Abstract
A plausible route for the spontaneous synthesis of an activated ribonucleotide that is poised for polymerization has been put forth (Powner et al., Nature 2009f, 459, 239–242). A key step in this route necessitates the regioselective phosphorylation of the secondary alcohol on C3′, of an anhydroarabinonucleoside in the presence of the primary alcohol on C5′. Here, we propose that this regioselectivity relies on electron delocalization between a lone pair (n) of O5′ and an antibonding orbital (π*) of C2=N3. This n→π* interaction modulates reactivity without the use of a protecting group. Thus, a stereoelectronic effect could have opened a gateway to the ‘RNA world’, the chemical milieu from which the first forms of life are thought to have emerged on Earth some 4 billion years ago.
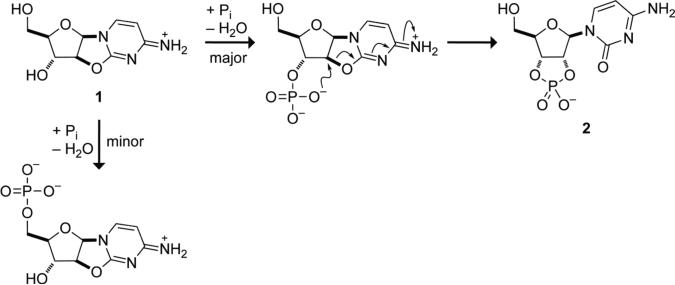
According to the ‘RNA world’ hypothesis (1), the extant DNA- and protein-based life on Earth was preceded by self-replicating RNA (2-4). This hypothesis is supported by the observation that RNA is capable of self-replication (5, 6), as well as executing the functions of both DNA and proteins. RNA, like DNA, can store genetic information (2-4) and, like protein-based enzymes, can catalyze reactions (7, 8). The validation of this hypothesis mandates the synthesis of RNA building blocks—activated ribonucleotides—under prebiotic conditions. Recently, some of us proposed a concise synthetic route to an activated ribonucleotide using plausible prebiotic feedstocks (9). The key intermediate in this route is anhydroarabinonucleoside 1. The last synthetic step requires the phosphorylation of 1 to yield cytidine 2′,3′-cyclic phosphate (2), an activated ribonucleotide poised to undergo polymerization (Scheme 1).
Scheme 1.
Phosphorylation of anhydroarabinonucleoside 1 to yield cytidine 2′,3′-cyclic phosphate (2) (Do not reduce. Print at 100%.)
Examination of anhydroarabinonucleoside 1 reveals two phosphorylation sites: the primary alcohol on C5′ and the secondary alcohol on C3′. On simple steric grounds alone, a primary alcohol should be phosphorylated faster than an otherwise similar secondary alcohol. Yet, under multiple reaction conditions, 3′-phosphorylation was found to proceed selectively over 5′-phosphorylation (9). This surprising regioselectivity is critical because 5′-phosphorylation would not yield an activated ribonucleotide. We sought to determine its origin.
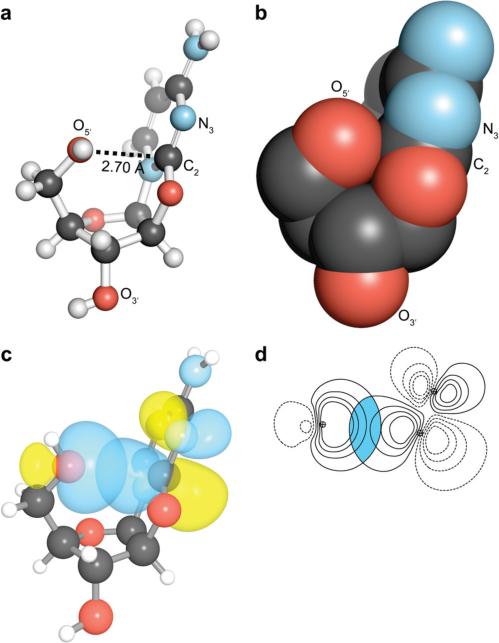
The crystal structure of anhydronucleoside 1 revealed that O5′, the oxygen of the primary alcohol, is in a short contact with C2 (Figure 1, panels a and b) (9, 10). Indeed, the van der Waals surfaces of O5′ (rO = 1.52 Å) and C2 (rC = 1.70 Å) interpenetrate to an extraordinary extent: 0.52 Å. To ascertain whether this intimacy was an artifact of crystal lattice forces, we optimized the geometry of 1 in the gas phase by using hybrid density functional theory at the B3LYP/6-311+G(2d,p) level of theory with Gaussian ’03 (11). The short contact observed in the crystal structure (rO···C = 2.70 Å) was preserved in the calculated structure (rO···C = 2.88 Å).
