Abstract
Previous studies conducted by our laboratory have demonstrated that suppression of transforming growth factor beta (TGFβ) mediated up-regulation of connective tissue growth factor (CTGF) by iloprost resulted in a greatly diminished oval cell response to 2-acetylaminofluorene/partial hepatectomy (2AAF/PH) in rats. We hypothesized that this effect is due to decreased activation of hepatic stellate cells. In order to test this hypothesis, we maintained rats on a diet supplemented with 2% L-cysteine as a means of inhibiting stellate cell activation during the oval cell response to 2AAF/PH. In vitro experiments demonstrate that L-cysteine did, indeed, prevent the activation of stellate cells while exerting no direct effect on oval cells. Desmin immunostaining of liver sections from 2AAF/PH animals indicated that maintenance on the L-cysteine diet resulted in an 11.1-fold decrease in the number of activated stellate cells within the periportal zones. The total number of cells proliferating in the periportal zones of livers from animals treated with L-cysteine was drastically reduced. Further analyses demonstrated a greater than four-fold decrease in the magnitude of the oval cell response in animals maintained on the L-cysteine diet as determined by immunostaining for both OV6 and alpha fetoprotein (AFP). Global liver expression of AFP as measured by real-time PCR was shown to be decreased 4.7-fold in the L-cysteine treated animals. These data indicate that the activation of hepatic stellate cells is required for an appropriate oval cell response to 2AAF/PH.
Keywords: hepatic stellate cell, L-cysteine, liver regeneration, oval cell activation, hepatic progenitor cell
Progenitor cell therapies for liver pathologies that result in massive loss of hepatic parenchyma are one alternative to liver transplantation. The “oval cell” is the facultative hepatic progenitor cell which aids in liver regeneration when the proliferation capacity of mature hepatocytes is compromised. These cells are bipotential (i.e. capable of differentiation down both the hepatocyte and biliary lineages) and are thought to reside within the portal zones of the liver at the canals of Hering1. A number of human conditions are associated with oval cell proliferation; among these are infections by hepatotropic viruses and terminal stages of liver cirrhosis2. Previous data from our laboratory suggest a coordinated interaction among the various hepatic cell types during the regeneration process, particularly between hepatic stellate cells and oval cells3. Their co-activation has been demonstrated in the 2-acetylaminofluorene/partial hepatectomy (2AAF/PH) model as well as several other liver remodeling processes, including hepatic fibrosis4 and liver regeneration following partial hepatectomy (PH) or D-galactosamine exposure5. Under physiological conditions stellate cells are quiescent, exhibiting vitamin A droplets and a star-like morphology. Their activation is followed by proliferation and differentiation into contractile myofibroblast-like cells. Known for their contribution to extracellular matrix (ECM) synthesis and remodeling, and as an important source of cytokines and growth factors, activated stellate cells are also responsible for the excessive fibrosis observed in liver cirrhosis6. Disruption of the TGFβ - CTGF signaling axis by the prostacyclin analogue iloprost resulted in a significant reduction of oval cell proliferation3. Since TGFβ has such a complex range of actions on various cell types, we further explored if these effects are the result of stellate cell inhibition, or are attributable to other interactions within the regenerating liver. L-cysteine is a non-essential amino acid, widely used as a nutritional supplement, antioxidant and mucolytic agent. Recent data suggest that it might also be a potent inhibitor of liver fibrosis, acting to prevent stellate cell activation7. Its complex effects on the liver are attributed to various mechanisms, including: synthesis of glutathione8,9; reactive oxygen species (ROS) mediated degradation of cyclin D1 with subsequent activation of manganese superoxide dismutase (MnSOD)10; downregulation of platelet derived growth factor receptor-beta (PDGFRβ), and inhibition of platelet derived growth factor (PDGF) signaling11,12. Despite the various putative mechanisms of action, it is widely accepted as a potent, harmless inhibitor of hepatic stellate cells and was proposed as an adjuvant therapy for human cirrhosis. In order to test our hypothesis that the stellate cells play a necessary role in facilitating oval cell proliferation, a diet supplemented with L-cysteine was combined with the well characterized 2AAF/PH protocol13, 14 for oval cell activation in rats. This study clearly demonstrates that hepatic stellate cell activation is required for a robust oval cell response to 2AAF/PH.
Materials and methods
Animal treatments
The animal procedures involved in this study were conducted with the approval of the University of Florida IACUC. 6 week old Fisher 344 male rats (Charles River Laboratories, Wilmington MA) were maintained on standard laboratory chow supplemented with 2% L-cysteine (Dyets Inc., Betlehem PA) for the duration of the experiment, according to a protocol established by Horie et al7. One month after initiation of the diet, 70 mg 2AAF pellets (Innovative Research of America, Sarasota, FL) were implanted intraperitoneally and, one week later, a 70% partial hepatectomy was performed as described by Higgins and Anderson15. The animals were sacrificed at defined time points indicated in Figure 1. Tissue samples were collected and preserved by formaldehyde fixation or frozen in OCT compound (Sakura Finetek USA Inc, Torrance CA).
