitsu S., Polakis P. The APC protein and E-cadherin form similar but independent complexes with alpha-catenin, beta-catenin, and plakoglobin. J. Biol. Chem. 1995;270:5549–5555. doi: 10.1074/jbc.270.10.5549. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B., Tice D. A., Polakis P. Axin-dependent phosphorylation of the adenomatous polyposis coli protein mediated by casein kinase 1epsilon. J. Biol. Chem. 2001;276:39037–39045. doi: 10.1074/jbc.M105148200. [DOI] [PubMed] [Google Scholar]
- Sansom O. J., et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004;18:1385–1390. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoor M., Parkos C. A. Disassembly of endothelial and epithelial junctions during leukocyte transmigration. Front. Biosci. 2008;13:6638–6652. doi: 10.2741/3178. [DOI] [PubMed] [Google Scholar]
- Shimoyama Y., Tsujimoto G., Kitajima M., Natori M. Identification of three human type-II classic cadherins and frequent heterophilic interactions between different subclasses of type-II classic cadherins. Biochem. J. 2000;349:159–167. doi: 10.1042/0264-6021:3490159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. J., Levy D. B., Maupin P., Pollard T. D., Vogelstein B., Kinzler K. W. Wild-type but not mutant APC associates with the microtubule cytoskeleton. Cancer Res. 1994;54:3672–3675. [PubMed] [Google Scholar]
- Stewart D. B., Nelson W. J. Identification of four distinct pools of catenins in mammalian cells and transformation-dependent changes in catenin distributions among these pools. J. Biol. Chem. 1997;272:29652–29662. doi: 10.1074/jbc.272.47.29652. [DOI] [PubMed] [Google Scholar]
- Townsley F. M., Bienz M. Actin-dependent membrane association of a Drosophila epithelial APC protein and its effect on junctional Armadillo. Curr. Biol. 2000;10:1339–1348. doi: 10.1016/s0960-9822(00)00770-3. [DOI] [PubMed] [Google Scholar]
- Vitorino P., Meyer T. Modular control of endothelial sheet migration. Genes Dev. 2008;22:3268–3281. doi: 10.1101/gad.1725808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votin V., Nelson W. J., Barth A. I. Neurite outgrowth involves adenomatous polyposis coli protein and beta-catenin. J. Cell Sci. 2005;118:5699–5708. doi: 10.1242/jcs.02679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y., Eng C. H., Schmoranzer J., Cabrera-Poch N., Morris E. J., Chen M., Wallar B. J., Alberts A. S., Gundersen G. G. EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat. Cell Biol. 2004;6:820–830. doi: 10.1038/ncb1160. [DOI] [PubMed] [Google Scholar]
- Wright M., Aikawa M., Szeto W., Papkoff J. Identification of a Wnt-responsive signal transduction pathway in primary endothelial cells. Biochem. Biophys. Res. Commun. 1999;263:384–388. doi: 10.1006/bbrc.1999.1344. [DOI] [PubMed] [Google Scholar]
- Wu G., He X., Yang Z., Guo L. [Influence of liposome-mediated recombinant plasmid pIRES-hBMP-2-hVEGF165 on osteogenic activity of hBMSCs in vitro] Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23:1124–1128. [PubMed] [Google Scholar]
- Yang J., Zhang W., Evans P. M., Chen X., He X., Liu C. Adenomatous polyposis coli (APC) differentially regulates beta-catenin phosphorylation and ubiquitination in colon cancer cells. J. Biol. Chem. 2006;281:17751–17757. doi: 10.1074/jbc.M600831200. [DOI] [PubMed] [Google Scholar]
- Yu X., Waltzer L., Bienz M. A new Drosophila APC homologue associated with adhesive zones of epithelial cells. Nat. Cell Biol. 1999;1:144–151. doi: 10.1038/11064. [DOI] [PubMed] [Google Scholar]
- Zumbrunn J., Kinoshita K., Hyman A. A., Nathke I. S. Binding of the adenomatous polyposis coli protein to microtubules increases microtubule stability and is regulated by GSK3 beta phosphorylation. Curr. Biol. 2001;11:44–49. doi: 10.1016/s0960-9822(01)00002-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.