Abstract
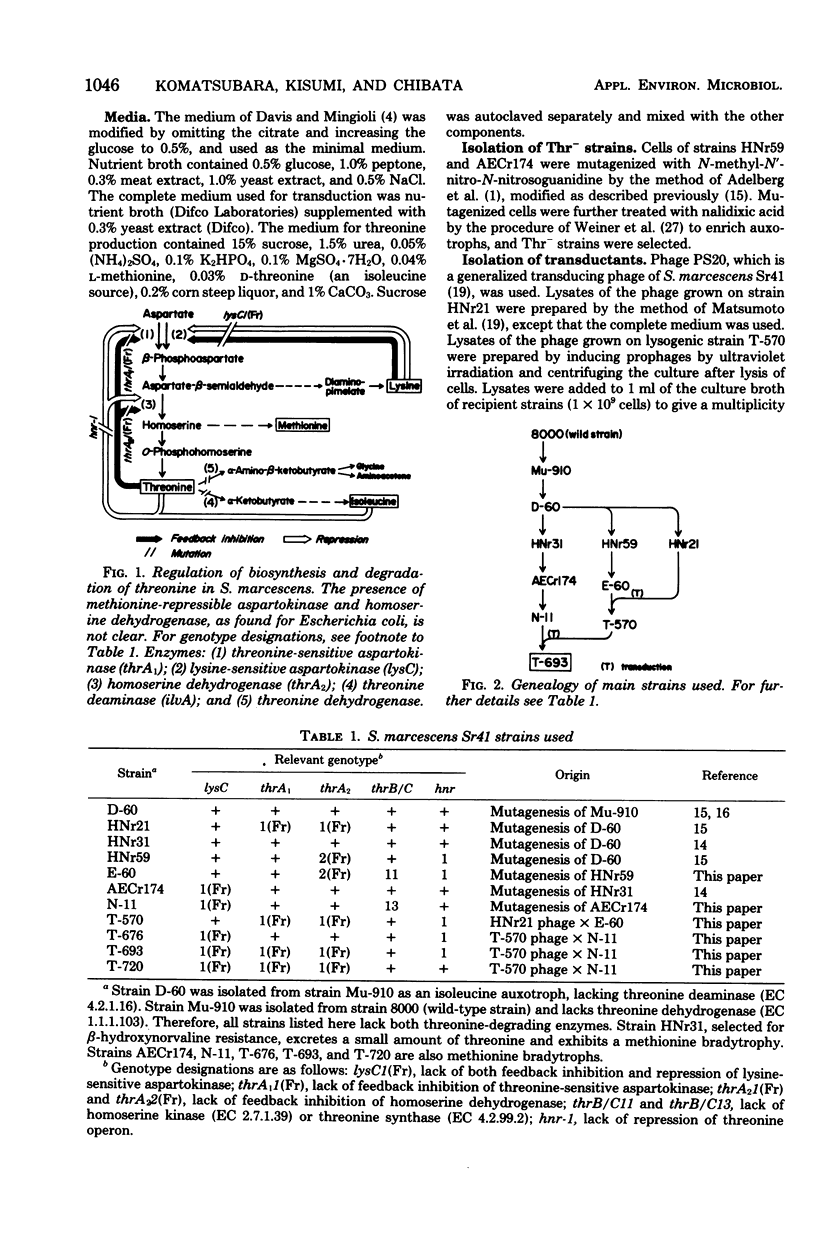
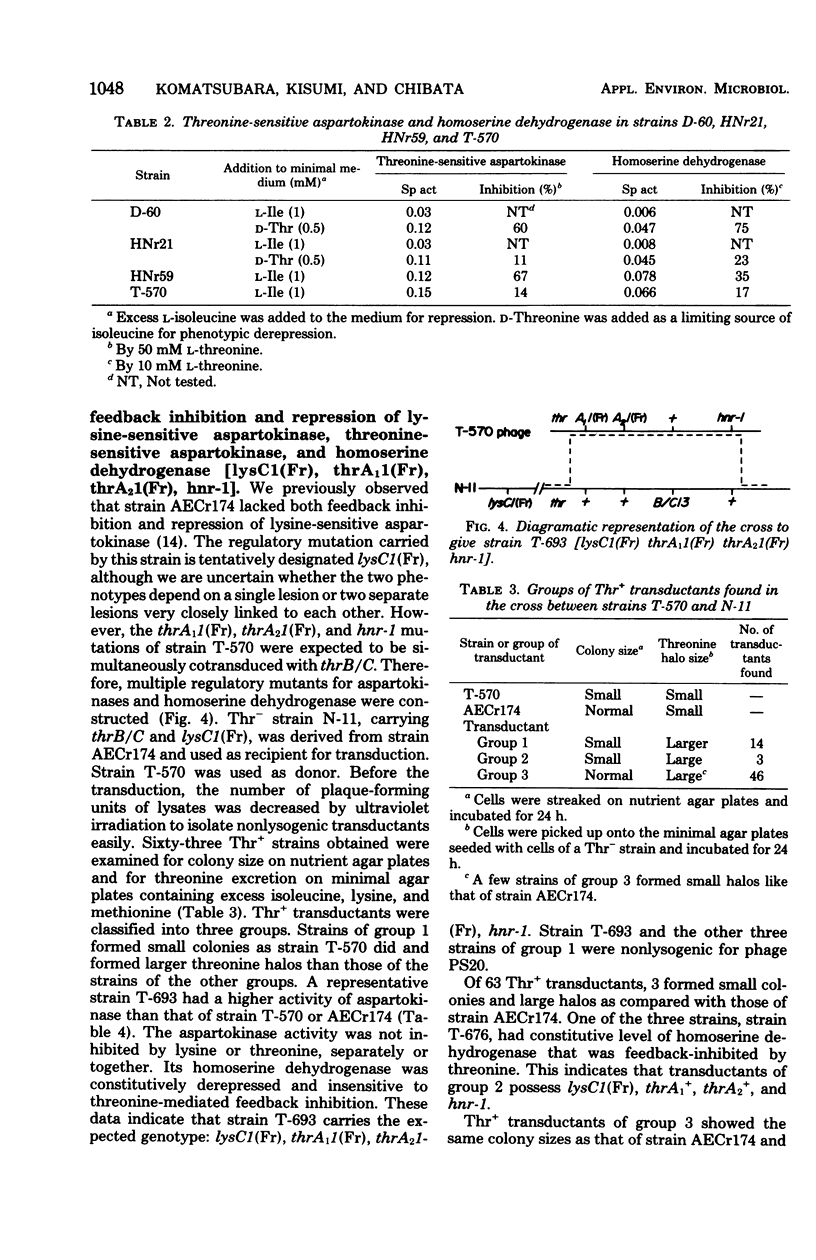
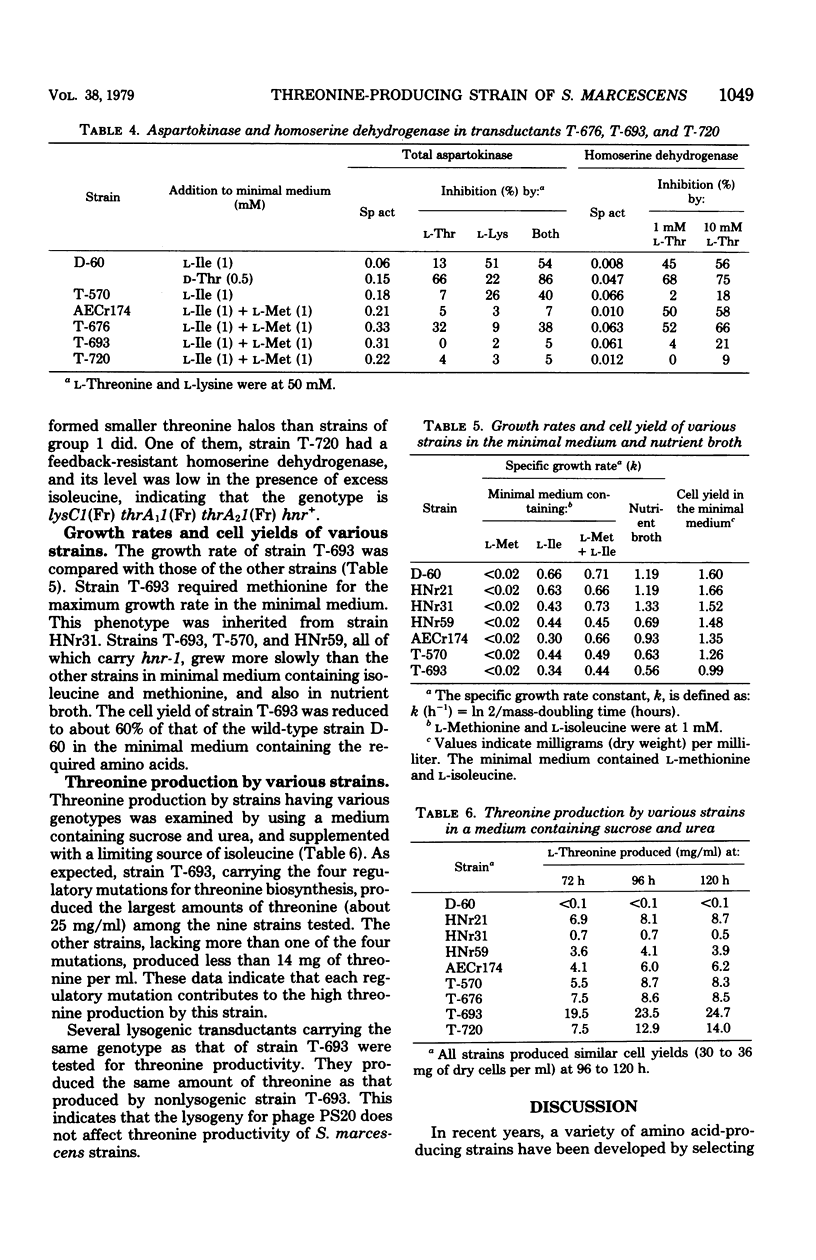
A threonine-producing strain of Serratia marcescens Sr41 was constructed according to the following process. Thr- strain E-60 was derived from strain HNr59 having constitutive levels of threonine-sensitive aspartokinase and homoserine dehydrogenase. Thr+ transductant T-570 was constructed from strain E-60 and phage grown on strain HNr21 having feedback-resistant threonine-sensitive aspartokinase and homoserine dehydrogenase. This transductant lacked both feedback inhibition and repression for the two enzymes. Thr- strain N-11 was derived from strain AECr174 lacking feedback inhibition and repression of lysine-sensitive aspartokinase. Subsequently, the threonine region of strain T-570 was transduced into strain N-11. One of the THR+ transductants, strain T-693, produced markedly high levels of the two aspartokinases and homoserine dehydrogenase, which were insensitive to feedback inhibition. This strain produced about 25 mg of threonine per ml in the medium containing sucrose and urea.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisumi M., Komatsubara S., Chibata I. Enhancement of isoleucine hydroxamate-mediated growth inhibition and improvement of isoleucine-producing strains of Serratia marcescens. Appl Environ Microbiol. 1977 Dec;34(6):647–653. doi: 10.1128/aem.34.6.647-653.