Figure 1.
Study design – diagrammatic representation of the hepatic stellate cell inhibition and oval cell induction model in ♂ Fisher 344 rats: 2% L-cysteine diet, 2-AAF pellet implantation, partial hepatectomy and selected time points for sacrifice (mo – month, w – week, d – day).
Cell culture and BrdU labeling
WB-F344 oval cells (kindly provided by Dr. William Coleman) were cultured at 37°C, 5% CO2 in RPMI-1640 medium (Mediatech Inc., Herndon VA) supplemented with 10% FBS (Sigma Aldrich, St. Louis MO) and 1% penicillin – streptomycin (Mediatech Inc., Herndon VA). Primary isolated portal fibroblasts and HSC T6 hepatic stellate cells (kind gifts from Drs. Rebecca Wells and Scott Friedman, respectively) were cultured under the same conditions in DMEM (Hyclone Lab Inc., Logan UT) with 10% FBS and 1% penicillin – streptomycin. When 30% confluence was achieved, they were synchronized for 24 hours with 0.4 μg/ml Demecolcine (Sigma Aldrich, St. Louis MO) and treated with 100 μM L-cysteine (MP Biomedicals LLC, Cleveland OH), thus inhibiting the hepatic stellate cell activation. After a three day exposure to L-cysteine, the chamberslides were treated with 10μM bromodeoxyuridine (BrdU) (Sigma Aldrich, St. Louis MO) and fixed in 4% paraformaldehyde.
Histology and immunohistochemistry
Paraffin embedded or frozen liver sections cut to 5 μm thickness were stained with Hematoxylin & Eosin or immunostained using anti OV6 (courtesy of Dr. Stewart Sell), anti Ki67 (BD Biosciences Pharmingen, Franklin Lakes NJ) or anti alpha-fetoprotein (Dako Cytochromatin, Carpinteria CA) antibodies. Anti desmin antibody (Dako Cytochromatin, Carpinteria CA) was also used for stellate cell immunostaining. Formalin fixed chamberslides were immunostained using anti BrdU antibody (Dako Cytochromatin, Carpinteria CA). Computer image analyses of immunostained sections were performed using Aperio ScanScope Image Analysis Platform (Aperio, Vista CA) and MetaMorph software (MDS Technologies, Concord ON, Canada) for histological evaluation and quantitation.
Real time quantitative PCR
The mRNA levels were assessed by 2-step quantitative real-time PCR reaction, using a DNA Engine Opticon 2 Continuously Fluorescence Detector (MJ Research Inc Waltham MA). Total RNA was extracted using the RNA Bee isolation kit (Tell-Test Inc. Friendswood TX), treated with DNase I (Ambion, Austin TX) and reverse transcribed with the Superscript III First Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad CA). Amplification was performed on a customized RT2Profiler PCR Array plate for the genes of interest (SA Biosciences, Frederick MD) using iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules CA). The primer pair used was: forward CAGGAGGAAGAAAGGACAAAAAA and reverse ATTCCTAAGGCATAGAAATCCCA. The amplification conditions were 10 min at 95°C, followed by 40 cycles of 15 seconds at 95°C, 30 seconds at 55°C, 30 seconds at 72°C. The comparative Ct threshold cycle method was used to assess the expression level, normalized to β-actin m RNA expression.
Statistical analysis
Values were expressed as mean +/- standard deviation (SD). Statistical significance was determined by ANOVA, and student t- test performed in Microsoft Excel. P values <0.05 were considered statistically significant.
Results
L-cysteine appears to be a selective in vitro inhibitor of hepatic mesenchymal populations
We first examined the in vitro effects of L-cysteine on several hepatic cell populations in culture. S-phase cells were identified by BrdU incorporation into newly synthesized DNA. In order to exclude the possibility that L-cysteine acts directly on oval cells, the hepatic progenitor cell line, WB F344 was cultured both with and without (Figures 2A and D, respectively) 100μM L-cysteine. As expected, treatment with L-cysteine had no effect on the proliferation rate of these cells (Figure 3). We next examined primary portal fibroblast cultures (Figures 2B and E), as well as the hepatic stellate cell line HSC-T6 (Figures 2C and F). In contrast to the progenitor cell line, both of the mesenchymal cell cultures demonstrated a significant reduction in proliferation rates when culture media was supplemented with 100μM L-cysteine. A 3.56-fold decrease in BrdU incorporation for HSC T6 and a 5.6-fold reduction for portal fibroblasts were observed (Figure 2 E and F). Taken together, quantitative image analysis data indicate that L-cysteine acts selectively on the mesenchymal cell populations examined (Figure 3).
