Abstract
The synthesis of precisely defined nanoscale hybrid materials remains a challenge at the frontier of chemistry and material science. In particular the assembly of diverse high aspect ratio one-dimensional materials such as gold nanorods and carbon nanotubes into functional systems is of ever increasing interest due to their electronic and sensing applications. To meet these challenges methods for interfacing gold nanorods with carbon materials such as single walled carbon nanotubes (SWCNTs) in a regio-controlled manner are needed. Herein, we report a method for the regiospecific synthesis of terminally linked gold nanorod-SWCNTs based on a nanotube surface protection strategy. Key to our approach is a SWCNT surface protection procedure allowing for selective functionalization of the SWCNT termini.
Keywords: Gold Nanorods, Gold Nanoparticles, Carbon Nanotubes, Heterojunctions
High aspect ratio building blocks such as Au-nanorods and carbon nanotubes are key elements to construct interconnected arrays of electronic materials. The high surface area and restricted electronic conduction pathways are of particular value to create materials that are very sensitive to chemical/physical stimuli for sensor applications.1-11 A wide range of carbon nanotube functionalization methods allow for sidewall immobilization12-15 of tailored metallic and/or semiconducting nanoparticles; however, regioselectivity remains a major problem. 6 The formation of heterojunctions between carbon nanotubes and metals has received increased attention due to the potential electronic and materials properties. Previous heterojunction fabrication methods have relied on vapor phase growth and solid-solid reactions at elevated temperatures >450 °C or ablative techniques using intense electron beam irradiation to destroy the nanotube wall around a metallic core.1,2,5 Heterojunctions between gold nanorods and carbon nanotubes have been reported; however, the current method relies on the nonspecific attachment of Au-NPs to the surface of CNTs, resulting in heterogenous structures with multiple random heterojunctions and unpredictable Au-nanorod growth patterns.6 Harsh experimental conditions and the control/precise positioning of the metal junctions have limited the widespread utility of these methods.1,2,5,6 Methods for the regioselective synthesis of well-defined Au-nanorod/SWCNT/Au-nanorod nanocomposites would represent a major step forward in the controlled functionalization of SWCNTs with precisely defined metallic heterojunctions. Herein, we report an operationally simple method for the regiospecific synthesis of terminally-linked Au-nanorod/SWCNT/Au-nanorod nanostructures. Our method allows for the growth of Au-nanorods from the termini of SWCNTs creating precisely positioned metallic heterojunctions. These nanocomposites are synthesized in solution and are found to be very stable under a variety of experimental conditions.
The Au-nanorod/SWCNT/Au-nanorod synthesis is depicted schematically in Fig. 1. HiPco SWCNTs were treated with a mixture of concentrated sulfuric and nitric acid (3:1, 98% and 70%, respectively), subjected to bath sonication for 35 min. at 40 °C, and then oxidized with a mixture of concentrated sulfuric acid and hydrogen peroxide (4:1, 98% and 30%, respectively, 35 min.) to functionalize the nanotube termini with carboxylic acid groups, and to remove extraneous carbon particles.16 Previous studies have demonstrated that the chemical shortening of SWCNTs by mixtures of strong acids leads to oxidation at the nanotube ends and can also introduce sidewall defect sites.16 The oxidized nanotubes were incubated with Triton X-100/PEG (Mr = 10,000) in an aqueous solution and sonicated for 4 h in an ice bath. This process results in a stable dispersion of SWCNTs wrapped with surfactant and polymer to prevent non-specific surface adsorption and/or unwanted covalent functionalization to defects on nanotube sidewalls.17,18 The oxidized termini of the shielded SWCNTs were then selectively coupled to the monofunctionalized 1.4 nm Au-nanoparticles (Au-NPs) bearing a single primary amine group. Efficient coupling was achieved using 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysulfosuccinimide (sulfo-NHS), resulting in SWCNTs functionalized with Au-NPs at their termini. The Au-NP diameter (~1.4 nm) is slightly larger than the diameter of HiPco SWCNTs (between ~0.7–1.2 nm) and the steric bulk of the Au-NPs provides a basis for monofunctionalization of the nanotube ends. The resulting Au-NP conjugated SWCNTs were used to seed the growth of Au-nanorods from the termini of the SWCNTs. Catalytic enlargement of the Au-nanoparticles was achieved using HAuCl4 and CTAB as a template (see Supporting Information).19,20
Figure 1.
