Abstract
Enterococcus faecalis is part of the commensal microbiota of humans and its main habitat is the gastrointestinal tract. Although harmless in healthy individuals, E. faecalis has emerged as a major cause of nosocomial infections. In order to better understand the transformation of a harmless commensal into a life-threatening pathogen, we developed a Recombination-based In Vivo Expression Technology for E. faecalis. Two R-IVET systems with different levels of sensitivity have been constructed in a E. faecalis V583 derivative strain and tested in the insect model Galleria mellonella, during growth in urine, in a mouse bacteremia and in a mouse peritonitis model. Our combined results led to the identification of 81 in vivo activated genes. Among them, the ef_3196/7 operon was shown to be strongly induced in the insect host model. Deletion of this operonic structure demonstrated that this two-component system was essential to the E. faecalis pathogenic potential in Galleria. Gene ef_0377, induced in insect and mammalian models, has also been further analyzed and it has been demonstrated that this ankyrin-encoding gene was also involved in E. faecalis virulence. Thus these R-IVET screenings led to the identification of new E. faecalis factors implied in in vivo persistence and pathogenic potential of this opportunistic pathogen.
Introduction
Enterococcus faecalis is a ubiquitous lactic acid bacterium and a core constituent of the intestinal flora of humans and many animals. The intrinsic ability of this bacterium to resist strongly against stressing environments may allow the bacterium to persist in hospital environments and to survive host defences [1], [2]. In the last decades, Enterococci have been recognized as one of the most common bacteria involved in hospital-acquired infections [3]. Indeed, these microorganisms can trigger serious infections such as sepsis, urinary tract infections, peritonitis and endocarditis and the species E. faecalis is still responsible for the majority of human enterococcal infections [3], [4]. For this reason, clinical strains have been studied for their virulence-associated factors: the Cytolysin CylL [5], the Aggregation substance Agg [6], the metallo-endopeptidase GelE [7], the Extracellular Surface Protein Esp [8], and the cell surface protein EfaA [9]. Despite studies characterizing these proteins, our knowledge of the mechanisms involved in infections, especially transcriptional modulation occurring in living hosts, remains incomplete. Thus, in order to further increase our understanding of enterococcal pathogenesis, it is necessary to identify genes that are specific to infection.
In an infected host, microorganisms are subjected to combined stresses. Although these conditions could be simulated in vitro by the study of each of these stresses independently, it is not possible to reproduce an exact mimic of the complex and dynamic environment encountered by bacteria in infected hosts. In a different approach, several In Vivo expression technologies (IVET) have been developed [10], [11]. With this technology, the living infected animal is the inducing-signal of virulence-associated genes expression. An IVET strategy was developed for the first time in Salmonella enterica serovar Typhimurium to identify in vivo highly expressed genes as compared to expression under laboratory conditions. Five in vivo-induced (ivi) operons have been identified using this strategy, originally based on the use of an auxotrophic marker. Three of them, corresponding to the carAB, pheST-himA and rfb operons, have been confirmed to play essential roles in virulence [12]. Other IVET approaches, using antibiotic resistance markers [13] or dual reporters [14]–[16], have also been employed. However, these strategies present common drawbacks due to the fact that weakly or transiently expressed ivi genes are difficult or impossible to detect. These disadvantages have led some authors to choose a fourth IVET approach, called R-IVET for Recombination-based IVET [17].
This molecular biology strategy functions as a genetic screening that allows the detection of the specific expression of genes in vivo. The method has been successfully used to identify 72 Lactobacillus plantarum genes induced in the gastrointestinal tract of mice [14], [17]. This approach is based on the irreversible activity of the site-specific recombinase Cre from the bacteriophage P1. Only a short or low pulse of recombinase expression is necessary to trigger a resolution leading to the permanent excision of an antibiotic marker flanked by two copies of site-specific recombination sequences (loxP) and hence to a phenotypic switch that enables a selection of resolved cells after recovery of bacteria from a host. Thus, R-IVET allows also the identification of transient or conditional promoter activations [17]. To this end, a genomic library of a given bacterium is created by cloning DNA fragments upstream from a promoterless copy of the recombinase-encoding gene. At the same time, the substrate for resolution is constructed by integrating the antibiotic resistance gene and the two loxP sites into the bacterial chromosome. These modified bacteria can then be screened under chosen conditions, for instance by inoculating them into a susceptible living host model such as mice or insects.
A R-IVET screen using the TnpR recombinase has been performed recently in Enterococcus faecalis OG1RF showing that R-IVET was a suitable strategy in this microorganism. This allowed the identification of 68 loci with a putative involvement in biofilm formation [18]. Some of these promoters were confirmed by quantitative RT-PCR to be induced under these conditions.
In this paper, we describe the construction of a novel R-IVET promoter-trap strategy and its application to the Enterococcus faecalis V19 strain, a plasmid-cured derivative of the clinical isolate V583. This is the first time that such an in vivo expression technology has been used in a context of virulence in Enterococci in order to study the in vivo behavior of this bacterium. Transcriptional activations during adaptation and response to host environment were firstly monitored after the injection of these bacteria into the hemocoel of Galleria mellonella larvae. This insect model has been shown to be a practical and well-adapted model for the in vivo screening of virulence-related genes [19]–[21]. To approach conditions encountered by these bacteria during human infections, further studies were performed during exposure to urine, in a peritonitis mouse model and in a mouse bacteremia mouse model thus leading to the identification of new genetic determinants putatively involved in the pathogenic potential of Enterococcus faecalis.
Results and Discussion
Construction of a R-IVET system in E. faecalis
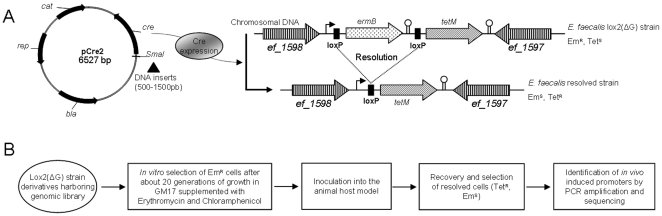
In order to identify bacterial genes that are induced during the persistence of E. faecalis in an animal host, a R-IVET strategy has been performed. The system previously developed in Lactobacillus plantarum [17] was adapted to E. faecalis and improved to allow a positive selection of resolved cells by an appropriate antibiotic selection. Therefore, a loxP-ermB-loxP-tetM cassette was integrated into the chromosome of the erythromycin sensitive E. faecalis V19 strain (Fig. 1A). To obtain this derivative strain, some intermediate plasmids had to be constructed (Fig. 2). The ermB gene and a loxP sequence separated by a strong terminator (ΔG = −36.3kcal±10%, [22]) were cloned between the first loxP site and tetM gene in plasmid pLox1. The resulting plasmid pLox2 thus carries the full loxP-ermB-loxP-tetM cassette in which the expression of the antibiotic resistance genes is controlled by the constitutive promoter from transposon Tn1545 [23] and the presence or absence of the terminator (see materials and methods part). Plasmid pLox2 was then introduced into strain V19 and subsequently integrated into the genome of this strain by a double crossover event. The V583 derivative strain thus obtained, harboring the loxP-ermB-loxP-tetM cassette was named Lox2. Southern blot experiments confirmed that the chromosomal construction was present in a single copy between genes ef_1597 and ef_1598, as expected. Our reporter system has been chosen to be integrated into this intergenic region of the E. faecalis chromosome in order to improve the stability of the construction. Both genes are convergent and a terminator located downstream from ef_1597 prevents the synthesis of an antisense-RNA attenuating the expression of the antibiotic resistance genes. Moreover, expression of the deoxyribodipyrimidine photolyase (ef_1598) would enhance the activity of the promoter cloned upstream from the first loxP site.
Figure 1. R-IVET strategy employed in E. faecalis to identify promoters that are induced in vivo.
A: Modification of Lox2 strain occurring when a promoter is active. Chromosome of V583 strain was modified by integrating ermB gene and a promoterless copy of tetM gene, first of which is flanked by two copies of site-specific recombination sequences (loxP). A V583 genomic library was constructed by cloning DNA fragments in plasmid pCre2 upstream from cre gene. If an active promoter is thus cloned, the recombinase Cre would be expressed resulting in a homologous recombination event between both loxP sites. This triggers the irreversible excision of the ermB gene and tetM becomes functional. B: In vivo screening of E. faecalis V583 genomic library. After elimination of cells containing an active promoter under in vitro conditions, the resulting library is administrated to the animal host model. After infection, changes in the antibiotic resistance phenotype allow to select resolved bacteria containing an in vivo induced promoter.
