Abstract
Aldose reductase, although identified initially as a glucose-reducing enzyme via polyol pathway, is believed to be an important component of antioxidant defense system as well as a key mediator of oxidative stress-induced molecular signaling. The dual role played by AR has made it a very important enzyme for the regulation of not only the cellular redox state by detoxifying the reactive lipid-aldehydes generated by lipid peroxidation which is crucial in the cellular homeostasis, but also in the regulation of molecular signaling cascade that may regulate oxidative stress-induced cytotoxic events. Search for the new molecular targets to restrain the oxidative stress-induced inflammation has resulted in the identification of AR as an unanticipated mediator of oxidative stress-induced signaling. Although, in last one decade or so AR has been implicated in various inflammation-related disease conditions ranging from diabetes, sepsis, cancer, cardiovascular and airway inflammation, however, a critical evaluation of the clinical efficacy of AR inhibitors awaits a better understanding of the role of AR in regulating inflammation, especially in ocular inflammation.
Keywords: Aldose reductase, inflammation, uveitis, NF-κB, GS-DHN, infection, autoimmunity
Introduction
Ocular inflammation, which includes inflammation affecting a part of the eye or surrounding ocular tissue, can range from allergic conjunctivitis to potentially blinding conditions such as scleritis, optic neuritis, keratitis, uveitis, retinal vasculitis, and chronic conjunctivitis. Inflammation due to infection or injury develops in the eyes or in the optic nerve, blood vessels or other tissues surrounding the eyes results in the dysfunction leading to an ocular inflammatory disease. The diagnosis of the ocular inflammatory disease is governed by the tissue involved in the inflammation, for example, uveitis involves inflammation in the uveal tract; scleritis, inflammation of the sclera, pars planitis, inflammation of the pars-plana and so on.
In general, inflammation is a physiological response to invading pathogens or antigens which involve the migration of specific types of inflammatory cells out of the bloodstream into affected tissue [1]. These cells release inflammatory agents such as cytokines, chemokines and other inflammatory markers to boost immune responses and kill invading bacteria, viruses, and parasites or any other antigen [1, 2]. The linking of the antibody to the antigens forms an immune complex which is removed quickly by phagocytic macrophages, however owing to excessive antigen exposure or compromised immune response, the pathogens or their toxins are lodged into tissues and cause severe inflammation. Excessive inflammatory response can damage the healthy tissues during this process. In excessive inflammation, the affected parts of the eye (the eyelids, sclera, iris, uvea, retina, optic nerve etc) become tender and inflamed. Chronic or sever ocular inflammation can damage the delicate tissues and blood vessels in and around the eye resulting in vision loss. Ocular inflammatory diseases occurs throughout the world independent of gender, race, ethnicity, or age [3] and can be caused due to various factors such as infection [4–6], auto-immunity [7, 8], trauma [9, 10], drugs [11, 12] or malignancy [13, 14].
Ocular infection may be caused by bacteria, parasites, fungus, viruses such as rubella, HIV; sexually transmitted diseases such as syphilis, chlamydia, or gonorrhea; or rare infections, such as tuberculosis, toxoplasmosis or Lyme disease [15–41]. Bacterial infection of the eye is one of the main eye infections among the infants worldwide resulting in the clinical features such as conjunctivitis, keratitis, cellulitis, blepharitis and endophthalmitis [42–44]. The autoimmune disease–induced ocular inflammation is systemic and affects specific parts of the eye. The ocular inflammatory disease is identified according to the inflamed part of the eye [45]. For example, juvenile rheumatoid or idiopathic arthritis is associated with inflammation in the anterior part of the eye, known as anterior uveitis or iritis [46, 47]. Autoimmune diseases can also affect the eye resulting in such ocular diseases as birdshot retinochoroidopathy [48–50], Fuchs’ heterochromic iridocyclitis [51], Vogt-Koyanagi – Harada [52–54], and ocular cicatricial pemphigoid [55, 56]. The other cause of noninfectious ocular inflammatory disease is termed “idiopathic” which is basically a diagnosis of exclusion i.e. no cause associated systemic illness can be identified at the time of the diagnosis [57]. Drugs or medication such as beta(2)-adrenergic agonists and anticholinergic agents, glucocorticoids, phenothiazines, antirheumatic drugs such as quinolines, and allopurinol, psychiatric drugs such as phenothiazine, and chlorpromazine; cardiovascular drugs such as practolol, and amiodarone can also cause ocular inflammatory diseases [11–12]. Also, certain cancers such as lymphoma [58, 59], lung cancer [60] and breast cancer [13, 61] can cause ocular inflammatory diseases [14].
These factors act as stimulus to induce oxidative stress which results in the formation of reactive oxygen species (ROS) that lead to the activation of transcription factors such as NF-κB which then transcribes various inflammatory markers. These inflammatory markers include various cytokines and chemokines which cause tissue damage leading to ocular inflammatory diseases (Fig. (1). This review focuses on recent identification of role of AR in ocular inflammatory disease such as uveitis and its pharmacological intervention by AR inhibition.
Fig. 1.
Mechanism of ROS-induced ocular pathogenesis. Various stimuli such as infectious pathogens including bacteria, viruses, fungi etc and cytokines, chemokine and other inflammatory markers produced by an autoimmune reaction result in reactive oxygen species formation which is known to activate redox-sensitive transcription factors including NF-κB. The activated transcription factors then enter nucleus and transcribe many inflammatory genes which cause ocular inflammation resulting in diseases such as uveitis.
Aldose reductase
Aldose reductase (AR), a member of the aldo-keto reductase super family of soluble, monomeric reduced nicotinamide adenine dinucleotide phosphate (NADPH)-dependent aldo-keto reductases (AKR1B1), is a rate-limitingenzyme of the polyol pathway (Fig. (2) of glucose metabolism that converts glucose to sorbitol during hyperglycemia in the presence of NADPH [62, 63]. AR is expressed in ocular tissues and its expression and activity have been shown to increase during hyperglycemia and other oxidative stress response diseases, including inflammatory diseases [64–67]. The AR catalyzed reduction of glucose to sorbitol, first described by H.G. Hers in 1956 [68], was thought to play a central role in cellular osmo-regulation. The observation that diabetic and galactosemic cataract are caused due to accumulation of AR catalyzed reduced product of aldehydic sugars glucose and galactose, to sorbitol and galactitol, respectively led to the concept of using AR inhibitor (ARI) to ameliorate the diabetic cataractogenesis, retinopathy and neuropathy [69–71]. However, the clinical trial failed to corroborate results obtained in rodents [72–74]. This difference could be because unlike in rats high doses of ARI required to inhibit AR in diabetic subjects could not be used in clinical trials [75]. Another reason could be that in case of diabetic retinopathy half of the study population had clinically evident retinopathy [76] and it is suggested that interventions are not as effective as prevention in diabetic retinopathy [77]. Moreover, reports indicating that sorbitol (polyols) accumulation could not be the only cause of diabetic retinopathy and cataract formation [78–80], led to the suggestion that other metabolic alterations along with AR activation could be responsible for diabetic complications [63, 81, 82]. Also, in vascular tissues such as kidney glomeruli, nerve, and retina, where sorbitol accumulation alone cannot account for the observedpathology suggest that AR may have other roles in the pathophysiology of these tissues [83]. For instance, AR mRNA is induced by oxidative stress suggesting that AR itself may be involved in cellular antioxidant defense mechanisms [84]. Being involved in detoxicification, AR has been implicated in drug resistance in tumor cells and its inhibition has been suggested to increase the cytotoxic effect of cancer drugs [85].