Figure 2.

In vitro effects of L-cysteine on proliferation of selected hepatic cell populations: (A, D) WB F344 cells, (B,E) primary portal fibroblasts, (C,F) HSC T6 cells. Panels A-C received no treatment while panels D-F were cultured in media supplemented with 2% L-cysteine; all shown at x10 magnification.
Figure 3.

BrdU index: quantitative analysis of L-cysteine effects on cultured mesenchymal and oval cells’ proliferation: WB 344F – rat oval cell line; PF – primary isolated portal fibroblasts; HSC T6 – hepatic stellate cell line; white columns – cells treated with 100 μM L-cysteine in culture medium; black columns – cells grown on culture medium w/out L-cysteine supplement.
Histological changes in oval cell activation under L-cysteine diet
Histological characterization of liver regeneration in the 2-AAF/PH model for oval cell activation in rats demonstrated the expected robust proliferation of small cells emanating from the periportal zone (Figure 4 B and E). These small oval shaped cells were not present in untreated rat liver (Figure 4 A and E). In animals that were maintained on the 2% L-cysteine diet, the small cell response in the portal zone remained quite modest (Figure 4 C and F). The disparity between the amplitude of the small cell response in the two groups was most evident on day 9 post partial hepatectomy. This time point is known to coincide with the peak of oval cell proliferation following 2AAF/PH activation protocol in rats. Aside from the reduced small cell presence in L-cysteine treated animals, there is also a notable difference in cell morphology. In the L-cysteine treated group, some cells tended to be larger (over 10μm diameter) with a slightly reduced nucleus to cytoplasm ratio, more rounded nuclei and basophilic vacuolar cytoplasm, bearing a resemblance to a small hepatocyte-like cell.
Figure 4.

Comparative histological exam of Hematoxylin & Eosin stained liver samples shows differences in the hepatic regeneration profile of L-cysteine fed animals: (A, D) normal animals, (B, E) 2-AAF/PH treated rats 9 days post PH, (C, F) L-cysteine/2-AAF/PH protocol 9 days after acute liver injury. BD – bile duct, PV – portal vein branch, HA – hepatic artery branch, OC – oval cell, SHL – small hepatocyte-like cell. Upper panels are shown at x20, lower panels at x40 magnification.
Hepatic stellate cell activation is inhibited by L-cysteine diet in the 2AAF/PH model
Definitive identification of activated hepatic stellate cells in microscopic liver sections was accomplished by immunostaining for desmin, a cytoskeleton intermediary filament which, in rat liver, is expressed only by activated stellate cells16.
Hepatic stellate cells are present in very low numbers in normal liver tissue (Figure 5 A and D). However, they proliferate and migrate in close proximity to the regenerating oval cells in the 2AAF/PH model (Figure 5 B and E). In animals treated with L-cysteine, considerably fewer desmin positive cells are apparent following 2AAF/PH (Figure 5 C and F). Quantitative computer image analysis showed a greater than eleven-fold decrease in the number of desmin positive cells in 2AAF/PH rats maintained on the L-cysteine diet as compared to 2AAF/PH animals that received the standard diet (Figure 5 G). These results suggest that the inhibitory effects of L-cysteine on hepatic stellate cell activation work as expected in our 2AAF/PH model of oval cell induction.
Figure 5.
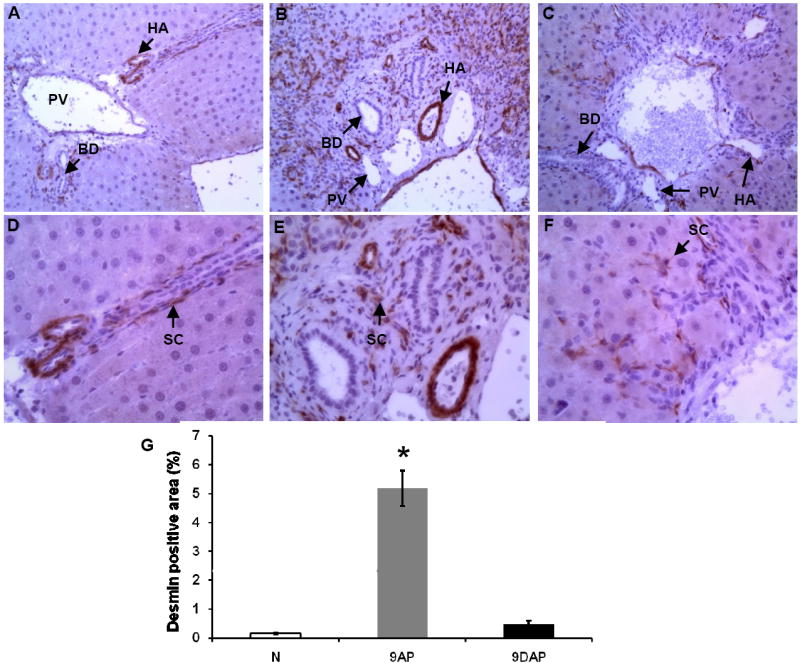
In vivo reduced activation of hepatic stellate cell population indicated by desmin immunostaining of liver sections from L-cysteine-fed rats: normal rat liver (A, D, N), liver at day 9 following 2AAF/PH (B, E, 9AP), same time point liver section from animals subjected to L-cysteine diet/2AAF/PH (C, F, 9DAP). BD – bile duct, PV – portal vein branch, HA – hepatic artery branch, SC – stellate cell. Upper panels are shown at x20, lower panels at x40 magnification. (G) Quantitative computer image analysis of desmin positive areas on the immunostained liver samples confirms the significant reduction of stellate cell activation on immunostained sections.