Assembly overview for regiospecific synthesis of Au-nanorod terminated SWCNT’s. Oxidation, shielding, Au-NP/nanotube/Au-NP conjugation, and growth of Au-nanorods from the seed termini of the SWCNTs via catalytic enlargement using HAuCl4 and CTAB as a template.
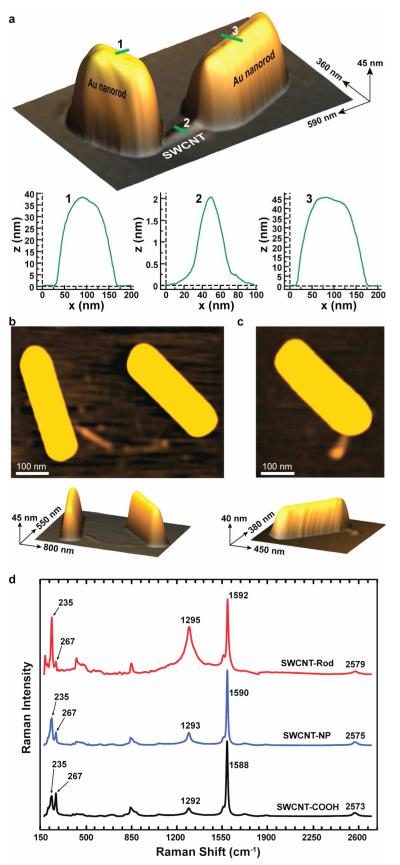
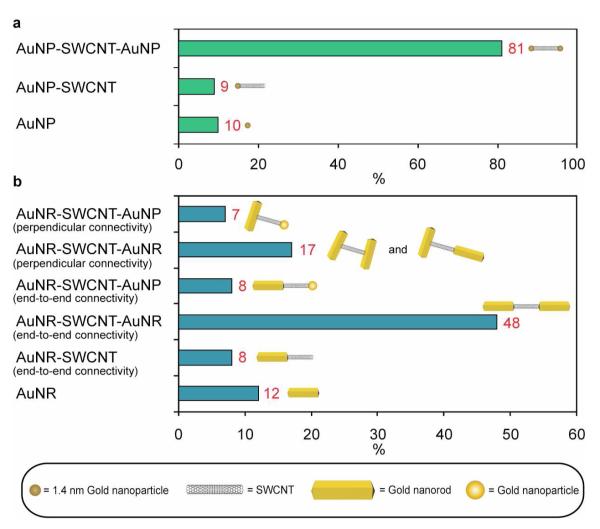
Characterization of the Au-nanorod/SWCNT/Au-nanorod nanostructures was performed using a combination of transmission electron microscopy (TEM) (Fig. 2), atomic force microscopy (AFM) (Fig. 3a-c, Supporting Information movie file), and confocal Raman spectroscopy (Fig. 3d) before and after Au-nanorod growth. AFM revealed that prior to Au-NP conjugation the oxidized shielded SWCNTs were well dispersed as unbundled monomeric species with average lengths of~200 nm (Supporting Information Fig. S1). The regiospecific Au-NP conjugation to the termini of the SWCNTs was confirmed by TEM and Figure 2a,b show images of individual SWCNTs labeled with a single gold nanoparticle at one end and both ends, respectively. A rare occurrence is shown in Fig. 2a, where two SWCNT’s proximate to each other are mono-functionalized with Au-NPs. Mono-functionalized nanotubes represented <10% of the structures observed prior to Au-nanorod growth as shown in figure 4a. The bis-functionalized SWCNTs were the predominate structures, representing ~90% of the structures observed by TEM and are shown in Fig. 2b and Fig. 4a. Dispersed individual nanotubes are difficult to see with TEM, since their local electron density is similar to the amorphous carbon supports. However, the individual Au-NPs attached directly to the tube termini are clearly visible and serve to unambiguously identify the images of the SWCNTs. Figure 2c-e are TEM images of the Au-nanorod/SWCNT/Au-nanorod nanostructures obtained after Au-nanorod growth from the Au-NP functionalized SWCNT termini and the distribution of observed nanostructures is shown in figure 4b. Au-nanorod lengths of ~200-400 nm are observed attached directly to the termini of short (~10 nm) and long (~100-250 nm) SWCNTs. The nanorod growth is also affected by nearby structures (i.e. SWCNT termini), and a majority of connections of the