Fig. 2.
Polyol pathway of glucose metabolism. In polyol pathway glucose is converted to sorbitol by a NADPH −dependent enzyme aldose reductase. Later sorbitol is converted to fructose by a NAD+ dependent enzyme sorbitol dehydrogenase. During hyperglycemia excess of sorbitol is formed and being impermeable through the membranes accumulates inside the cells and cause osmotic stress. Increase in osmotic stress during hyperglycemia has been shown to be the major cause of secondary diabetic complications. Further, decrease in the ratio of NAPDH/NAD+ leads to oxidative stress which could be responsible for changes in inflammatory signaling.
In recent years, reports from our laboratory and others have shown that AR is an essential mediator of oxidative stress and inflammation that are induced by varied sources such as hyperglycemia [86, 87], growth factors [88], cytokines [87, 89], endotoxins [67, 90, 91], allergens [92] and certain tumors including the colorectal tumors [93]. These reports have generated a shift in the paradigm to treat the oxidative stress and inflammation-induced disease conditions and suggest the use of AR inhibitors as anti-inflammatory agents. Spicher et al [84] have shown that oxidative stress induced by different conditions led to the expression of AR in vascular smooth muscle cells (VSMC) suggesting that glucose (an aldehyde sugar; Km: >50 mM)) may not be the only inducer of AR expression. On the other hand, based on the lower Km value of a lipid aldehyde, 4-hydroxynonenal (HNE) (Km: 10–30μM), a highly electrophilic aldehyde formed by ROS-induced lipid peroxidation of poly-unsaturated fatty acids in the plasma membrane, could be the better substrate for AR [94, 95]. HNE has been shown to have many cytotoxic and genotoxic effects at micro-molar concentrations in different cellular models of oxidative stress-induced inflammation [96, 97]. Thus, AR expression during oxidative stress could be a part of defense mechanism, in which AR reduces the aldehydes generated during stress conditions. Interestingly, we have shown that reduced product of HNE activate the redox-signaling that cause cytotoxicity [90, 97].
Aldose reductase in oxidative stress-induced inflammatory signaling
Oxidative stress is the underlying factor in a number of disease conditions including the ocular inflammatory diseases [98–101]. The key mediators of oxidative injury are H2O2, superoxide anions and peroxinitrite (NOO−), collectively termed as ROS [102–104]. ROS are believed to underlie many of the oxidative changes in varied pathological conditions and are known to enhance various mediators including increased expression of AR and activation of protein kinase C and redox-sensitive transcription factors such as NF-κB and AP-1 [63]. In the normal growth and development of an organism, these regulations are necessary and required for organ homeostasis [105–107]. However, in the disease conditions excessive ROS causes disorder in the normal tissue metabolism and molecular signaling leading to enhanced activation and over-expression of many proteins generating a pathological condition [108]. Inhibition and suppression of altered molecular targets are mandatory to restore the homeostasis.
We have shown that AR is an essential mediator of the ROS-induced signaling by various stimuli including hyperglycemia [86–87], growth factors [88], cytokines [87, 89], endotoxins [67, 90, 91]. Our in-vitro studies show that AR catalyzes the reduction of a large series of saturated and unsaturated aldehydes with 103- to 104-fold higher efficiency than that of glucose, particularly medium to long chain (C-6 to C-18) aldehydes such as those generated abundantly during lipid peroxidation [109]. AR also catalyzes the reduction of the glutathione conjugates of unsaturated aldehydes, in many cases with higher efficiency than that of the parent aldehydes [94, 95]. Conjugation of glutathione with electrophilic metabolites, including lipid peroxidation products such as HNE, leads to detoxification of hydrophobic xenobiotics and metabolites by increasing their solubility in water and extrusion from the cell. However, we have shown that conjugation with glutathione (GSH) does not abolish the mitogenic and signaling effects of HNE [97]. In fact the glutathione conjugates, when delivered to the cell as hydrolysable esters were as potent as HNE in stimulating VSMC growth and cell-signaling. Furthermore, we have shown that AR catalyzed reduced product of glutathione-HNE conjugates (GS-HNE), i.e. Glutathione-1,4-dihydroxynonene (GS-DHN), has mitogenic effect on VSMC. We have demonstrated that HNE, GS-HNE and GS-DHN all three activate PKC and NF-κB, and inhibition of AR could only prevent cell-growth and activation of PKC, NF-κB and AP-1 by HNE and GS-HNE, but not by GS-DHN [90]. These results indicated that reduced product of glutathione-lipid aldehyde conjugate, GS-DHN could be the transducer of inflammatory signaling that cause cellular toxicity, tissue damage and dysfunction leading to inflammatory pathologies. Though the exact mechanism of the involvement of AR in oxidative stress-induced signaling in ocular inflammation is under investigation, the preliminary indication from our study suggests that reduced product of glutathione-aldehyde conjugates i.e. GS-DHN could be the effecter molecules, which could induce the inflammatory response [90, 97]. These results clearly indicate a link between the oxidative stress-induced lipid peroxidation and generation of lipid aldehydes which are then metabolized by AR and the product could activate the signaling proteins (most probably redox-sensitive protein kinases) leading to activation of redox-sensitive transcription factors NF-κB and AP-1. This leads to the synthesis and release of NF-κB and AP-1-transcribed cytokines, chemokine, and other inflammatory markers, which further induced oxidative stress and perturbed tissues homeostasis resulting in various pathological conditions including inflammation (Fig. 3).
Fig. 3.
Role of AR in ocular inflammatory signaling. AR has been shown to be a key mediator of the signaling initiated by different stimuli including high glucose, growth factors, cytokines, or bacterial endotoxins which induce increased reactive oxygen species (ROS). ROS then oxidize membrane lipids into lipid aldehydes such as 4-hydroxynonenal (HNE), which readily reacts with glutathione and converted into GS-HNE conjugate. AR then catalyzes the reduction of GS-HNE into GS-DHN, which we have shown could be the key molecule that activates PLC and PKC kinases and various downstream protein kinases, eventually activating transcription factors NF-kB and AP-1. The activated transcription factors then enter nucleus and transcribe various inflammatory marker genes which by autocrine and paracrine manner cause inflammation including ocular inflammation such as uveitis.