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisumi M., Komatsubara S., Sugiura M., Chibata I. Isoleucine accumulation by regulatory mutants of Serratia marcescens: lack of both feedback inhibition and repression. J Bacteriol. 1972 May;110(2):761–763. doi: 10.1128/jb.110.2.761-763.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisumi M., Komatsubara S., Sugiura M., Chibata I. Isoleucine hydroxamate, an isoleucine antagonist. J Bacteriol. 1971 Sep;107(3):741–745. doi: 10.1128/jb.107.3.741-745.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisumi M., Komatsubara S., Sugiura M., Chibata I. Properties of isoleucine hydroxamate-resistant mutants of Serratia marcescens. J Gen Microbiol. 1971 Dec;69(3):291–297. doi: 10.1099/00221287-69-3-291. [DOI] [PubMed] [Google Scholar]
- Kisumi M., Nakanishi N., Takagi T., Chibata I. Construction of a urocanic acid-producing strain of Serratia marcescens by transduction. Appl Environ Microbiol. 1978 Feb;35(2):231–236. doi: 10.1128/aem.35.2.231-236.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisumi M., Nakanishi N., Takagi T., Chibata I. L-Histidine production by histidase-less regulatory mutants of Serratia marcescens constructed by transduction. Appl Environ Microbiol. 1977 Nov;34(5):465–472. doi: 10.1128/aem.34.5.465-472.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisumi M., Sugiura M., Takagi T., Chibata I. Norvaline accumulation by regulatory mutants of Serratia marcescens. J Antibiot (Tokyo) 1977 Jan;30(1):111–117. doi: 10.7164/antibiotics.30.111. [DOI] [PubMed] [Google Scholar]