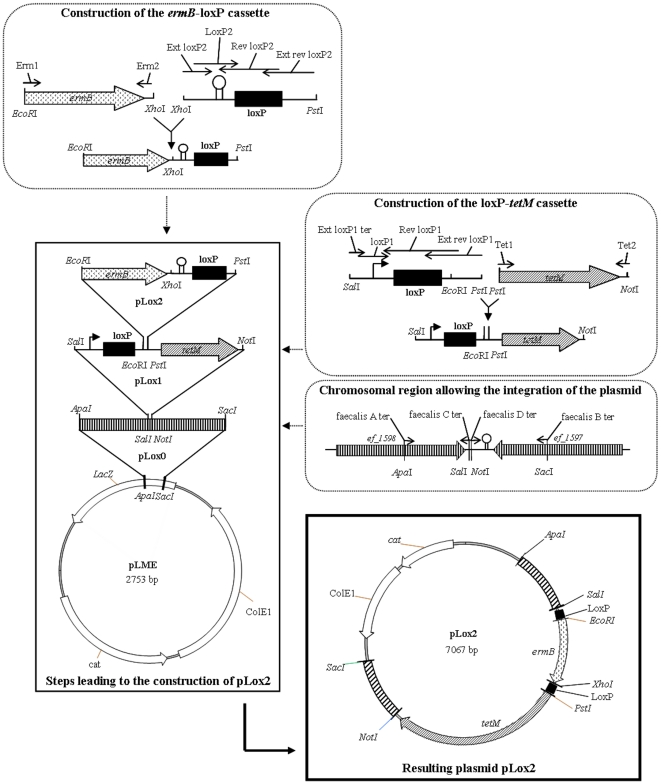
Figure 2. Schematic representation of cloning steps leading to the construction of the integration vector pLox2.
An amplicon resulting from the amplification of a genomic fragment was first subcloned into pLME vector in order to allow the integration of the loxP-ermB-loxP-tetM cassette in the intergenic region between loci ef_1597 and ef_1598. The resulting plasmid, pLox0, is then used to add a loxP-tetM cassette between these both genes. The obtained vector was named pLox1. A cassette ermB-loxP was finally cloned into pLox1 to obtain the integration plasmid pLox2. Only relevant restriction sites are indicated. ColE1 = colE1 origin; cat = CmR encoding gene from pNZ7125 [17]; ermB = erythromycin rRNA methylase gene from pUCB30 [51]; tetM = TetR encoding gene from p3Tet [27]. Thin arrows represent primers used in these constructions.
Growth experiments in liquid GM17 or isolation of strains on agar plates containing GM17 supplemented with appropriate antibiotics confirmed the suitability of the construction in E. faecalis. Indeed, Lox2 strain can grow if erythromycin is present but not in the presence of tetracycline contrary to Lox1 strain. This latter strain was constructed by integrating plasmid pLox1 into the chromosome of strain V19 by a double cross-over event and used as an excision control because the integrated region in this strain is identical to that of the resolved Lox2 strain (Fig. 1A). The Lox1 strain was shown to be able to grow in the presence of tetracycline but not if erythromycin is present in the medium. The whole loxP-ermB-loxP-tetM cassette was finally sequenced.
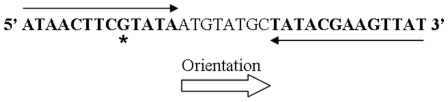
In order to allow us to identify promoters that are weakly expressed under in vitro conditions but that are strongly activated in vivo, we decided to use also another strain that contained a 1 base-deletion in the second loxP site, as shown in Fig. 3. This strategy has already been used and it has been demonstrated that mutations in loxP reduce the affinity of the enzyme Cre for these sites thus decreasing the level of sensitivity of the reporter system [24]. Both strains, Lox2 (without mutation) and Lox2ΔG (with mutation) were used in this study thus providing a R-IVET system with two different levels of sensitivity.
Figure 3. LoxP sequence.
LoxP sites are composed of two 13 pb inverted sequences separated by an 8 pb spacer region which gives the orientation of the LoxP site [56]. The base indicated by * is deleted in the second loxP site of Lox2ΔG strain.
Construction of a E. faecalis V583 genomic library in Lox2(ΔG) strains
To generate the V583 genomic library, the replicative vector pCre2 has been used. This plasmid contains a cat gene, conferring resistance to chloramphenicol, and a promoterless copy of the cre gene, encoding the site-specific recombinase Cre. AluI-digested E. faecalis genomic fragments were cloned upstream from cre gene using E. coli VE14188 as an intermediate host. PCre2 derivatives thus obtained from 57,600 E.coli colonies were extracted to be introduced in Lox2 and Lox2ΔG strains. 58,000 independent clones were recovered with Lox2 strain. PCR analysis of 90 clones suggested that 68% of them contained inserts with an average size of 1.1 kb. In the Lox2ΔG strain, about 85,000 clones were recovered of which, 74% had an insert with an average size of 900 pb. These results showed that the libraries covered in both strains more than 99% of the E. faecalis V583 genome. To evaluate the libraries redundancy, 22 inserts were sequenced suggesting that libraries are random, not redundant and that no specific genomic region was over- or underrepresented. It can also be noticed that fragments of plasmids pTEF1 and pTEF2 were represented in the libraries. The percentage of clones active under laboratory conditions was also evaluated by calculating the resolution frequency in the library before subcultures in an erythromycin-containing medium. It was thus shown that 8% of clones were resolved suggesting that 8% of the library inserts contained active promoters.
R-IVET screen in the Galleria mellonella host model
Several R-IVET screenings have been performed in this study in order to identify genes involved in E. faecalis pathogenesis. First we used a surrogate virulence model based on the insect G. mellonella. This model is increasingly used since the immune system of the larvae used for infection exhibits a high degree of structural and functional similarity with the innate immune system of mammals [19], [21]. Recently it has been demonstrated that this model is also useful to evaluate virulence of E. faecalis [25], [26].
Because this approach aims to highlight promoters that are specifically activated during infection in comparison with in vitro growth conditions, the first step consisted in eliminating cells in which promoters that are active under laboratory conditions were cloned upstream from cre gene. These pCre2 derivatives were removed from screening by subculturing R-IVET libraries for 20 generations on GM17 supplemented with erythromycin and cells were plated on an agar medium containing tetracycline (Fig. 1B). No resolved clone was observed after this subculturing step in the Lox2ΔG library, but it was noticed that a percentage of clones from the Lox2 library was spontaneously resolved (reaching 0.12%±0.12 clones). This rate of spontaneous excision in the Lox2 library called into question the reliability of our R-IVET system. TetR and EmS colonies from the Lox2 library were used as a template for PCR amplification of the insert cloned upstream from cre and amplicons were finally used for sequencing. Interestingly, upon more than 50 spontaneously resolved colonies analyzed, always the same three inserts have been found. Indeed, clones containing the promoters of ef_0020, ef_0092 (and ef_0093) and ef_0573 became EmS and TetR at any time even though any stress had already been applied to bacteria. Therefore these genes were to remove from results and from further studies because of their non-specificity to in vivo conditions.