Many inflammatory mediators, such as TNF-α, IL-1β, IL-6, and inducible-nitric oxide synthase (iNOS) require NF-κB activation for their expression as their genes possess NF-κB binding sequences in promoter regions [110, 111]. Since the discovery of NF-κB, a number of paradigms for its function have been established including its key role in the inflammatory and immune responses. NF-κB stimulates immune cell function and acts in a pro-inflammatory manner by inducing the expression of cytokines, chemokines and their receptors [112, 113]. These aspects of NF-κB function are undoubtedly central to the understanding of the overall action of this family of transcription factors, and they provide a foundation for therapeutic intervention in inflammatory diseases based on NF-κB inhibition [114–116]. Since, we have shown that AR mediates the activation of NF-κB during oxidative stress caused by varied stimuli and that inhibition of AR attenuates the activation of key signaling kinases leading to deactivation of NF-κB, it is plausible that AR inhibitors could be potential therapeutic agents to treat the oxidative stress-induced ocular inflammation.
We and others have demonstrated that NF-κB is indeed activated due to various stimuli in different cellular models of ocular inflammatory diseases and in the iris-ciliary body (ICB) during endotoxin-induced uveitis [67, 117]. Treatment with anti-inflammatory agents such as PDTC, an antioxidant NF-κB inhibitor, decreased the gene expression of these pro-inflammatory cytokines in the ICB as detected by decrease in the inflammatory marker proteins in the aqueous humor [117]. However, using anti-oxidants as therapeutic agents is not a potential anti-inflammatory approach in the treatment of inflammatory diseases because the antioxidants become pro-oxidants at higher concentration or in presence of metal ions (118–120]. Alternatively, we have shown that inhibition of AR prevents NF-κB activation in both cellular as well as animal models of ocular inflammation thereby regulating the synthesis and secretion of pro-inflammatory markers [67, 87, 91], suggesting that inhibition of AR by gene silencing or pharmacological agents could be an important strategy to treat ocular inflammatory diseases.
Aldose reductase in ocular inflammation
The evidence showing that lipid peroxidation products are excellent substrates of AR has led to this enzyme being increasingly linked to inflammation and auto-immune mediated oxidative stress, besides diabetic complications. Many studies with cell-culture and animal models of ocular inflammation have shown that inhibition of AR could ameliorate the inflammation induced by various stimuli including bacterial endotoxin, lipopolysachharide (LPS), high glucose, and cytokines [67, 86, 87, 89–91]. The distribution of AR in human cornea, lens, retina, and optic nerve has been shown in a number of studies using specific antibodies against purified human placental AR [65] whose expression gets elevated during oxidative stress. The conversion of glucose to sorbitol by AR and its subsequent intracellular accumulation have been implicated in the pathogenesis of diabetic cataracts [121] however same can not be attributed to the pathogenesis of retina, cornea, and other eye tissues. For instance, ocular surface disorders, such as corneal epithelites, superficial punctate keratopathy, recurrent corneal erosion, persistent epithelial defects are thought to result from the disease progression of diabetes mellitus itself [122–124], and studies describe the effectiveness of AR inhibitors in promoting corneal re-epithelialisation [125, 126]. More recent reports suggest an unanticipated link between AR and ocular inflammation, e.g. studies in our laboratory have shown that inhibition of AR by pharmacological agents or by mRNA ablation leads to the prevention of high glucose-, TNF-α-, and LPS-induced oxidative stress in human lens epithelial cells [91], suggesting that AR could be a molecular target for the treatment of oxidative stress-induced ocular inflammation.
Being constantly exposed to various agents the eye can be the target of infections, cancers, and systemic or localized autoimmune diseases. Although an immunologically privileged organ, the eyes can also get damaged from the excessive immune response of the body when blood ocular barrier function is compromised [127]. Ocular inflammation, when untreated, could result in serious vision loss. In ocular inflammatory disorders, activation of redox-sensitive transcription factors has been implicated [67, 91, 117, 128]. Since we have shown in our both in-vitro and in-vivo studies that AR inhibition or ablation prevents the activation of PKC/NF-κB thereby attenuate cytotoxicity and tissue damage [67, 86–93, 128] it is can be suggested that AR inhibition could be a potential molecular target to contain the ocular inflammatory diseases caused due to infections, autoimmune or other etiological factors. We have shown that AR inhibition in rodent endotoxin-induced uveitis model leads to attenuation of ocular inflammation as characterized by decreased protein extravasations and cellular infiltration into the anterior chamber [67]. These results demonstrated that AR inhibition leads to in-vivo suppression of NF-κB that can attenuate inflammation in the eye. Besides, we have shown in our cell-culture studies that AR inhibition/ablation in the ocular epithelial cells prevents the cytotoxicity induced by various stimuli including high glucose, TNF-α, and LPS [91].
Cell-culture studies
AR over-expression in the retinal pigment epithelium has been associated with diabetic retinopathy [75]. The regulation of AR gene expression and associated changes in sorbitol and myo-inositol were increased >11 fold by hyper-osmolarity in human retinal pigment epithelial cells in culture [129]. In another study Obrosova et al [130] showed that in Bovine retina endothelial cells, increased AR activity contributes to retinal oxidative stress during hyperglycemia and vascular endothelial growth factor (VEGF) protein over-expression in early diabetes and ARI, fidarestat, was able to correct this. In the human lens epithelial cells (HLEC), we have recently shown that AR mediates the LPS-induced cytotoxicity via the activation of redox sensitive transcription factors NF-κB and AP-1, and inhibition of AR by pharmacological inhibitor or by silencing the AR expression by AR siRNA prevented the cytotoxicity caused by LPS [91]. These findings are significant as LPS – induced ocular inflammation, such as endogenous endophthalmitis in which infection reaches the eye via circulation and infection-induced uveitis, are well known threats to vision in humans [4, 131, 132]. Inhibition of such inflammation by AR inhibitors provides a novel therapeutic approach for infection –induced ocular diseases. Further, a study by Kubo et al showed that over-expression AR in HLEC led to the increased oxidative stress and apoptosis, and inhibition of AR prevented the cells from oxidative stress [133]. We have also presented evidence that over-expression of AR in HLEC led to increased cell mortality by various aldehydes [134]. In a recent study, we have demonstrated that AR mediates LPS-induced cytotoxicity in non-pigmented ciliary epithelial cells and disturbs the aqueous humor dynamics by altering the expression of channel proteins such as Na-K-ATPase [unpublished data]. This study has greater implication in the infection-induced ocular inflammation as reduced flow of aqueous humor could result in the augmentation of oxidative stress resulting in the severe vision impairment or vision loss. Attraction of macrophages to the ocular tissues or activation of resident macrophages e.g. dendritic cells during inflammation causes severe tissue damage as observed in infection or auto-immune-induced uveitis where damage to the ciliary body and retinal layer have been reported [67, 131, 132, 135, 136]. We have shown that AR mediates the molecular signaling induced by varied stimuli including bacterial toxin, that activates macrophages and cause inflammation and inhibition as well as genetic ablation of AR blocks the inflammatory circuitry preventing the tissue damage [67, 91]. Indeed, in murine macrophages we have demonstrated that AR mediates LPS-induced expression of inflammatory cytokines (TNF-α, IL-1β, IL-6), chemokines (MCP-1) and other inflammatory markers such as Cox-2 at both protein and mRNA levels via activation of PKC/NF-κB pathway and inhibition/ablation of AR reversed these changes [90].