L-cysteine exposure is associated with reduced cell proliferation in periportal areas
We next sought to determine the proliferation status of the cells within the periportal zones of livers from 2AAF/PH treated animals both with, and without the L-cysteine diet. Proliferation was determined by immunostaining for Ki67 nuclear antigen on day 9 following PH. Ki67 identifies all cells that are not in G0. In the normal liver very few hepatocytes proliferate in the absence of an injury (Figure 6 A). A large number of proliferating cells were present in the portal zones of the livers of rats subjected to the 2AAF/PH protocol (Figure 6 B). A greater than three-fold reduction in the number of proliferating cells was measured in the portal zones of the liver from rats maintained on the L-cysteine diet (Figures 6 C and D).
Figure 6.

Assessment or proliferation status of hepatic cell populations by quantitative analysis of Ki67 positive nuclei on random microscopic fields: normal liver (A, N) and samples collected 9 days after the acute liver injury; (B, 9AP) rats subjected to the 2AAF/PH protocol, (C, 9DAP) animals fed with L-cysteine during the oval cell activation treatment. All shown at x20 magnification.
Reduced cell proliferation in periportal areas during L-cysteine exposure is associated with diminished desmin presence in the regenerating liver
Double immunofluorescent staining for Ki67 nuclear antigen and type III intermediate filament desmin was performed. Specifically expressed by activated stellate cells, desmin is present in portal myofibroblasts and Ito cells in normal liver (Figure 7 A, D). The numerous proliferating cells in the periportal areas of 2AAF/PH exposed animals were surrounded by desmin positive cytoplasmic projections (Figure 7 B, E). Sections collected from animals kept on L-cysteine diet exhibited a reduced presence of proliferating cells accompanied by few desmin positive cells in acinar zone I of regenerating liver (Figure 7 C, E).
Figure 7.
Concomitant reductions in proliferation of hepatic cell populations and oval cell activation, reflected by Ki67 and desmin immunofluorencent staining, respectively: normal liver (A, D); sections collected 9 days post hepatectomy from animals subjected to 2AAF/PH protocol (B, E) and rats maintained on L-cysteine/2AAF/PH (C, F). PV – portal vein branch, HA – hepatic artery branch. Upper panels are shown at x20, lower panels at x40 magnification.
Regenerating cells in the periportal areas are AFP and OV6 positive
To identify the oval cell population within the portal zone on day 9 following 2AAF/PH, paraffin sections were stained for alpha fetoprotein (AFP). On the normal liver sections no AFP positive cells were apparent (Figure 8 A and D). As expected, the portal zones of livers from 2AAF/PH treated animals contained a large population of AFP positive cells (Figure 8 B and E). Animals exposed to L-cysteine showed a greater than four-fold decrease in AFP positive cells (Figure 8 C, F and G). It is worth noting that a greater percentage of the cells within the portal zones of livers from animals maintained on the L-cysteine diet appeared to be transitional hepatocytes (i.e. larger, hepatocyte-like cells with weak expression of AFP).
Figure 8.
Reduced oval cell presence on AFP immunostained liver sections suggested by quantitative image analysis (G): normal liver (A,D, N), 2AAF/PH treated rats day 9 following PH (B,E, 9AP) and 2AAF/PH treated rats maintained on the 2% L-cysteine diet 9 days following PH (C,F, 9DAP), OC – oval cell, TC – transitional hepatocytes. Upper panels are shown at x10, lower panels at x20 magnification.
Global AFP expression in the livers of animals subjected to 2AAF/PH was determined by quantitative real-time PCR (Figure 9 A). AFP message was measured relative to the normal liver and normalized to beta actin. Animals that were exposed to L-cysteine demonstrated a 4.7-fold decrease in total liver expression of AFP as compared to animals that were fed the normal diet. These results suggest a significant reduction of oval cell contribution to hepatic mass recovery.
Figure 9.