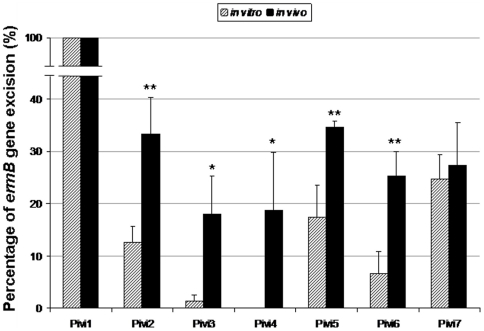
Both libraries (Lox2 and Lox2ΔG) were then screened in the G. mellonella insect model. The infected larvae were incubated at 37°C for 16 hours. Melanized larvae were sacrificed, haemolymph was recovered, diluted and plated. Replica plating of colonies then allowed the selection of bacteria in which a promoter cloned upstream from cre was activated during persistence in the insect host. Indeed, clones harboring a pCre2 derivative in which a promoter was activated at any time during passage in the host model are able to grow on plates containing tetracycline and chloramphenicol but not erythromycin (Fig. 1B). This resulted in the identification of 16 Lox2 resolved clones and 3 Lox2ΔG resolved clones, respectively corresponding to 0.74 and 0.26% of the screened colonies. According to the genome annotation database, only one of the inserts did not contain an untranslated region upstream from a coding sequence whereas the 18 other candidates corresponded to 7 redundant putative promoter regions. These promoters were checked for in vivo induction by individual screening in vitro and in the insect host. Plasmid pCre2 derivatives containing a putative in vivo induced promoter were extracted from resolved EmS and TetR cells before being introduced into the unresolved EmR and TetS Lox2ΔG or Lox2 strains. To compare expression of putative promoters in in vitro conditions and during infection in G. mellonella, resolution frequencies of the reporter cassette were measured by determining the rate of ermB gene excision as described in the experimental procedures (Fig. 4). The resolution rate was measured for each putative in vivo induced promoter. For 5 of the 7 clones, promoters were thus subsequently confirmed to be significantly induced in the insect host model, with an in vivo resolution at least twice greater than in vitro resolution. This suggests that the corresponding genes are important for the infection process and hence for virulence. The genomic locations of these promoting regions were mapped by comparing insert sequences to the genomic sequence of E. faecalis V583 (Fig. 5). The genetic organization of three regions (Pivi4, 5 and 6) suggests that the identified promoters could control the expression of bicistronic operons. According to the control experiments described above, the promoter of the operon ef0092/3 which corresponded to Pivi5 was removed from results and the genes controlled by the other identified promoters are listed in Table 1. Functions of three of these genes remain unknown but the three others are involved in protein degradation or signal transduction.
Figure 4. Individual resolution analysis of R-IVET identified promoters.
Plasmids containing a putative in vivo induced promoter cloned upstream from cre gene were extracted from resolved strain to be introduced in the unresolved Lox2 (or Lox2ΔG) strain. Resolution rate was measured after 15 generations of in vitro growth or after in vivo persistence by calculating the percentage of EmS, CmR and TetR CFU. Values correspond to the mean of three measurements obtained after independent experiments. Error bars indicate standard deviations. A Student test was used to determine the following P values when comparing in vitro resolution to in vivo induction: for Pivi1, nd; for Pivi2, 0.009; for Pivi3, 0.017; for Pivi4, 0.043; for Pivi5, 0.008; for Pivi6, 0.007; and for Pivi7, 0.646. All promoters indicated by an asterisk are significantly induced during in vivo persistence (*, p≤0.05; **, p≤0.001).
Figure 5. Locations of in vivo induced promoters on E. faecalis chromosome.
Promoters that were shown to be induced during persistence in Galleria mellonella larvae are located in open boxes and arrows below the boxes indicate the orientation in which promoters were cloned upstream from the recombinase gene in plasmid pCre2. Thick arrows represent Open Reading Frames located in the region neighboring these promoters. A: Pivi2 is located within a 1.2 kb-long region overlapping ef_0040, ef_0041 and the untranslated region between them. Pivi2 probably regulates ef_0041 expression. B: Pivi3 is located within a 1.1 kb-long fragment overlapping ef_3282 and ef_3283 and should control ef_3282 expression. C: Pivi4 is included in a 550 pb-long region overlapping ef_3198 and a small part of untranslated region between ef_3197 and ef_3198. This suggests that Pivi4 regulates the expression of the two-component system encoding operon ef_3196/7. D: Pivi5 is located in a 1.2 kb-long fragment overlapping ef_0091, ef_0092 and the untranslated region between them. Pivi5 could regulate ef_0092 and/or ef_0093 expression. E: Pivi6 is included in a 620 pb-long region overlapping ef_0376 and the untranslated fragment between this gene and ef_0377. So Pivi6 could control expression of ef_0377 and ef_0378 which seem to be co-transcribed.
Table 1. E. faecalis genes induced during persistence in the Galleria mellonella host model.
| N° | ORFa | Annotationb | Cellular roleb |
| Pivi2 | ef_0041 | PIN domain protein | Unknown function |
| Pivi3 | ef_3282 | ATP-binding subunit ClpC | Protein fate |
| Pivi4 | ef_3196 | Response regulator | Signal transduction |
| ef_3197 | Sensor histidine kinase | Signal transduction | |
| Pivi6ΔG | ef_0377 | Ankyrin repeat protein | Unknown function |
| ef_0378 | N-acyl-D-amino-acid deacylase family protein | Unkonwn function |
Promoter region mapping led to the identification of genes putatively controlled by in vivo induced promoters.
Annotation and cellular role category of genes were determined as specified in TIGR genome database.
Genes identified in Lox2ΔG library.
As a control, plasmid pCre2 without any insert cloned upstream from cre was introduced into Lox2 and Lox2ΔG strains in order to confirm that induction is not due to the presence of the plasmid. No resolution was detected before or after infection for the Lox2ΔG:pCre2 strain and resolution rate was not significantly different before and after the larvae infection (0.56%±0.97 and 1.55%±2.56, respectively) for the Lox2:pCre2 strain confirming that the promoter activation is necessary for ermB gene excision.
Even if the spontaneous excision rate of the Lox2:pCre2 strain remains unexplained, it was shown that the vast majority of identified promoters were significantly induced in vivo thus confirming the suitability of our R-IVET system in E. faecalis.
Involvement of an in vivo induced two-component system in the pathogenic potential of E. faecalis in the Galleria mellonella infection model
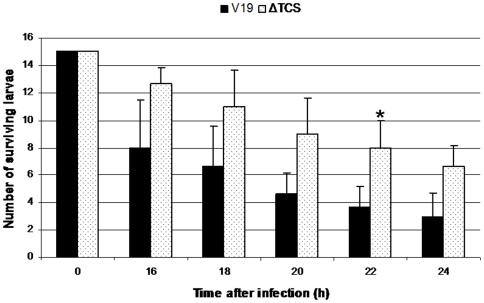
Independent individual analysis of each promoter has shown that Pivi4 was not active under in vitro conditions whereas it was strongly induced during insect infection (Fig. 4). Thus transcriptional activation controlled by Pivi4 seems to be specific to in vivo persistence suggesting that the two-component system regulated by Pivi4 could be essential to the pathogenic potential of E. faecalis. To confirm this hypothesis, a deletion mutant of this operon, named ΔTCS, was constructed and used to evaluate its virulence in comparison to the wild type strain in the G. mellonella model (Fig. 6). The number of surviving larvae was followed from 16 to 24 hours after infection. The results showed that the ΔTCS mutant was significantly less virulent than the wild type. We conducted this experiment also with the insertional mutant RR02 used in a previous study [27]. Similar results as with the ΔTCS mutant have been obtained. These experiments demonstrated that ef_3196/7 operon seems indeed to be implicated in virulence confirming that R-IVET is a valuable tool to identify new fitness/virulence factors in E. faecalis. Of note, the previous work by Hancock and Perego did not identify a significant phenotype neither in stressing environments not in biofilm formation of the RR02 insertional mutant [27].
Figure 6. Effect of the deletion of the ef_3196/7 operon on virulence.
Deletion mutant of the in vivo induced genes encoding a two-component system (ef_3196/7 operon) has been constructed. Surviving G. mellonella larvae are counted from 16 to 24 hours after infection with E. faecalis V19 (black bar) and E. faecalis ΔTCS (white bar). Bars indicated by an asterisk show a significant difference in comparison with wild type (*, p≤0.05). Experiments were repeated at least three times.