To further understand the role of AR in activation of transcription factors, we incubated murine macrophages with HNE, GS-HNE, and GS-DHN and observed that while HNE-and GS-HNE-induced activation of PKC and NF-κB was inhibited by AR inhibition and/or ablation, GS-DHN-induced activation of these signaling molecules remained unchanged, suggesting a novel role for GS-DHN in the induction of inflammation [90]. The role of AR in LPS-induced inflammation and macrophage activation is important because macrophages play an important role in the ocular inflammation such as uveitis. As described elsewhere, macrophages infiltrate the vasculature and enter the aqueous humor in anterior segment releasing enormous amount of cytokines and chemokines that result in the pathological symptoms of uveitis such as flair, edema, and vasodilation [67, 117]. Macrophages have also been implicated in the chronic degenerative ocular pathogenesis such as macular degeneration [137]. Various investigators have presented evidence that flavonoids [138–140] and vitamin supplements [101, 141, 142] could be beneficial in the ocular inflammation including uveitis and many flavonoids are known to be good AR inhibitors [143–145]. Therefore, our observation is significant in the way that AR inhibition provides a novel therapeutic target in ocular inflammatory diseases. Interestingly, we have recently demonstrated that AR inhibition prevented the growth and trans-differentiation of lens epithelial cells in pig eye capsular bag model as well as FGF-induced growth and differentiation/trans-differentiation of human lens epithelial cells in vitro [128]. These results suggest that AR could be involved in the secondary cataract induced by post cataract surgery-induced inflammation leading to opacity of the posterior capsule. These studies in the cellular models provide an evidence of an unanticipated role of AR in mediating acute inflammatory responses and also that inhibition of AR could be therapeutically useful in preventing ocular inflammation induced by oxidative stress especially in various pathological conditions.
Animal model studies
Beside cell culture studies, a number of animal models and human studies also corroborate the involvement of AR in ocular inflammatory diseases. An eye drop containing ARI was effective in improving the corneal epithelial barrier dysfunction of galactose-fed rats with cell membrane disruption in the superficial corneal cells and lost intercellular junction integrity [146]. Similarly, in a human study, topical use of ARI improvedthe corneal epithelial barrier function in diabetic patients [147].
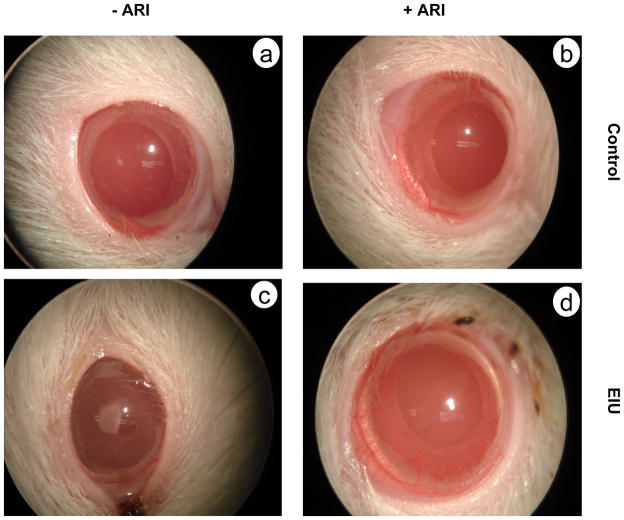
We have shown that in chronic and acute forms of uveitis in rodents, systemic treatment with ARI significantly prevented the development of inflammation in rat eye [67]. In the acute form of endotoxin-induced uveitis (Fig. 4), LPS injection led to increased inflammatory cell infiltration in the aqueous humor of rat eye along with increased protein concentration, inflammatory cytokine TNF-α, and other inflammatory markers PGE2 and NO and pretreatment of rats with ARI prevented LPS-induced enhancement in these parameters [67]. Also, LPS-induced activation of NF-κB, and expression of inflammatory proteins such as COX2 and iNOS were inhibited by pre treatment with ARI [67]. Similarly, in the chronic rodent model a short synthetic peptide, interphotoreceptor retinoid-binding protein (IRBP1169–1191)-induced experimental autoimmune uveitis, treatment with AR-inhibitor decreased the infiltration of inflammatory cells in vitreous chamber as well as significantly reduced the histopathological score [unpublished data]. Further, we have also demonstrated that LPS administration in mice resulted in severe elevation of cytokines (TNF-α, IL-6 and IL-12) and chemokines (MCP-1) in the serum as well as in vital organs such as heart, spleen and liver which were significantly blocked by ARI treatment suggesting that AR mediates LPS-induced inflammation in mice and that ARI could be anti-inflammatory [148]. These results clearly indicate that AR is crucial in the mediation of ocular inflammation-induced either by bacterial infection or by autoimmune response. Further studies are underway to establish ARI as potential therapeutic agent and to sketch the exact role of AR in the induction of inflammatory process that affects the eye.
Fig. 4.
AR inhibition prevents endotoxin-induced uveitis in rats. Bacterial endotoxin-induced uveitis is a well known experimental model to study uveitis in humans. After 24 h of subcutaneous LPS injection, rats develop severe symptoms of anterior uveitis which includes vasodilatation, synechia, presence of exudates at the pupil rim, and miosis (c). Treatment with AR inhibitor results in prevention of these symptoms (d). The control rats treated without (a) or with (b) AR inhibitor showed no change.
In a human study, polymorphism in the Z-2 allele of the AR gene has been implicated as risk factor for the development of retinopathy in diabetic patients [149, 150]. Another study has also implicated AR polymorphism in the development of retinopathy in type 1 diabetes [151]. Given the increased understanding of AR’s role in the oxidative stress-induced inflammation and better understanding of why the earlier clinical trials failed in case of diabetic complications should provide enough enthusiasm to design trials with better, efficient, selective inhibitors with optimized doses for clinical intervention in ocular inflammation.