SWCNTs to the ends of the Au-nanorods suggest that the nanorod growth is mainly in a direction opposite from the point of Au-NP conjugation. A small percentage (~24%) of the SWCNT structures contained perpendicularly connected Au-nanorods, and are produced from the seed-mediated growth in two directions orthogonal to the lengthwise axial edge of the SWCNTs (Fig. 4b).19 Additionally, we observe a few (~15%) of structures where a SWCNT has one terminus connected to a single Au-nanorod and the other to a large Au-nanoparticle (Fig. 4b and Supporting Information Fig. S2) produced by non-directional growth. We expect that as improved methods for controlling Au-nanorod growth emerge even more homogenous materials will be possible.
Figure 2.
TEM characterization of Au-nanorod terminated SWCNT hetrojunctions. (a,b) TEM images showing SWCNT’s with regiospecific Au-nanoparticle functionalization at their termini. NPs are bordered with yellow circles. (c) TEM image showing a short SWCNT between Au-nanorods. Right image displays an enlarged view of the heterojunction from the image on the left. (d,e) TEM images showing long SWCNT’s between Au-nanorods. (f) TEM images showing Au-nanorods perpendicularly connected to the SWCNT terminus. All images are shown with their respective scale bars.
Figure 3.
AFM characterization of Au-nanorod terminated SWCNT hetrojunctions. (a) Topographical AFM image of a gold nanorod-linked SWCNT with the associated height profiles. (b) 2D and topographical AFM images of a perpendicularly connected Au-nanorod/SWCNT/Au-nanorod nanostructure. (c) 2D and topographical AFM images of a Au-nanorod/SWCNT/Au-nanoparticle nanostructure. (d) Confocal Raman spectra of Au-nanorod/SWCNT/Au-nanorods after oxidation (black,bottom spectrum), after Au-NP conjugation (blue, middle spectrum), and after Au-nanorod growth (red,top spectrum) using a laser excitation wavelength of 784.4 nm (1.58 eV).
Figure 4.
Distribution of nanostructures before and after Au-nanorod growth procedure. (a) Distribution of mono- and bis-functionalized CNTs and free gold nanoparticles observed prior to Au-nanorod growth procedure. (b) Distribution of nanostructures observed after Au-nanorod growth procedure.