R-IVET screening during exposure to urine
To identify genes specifically activated during urinary tract infections, R-IVET libraries were screened during a 3 hours challenge in urine, as described in experimental procedures. Whereas no resolved colony has been obtained with the Lox2ΔG library, 18 CmR, EmS and TetR colonies (1.01% of the screened bacteria) were identified from Lox2 library. Sequencing revealed that 3 of them did not correspond to obvious promoting regions. The 15 remaining sequences corresponded to 12 loci containing one or several promoters controlling a total of 17 ORFs. Genes controlled by these specifically in vivo induced promoters are listed in Table 2. Two inserts contained two AluI restriction fragments (Uivi7 and Uivi8) and harbored two putative promoting regions. Concerning Uivi7, one of the two fragments contained the putative promoter of ef_0839 or ef_2975 (these both genes are strictly identical). Even though the functions of the majority of these genes remain unknown, it can be noticed that the other genes are involved in transport or binding to substrates and cell envelope composition. Quantitative RT-PCR (qRT-PCR) was then used to confirm that clones, identified in the R-IVET screening, contained promoters that were significantly activated during exposure to urine compared to growth in a rich medium. For this experiment, RNAs were extracted from E. faecalis V19 cells after 3 hours of exposure to filter-sterilized urine or in GM17 under the same conditions used for the R-IVET screening. Expression levels were measured for 17 genes and the expression of ef_2959, ef_2960, ef_3324 and ef_3325, encoding a putative ribose uptake protein, a ribose transporter, the beta-subunit and the biotin carboxyl carrier of a sodium ion-translocating decarboxylase, respectively, was shown to be expressed at a higher level at 3 hours of exposure to urine (induction factors were 13.1±5.2, 4.3±1.9, 5.6±4.4 and 12.9±1.5 respectively). This suggests that bacteria overexpress new transport pathways in order to adapt to nutritional conditions encountered in urine. Since qRT-PCR allows the quantification of a given transcript at a defined time (in this case, 3 hours), the other genes identified by R-IVET screening and not confirmed by qRT-PCR might be induced at another moment during urine exposure. An analysis of the transcriptome of E. faecalis V583 in response to growth in blood has recently been performed [28]. Cluster of citrate catabolism, including ef_3324 and ef_3325, has been demonstrated to be up-regulated in response to blood exposure. Both transcriptional studies, in blood and in urine, suggest that these genes are important for E. faecalis to grow in biofluids.
Table 2. E. faecalis genes induced during a challenge in urine.
| N° | ORFa | Annotationb | Cellular role categoryb |
| Uivi2 | ef_2959 | Ribose uptake protein, putative | Transport and binding proteins |
| ef_2960 | Ribose transporter protein RbsD | Transport and binding proteins | |
| Uivi3 | ef_3324 | Sodium ion-translocating decarboxylase, Beta subunit | Transport and binding proteins, Energy metabolism |
| ef_3325 | Sodium ion-translocating decarboxylase, Biotin carboxyl carrier protein | Transport and binding proteins, Energy metabolism | |
| Uivi4 | ef_0153 | Cell wall surface anchor protein | Cell envelope |
| ef_0154 | Conserved hypothetical protein | Unknown function | |
| Uivi5 | ef_0330 | SNF2 domain protein | Unknown function |
| Uivi6 | ef_0418 | ABC transporter, ATP-binding protein | Transport and binding proteins |
| Uivi7 | ef_0839 | Conserved hypothetical protein | Unknown function |
| ef_1434 | DnaD domain protein | Unknown function | |
| ef_2975 | Conserved hypothetical protein | Unknown function | |
| Uivi8 | ef_0528 | Cytolysin B transporter protein, truncation | Toxin production and resistance, Protein fate |
| ef_3251 | Hypothetical protein | Unknown function | |
| Uivi10 | ef_0175 | Cytidine deaminase cdd | Salvage of nucleosides and nucleotides |
| ef_0176 | Basic membrane family protein | Cell envelope | |
| Uivi11 | ef_2987 | Conserved hypothetical protein | Unknown function |
| ef_2988 | Conserved hypothetical protein | Unknown function | |
| Uivi12 | ef_1201 | Conserved hypothetical protein | Unknown function |
Insert identification and comparison to the E. faecalis V583 genome database.
Annotation and cellular role category of genes were determined as specified in TIGR genome database.
Highlighting of E. faecalis genes activated during bacteremia and peritonitis infections
Next we were interested in identifying genetic determinants that are activated during bacteremia and peritonitis. Therefore, 5×108 CFU and 1.7×1010 CFU of Lox2 or Lox2ΔG libraries were injected intravenously via the tail vein and into the peritoneal cavity of 8 BALB/c mice, respectively. In the case of bacteremia, 2, 4, 6 and 24 hours after inoculation, mice were sacrificed, blood and kidneys were obtained, homogenized and plated on a tetracycline containing medium. Four and 2 EmS, TetR and CmR clones were obtained from blood recovered 2 and 4 hours after inoculation, respectively. After 6 and 24 hours, no bacteria could be cultured from the blood. However, additional clones were isolated from kidneys of these animals. Indeed, a total of 50 EmS, TetR and CmR colonies was recovered after 2, 4, 6 or 24 hours of infection. In the peritonitis model, the relatively high number of inoculated bacteria resulted in 280, 219 and 265 EmS, TetR and CmR colonies that were recovered from blood, kidneys and after a peritoneal lavage, respectively. Because of the high concentration of bacteria inoculated into animals, the experiment was conducted for only 6 hours to avoid death of the animals during the experiment. As described above, resolved colonies were used as a template for PCR amplification of the insert cloned in plasmid pCre2 upstream from cre gene. Both R-IVET screenings using the bacteremia and peritonitis mouse models led to the identification of 64 putative genes that are activated during the pathogenic process. These in vivo induced genes were functionally listed in genes involved in nutrient absorption and metabolism (23 ORFs), cell wall composition (4 ORFs), extracellular functions (1 ORF), transport (4 ORFs), regulation (7 ORFs) and other functions (6 ORFs). The 19 remaining genes encode hypothetical proteins (Table S1). As already observed in previous studies [17], [20], [29], a large part of these ORFs are involved in metabolism and transport. This suggests that microorganisms have to adapt to the in vivo nutrient specific conditions. Moreover, 7 transcriptional regulators have been identified, thus indicating that bacteria develop a specific and adaptive response to its host-associated environment.
Identification of ef_0377 as a gene induced during in vivo persistence in insect and mice infection models and involved in the pathogenesis of E. faecalis
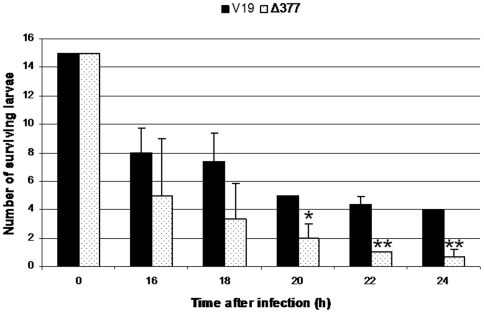
Lists of induced genes resulting from the infection models were compared, thus highlighting 21 ORFs whose expressions were found to be activated in at least two different conditions (Table 3). One of these genes was chosen for further investigations. A promoter controlling the expression of ef_0377, encoding an ankyrin repeat protein, had been demonstrated to be strongly induced in the hemocoel of G. mellonella larvae and had also been identified in the peritonitis mouse model. In some Gram-negative pathogens, ankyrin-like proteins are involved in intracellular proliferation or persistence within macrophages [30], [31] and in oxidative stress response [32]. From these combined results, we suggested that ef_0377 could be involved in E. faecalis pathogenicity. To test this hypothesis, a deletion mutant was constructed in E. faecalis strain V19. This strain, named Δ377, was used to evaluate its virulence in the G. mellonella model. Obtained results showed that Δ377 mutant was somewhat more virulent than the wild type (Fig. 7). Similar results have been obtained with an insertional 377::Ery mutant. These results suggest that the ankyrin protein encoded by this gene could be essential to the bacterial in vivo persistence or the host colonization even though its expression affects the virulence potential of E. faecalis. This has already been observed by Hollands et al in Streptococcus in which a mutation in the CovRS two-component system enhances the pathogenic potential of the bacteria whereas it reduces its ability to colonize the host [33].