Future Directions
Since AR has been advocated as an important therapeutic target to treat oxidative stress-induced inflammatory disorders including ocular inflammation, detailed studies of the molecular events and clear understanding of AR’s involvement in the pathogenesis of inflammation is required. Understanding this role of AR should provide pharmacological tools for eventual therapeutic interventions to control cell proliferation, apoptosis, tissue repair, and prevention of the cytotoxicity of cytokines and chemokines which are elevated during ocular inflammation. More importantly, these studies will provide a mechanistic link between AR with ocular inflammation. Studies using various animal models are required to clearly understand the mechanism of AR’s involvement in the inflammation and related pathologies which in turn will help in the design and synthesis of more specific inhibitors. We have observed that reduced product of GS-HNE, i.e. GS-DHN could be the key signaling molecule that activates the inflammatory signaling [90, 97, 148], however its exact mode of action is still not clear. Based on our preliminary results we postulate that GS-DHN either by direct interaction or indirectly activates a protein kinase upstream of PLC/PKC phosphorylation event [90]. Identification of probable association of GS-DHN with the key protein kinase molecule in cytoplasm will be key in understanding to role of AR in oxidative stress-induced pathogenesis. Also, since AR inhibition has been shown to prevent the intracellular ROS generation, studies are required to explore the involvement of ROS producing machinery NADPH oxidase. Several reports have demonstrated that diabetes is a major risk factor in developing ocular diseases [152–154], increasing their prevalence and severity. The progression of severe eye pathologies in diabetes is mediated by several factors such as increased oxidative stress, free radicals, inflammatory cytokines and chemokines, and infections. Although it is not clear if the increased incidence of ocular inflammation is due to the high glucose levels in diabetics or to increased cytokines and chemokines, but the later are considered to be the major cause. Further, the molecular understating of why ocular inflammation is more prevalent in diabetics is not well understood and it is not known how increased expression of AR during hyperglycemia could lead to increased ocular inflammation. It is likely that already increased levels of cytokines and AR in diabetes could contribute to the increased risk of uveitis in diabetes, if so; the inhibition of AR should prevent uveitis in diabetics since it will prevent the increase of inflammatory cytokines as well. AR inhibitors such as sorbinil, tolrestat, zopolrestat, fidarestat, etc have been investigated as potential pathogenetic therapies for diabetic neuropathy for many years, but only one agent in this drug class, epalrestat, is currently marketed and available, in Japan. Common limitations for some of the earlier drugs such as sorbinil and tolrestat include critical hepatic and renal toxicity for long-term use. Newer AR inhibitors such as zopolrestat, raneristat and fidarestat are now being tested for their ability to prevent the progression of diabetic neuropathy. Since these drugs have already passed in the Food and Drug Administration (FDA)’s phase I and II clinical trials and have been found to be safe without any major irreversible side effects, we expect that ARI such as fidarestat could be developed as novel therapy for preventing ocular inflammation especially uveitis in a relatively shorter time.
Acknowledgments
This study was supported in parts by NIH grants GM71036 and EY08591 to KVR and DK36118 to SKS.
List of Abbreviations
- AR
Aldose reductase
- ARI
AR inhibitor
- ROS
Reactive oxygen species
- VSMC
Vascular smooth muscle cells
- HLEC
Human lens epithelial cells
- HNE
4-hydroxynonenal
- GS-HNE
Glutathione-HNE conjugate
- GS-DHN
Glutathione-1,4-dihydroxynonene conjugate
- LPS
Lipopolysachharides
- NADPH
Reduced nicotinamideadenine dinucleotide phosphate
- ICB
Iris-ciliary body
- IRBP
Interphotoreceptor retinoid-binding protein
- PKC
Protein kinase C
References
- 1.Schmid-Schonbein GW. Annu Rev Biomed Eng. 2006;8:93–151. doi: 10.1146/annurev.bioeng.8.061505.095708. [DOI] [PubMed] [Google Scholar]
- 2.Serbina NV, Jia T, Hohl TM, Pamer EG. Annu Rev Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson GJ, Minassian DC, Weale R. The Epidemiology of Eye Disease. Chapman & Hall; New York: 1998. [Google Scholar]
- 4.Durand ML. Curr Infect Dis Rep. 2009;11:283–288. doi: 10.1007/s11908-009-0042-2. [DOI] [PubMed] [Google Scholar]
- 5.Okada AA, Johnson RP, Liles WC, D’Amico DJ, Baker AS. Ophthalmology. 1994;101:832–838. [PubMed] [Google Scholar]
- 6.Wong JS, Chan TK, Lee HM, Chee SP. Ophthalmology. 2000;107:1483–1491. doi: 10.1016/s0161-6420(00)00216-5. [DOI] [PubMed] [Google Scholar]
- 7.Gery I, Streilein JW. Curr Opin Immunol. 1994;6:938–945. doi: 10.1016/0952-7915(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 8.Forrester JV, McMenamin PG. Chem Immunol. 1999;73:159–185. doi: 10.1159/000058745. [DOI] [PubMed] [Google Scholar]
- 9.Cho H, Wolf KJ, Wolf EJ. Clin Ophthalmol. 2009;3:199–210. doi: 10.2147/opth.s4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagoner MD. Surv Ophthalmol. 1997;41:275–313. doi: 10.1016/s0039-6257(96)00007-0. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Tripathi RC, Tripathi BJ. Drug Saf. 2008;31:127–141. doi: 10.2165/00002018-200831020-00003. [DOI] [PubMed] [Google Scholar]
- 12.Taylor F. Aust Fam Physician. 1985;14:744–745. [PubMed] [Google Scholar]