The Au-nanorod/SWCNT/Au-nanorod nanostructures were further characterized by AFM (Fig. 3a-c). Long nanorods 40-45 nm in height and 200-400 nm in length, with SWCNT 0.7-2.2 nm in height and 10-500 in length, were observed after drop-casting of the solution onto a freshly cleaved mica surface. A topographical AFM image of a gold nanorod-linked SWCNT is shown in Fig. 3a with the associated height profiles. Figure 3a shows an individual nanostructure where two Au-nanorods are attached regiospecifically to the termini of a SWCNT. The Au-nanorod height and length dimensions range from 40×200 to 45×300 nm, respectively. The SWCNT fixed between the two Au-nanorods has a height of ~2.2 nm and a length of ~100 nm (Fig. 3a). Figure 3b shows the AFM image of a perpendicularly connected Au-nanorod/SWCNT/Au-nanorod nanostructure. The height and length dimensions of the Au-nanorods varied from 40×400 to 45×400 nm, respectively. The SWCNT positioned between the two rods has a height of ~0.7-1.2 nm and lenth of ~ nm The Au-nanorod on the left is perpendicularly connected to the SWCNT terminus. Whereas. The other nanorod is connected in an end-to-end fashion with the SWCNT. Figure 3c shows a SWCNT with one end connected to a gold nanorod and the other terminated by a gold nanoparticle, which failed to seed a nanorod. Inhibition of nanorod growth could be attributed to interference from nearby objects.19,20
Further characterization of the Au-nanorod/SWCNT/Au-nanorod nanocomposites at each step in the synthesis was performed by laser scanning Raman confocal microscopy. Previous resonance Raman studies have demonstrated that SWCNTs filled with metallic silver altered the Fermi level with silver behaving as an electron donor increasing the electron carrier density.21-23 Utilizing laser scanning confocal Raman microscopy and a laser excitation wavelength of 784.4 nm (1.58 eV), we characterized our Au-nanorod/SWCNT/Au-nanorods after oxidation, after Au-NP conjugation, and after Au-nanorod growth (Fig. 3d). Gold nanorod growth results in significant changes in the Raman spectra and the RBM region showed major peaks at 235, and 267 cm−1. The RBM peak at 235 cm−1 shows a significant increase in intensity after Au-nanorod growth whereas the peak at 267 cm−1 shows a significant decrease, implying an increased isolation of individual carbon nanotubes by Au-nanorods.21 The intensity of the D-band increases with significant broadening upon Au-nanorod formation and shows a slight ~4 cm−1 shift to higher frequency from 1588 cm−1 consistent with previous studies of SWCNTs on silver surfaces.24 Increases in the D/G ratio upon Au-nanorod formation reflects a modification of the nanotube surface and/or a plasmonic enhancement of scattering from sights proximate to the Au-nanorods. Additionally, the peak in the G’ region shows a 6 cm−1 shift to higher wavenumbers. Control spectra for CTAB, CTAB-coated gold nanorods, and 1.4 nm gold nanoparticles are shown in figure S3. The control spectra show no significant resolvable signals above the baseline when compared to the spectra in figure 3d.
An operationally simple and controllable method for the regioselective synthesis of well-defined Au-nanorod/SWCNT/Au-nanorod nanostructures represents a major step forward in the controlled functionalization of SWCNTs with precisely defined metallic heterojunctions. Control of gold nanorod length, growth direction, and alignment are under investigation in addition to the use of alternate metals such as silver as well as the use of multiwalled CNTs as a seed scaffold. Applications utilizing the optical and electronic properties are under investigation and range from field effect transistors to chemical sensors and Raman active nanodevices.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by NSF ECCS-0731100 and ARO W911NF-07-D-0004. D.M.C. is grateful for an NIH-NIGMS postdoctoral fellowship (1-F32-GM087028-01A1).