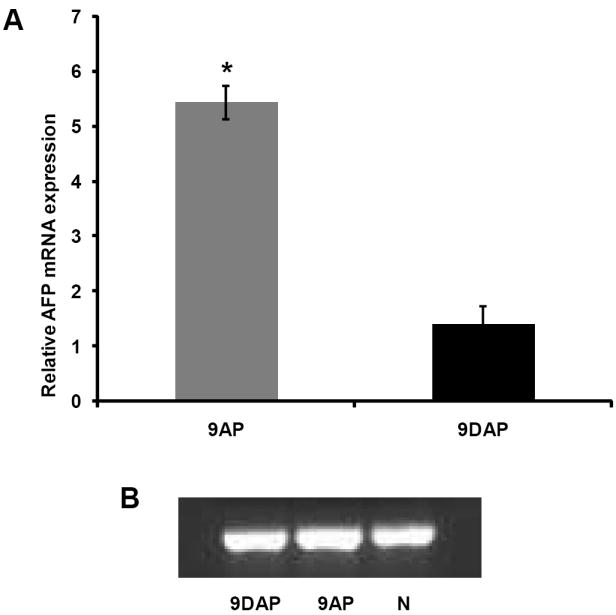
Magnitude of oval cell response quantified by real time PCR analysis of AFP mRNA, relative to normal liver tissue expression and normalized to beta actin (A), 9AP – animals fed normal rat chow and subjected to 2AAF/PH protocol; 9DAP – animals maintained on the L-cysteine diet associated to 2AAF/PH treatment. GAPDH was used as a loading control (B).
An alternative oval cell marker was used to confirm the findings of the AFP immunostaining. OV6 is a well characterized marker for oval cells and bile duct cells. In normal liver, only the bile ducts within the portal triad were positive for OV6 (Figure 10 A and D). Liver samples from animals subjected to 2AAF/PH contained a large population of OV6 positive cells within the periportal zone that radiated out toward the central vein (Figure 10 B and E). This is consistent with a normal oval cell response at day 9 following PH in the 2AAF/PH model. In contrast to this, the liver sections from animals maintained on the L-cysteine diet displayed a very modest oval cell response at day 9 following PH (Figure 10 C, F). Computer image analysis of scanned slides confirmed the 3.5-fold disparity in the magnitude of oval cell response in animals that were fed the normal rat food, as compared to animals that were administered L-cysteine (Figure 10 G).
Figure 10.
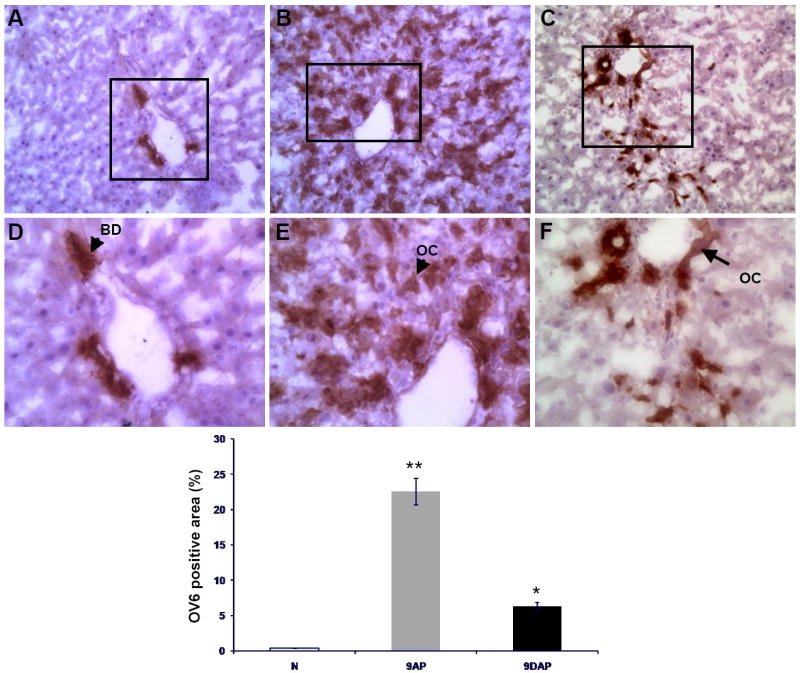
Oval cell response reduction assessed by quantitative image analysis (D) of OV6 positive areas on tissue sections obtained from normal liver (A, N) and animals sacrificed 9 days after the acute liver injury: (B, 9AP) subjected to the 2-AAF/PHx protocol, and (C, 9DAP) fed L-cysteine during the oval cell activation treatment. OC – oval cell, BD – bile duct. Upper panels are shown at x20, lower panels at x40 magnification.