Figure 1.
n→π* interaction in anhydroarabinonucleoside 1. (a) Ball-and-stick and (b) space-filling (without hydrogens) representation of crystalline 1 (9). (c) Overlap between n of O5′ and π* orbital of C2=N3 in the preferred conformation of 1. (d) Overlap integral (0.1295) from panel c.
Some of us have shown that a short contact between the lone pair of an oxygen donor and an sp2 carbon lead to electron delocalization (12). To reveal contributions from electron delocalization in anhydronucleoside 1, we resorted to Natural Bond Orbital (NBO) analysis (13-15). Geometry optimization and NBO analyses were performed at the B3LYP/6-311+G(2d,p) level of theory. The stabilization afforded by the various donor-acceptor orbital interactions, such as En→π*, was calculated using second-order perturbation theory, as implemented in NBO 5.0.
We found that the lone pair (n) of O5′ is delocalized over the antibonding orbital (π*) of the C2=N3 bond (Figure 1, panels c and d) with En→π* = 1.09 kcal/mol. Analogous electron delocalization between two carbonyl groups has been reported previously (12). Such n→π* electronic delocalization is reminiscent of the nucleophilic attack on carbonyl groups along the Bürgi–Dunitz trajectory (16), and is accompanied by pyramidalization of the acceptor carbon (12). Both of these signatures are apparent in the crystal structure of anhydronucleoside 1. The O5′···C2=N3 angle is 99.2°, which is close to the Bürgi–Dunitz trajectory; C2 is displaced towards O5′ by 0.01 Å from the plane formed by its three pendant atoms.
Engaging O5′ in an n→π* interaction is likely to diminish its reactivity in two distinct ways. First, the enforced proximity of O5′ and C2 increases steric crowding near O5′. Secondly, the delocalization of electron density from n into π* decreases the intrinsic nucleophilicity of O5′. Neither of these factors affects the reactivity of O3′, which hence undergoes selective phosphorylation (9).
Complementary support for the existence of an n→π* interaction in arabinose anhydronucleoside 1 comes from its ribo-diastereomer, 3, which undergoes deleterious phosphate-mediated hydrolysis much more rapidly (Scheme 2) (9). An inspection of the crystal structure and gas-phase optimized geometry of 3 indicates that its O5′ cannot participate in an n→π* interaction—the donor and acceptor groups are too distal. Thus, whereas both faces of the C2=N3 bond of 3 are accessible to inorganic phosphate, only one face is accessible in 1 (cf: Figure 1C and Figure 2). In addition to this steric effect, an electronic effect is also operative. An n→π* interaction in 1 increases the energy of the π* orbital of its C2=N3 bond, thereby reducing the electrophilicity of C2. As in the regioselectivity of the phosphorylation reaction (Scheme 1), the differing rates of the hydrolysis reaction (Scheme 2) are a manifestation of the steric and electronic effects that arise from an n→π* interaction. Notably, ribose anhydronucleoside 3, which lacks the n→π* interaction of arabinose anhydronucleoside 1, is phosphorylated primarily on O5′ (9), as expected on simple steric grounds.
Scheme 2.
Phosphate-mediated hydrolysis of anhydroarabinonucleoside 1 and anhydroribonucleoside 3 (Do not reduce. Print at 100%.)
Figure 2.
π* orbital of C2=N3 in the preferred conformation of anhydroribonucleoside 2.