- Kisumi M., Takagi T., Chibata I. Construction of an L-arginine-producing mutant in Serratia marcescens. Use of the wide substrate specificity of acetylornithinase. J Biochem. 1978 Oct;84(4):881–890. doi: 10.1093/oxfordjournals.jbchem.a132200. [DOI] [PubMed] [Google Scholar]
- Komatsubara S., Kisumi M., Chibata I. Participation of lysine-sensitive aspartokinase in threonine production by S-2-aminoethyl cysteine-resistant mutants of Serratia marcescens. Appl Environ Microbiol. 1979 Nov;38(5):777–782. doi: 10.1128/aem.38.5.777-782.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsubara S., Kisumi M., Murata K., Chibata I. Threonine production by regulatory mutants of Serratia marcescens. Appl Environ Microbiol. 1978 May;35(5):834–840. doi: 10.1128/aem.35.5.834-840.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsubara S., Murata K., Kisumi M., Chibata I. Threonine degradation by Serratia marcescens. J Bacteriol. 1978 Aug;135(2):318–323. doi: 10.1128/jb.135.2.318-323.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsumoto H., Hosogaya S., Suzuki K., Tazaki T. Arginine gene cluster of Serratia marcescens. Jpn J Microbiol. 1975 Feb;19(1):35–44. doi: 10.1111/j.1348-0421.1975.tb00845.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto H., Tazaki T., Hosogaya S. A generalized transducing phage of Serratia marcescens. Jpn J Microbiol. 