Discussion
Progenitor cell mediated liver regeneration is an alternative compensatory hyperplasia, able to restore hepatic mass when hepatocyte proliferation is severely impaired by massive liver necrosis or chronic cirrhogenic conditions17. Oval cells and stellate cells are among the first cells to enter the cell cycle following 2AAF/PH progenitor activation protocol18. Less is known regarding the exact temporal relationship between oval and stellate cell activation in the regenerating liver. It is for this reason that we chose to begin the L-cysteine diet well before initiation of the 2AAF/PH protocol (Figure 1). The daily food intake and the body weight of all animals were monitored for the duration of the study in order to identify any potential effects attributable to the diet, and no significant differences were seen between animals fed the experimental and control diets. Our in vitro studies also demonstrate that L-cysteine does not directly govern oval cell proliferation; nor does it induce any alterations of their phenotype (Figure 2). Taken together, these facts indicate that the effects of L-cysteine in our model result from the specific inhibition of hepatic stellate cells.
The hepatocyte proliferation inhibitor 2-AAF is activated and detoxified by the liver through rounds of hydroxylation and conjugation19 which lead to renal excretion of water-soluble derivatives 20, 21. Being a precursor for glutathione synthesis, it is reasonable to consider the possibility that L-cysteine could increase the rate of 2AAF detoxification. This would potentially lead to incomplete suppression of hepatocyte proliferation in the 2AAF/PH model. However, examination of Ki67 stained liver sections from the L-cysteine treated group showed no signs of mature hepatocyte proliferation, the only Ki67 positive cells being oval cells and small hepatocyte-like phenotype (Figure 6). This would seem to exclude differential 2AAF metabolism as a complicating factor in these studies. The reduced Ki67 presence in the animals maintained on L-cysteine was associated with a diminished presence of desmin in the periportal spaces, indicating a reduced activation of hepatic stellate cells. Under these circumstances, the number of proliferating cells in the periportal areas was also reduced, suggesting an association between a reduced contribution of stellate cells and diminished hepatic regeneration (Figure 7 C, F as opposed to Figure 7 B, E).
Progenitor cell mediated liver regeneration is a complex process, involving sequential waves of cytokine secretion and remodeling of the extracellular matrix. These two processes are intimately coupled, as the matrix can liberate chemical signals when degraded, and concentrate chemical signals22 that bind to the matrix within specific regions 23, 24. Thus ECM functions as a primary reservoir of biologically active molecules in the liver18. During hepatic regeneration the presence of increased numbers of hepatic stellate cells was noted in close proximity to the oval cells25. Activated hepatic stellate cells are the main source of MMPs and TIMPS that participate in matrix remodeling and release of bound cytokines18, 26. Matrix remodeling results in the establishment of a unique microenvironment, conducive for the proliferation and migration of cells within the regenerating zone. This renders the activation of hepatic stellate cells a critical component of progenitor cell mediated liver regeneration process. Previous research conducted by our group demonstrates that during progenitor cell mediated liver regeneration, a fibronectin rich provisional matrix is synthesized in the portal zone23. We feel that it is likely that this provisional matrix contributes to the oval cell response, acting as a required substrate on which oval cells may proliferate and providing binding sites for signaling molecules that regulate oval cell phenotype. One such example is CTGF which binds to the fibronectin rich provisional matrix of the periportal zone, where it is concentrated and made available to the oval cells which are known to express CTGF receptors23.
Several recently published studies clearly demonstrate that stellate cells within the liver may, through a process of mesenchymal to epithelial transition, give rise to hepatocytes27. It is possible that this phenomenon involves an intermediate cell type with oval cell properties. It is impossible to determine if decreased mesenchymal to epithelial transition contributes to the reduction in oval cell proliferation seen in our model. However, this possibility deserves mention.
Another interesting feature of the L-cysteine treated livers is the apparent accumulation of transitional or intermediate hepatocytes (Figure 4), displaying a phenotype characteristic for the regeneration process, without any alterations induced by the diet.
These cells are morphologically similar to hepatocytes, but are much smaller and weakly express AFP. In our model, we speculate that these cells are oval cells that have failed to complete the differentiation program, but at this stage the absence of reliable markers have made impossible their precise identification. This would suggest that inhibition of stellate cell activation following 2AAF/PH not only affects oval cell proliferation, but also oval cell differentiation. Once again, this would most likely result from the lack of an appropriate microenvironment within the regenerating zone and advocates the “nurturing role” of stellate cells during the regeneration process. While the cellular response appears to be attenuated quantitatively in response to stellate cell inhibition, we have not noticed any phenotype alterations of the oval cells during the regeneration process. Overall, the blunted oval cell response in animals fed the L-cysteine diet is likely due to a combination of the loss of a major cytokine source (i.e. activated stellate cells), coupled with the loss of an appropriate microenvironment for expansion of the oval cell population (i.e. the fibronectin rich provisional matrix). The end result of L-cysteine treatment is a rough doubling of the time required for regeneration of the liver following 2AAF/PH (data not shown). Because the injury does eventually resolve in the L-cysteine treated group, redundant pathways for the regulation of oval cell phenotype likely exist. However, the significant reduction in oval cells seen at what would normally be considered the time of maximal oval cell proliferation proves, for the first time, the critical role of stellate cells in oval cell mediated liver regeneration.