Our analysis supports the hypothesis that an n→π* interaction is responsible for the phosphorylation of anhydronucleoside 1 on C3, as well as its resistance to hydrolysis. We note that the use of an n→π* interaction had not been invoked as a means to control the reactivity of a nucleic acid. In effect, the ensuing electron delocalization obviates the need for a protecting group. We propose that a stereoelectronic effect played a key role in the prebiotic synthesis of activated ribonucleotides.
Acknowledgement
This work was supported by grant R01 AR044276 (NIH) and the UK Engineering and Physical Sciences Research Council.
REFERENCES
- 1.Gilbert W. The RNA World. Nature. 1986;319:618. [Google Scholar]
- 2.Woese CR. The Genetic Code. Harper & Row; New York: 1967. [Google Scholar]
- 3.Crick FHC. The origin of the genetic code. J. Mol. Biol. 1968;38:367–379. doi: 10.1016/0022-2836(68)90392-6. [DOI] [PubMed] [Google Scholar]
- 4.Orgel LE. Evolution of the genetic apparatus. J. Mol. Biol. 1968;38:381–393. doi: 10.1016/0022-2836(68)90393-8. [DOI] [PubMed] [Google Scholar]
- 5.Johnston WK, Unrau PJ, Lawrence MS, Glasner ME, Bartel DP. RNA-catalyzed RNA polymerization: Accurate and general RNA-template primer extension. Science. 2001;292:1319–1325. doi: 10.1126/science.1060786. [DOI] [PubMed] [Google Scholar]
- 6.Lincoln TA, Joyce GF. Self-sustained replication of an RNA enzyme. Science. 2009;323:1229–1232. doi: 10.1126/science.1167856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altman S. Enzymatic cleavage of RNA by RNA (Nobel lecture) Angew. Chem. Int. Ed. 1990;29:749–758. [Google Scholar]
- 8.Cech TR. Self-splicing and enzymatic activity of an intervening sequence RNA from Tetrahymena (Nobel lecture) Angew. Chem. Int. Ed. 1990;29:759–768. doi: 10.1007/BF01117241. [DOI] [PubMed] [Google Scholar]
- 9.Powner MW, Gerland B, Sutherland JD. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature. 2009;459:239–242. doi: 10.1038/nature08013. [DOI] [PubMed] [Google Scholar]
- 10.Brennan T, Sundaralingam M. Molecular structure of 2,2′-anhydro-1-β-d-arabinofuranosyl cytosine hydrochloride (cyclo ara-C): A highly rigid nucleoside. Biochem. Biophys. Res. Commun. 1973;52:1348–1353. doi: 10.1016/0006-291x(73)90649-9. [DOI] [PubMed] [Google Scholar]
- 11.Frisch MJ, et al. Gaussian 03, Revision C.02. Gaussian, Inc.; Wallingford, CT: 2004. [Google Scholar]
- 12.Choudhary A, Gandla D, Krow GR, Raines RT. Nature of amide carbonyl–carbonyl interactions in proteins. J. Am. Chem. Soc. 2009;131:7244–7246. doi: 10.1021/ja901188y. and references therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinhold F. Natural Bond Orbital Methods. In: Schleyer P. v. R., Allinger NL, Clark T, Gasteiger J, Kollman PA, Shaefer HF, III, Schreiner PR., editors. Encyclopedia of Computational Chemistry. John Wiley & Sons; Chichester, UK: 1998. pp. 1792–1811. [Google Scholar]
- 14.Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Weinhold F. NBO 5.0, NBO 5.0. 2001 [Google Scholar]
- 15.Weinhold F, Landis CR. Valency and Bonding: A Natural Bond Orbital Donor–Acceptor Perspective. Cambridge University Press; Cambridge, UK: 2005. [Google Scholar]
- 16.Bürgi HB, Dunitz JD, Shefter E. Geometrical reaction coordinates. II. Nucleophilic addition to a carbonyl group. J. Am. Chem. Soc. 1973;95:5065–5067. [Google Scholar]