1973 Nov;17(6):473–479. doi: 10.1111/j.1348-0421.1973.tb00933.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto H., Tazaki T., Hosogaya S. Transductional analysis of the leu-thr region of chromosome of Serratia marcescens. J Bacteriol. 1974 Mar;117(3):1365–1367. doi: 10.1128/jb.117.3.1365-1367.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shailaja M. S., Rao M. R. Lysine-sensitive aspartate kinase of Serratia marcescens. Indian J Biochem Biophys. 1975 Mar;12(1):17–20. [PubMed] [Google Scholar]
- Thèze J., Margarita D., Cohen G. N., Borne F., Patte J. C. Mapping of the structural genes of the three aspartokinases and of the two homoserine dehydrogenases of Escherichia coli K-12. J Bacteriol. 1974 Jan;117(1):133–143. doi: 10.1128/jb.117.1.133-143.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thèze J., Saint-Girons I. Threonine locus of Escherichia coli K-12: genetic structure and evidence for an operon. J Bacteriol. 1974 Jun;118(3):990–998. doi: 10.1128/jb.118.3.990-998.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbarger H. E. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]
- Weiner R. M., Voll M. J., Cook T. M. Nalidixic acid for enrichment of auxotrophs in cultures of Salmonella typhimurium. Appl Microbiol. 1974 Oct;28(4):579–581. doi: 10.1128/am.28.4.579-581.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