Acknowledgments
The authors would like to thank Dr. Rebecca Wells from University of Pennsylvania, Dr. William Coleman from University of North Carolina and Dr. Scott Friedman from Mount Sinai School of Medicine for their kind gifts of primary isolated portal fibroblasts, WB F344 cells and HSC T6 cells, respectively and to Dr. Stuart Sell from Ordway Research Institute for generously providing the OV6 antibody.
Grant support DK58614; DK65096 awarded to BEP
Abbreviations
- 2AAF/PH
2-acetylaminofluorene/partial hepatectomy
- ECM
extracellular matrix
- TGF-b
transforming growth factor-b
- CTGF
connective tissue growth factor
- AFP
alpha fetoprotein ROS reactive oxygen species
- MnSOD
manganese superoxide dismutase
- PDGFR-b
platelet derived growth factor receptor-beta
- PDGF
platelet derived growth factor
- BrdU
bromodeoxyuridine
- MMPs
matrix metalloproteinases
- TIMPs
tissue-specific inhibitor of metalloproteinases
References
- 1.Kuwahara R, Kofman AV, Landis CS, Swenson ES, Barendswaard E, Theise ND. The hepatic stem cell niche: identification by label-retaining cell assay. Hepatology. 2008;47(6):1994–2002. doi: 10.1002/hep.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiao JC, Jin XL, Ruck P, Adam A, Kaiserling E. Hepatic progenitor cells in human liver cirrhosis: immunohistochemical, electron microscopic and immunofluorencence confocal microscopic findings. World J Gastroenterol. 2004;10(8):1208–11. doi: 10.3748/wjg.v10.i8.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pi L, Oh SH, Shupe T, Petersen BE. Role of connective tissue growth factor in oval cell response during liver regeneration after 2-AAF/PHx in rats. Gastroenterology. 2005;128(7):2077–88. doi: 10.1053/j.gastro.2005.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman SL. Liver fibrosis – from bench to bedside. J Hepatol. 2003;38(Suppl 1):S38–S53. doi: 10.1016/s0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 5.Ujike K, Shinji T, Hirasaki S, Shiraha H, Nakamura M, Tsuji T, Koide N. Kinetics of expression of connective tissue growth factor gene during liver regeneration after partial hepatectomy and D-galactosamine-induced liver injury in rats. Biochem Biophys Res Commun. 2000;277:448–454. doi: 10.1006/bbrc.2000.3693. [DOI] [PubMed] [Google Scholar]
- 6.Friedman SL. Mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol. 2004;1:98–105. doi: 10.1038/ncpgasthep0055. [DOI] [PubMed] [Google Scholar]
- 7.Horie T, Sakaida I, Yokoya F, Nakajo M, Sonaka I, Okita K. L-cysteine administration prevents liver fibrosis by suppressing hepatic stellate cell proliferation and activation. Biochem Biophys Res Commun. 2003;305(1):94–100. doi: 10.1016/s0006-291x(03)00691-0. [DOI] [PubMed] [Google Scholar]
- 8.Kim KI, Rhim T, Choi I, Kim SS. N-acetylcysteine induces cell cycle arrest in hepatic stellate cells through its reducing activity. J Biol Chem. 2001;276:40591–40598. doi: 10.1074/jbc.M100975200. [DOI] [PubMed] [Google Scholar]
- 9.Zheng S, Yumei F, Chen A. De novo synthesis of glutathione is a prerequisite for curcumin to inhibit hepatic stellate cell (HSC) activation. Free Radic Biol Med. 2007;43(3):444–53. doi: 10.1016/j.freeradbiomed.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menon SG, Sarsour EH, Kalen AL, Venkataraman S, Hitchler MJ, Domann FE, Oberley LW, Goswami PC. Superoxide signaling mediates N-acetyl-L-cysteine-induced G1 arrest: regulatory role of cyclin D1 and manganese superoxide dismutase. Cancer Res. 2007;67(13):6392–9. doi: 10.1158/0008-5472.CAN-07-0225. [DOI] [PubMed] [Google Scholar]
- 11.Okuyama H, Shimahara Y, Kawada N, Seki S, Kristensen DB, Yoshizato K, Uyama N, Yamaoka Y. Regulation of cell growth by redox-mediated extracellular proteolysis of platelet derived growth factor receptor b. J Biol Chem. 2001;276:28274–28280. doi: 10.1074/jbc.M102995200. [DOI] [PubMed] [Google Scholar]