Footnotes
Supporting Information Available. Materials, methods, movie_1, and Figure S1-S3. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).Zhang Y, Ichihashi T, Landree E, Nihey F, Iijima S. Science. 1999;285:1719–1722. doi: 10.1126/science.285.5434.1719. [DOI] [PubMed] [Google Scholar]
- (2).Hu J, Ouyang M, Yang P, Lieber CM. Nature. 1999;399:48–51. [Google Scholar]
- (3).Luo J, Zhang L, Zhang Y, Zhu J. Advanced Materials. 2002;14:1413–1414. [Google Scholar]
- (4).Asaka K, Nakahara H, Saito Y. Applied Physics Letters. 2008;92:023114. [Google Scholar]
- (5).Rodríguez-Manzo JA, Banhart F, Terrones M, Terrones H, Grobert N, Ajayan PM, Sumpter BG, Meunier V, Wang M, Bando Y, Golberg D. Proc Natl Acad Sci U S A. 2009;106:4591–4595. doi: 10.1073/pnas.0900960106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Mieszawska AJ, Jalilian R, Sumanasekera GU, Zamborini FP. J Am Chem Soc. 2005;127:10822–10823. doi: 10.1021/ja0521122. [DOI] [PubMed] [Google Scholar]
- (7).Gole A, Murphy CJ. Chem. Mater. 2004;16:3633–3640. [Google Scholar]
- (8).Jana NR, Gearheart L, Murphy CJJ. Phys. Chem. B. 2001;105:4065–4067. [Google Scholar]
- (9).Kauffman DR, Star A. Angew Chem Int Ed Engl. 2008;47:6550–6570. doi: 10.1002/anie.200704488. [DOI] [PubMed] [Google Scholar]
- (10).Lobez JM, Swager TM. Angew Chem Int Ed Engl. 2010;49:95–98. doi: 10.1002/anie.200904936. [DOI] [PubMed] [Google Scholar]
- (11).Wang F, Gu H, Swager TM. J Am Chem Soc. 2008;130:5392–5393. doi: 10.1021/ja710795k. [DOI] [PubMed] [Google Scholar]
- (12).Zhang W, Swager TM. J Am Chem Soc. 2007;129:7714–7715. doi: 10.1021/ja0717212. [DOI] [PubMed] [Google Scholar]
- (13).Zhang W, Sprafke JK, Ma M, Tsui EY, Sydlik SA, Rutledge GC, Swager TM. J Am Chem Soc. 2009;131:8446–8454. doi: 10.1021/ja810049z. [DOI] [PubMed] [Google Scholar]
- (14).Bahr JL, Tour JM. J. Mater. Chem. 2002;12:1952–1958. [Google Scholar]
- (15).Banerjee S, Hemraj-Benny T, Wong SS. New Reference. 2005;17:17–29. [Google Scholar]
- (16).Liu J, Rinzler AG, Dai H, Hafner JH, Bradley RK, Boul PJ, Lu A, Iverson T, Shelimov K, Huffman CB. Science. 1998;280:1253. doi: 10.1126/science.280.5367.1253. others. [DOI] [PubMed] [Google Scholar]
- (17).Chen RJ, Bangsaruntip S, Drouvalakis KA, Kam NW, Shim M, Li Y, Kim W, Utz PJ, Dai H. Proc Natl Acad Sci U S A. 2003;100:4984–4989. doi: 10.1073/pnas.0837064100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Rastogi R, Kaushal R, Tripathi SK, Sharma AL, Kaur I, Bharadwaj LM. J Colloid Interface Sci. 2008;328:421–428. doi: 10.1016/j.jcis.2008.09.015. [DOI] [PubMed] [Google Scholar]
- (19).Wei Z, Zamborini FP. Langmuir. 2004;20:11301–11304. doi: 10.1021/la047408k. [DOI] [PubMed] [Google Scholar]
- (20).Wei Z, Mieszawska AJ, Zamborini FP. Langmuir. 2004;20:4322–4326. doi: 10.1021/la049702i. [DOI] [PubMed] [Google Scholar]
- (21).Corio P, Santos AP, Santos PS, Temperini MLA, Brar VW, Pimenta MA, Dresselhaus MS. Chem Phys Lett. 2004;383:475–480. [Google Scholar]
- (22).Dresselhaus MS, Dresselhaus G, Saito R, Jorio A. Phys Reports. 2005;409:47. [Google Scholar]
- (23).Dresselhaus MS, Dresselhaus G, Hofmann M. Vibrational Spectroscopy. 2007;45:71–81. [Google Scholar]
- (24).Wu B, Zhang J, Wei Z, Cai S, Liu Z. J Phys Chem B. 2001;105:5075–5078. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.