- 13.Miller J. Optometry. 2008;79:189–192. doi: 10.1016/j.optm.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 14.De Potter P. Curr Opin Ophthalmol. 1998;9:100–104. doi: 10.1097/00055735-199812000-00018. [DOI] [PubMed] [Google Scholar]
- 15.Seal DV, Hay J, Bron AJ. Ocular Infection: management and treatment in practice. Martin Dunitz Ltd; London: 1998. [Google Scholar]
- 16.Khosravi AD, Mehdinejad M, Heidari M. Singapore Med J. 2007;48:741–743. [PubMed] [Google Scholar]
- 17.Klotz SA, Penn CC, Negvesky GJ, Butrus SI. Clin Microbiol Rev. 2000;13:662–685. doi: 10.1128/cmr.13.4.662-685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas PA. Clin Microbiol Rev. 2003;16:730–797. doi: 10.1128/CMR.16.4.730-797.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas PA. Eye. 2003;17:852–862. doi: 10.1038/sj.eye.6700557. [DOI] [PubMed] [Google Scholar]
- 20.Chong EM, Wilhelmus KR, Matoba AY, Jones DB, Coats DK, Paysse EA. Am J Ophthalmol. 2004;138:474–475. doi: 10.1016/j.ajo.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 21.Wilhelmus KR, Dawson CR, Barron BA, Bacchetti P, Gee L, Jones DB, Kaufman HE, Sugar J, Hyndiuk RA, Laibson PR, Stulting RD, Asbell PA. Br J Ophthalmol. 1996;80:969–972. doi: 10.1136/bjo.80.11.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sudesh S, Laibson PR. Curr Opin Ophthalmol. 1999;10:230–233. doi: 10.1097/00055735-199908000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Santos C. Bol Asoc Med P R. 2004;96:71–74. [PubMed] [Google Scholar]
- 24.De Groot-Mijnes JD, de Visser L, Rothova A, Schuller M, van Loon AM, Weersink AJ. Am J Ophthalmol. 2006;141:212–214. doi: 10.1016/j.ajo.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 25.Siemerink MJ, Sijssens KM, de Groot-Mijnes JD, de Boer JH. Am J Ophthalmol. 2007;143:899–900. doi: 10.1016/j.ajo.2006.11.052. [DOI] [PubMed] [Google Scholar]
- 26.de Visser L, Braakenburg A, Rothova A, de Boer JH. Am J Ophthalmol. 2008;146:292–297. doi: 10.1016/j.ajo.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Gharai S, Venkatesh P, Garg S, Sharma SK, Gharai PG, Vohra R. AIDS Read. 2009;19:241–244. [PubMed] [Google Scholar]
- 28.Jabs DA, Green WR, Fox R, Polk BF, Bartlett JG. Ophthalmology. 1989;96:1092–1099. doi: 10.1016/s0161-6420(89)32794-1. [DOI] [PubMed] [Google Scholar]
- 29.Holland GN. Am J Ophthalmol. 2008;145:397–408. doi: 10.1016/j.ajo.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Lee L. Clin Ophthalmol. 2008;2:669–673. doi: 10.2147/opth.s2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chao JR, Khurana RN, Fawzi AA, Reddy HS, Rao NA. Ophthalmology. 2006;113:2074–2079. doi: 10.1016/j.ophtha.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 32.Tran TH, Cassoux N, Bodaghi B, Fardeau C, Caumes E, Lehoang P Graefes Arch Clin Exp Ophthalmol. 2005;243:863–869. doi: 10.1007/s00417-005-1137-6. [DOI] [PubMed] [Google Scholar]
- 33.Doris JP, Saha K, Jones NP, Sukthankar A. Eye. 2006;20:703–705. doi: 10.1038/sj.eye.6701954. [DOI] [PubMed] [Google Scholar]
- 34.Mathew AA, Turner A, Taylor HR. Drugs. 2009;69:953–970. doi: 10.2165/00003495-200969080-00002. [DOI] [PubMed] [Google Scholar]
- 35.Dean D, Kandel RP, Adhikari HK, Hessel T. PLoS Med. 2008;5:e14. doi: 10.1371/journal.pmed.0050014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moss TR, Van Der Pol B. Int J STD AIDS. 2009;20:143–144. doi: 10.1258/ijsa.2008.008408. [DOI] [PubMed] [Google Scholar]
- 37.Lee JS, Choi HY, Lee JE, Lee SH, Oum BS. Eye. 2002;16:646–649. doi: 10.1038/sj.eye.6700112. [DOI] [PubMed] [Google Scholar]
- 38.Chen FK, White A, Harney BA. Br J Ophthalmol. 2009 doi: 10.1136/bjo.2008.153445. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 39.Holland GN, Crespi CM, ten Dam-van Loon N, Charonis AC, Yu F, Bosch-Driessen LH, Rothova A. Am J Ophthalmol. 2008;145:1007–1013. doi: 10.1016/j.ajo.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 40.Lesser RL. Am J Med. 1995;98:60S–62S. doi: 10.1016/s0002-9343(99)80045-x. [DOI] [PubMed] [Google Scholar]
- 41.Bodaghi B. Med Mal Infect. 2007;37:518–522. doi: 10.1016/j.medmal.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 42.Behrman RE, Vaughan VC. Nelson textbook of pediatrics. WB Saunders Company; Philadelphia: 1989. [Google Scholar]
- 43.Vaughan D, Asbury T. General ophthalmology. Appleton and Lange; Stamford: 1992. [Google Scholar]
- 44.Dannevig L, Strom B, Melby M. Ophthalmia neonatarum in northern Norway I Epidemiology and risk factors. Acta ophthalmologica. 1992;70:14–18. doi: 10.1111/j.1755-3768.1992.tb02086.x. [DOI] [PubMed] [Google Scholar]
- 45.Patel SJ, Lundy DC. American Family Physician. 2002;66:991–998. [PubMed] [Google Scholar]
- 46.Billson FA. Aust Fam Physician. 1978;7:1413–1421. [PubMed] [Google Scholar]
- 47.Schaller J, Kupfer C, Wedgwood RJ. Pediatrics. 1969;44:92–100. [PubMed] [Google Scholar]
- 48.Nussenblatt RB, Mittal KK, Ryan S, Green WR, Maumenee AE. Am J Ophthalmol. 1982;94:147–158. doi: 10.1016/0002-9394(82)90069-1. [DOI] [PubMed] [Google Scholar]
- 49.Baarsma GS, Priem HA, Kijlstra A. Curr Eye Res. 1990;9(Suppl):63–68. doi: 10.3109/02713689008999422. [DOI] [PubMed] [Google Scholar]
- 50.LeHoang P, Ozdemir N, Benhamou A, Tabary T, Edelson C, Betuel H, Semiglia R, Cohen JH. Am J Ophthalmol. 1992;113:33–35. doi: 10.1016/s0002-9394(14)75749-6. [DOI] [PubMed] [Google Scholar]
- 51.La Hey E, Broersma L, van der Gaag R, Baarsma GS, Rothova A, Kijlstra A. Br J Ophthalmol. 1993;77:436–439. doi: 10.1136/bjo.77.7.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Read RW, Rao NA, Cunningham ET. Curr Opin Ophthalmol. 2000;11:437–442. doi: 10.1097/00055735-200012000-00009. [DOI] [PubMed] [Google Scholar]