Table 3. E. faecalis genes induced in at least two different conditionsa as compared to in vitro growth.
| Locus | Annotation | Insect | Bacteremia | Peritonitis | Urine | |
| ef_0041 | PIN domain protein | x | x | |||
| ef_0104 | Arginine deiminase, ArcA | x | ||||
| ef_0105 | Ornithine carbamoyltransferase, ArcF-1 | x | ||||
| ef_0107 | Transcriptional regulator Crp/Fnr, ArcR | x | x | |||
| ef_0108 | C4 dicarboxylate transporter putative, ArcD | x | x | |||
| ef_0175 | Cytidine deaminase, Cdd | x | x | |||
| ef_0176 | Basic membrane family protein | x | x | |||
| ef_0185 | Phosphopentomutase, DeoB | x | x | |||
| ef_0186 | Purine nucleoside phosphorylase, DeoD-1 | x | x | |||
| ef_0349 | Tail protein | x | x | |||
| ef_0377 | Ankyrin repeat protein | x | x | |||
| ef_0378 | N-acyl-D-amino-acid deacylase family protein | x | x | |||
| ef_0528 | Cytolysin B transport protein, truncation | x | x | |||
| ef_1072 | Operon galactose repressor, GalR | x | x | |||
| ef_2405 | Hypothetical protein | x | ||||
| ef_2424 | Pyrroline-5-carboxylate reductase, putative | x | ||||
| ef_2987 | Conserved hypothetical protein | x | x | |||
| ef_2988 | Rhodanese family protein | x | x | |||
| ef_2996 | Conserved hypothetical protein | x | ||||
| ef_3060 | Putative secreted lipase | x | ||||
| ef_3251 | Hypothetical protein | x | x | |||
| ef_3282 | ATP-binding subunit ClpC, protease | x | x | |||
In mouse infection models, resolved cells were recovered from blood, kidneys (and peritoneal lavage fluid), 2, 4, 6 (or 24) hours after infection.
Figure 7. Effect of the deletion of the ef_0377 gene on virulence.
Deletion mutant of the in vivo induced genes encoding an ankyrin repeat protein (ef_0377) has been constructed. Surviving insects are counted from 16 to 24 hours after infection with E. faecalis V19 (black bar) and E. faecalis Δ377 (white bar). Bars indicated by an asterisk show a significant difference in comparison with wild type (*, p≤0.05; **, p≤0.01). Experiments were repeated at least three times.
Our R-IVET reporter system was based on two strains, one of which contained a single deletion in the first loxP site. This would allow trapping of two types of promoters. Indeed, in the strain without mutation, the expression threshold necessary to excision is very low. Thus the identified promoters have to be not expressed at all under in vitro growth conditions to not being eliminated during pre-cultures. However even a weak activation of their expression in vivo enables their identification. On the other hand, the mutant strain allows the trapping of promoters that are not or weakly expressed in vitro but strongly induced in vivo. The combination of both systems gives a powerful tool to identify gene activation. However, our results also show that the vast majority of genes were identified within the Lox2 strain suggesting that the recombination activity was too weak in the Lox2ΔG strain to be fully efficient. Nevertheless, ef_0377 has been shown to be activated via both Lox2ΔG and Lox2 libraries in two infection models. This demonstrates that ef_0377 is probably weakly expressed in vitro and strongly induced in vivo.
Confirmation of previously identified in vivo expressed genes by our R-IVET screens
Combined R-IVET results led to the identification of genes that had already been described as in vivo induced genes. Indeed, ef_0104 [34], [29] and ef_0105 [20], both involved in arginine catabolism, are induced in Vibrio cholerae or Bacillus cereus during intestinal infection of mice and during an oral infection in insects, respectively. The ClpC encoding gene, ef_3282, had also been identified by R-IVET screenings in E. faecalis during biofilm formation [18], in Lb. plantarum in the gastrointestinal tract of mice [17] and in Bacillus cereus in insects [20]. Moreover, it has been shown that ClpC is required for growth of Streptococcus pneumoniae in the lung and blood in a murine pneumonia model [35]. This ATP-binding protease plays a role in autolysis and adherence to human cells [36], [37] thus being essential for pathogenesis [38].
In vivo regulation of the Pathogenicity Island of E. faecalis V583
Interestingly, the analysis of results in their entirety revealed that only a few number of ORFs from the PAI of E. faecalis were demonstrated to be up-regulated (ef_0486, ef_0502, ef_0528 and ef_0530). However, gene ef_0528 (cylB), encoding the cytolysin B transport protein, was found to be activated in two different models, suggesting that cyl operon, contributing to enterococcal virulence [39]–[41], could be induced in response to the host environment. The 3′-end of ef_0528 and genes encoding cylA, and cylI, are missing in E. faecalis V583 strain [42]. However, the two-component regulatory system encoded by cylR1 and cylR2 genes modulating the autoinduction of the expression of this operon in response to a quorum-sensing [43] is present in this strain. A previous study demonstrated that the expression of the cyl operon was induced in urine during stationnary phase [44]. In these conditions, it was shown that abundances of mRNAs encoding CylM and CylB seem to evolve differently from each other. The author suggested that this was due to a difference in stability of different segments of the polycistronic transcript. However, according to our R-IVET results, we alternatively propose the presence of a cylM internal promoter responsible for a conditional modulation of expression of cylB in vivo or in biofluids. Another gene potentially induced during peritonitis is ef_0530 encoding an AraC-type regulator. In a recent study, it was shown that this gene is involved in biofilm formation, survival within macrophages and virulence in a mouse peritoneal infection model [45]. Thus, this last finding is in perfect accordance with our R-IVET results again demonstrating the usefulness of our tool. However, even though the vast majority of sequenced fragments cloned upstream from cre gene corresponded to obvious promoters, some inserts contained intragenic fragments or were obviously cloned in an opposite orientation. These fragments might encode small non-coding RNAs or antisense-RNAs, suggesting that our R-IVET reporter system may also be a useful and convenient tool to identify the expression of these regulatory RNAs. Work is in progress to examine this possibility.
Materials and Methods
Bacterial strains and growth conditions
Bacterial strains and plasmids used in this study are listed in Table S2. E. faecalis strains were cultivated at 37°C without shaking in M17 medium [46] supplemented with 0.5% glucose (w/vol) (GM17) and with erythromycin (150 µg.ml-1), tetracycline (10 µg.ml-1) or chloramphenicol (15 µg.ml-1) if needed. E. coli strains were grown at 37°C under vigorous agitation in LB medium [47] with ampicillin (100 µg.ml-1), erythromycin (150 µg.ml-1) or tetracycline (10 µg.ml-1) when required.
General molecular methods and sequence analysis
Restriction endonucleases, shrimp alkaline phosphatase and T4 DNA ligase were obtained from GE Healthcare and Promega, and used according to the manufacturers' instructions. PCR was performed with the thermocycler “Master Cycler Gradient” (Eppendorf, Hambourg, Germany) using GoTaq DNA polymerase (Promega, Madison, WI, USA) or “Triple Master Mix” (Eppendorf). Primers are listed in Table S2. If needed, PCR products were purified using the Nucleospin® Extract II kit (Macherey-Nagel, Düren, Germany). E. coli and E. faecalis were transformed by electroporation with the Gene Pulser Apparatus (Bio-Rad), as described by Dower et al [48] and Holo & Nes [49], respectively. Plasmids were extracted from E. coli by using the Nucleospin® Plasmid kit (Macherey-Nagel). Plasmids in E. faecalis were extracted as previously described [50].
Construction of a chromosomal reporter E. faecalis strain of cre expression
The non-encoding region flanked by ef_1598 and ef_1597 encoding a deoxyribodipyrimidine photolyase and the catalase KatA, respectively, has been chosen to integrate the loxP-ermB-loxP-tetM cassette into the chromosome of E. faecalis. First, this region was amplified by using genomic DNA of E. faecalis V583 as a template with two primers combinations faecalis A ter and faecalis C ter, and faecalis B ter and faecalis D ter, thus introducing ApaI and SalI, and SacI and NotI restriction sites, respectively. A mix of both amplicons was then used as a template to amplify the whole region with the primers faecalis A ter and faecalis B ter. The resulting PCR product is thus flanked by ApaI and SacI restriction sites and includes SalI and NotI sites in its middle. This amplicon was digested by ApaI and SacI and cloned into a dephosphorylated and similarly digested plasmid, pLME (vector constructed by replacing the bla gene of pBlueScript 2 SK+ (Stratagene, La Jolla, US) with a cat gene amplified from pNZ7125 [17], data not shown). The resulting plasmid was designated pLox0. The LoxP1 cassette includes a strongly active promoter [23] followed by a loxP site. This cassette was constructed by annealing and ligating the oligonucleotides ext lox P1 ter, ext rev lox P1, lox P1 and rev loxP1. P3Tet [27] was used as template to amplify the gene tetM. This antibiotic resistance marker and loxP1 cassette share the same PstI restriction site allowing us to join them after restriction, ligation and amplification. The resulting amplicon digested by SalI and NotI was then subcloned in the similarly digested and dephosphorylated pLox0 to obtain a new plasmid named pLox1. LoxP2 cassette was constructed by annealing and ligating the primers ext loxP2, loxP2, rev loxP2 and ext rev loxP2 thus introducing a second loxP site and a strong terminator (ΔG = −36.3kcal±10%)[22]. Gene ermB was amplified from pUCB30 [51] and ligated to loxP2 cassette via a XhoI restriction site. The ligation product was then amplified and subcloned into pLox1 between EcoRI and PstI sites. The resulting plasmid was designated pLox2 and includes a loxP-ermB-loxP-tetM reporter cassette flanked by two fragments of E. faecalis chromosome allowing the integration of the cassette into the bacterial genome via a double crossover event.