- 12.Matsui H, Ikeda K, Nakajima Y, Horikawa S, Imanishi Y, Kawada N. Sulfur-containing amino acids attenuate the development of liver fibrosis in rats through down-regulation of stellate cell activation. J Hepatol. 2004;40(6):917–25. doi: 10.1016/j.jhep.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Solt DB, Farber E. New principle for the analysis of chemical carcino-genesis. Nature (Lond) 1976;263:702–703. [Google Scholar]
- 14.Petersen BE, Goff JP, Greenberger JS, Michalopoulos GK. Hepatic oval cells express the hematopoietic stem cell marker Thy-1 in the rat. Hepatology. 1998;27(2):433–45. doi: 10.1002/hep.510270218. [DOI] [PubMed] [Google Scholar]
- 15.Higgins GM, Anderson RM. Experimental pathology of the liver. Arch Pathol. 1931;12:186–201. [Google Scholar]
- 16.Tsusumi M, Takada A, Takase S. Characterization of desmin-positive rat liver sinusoidal cells. Hepatology. 1987;7(2):277–284. doi: 10.1002/hep.1840070212. [DOI] [PubMed] [Google Scholar]
- 17.Lowes KN, Brennan BA, Yeoh GC, Olynyk JK. Oval cell numbers in human chronic liver diseases are directly related to disease severity. Am J Pathol. 1999;154(2):537–41. doi: 10.1016/S0002-9440(10)65299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evarts RP, Nakatsukasa H, Marsden ER, Hu Z, Thorgeirsson SS. Expression of transforming growth factor-a in regenerating liver and during hepatic differentiation. Mol Carcinog. 1992;5:25–31. doi: 10.1002/mc.2940050107. [DOI] [PubMed] [Google Scholar]
- 19.Miller EC, Miller JA, Hartmann HA. N-Hydroxy-2-acetylaminofluorene: a metabolite of 2-acetylaminofluorene with increased carcinogenic activity in the rat. Cancer Res. 1961;21:815–24. [PubMed] [Google Scholar]
- 20.Weisburger JH, Weisburger EK. Biochemical formation and pharmacological, toxicological, and pathological properties of hydroxylamines and hydroxamic acids. Pharmacol Rev. 1973 Mar;25(1):1–66. Review. [PubMed] [Google Scholar]
- 21.Lotlikar PD, Hong YS. Microsomal N- and C-oxidations of carcinogenic aromatic amines and amides. Natl Cancer Inst Monogr. 1981;(58):101–7. [PubMed] [Google Scholar]
- 22.Ellerbroek SM, Wu YI, Stack MS. Type I collagen stabilization of matrix 695 metalloproteinase-2. Arch Biochem Biophys. 2001;390:51–56. doi: 10.1006/abbi.2001.2345. [DOI] [PubMed] [Google Scholar]
- 23.Pi L, Ding X, Jorgensen M, Pan JJ, Oh SH, Pintilie D, Brown A, Song WY, Petersen BE. Connective tissue growth factor with a novel fibronectin binding site promotes cell adhesion and migration during rat oval cell activation. Hepatology. 2008;47(3):996–1004. doi: 10.1002/hep.22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freise C, Erben U, Muche M, Farndale R, Zeitz M, Somasundaram R, Ruehl M. The alpha 2 chain of collagen type VI sequesters latent proforms of matrix-metalloproteinases and modulates their activation and activity. Matrix Biol. print copy in press 2009 Aug. Available from URL: www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6VPM-4X1SB84-2&_user=2139813&_rdoc=1&_fmt=&_orig=search&_sort=d&_docanchor=&view=c&_acct=C000054276&_version=1&_urlVersion=0&_userid=2139813&md5=f0621d8c889048dbf732074d8db11c6b. [DOI] [PubMed]
- 25.Paku S, Schnur J, Nagy P, Thorgeirsson SS. Origin and structural evolution of the early proliferating oval cells in rat liver. Am J Pathol. 2001;158:1313–1323. doi: 10.1016/S0002-9440(10)64082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knittel T, Mehde M, Kobold D, Saile B, Dinter C, Ramadori G. Expression patterns of matrix metalloproteinases and their inhibitors in parenchymal and non-parenchymal cells of rat liver: regulation by TNF-alpha and TGF-beta1. J Hepatol. 1999;30:48–60. doi: 10.1016/s0168-8278(99)80007-5. [DOI] [PubMed] [Google Scholar]
- 27.Yang L, Jung Y, Omenetti A, Witek RP, Choi S, Vandongen HM, Huang J, Alpini GD, Diehl AM. Fate-mapping evidence that hepatic stellate cells are epithelial progenitors in adult mouse livers. 2008 Aug;26(8):2104–13. doi: 10.1634/stemcells.2008-0115. Epub 2008 May 29. [DOI] [PMC free article] [PubMed] [Google Scholar]