- 53.Rao NA. Eye. 1997;11:213–216. doi: 10.1038/eye.1997.54. [DOI] [PubMed] [Google Scholar]
- 54.Damico FM, Bezerra FT, da Silva GC, Gasparin F, Yamamoto JH. Arq Bras Oftalmol. 2009;72:413–420. doi: 10.1590/s0004-27492009000300028. [DOI] [PubMed] [Google Scholar]
- 55.Rice BA, Foster CS. Ophthalmology. 1990;97:1476–1483. doi: 10.1016/s0161-6420(90)32402-8. [DOI] [PubMed] [Google Scholar]
- 56.Elder MJ, Lightman S. Eye. 1994;8:196–199. doi: 10.1038/eye.1994.45. [DOI] [PubMed] [Google Scholar]
- 57.Gupta R, Murray PI. Drugs Aging. 2006;23:535–558. doi: 10.2165/00002512-200623070-00001. [DOI] [PubMed] [Google Scholar]
- 58.Buggage RR, Chan CC, Nussenblatt RB. Curr Opin Oncol. 2001;13:137–142. doi: 10.1097/00001622-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 59.Chan CC, Wallace DJ. Cancer Control. 2004;11:285–295. doi: 10.1177/107327480401100502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vroman DT, Breckenridge RR, Solomon KD, Sandoval HP, Fernández de Castro LE. Cornea. 2006;25:611–613. doi: 10.1097/01.ico.0000196447.79817.ce. [DOI] [PubMed] [Google Scholar]
- 61.Merrill CF, Kaufman DI, Dimitrov NV. Cancer. 1991;68:623–627. doi: 10.1002/1097-0142(19910801)68:3<623::aid-cncr2820680330>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 62.Suzen S, Buyukbingol E. Curr Med Chem. 2003;10:1329–1352. doi: 10.2174/0929867033457377. [DOI] [PubMed] [Google Scholar]
- 63.Srivastava SK, Ramana KV, Bhatnagar A. Endocr Rev. 2005;26:380–392. doi: 10.1210/er.2004-0028. [DOI] [PubMed] [Google Scholar]
- 64.Kern TS, Engerman RL. Exp Eye Res. 1981;33:175–182. doi: 10.1016/s0014-4835(81)80066-8. [DOI] [PubMed] [Google Scholar]
- 65.Akagi Y, Yajima Y, Kador PF, Kuwabara T, Kinoshita JH. Diabetes. 1984;33:562–566. doi: 10.2337/diab.33.6.562. [DOI] [PubMed] [Google Scholar]
- 66.Vinores SA, Campochiaro PA, Williams EH, May EE, Green WR, Sorenson RL. Diabetes. 1988;37:1658–1664. doi: 10.2337/diab.37.12.1658. [DOI] [PubMed] [Google Scholar]
- 67.Yadav UC, Srivastava SK, Ramana KV. Invest Ophthalmol Vis Sci. 2007;48:4634–4642. doi: 10.1167/iovs.07-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hers HG. Biochim Biophys Acta. 1956;22:202–203. doi: 10.1016/0006-3002(56)90247-5. [DOI] [PubMed] [Google Scholar]
- 69.Cogan DG, Kinoshita JH, Kador PF, Robison G, Datilis MB, Cobo LM, Kupfer C. Ann Intern Med. 1984;101:82–91. doi: 10.7326/0003-4819-101-1-82. [DOI] [PubMed] [Google Scholar]
- 70.Kador PF, Kinoshita JH, Sharpless NE. J Med Chem. 1985;28:841–849. doi: 10.1021/jm00145a001. [DOI] [PubMed] [Google Scholar]
- 71.Kinoshita JH. Am J Ophthalmol. 1986;102:685–692. doi: 10.1016/0002-9394(86)90394-6. [DOI] [PubMed] [Google Scholar]
- 72.Handelsman DJ, Turtle JR. Diabetes. 1981;30:459–464. doi: 10.2337/diab.30.6.459. [DOI] [PubMed] [Google Scholar]
- 73.Lewin IG, O’Brien IA, Morgan MH, Corrall RJ. Diabetologia. 1984;26:445–448. doi: 10.1007/BF00262218. [DOI] [PubMed] [Google Scholar]
- 74.Jennings PE, Nightingale S, Le Guen C, Lawson N, Williamson JR, Hoffman P, Barnett AH. Diabet Med. 1990;7:63–68. doi: 10.1111/j.1464-5491.1990.tb01310.x. [DOI] [PubMed] [Google Scholar]
- 75.Dagher Z, Park YS, Asnaghi V, Hoehn T, Gerhardinger C, Lorenzi M. Diabetes. 2004;53:2404–2411. doi: 10.2337/diabetes.53.9.2404. [DOI] [PubMed] [Google Scholar]
- 76.Sorbinil Retinopathy Trial Research Group. Arch Ophthalmol. 1990;108:1234–1244. doi: 10.1001/archopht.1990.01070110050024. [DOI] [PubMed] [Google Scholar]
- 77.The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291410. [DOI] [PubMed] [Google Scholar]
- 78.Srivastava SK, Ansari NH. Diabetes. 1988;37:1505–1508. doi: 10.2337/diab.37.11.1505. [DOI] [PubMed] [Google Scholar]
- 79.Ansari NH, Srivastava SK. Biochem Biophys Res Commun. 1990;168:939–943. doi: 10.1016/0006-291x(90)91119-d. [DOI] [PubMed] [Google Scholar]
- 80.Ansari NH, Bhatnagar A, Fulep E, Khanna P, Srivastava SK. Res Commun Chem Pathol Pharmacol. 1994;84:93–104. [PubMed] [Google Scholar]
- 81.Bhatnagar A, Srivastava SK. Biochem Med MetabBiol. 1992;48:91–121. doi: 10.1016/0885-4505(92)90055-4. [DOI] [PubMed] [Google Scholar]
- 82.Sheetz MJ, King GL. JAMA. 2002;288:2579–2588. doi: 10.1001/jama.288.20.2579. [DOI] [PubMed] [Google Scholar]
- 83.Boel E, Selmer J, Flodgaard HJ, Jensen T. J Diabetes Complications. 1995;9:104–129. doi: 10.1016/1056-8727(94)00025-j. [DOI] [PubMed] [Google Scholar]
- 84.Spycher SE, Tabataba-Vakili S, O’Donnell VB, Palomba L, Azzi A. FASEB J. 1997;11:181–188. doi: 10.1096/fasebj.11.2.9039961. [DOI] [PubMed] [Google Scholar]
- 85.Lee EK, Regenold WT, Shapiro P. Anticancer Drugs. 2002;13:859–868. doi: 10.1097/00001813-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 86.Ramana KV, Friedrich B, Tammali R, West MB, Bhatnagar A, Srivastava SK. Diabetes. 2005;54:818–829. doi: 10.2337/diabetes.54.3.818. [DOI] [PubMed] [Google Scholar]
- 87.Ramana KV, Friedrich B, Bhatnagar A, Srivastava SK. FASEB J. 2003;17:315–317. doi: 10.1096/fj.02-0568fje. [DOI] [PubMed] [Google Scholar]
- 88.Ramana KV, Chandra D, Bhatnagar A, Aggarwal BB, Srivastava SK. J Biol Chem. 2002;275:32063–32070. doi: 10.1074/jbc.M202126200. [DOI] [PubMed] [Google Scholar]
- 89.Ramana KV, Bhatnagar A, Srivastava SK. FEBS Lett. 2004;570:189–194. doi: 10.1016/j.febslet.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 90.Ramana KV, Fadl AA, Tammali R, Reddy AB, Chopra AK, Srivastava SK. J Biol Chem. 2006;28:33019–33029. doi: 10.1074/jbc.M603819200. [DOI] [PubMed] [Google Scholar]
- 91.Pladzyk A, Reddy ABM, Yadav UCS, Tammali R, Ramana KV, Srivastava SK. Invest Ophthalmol Vis Sci. 2006;47:5395–5403. doi: 10.1167/iovs.06-0469. [DOI] [PubMed] [Google Scholar]