The integrative vector pLox2 was introduced in E. faecalis V19 strain and integrants were first selected on GM17 plates containing 150 µg/ml erythromycin. Integrants were checked by PCR before being subcultured in GM17 without erythromycin. After about 30 generations of growth, replica plating allowed the identification of a few colonies which were both sensitive to chloramphenicol and resistant to erythromycin. The double crossover event was confirmed by PCR and Southern blotting. The resulting strain containing the loxP-ermB-loxP-tetM construction between loci ef_1597 and ef_1598 was designated E. faecalis Lox2.
Construction of the E. faecalis genomic library
Total genomic DNA was extracted from a late log-phase culture of E. faecalis V583. Pelleted cells were treated with lysozyme (5 mg/ml) and RNAse A (80 µg/ml) at 37°C for 10 minutes and with a solution of Proteinase K (20 mg/ml) containing Sodium Dodecyl Sulfate (5 mg/ml) at 60°C. Nucleic acids are then purified by phenol-chloroform extraction. A sufficient amount of partially digested genomic DNA from E. faecalis strain V583 was obtained by repeating the digestion of 2.5 µg DNA with 0.375 units of AluI for 1 h at 37°C. This condition provides a maximum amount of fragments ranging from 0.5 to 1.5 kb in size. Purified DNA fragments were ligated to SmaI digested and dephosphorylated pCre2. This vector was constructed by adding the gene cre amplified from pNZ7125 [17] into plasmid pCU1 and replacing the BglII restriction site with a SmaI site upstream from cre. The resulting plasmids were introduced into E. coli VE14188. Transformants were plated on LB agar plates with ampicillin and incubated at 37°C. The 57,600 obtained colonies were collectively resuspended in LB. Plasmids were extracted from these cells to be introduced into Lox2 and Lox2ΔG strains. The approximative 85,000 (in Lox2ΔG strain) and 58,000 (in Lox2 strain) resulting colonies were resuspended in GM17 containing 15% glycerol (w/vol) and stored in aliquots at −80°C.
Screening of the genomic library in the insect host model
To counter-select against clones in Lox2 and Lox2ΔG libraries that harbor pCre2 derivatives containing a promoter active during in vitro growth, the libraries were sub-cultured for about 20 generations in GM17 supplemented with chloramphenicol and erythromycin. Infection of G. mellonella larvae with E. faecalis was then performed as previously described by Park et al. [7]. Using a syringe pump (KD Scientific, Holliston, MA, USA), larvae (about 0.3 g and 3 cm in length) were infected subcutaneously with washed E. faecalis strains from over-night culture in GM17 (about 3.5×106 CFU per larvae) in 10 µl of sterile saline buffer using a sterilized micro syringe, and incubated at 37°C. Sixteen hours after inoculation, bacteria were recovered from haemolymph of 15 melanized larvae. Serial dilutions were plated on GM17 supplemented with chloramphenicol. In this specific case of infection in the insect_A replica plating strategy was used, contrary to the mouse and urine experiments, because a tetracycline-resistant contaminating flora was present in the insect preventing us to select resolved bacteria on this antibiotic. After 72 h growth, colonies were replica-plated onto plates containing GM17 supplemented with chloramphenicol, erythromycin or tetracycline. After 24 h incubation at 37°C, plates were compared to identify erythromycin-sensitive and tetracycline-resistant colonies. Inserts cloned upstream from cre gene of these resolved cells were amplified by PCR and resulting amplicons were sequenced.
Screening of the genomic library during exposure to urine
As explained above, genomic libraries were sub-cultured in presence of erythromycin to eliminate clones harboring a promoter active under in vitro conditions. E. faecalis V19 strain was tested during growth experiments in filter-sterilized urine. This showed that this strain was not able to double its population under these conditions (data not shown), contrary to strains MMH594 [44] and JH2.2 (personal communication). Thus, libraries were inoculated into urine without the supplementation of exogenous carbon sources or micronutrients for an overnight growth. Cells were then pelleted and resuspended into fresh urine for 3 hours at 37°C without aeration. Serial dilutions were then plated onto plates containing tetracycline. Colonies were then checked for their phenotypic switch by replica-plating on plates containing chloramphenicol, erythromycin or tetracycline. CmR, EmS and TetR colonies were then used as a template for PCR amplification of the insert cloned upstream from cre. Resulting amplicons were then sequenced.
E. faecalis V19 strain was also inoculated into filter-sterilized urine, as described previously. Three hours after exposure to fresh urine, RNAs were isolated and used for qRT-PCR experiments.
Screening of the genomic library in mice infection models
Clones harboring active promoters under in vitro conditions were eliminated as explained above. The mouse bacteraemia model was then adapted from the method previously described by Hufnagel et al. [52], [53]. Eight 6–8 weeks-old female BALB/c mice were challenged by intravenous injection of Lox2 or Lox2ΔG libraries (5.108 CFU per mouse) via the tail vein. Two, 4, 6 or 24 h after inoculation, mice were sacrificed and exsanguinated. Kidneys were removed and homogenized in 500 µl TSB. The murine peritonitis model was performed using 4–6 weeks-old female BALB/c mice that were injected i.p. with 1.7×1010 CFU of Lox2 or Lox2ΔG libraries in 200 µl sterile saline. Two, four and six hours after injection, mice were sacrificed, the peritoneal cavity was washed by injecting 5 ml sterile PBS, and bacteria in the resulting liquid were pelleted before being resuspended in 500 µl sterile PBS. After the peritoneal wash, rodents were also exsanguinated and kidneys were removed as described above. Serial dilutions of blood, homogenized kidneys and peritoneal wash were plated on GM17 containing tetracycline. After 72 h growth, colonies were replica plated onto plates containing GM17 with erythromycin, tetracycline or chloramphenicol. Plates were compared 24 h later to identify colonies that were sensitive to erythromycin and resistant to chloramphenicol and tetracycline. Colonies harboring such a phenotype were then used as a template to amplify insert cloned upstream from cre gene in pCre2. The resulting amplicons were subsequently sequenced.
Resolution frequencies of the reporter cassette
To assess the validity of the G. mellonella R-IVET screen, the rate of ermB gene excision by the ivi promoters activity was determined by extracting pCre2 derivatives from ivi clones and using them to electroporate strain Lox2 or Lox2ΔG. The resolution rate after a about 15 generations in vitro growth was measured by plating serial dilution of culture and transferring 50 CmR colonies to GM17 plates containing chloramphenicol, erythromycin or tetracycline. The excision rate thus corresponds to the percentage of EmS, CmR and TetR colonies. Resolution frequencies after persistence in the animal host model were determined by injecting bacteria as described in animal experiments. Recovered bacteria in haemolyph were then plated onto GM17 plates supplemented with chloramphenicol. The excision rate was then determined as for in vitro growth.
Construction of E. faecalis mutant strains
The RR02 mutant was constructed by Hancock and Perego [27]. The 377::Ery mutant was constructed using the insertional inactivation vector pOri19 as previously described [54]. Briefly, an internal fragment of ef_0377 gene amplified from the chromosome of V583 strain was cloned into pOri19 vector. The recombinant plasmid was then electroporated into E. faecalis VE14412 and cells were plated on GM17 containing erythromycin and incubated at 30°C. Bacteria that received the plasmid were identified by colony PCR and then cultured at 42°C. Insertional mutants were identified by PCR. E. faecalis ΔTCS and Δ377 deletion mutants were constructed using the shuttle vector pMAD [55]. Upstream and downstream regions of the fragment to be deleted were cloned in the plasmid. After the transformation into the host, integration of the plasmid into the chromosome was selected during growth at a non-permissive temperature in the presence of erythromycin. The second cross-over event resulting in the deletion of the chosen region was induced by growth at the permissive temperature 30°C.
Virulence assays
Virulence assays were performed in the insect model Galleria mellonella adapted from the method described by Park et al. [7]. Larvae were infected as explained above with E. faecalis overnight cultures in GM17. For each experiment, 15 larvae were used and tests were repeated at least three times. Larvae mortality was then monitored over 16 to 24 h post infection.
Ethics statement
The study was approved by the Regierungspräsidium Freiburg, Az 35/9185.81/G-07/15.
Supporting Information
(0.02 MB XLS)
(0.02 MB XLS)
Acknowledgments
The authors wish to thank Dr L. Hancock and Dr P. Serror for providing RR02 mutant and Dr A. Kropec, I. Toma, J-C Giard and A. Rincé for their technical support and critical review of the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Auralie Hanin is a PhD student whose thesis was granted by the French Ministre de l'Enseignement suparieur et de la Recherche. This work was partly supported by grants from the Agence Nationale de la Recherche in the frame of a transnational ERA-NET PathoGenoMics program (ANR-06-PATHO-008-01). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Giard J, Rincé A, Benachour A, Hartke A, Laplace J, et al. The response to environmental stresses in Enterococcus faecalis. Recent Res Devel Microbiology. 2003;7:325–39. [Google Scholar]
- 2.Riboulet E, Verneuil N, La Carbona S, Sauvageot N, Auffray Y, et al. Relationships between oxidative stress response and virulence in Enterococcus faecalis. J Mol Microbiol Biotechnol. 2007;13:140–6. doi: 10.1159/000103605. [DOI] [PubMed] [Google Scholar]
- 3.Hancock LE, Gilmore MS. 2000. Pathogenicity of Enterococci. Dans: Gram-Positive Pathogens (In Fischetti, et al. (ed.)). ASM Press, Washington D. C. p. 251-258 Available at: http://www.enterococcus.ouhsc.edu/lynn_revirew.asp.
- 4.Sood S, Malhotra M, Das BK, Kapil A. Enterococcal infections & antimicrobial resistance. Indian J Med Res. 2008;128:111–21. [PubMed] [Google Scholar]
- 5.Shankar N, Coburn P, Pillar C, Haas W, Gilmore M. Enterococcal cytolysin: activities and association with other virulence traits in a pathogenicity island. Int J Med Microbiol. 2004;293:609–18. doi: 10.1078/1438-4221-00301. [DOI] [PubMed] [Google Scholar]
- 6.Kreft B, Marre R, Schramm U, Wirth R. Aggregation substance of Enterococcus faecalis mediates adhesion to cultured renal tubular cells. Infect Immun. 1992;60:25–30. doi: 10.1128/iai.60.1.25-30.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park SY, Kim KM, Lee JH, Seo SJ, Lee IH. Extracellular gelatinase of Enterococcus faecalis destroys a defense system in insect hemolymph and human serum. Infect Immun. 2007;75:1861–9. doi: 10.1128/IAI.01473-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shankar N, Lockatell CV, Baghdayan AS, Drachenberg C, Gilmore MS, et al. Role of Enterococcus faecalis surface protein Esp in the pathogenesis of ascending urinary tract infection. Infect Immun. 2001;69:4366–72. doi: 10.1128/IAI.69.7.4366-4372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh KV, Coque TM, Weinstock GM, Murray BE. In vivo testing of an Enterococcus faecalis efaA mutant and use of efaA homologs for species identification. FEMS Immunol Med Microbiol. 1998;21:323–31. doi: 10.1111/j.1574-695X.1998.tb01180.x. [DOI] [PubMed] [Google Scholar]
- 10.Angelichio MJ, Camilli A. In vivo expression technology. Infect Immun. 2002;70:6518–23. doi: 10.1128/IAI.70.12.6518-6523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rainey PB, Preston GM. In vivo expression technology strategies: valuable tools for biotechnology. Curr Opin Biotechnol. 2000;11:440–4. doi: 10.1016/s0958-1669(00)00132-4. [DOI] [PubMed] [Google Scholar]
- 12.Mahan MJ, Slauch JM, Mekalanos JJ. Selection of bacterial virulence genes that are specifically induced in host tissues. Science. 1993;259:686–8. doi: 10.1126/science.8430319. [DOI] [PubMed] [Google Scholar]
- 13.Mahan MJ, Tobias JW, Slauch JM, Hanna PC, Collier RJ, et al. Antibiotic-based selection for bacterial genes that are specifically induced during infection of a host. Proc Natl Acad Sci U S A. 1995;92:669–73. doi: 10.1073/pnas.92.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bachmann H, Kleerebezem M, van Hylckama Vlieg JET. High-throughput identification and validation of in situ-expressed genes of Lactococcus lactis. Appl. Environ. Microbiol. 2008;74:4727–4736. doi: 10.1128/AEM.00297-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bachmann H, de Wilt L, Kleerebezem M, van Hylckama Vlieg JET. Time-resolved genetic responses of Lactococcus lactis to a dairy environment. Environ Microbiol. 2010 doi: 10.1111/j.1462-2920.2010.02168.x. [DOI] [PubMed] [Google Scholar]
- 16.Gahan CG, Hill C. The use of listeriolysin to identify in vivo induced genes in the gram-positive intracellular pathogen Listeria monocytogenes. Mol Microbiol. 2000;36:498–507. doi: 10.1046/j.1365-2958.2000.01869.x. [DOI] [PubMed] [Google Scholar]
- 17.Bron PA, Grangette C, Mercenier A, de Vos WM, Kleerebezem M. Identification of Lactobacillus plantarum genes that are induced in the gastrointestinal tract of mice. J Bacteriol. 2004;186:5721–9. doi: 10.1128/JB.186.17.5721-5729.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ballering KS, Kristich CJ, Grindle SM, Oromendia A, Beattie DT, et al. Functional genomics of Enterococcus faecalis: multiple novel genetic determinants for biofilm formation in the core genome. J Bacteriol. 2009;191:2806–2814. doi: 10.1128/JB.01688-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brennan M, Thomas DY, Whiteway M, Kavanagh K. Correlation between virulence of Candida albicans mutants in mice and Galleria mellonella larvae. FEMS Immunol Med Microbiol. 2002;34:153–7. doi: 10.1111/j.1574-695X.2002.tb00617.x. [DOI] [PubMed] [Google Scholar]
- 20.Fedhila S, Daou N, Lereclus D, Nielsen-LeRoux C. Identification of Bacillus cereus internalin and other candidate virulence genes specifically induced during oral infection in insects. Mol Microbiol. 2006;62:339–55. doi: 10.1111/j.1365-2958.2006.05362.x. [DOI] [PubMed] [Google Scholar]
- 21.Jander G, Rahme LG, Ausubel FM. Positive correlation between virulence of Pseudomonas aeruginosa mutants in mice and insects. J Bacteriol. 2000;182:3843–3845. doi: 10.1128/jb.182.13.3843-3845.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tinoco I, Borer PN, Dengler B, Levin MD, Uhlenbeck OC, et al. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973;246:40–1. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- 23.Trieu-Cuot P, Poyart-Salmeron C, Carlier C, Courvalin P. Nucleotide sequence of the erythromycin resistance gene of the conjugative transposon Tn1545. Nucleic Acids Res. 1990;18:3660. doi: 10.1093/nar/18.12.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castillo AR, Woodruff AJ, Connolly LE, Sause WE, Ottemann KM. Recombination-based in vivo expression technology identifies Helicobacter pylori genes important for host colonization. Infect Immun. 2008;76:5632–5644. doi: 10.1128/IAI.00627-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaspar F, Teixeira N, Rigottier-Gois L, Marujo P, Nielsen-LeRoux C, et al. Virulence of Enterococcus faecalis dairy strains in an insect model: the role of fsrB and gelE. Microbiology (Reading, Engl.) 2009;155:3564–3571. doi: 10.1099/mic.0.030775-0. [DOI] [PubMed] [Google Scholar]
- 26.Lebreton F, Riboulet-Bisson E, Serror P, Sanguinetti M, Posteraro B, et al. ace, Which encodes an adhesin in Enterococcus faecalis, is regulated by Ers and is involved in virulence. Infect Immun. 2009;77:2832–2839. doi: 10.1128/IAI.01218-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hancock LE, Perego M. Systematic inactivation and phenotypic characterization of two-component signal transduction systems of Enterococcus faecalis V583. J Bacteriol. 2004;186:7951–8. doi: 10.1128/JB.186.23.7951-7958.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vebø HC, Snipen L, Nes IF, Brede DA. The transcriptome of the nosocomial pathogen Enterococcus faecalis V583 reveals adaptive responses to growth in blood. PLoS ONE. 2009;4:e7660. doi: 10.1371/journal.pone.0007660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osorio CG, Crawford JA, Michalski J, Martinez-Wilson H, Kaper JB, et al. Second-generation recombination-based in vivo expression technology for large-scale screening for Vibrio cholerae genes induced during infection of the mouse small intestine. Infect Immun. 2005;73:972–980. doi: 10.1128/IAI.73.2.972-980.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Khodor S, Price CT, Habyarimana F, Kalia A, Abu Kwaik Y. A Dot/Icm-translocated ankyrin protein of Legionella pneumophila is required for intracellular proliferation within human macrophages and protozoa. Mol Microbiol. 2008;70:908–23. doi: 10.1111/j.1365-2958.2008.06453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan X, Lührmann A, Satoh A, Laskowski-Arce MA, Roy CR. Ankyrin repeat proteins comprise a diverse family of bacterial type IV effectors. Science. 2008;320:1651–4. doi: 10.1126/science.1158160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howell ML, Alsabbagh E, Ma JF, Ochsner UA, Klotz MG, et al. AnkB, a periplasmic ankyrin-like protein in Pseudomonas aeruginosa, is required for optimal catalase B (KatB) activity and resistance to hydrogen peroxide. J Bacteriol. 2000;182:4545–56. doi: 10.1128/jb.182.16.4545-4556.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hollands A, Pence MA, Timmer AM, Osvath SR, Turnbull L, et al. Genetic switch to hypervirulence reduces colonization phenotypes of the globally disseminated group A Streptococcus M1T1 clone. J Infect Dis. 2010;202:11–19. doi: 10.1086/653124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Camilli A, Mekalanos JJ. Use of recombinase gene fusions to identify Vibrio cholerae genes induced during infection. Mol Microbiol. 1995;18:671–683. doi: 10.1111/j.1365-2958.1995.mmi_18040671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ibrahim YM, Kerr AR, Silva NA, Mitchell TJ. Contribution of the ATP-dependent protease ClpCP to the autolysis and virulence of Streptococcus pneumoniae. Infect Immun. 2005;73:730–40. doi: 10.1128/IAI.73.2.730-740.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charpentier E, Novak R, Tuomanen E. Regulation of growth inhibition at high temperature, autolysis, transformation and adherence in Streptococcus pneumoniae by clpC. Mol Microbiol. 2000;37:717–726. doi: 10.1046/j.1365-2958.2000.02011.x. [DOI] [PubMed] [Google Scholar]
- 37.Nair S, Milohanic E, Berche P. ClpC ATPase is required for cell adhesion and invasion of Listeria monocytogenes. Infect Immun. 2000;68:7061–7068. doi: 10.1128/iai.68.12.7061-7068.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polissi A, Pontiggia A, Feger G, Altieri M, Mottl H, et al. Large-scale identification of virulence genes from Streptococcus pneumoniae. Infect Immun. 1998;66:5620–5629. doi: 10.1128/iai.66.12.5620-5629.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chow JW, Thal LA, Perri MB, Vazquez JA, Donabedian SM, et al. Plasmid-associated hemolysin and aggregation substance production contribute to virulence in experimental enterococcal endocarditis. Antimicrob Agents Chemother. 1993;37:2474–2477. doi: 10.1128/aac.37.11.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ike Y, Hashimoto H, Clewell DB. Hemolysin of Streptococcus faecalis subspecies zymogenes contributes to virulence in mice. Infect Immun. 1984;45:528–530. doi: 10.1128/iai.45.2.528-530.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jett BD, Jensen HG, Nordquist RE, Gilmore MS. Contribution of the pAD1-encoded cytolysin to the severity of experimental Enterococcus faecalis endophthalmitis. Infect Immun. 1992;60:2445–2452. doi: 10.1128/iai.60.6.2445-2452.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shankar N, Baghdayan AS, Gilmore MS. Modulation of virulence within a pathogenicity island in vancomycin-resistant Enterococcus faecalis. Nature. 2002;417:746–50. doi: 10.1038/nature00802. [DOI] [PubMed] [Google Scholar]
- 43.Haas W, Shepard BD, Gilmore MS. Two-component regulator of Enterococcus faecalis cytolysin responds to quorum-sensing autoinduction. Nature. 2002;415:84–87. doi: 10.1038/415084a. [DOI] [PubMed] [Google Scholar]
- 44.Shepard BD, Gilmore MS. Differential expression of virulence-related genes in Enterococcus faecalis in response to biological cues in serum and urine. Infect Immun. 2002;70:4344–4352. doi: 10.1128/IAI.70.8.4344-4352.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coburn PS, Baghdayan AS, Dolan GT, Shankar N. An AraC-type transcriptional regulator encoded on the Enterococcus faecalis pathogenicity island contributes to pathogenesis and intracellular macrophage survival. Infect Immun. 2008;76:5668–5676. doi: 10.1128/IAI.00930-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terzaghi BE, Sandine WE. Improved Medium for Lactic Streptococci and Their Bacteriophages. Appl Microbiol. 1975;29:807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bertani G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951;62:293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dower WJ, Miller JF, Ragsdale CW. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988;16:6127–45. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holo H, Nes IF. High-Frequency Transformation, by Electroporation, of Lactococcus lactis subsp. cremoris Grown with Glycine in Osmotically Stabilized Media. Appl Environ Microbiol. 1989;55:3119–23. doi: 10.1128/aem.55.12.3119-3123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Birnboim HC, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benachour A, Auffray Y, Hartke A. Construction of plasmid vectors for screening replicons from gram-positive bacteria and their use as shuttle cloning vectors. Curr Microbiol. 2007;54:342–7. doi: 10.1007/s00284-006-0358-1. [DOI] [PubMed] [Google Scholar]
- 52.Hufnagel M, Koch S, Creti R, Baldassarri L, Huebner J. A putative sugar-binding transcriptional regulator in a novel gene locus in Enterococcus faecalis contributes to production of biofilm and prolonged bacteremia in mice. J Infect Dis. 2004;189:420–430. doi: 10.1086/381150. [DOI] [PubMed] [Google Scholar]
- 53.Theilacker C, Sanchez-Carballo P, Toma I, Fabretti F, Sava I, et al. Glycolipids are involved in biofilm accumulation and prolonged bacteraemia in Enterococcus faecalis. Mol Microbiol. 2009;71:1055–1069. doi: 10.1111/j.1365-2958.2008.06587.x. [DOI] [PubMed] [Google Scholar]
- 54.Law J, Buist G, Haandrikman A, Kok J, Venema G, et al. A system to generate chromosomal mutations in Lactococcus lactis which allows fast analysis of targeted genes. J Bacteriol. 1995;177:7011–7018. doi: 10.1128/jb.177.24.7011-7018.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arnaud M, Chastanet A, Débarbouillé M. New vector for efficient allelic replacement in naturally nontransformable, low-GC-content, gram-positive bacteria. Appl Environ Microbiol. 2004;70:6887–6891. doi: 10.1128/AEM.70.11.6887-6891.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoess RH, Abremski K. Interaction of the bacteriophage P1 recombinase Cre with the recombining site loxP. Proc Natl Acad Sci U S A. 1984;81:1026–9. doi: 10.1073/pnas.81.4.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sahm DF, Kissinger J, Gilmore MS, Murray PR, Mulder R, et al. In vitro susceptibility studies of vancomycin-resistant Enterococcus faecalis. Antimicrob. Agents Chemother. 1989;33:1588–1591. doi: 10.1128/aac.33.9.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Konarska-Kozlowska M, Iyer VN. Physical and genetic organization of the IncN-group plasmid pCU1. Gene. 1981;14:195–204. doi: 10.1016/0378-1119(81)90115-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(0.02 MB XLS)
(0.02 MB XLS)