- 92.Yadav UC, Ramana KV, Aguilera-Aguirre L, Boldogh I, Boulares HA, Srivastava SK. PLoS One. 2009;4:e6535. doi: 10.1371/journal.pone.0006535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tammali R, Reddy AB, Ramana KV, Petrash JM, Srivastava SK. Carcinogenesis. 2009;30:799–807. doi: 10.1093/carcin/bgn246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Srivastava S, Liu SQ, Conklin DJ, Zacarias A, Srivastava SK, Bhatnagar A. Chem Biol Interact. 2001;130–132:563–571. doi: 10.1016/s0009-2797(00)00299-4. [DOI] [PubMed] [Google Scholar]
- 95.Srivastava S, Chandrasekar B, Bhatnagar A, Prabhu SD. Am J Physiol Heart Circ Physiol. 2002;283:H2612–H2619. doi: 10.1152/ajpheart.00592.2002. [DOI] [PubMed] [Google Scholar]
- 96.Yadav UC, Ramana KV, Awasthi YC, Srivastava SK. Toxicol Appl Pharmacol. 2008;227:257–264. doi: 10.1016/j.taap.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ramana KV, Bhatnagar A, Srivastava S, Yadav UC, Awasthi S, Awasthi YC, Srivastava SK. J Biol Chem. 2006;281:17652–17660. doi: 10.1074/jbc.M600270200. [DOI] [PubMed] [Google Scholar]
- 98.Favier A. Ann Pharm Fr. 2006;64:390–396. doi: 10.1016/s0003-4509(06)75334-2. [DOI] [PubMed] [Google Scholar]
- 99.Ohira A, Ueda T, Ohishi K, Hiramitsu T, Akeo K, Obara Y. Nippon Ganka Gakkai Zasshi. 2008;112:22–29. [PubMed] [Google Scholar]
- 100.Kubota S, Kurihara T, Mochimaru H, Satofuka S, Noda K, Ozawa Y, Oike Y, Ishida S, Tsubota K. Invest Ophthalmol Vis Sci. 2009;50:3512–3519. doi: 10.1167/iovs.08-2666. [DOI] [PubMed] [Google Scholar]
- 101.Yadav UC, Subramanyam S, Ramana KV. Invest Ophthalmol Vis Sci. 2009;50:2276–2282. doi: 10.1167/iovs.08-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mittag T. Exp Eye Res. 1984;39:759–769. doi: 10.1016/0014-4835(84)90075-7. [DOI] [PubMed] [Google Scholar]
- 103.Rao NA, Romero JL, Fernandez MA, Sevanian A, Marak GE., Jr Surv Ophthalmol. 1987;32:209–213. doi: 10.1016/0039-6257(87)90096-8. [DOI] [PubMed] [Google Scholar]
- 104.Rao NA. Trans Am Ophthalmol Soc. 1990;88:797–850. [PMC free article] [PubMed] [Google Scholar]
- 105.Gałecka E, Mrowicka M, Malinowska K, Gałecki P. Pol Merkur Lekarski. 2008;24:446–448. [PubMed] [Google Scholar]
- 106.Turpaev KT. Biochemistry (Mosc) 2002;67:281–292. doi: 10.1023/a:1014819832003. [DOI] [PubMed] [Google Scholar]
- 107.Dröge W. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 108.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 109.Srivastava S, Watowich SJ, Petrash JM, Srivastava SK, Bhatnagar A. Biochemistry. 1999;38:42–54. doi: 10.1021/bi981794l. [DOI] [PubMed] [Google Scholar]
- 110.Ahn KS, Sethi G, Aggarwal BB. Curr Mol Med. 2007;7:619–637. doi: 10.2174/156652407782564363. [DOI] [PubMed] [Google Scholar]
- 111.Srivastava SK, Ramana KV. Exp Eye Res. 2009;88:2–3. doi: 10.1016/j.exer.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tak PP, Firestein GS. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li Q, Verma IM. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 114.Makarov SS. Mol Med Today. 2000;6:441–448. doi: 10.1016/s1357-4310(00)01814-1. [DOI] [PubMed] [Google Scholar]
- 115.Uwe S. Biochem Pharmacol. 2008;75:1567–1579. doi: 10.1016/j.bcp.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 116.Sarkar FH, Li Y, Wang Z, Kong D. Int Rev Immunol. 2008;27:293–319. doi: 10.1080/08830180802276179. [DOI] [PubMed] [Google Scholar]
- 117.Ohta K, Nakayama K, Kurokawa T, Kikuchi T, Yoshimura N. Invest Ophthalmol Vis Sci. 2002;43:744–750. [PubMed] [Google Scholar]
- 118.Yeh S, Hu M. J Nutr Biochem. 2000;11:548–554. doi: 10.1016/s0955-2863(00)00117-0. [DOI] [PubMed] [Google Scholar]
- 119.Kim SH, Han SI, Oh SY, Chung HY, Kim HD, Kang HS. Biochem Biophys Res Commun. 2001;281:367–372. doi: 10.1006/bbrc.2001.4376. [DOI] [PubMed] [Google Scholar]
- 120.Poljsak B, Raspor P. J Appl Toxicol. 2008;28:183–188. doi: 10.1002/jat.1264. [DOI] [PubMed] [Google Scholar]
- 121.Kinoshita JH, Kador P, Catiles M. JAMA. 1981;246:257–261. [PubMed] [Google Scholar]
- 122.Schultz RO, Van Horn DL, Peters MA, Klewin KM, Schutten WH. Trans Am Ophthalmol Soc. 1981;79:180–199. [PMC free article] [PubMed] [Google Scholar]
- 123.Friend J, Ishii Y, Thoft RA. Ophthalmic Res. 1982;14:269–278. doi: 10.1159/000265202. [DOI] [PubMed] [Google Scholar]
- 124.Hyndiuk RA, Kazarian EL, Schultz RO, Seideman S. Arch Ophthalmol. 1977;95:2193–2196. doi: 10.1001/archopht.1977.04450120099012. [DOI] [PubMed] [Google Scholar]
- 125.Fukushi S, Merola LO, Tanaka M, Datiles M, Kinoshita JH. Exp Eye Res. 1980;31:611–621. doi: 10.1016/s0014-4835(80)80020-0. [DOI] [PubMed] [Google Scholar]
- 126.Datiles MB, Kador PF, Fukui HN, Hu TS, Kinoshita JH. Invest Ophthalmol Vis Sci. 1983;24:563–569. [PubMed] [Google Scholar]
- 127.Streilein JW, Ohta K, Mo JS, Taylor AW. DNA Cell Biol. 2002;21:453–459. doi: 10.1089/10445490260099746. [DOI] [PubMed] [Google Scholar]
- 128.Yadav UC, Ighani-Hosseinabad F, van Kuijk FJ, Srivastava SK, Ramana KV. Invest Ophthalmol Vis Sci. 2009;50:752–759. doi: 10.1167/iovs.08-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Henry DN, Del Monte M, Greene DA, Killen PD. J Clin Invest. 1993;93:617–623. doi: 10.1172/JCI116629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Obrosova IG, Minchenko AG, Vasupuram R, White L, Abatan OI, Kumagai AK, Frank RN, Stevens MJ. Diabetes. 2003;52:864–871. doi: 10.2337/diabetes.52.3.864. [DOI] [PubMed] [Google Scholar]
- 131.Yang P, de Vos AF, Kijlstra A. Br J Ophthalmol. 1997;81:396–401. doi: 10.1136/bjo.81.5.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.McMenamin PG, Crewe J. Invest Ophthalmol Vis Sci. 1995;36:1949–1959. [PubMed] [Google Scholar]
- 133.Kubo E, Urakami T, Fatma N, Akagi Y, Singh DP. Biochem Biophys Res Commun. 2004;314:1050–1056. doi: 10.1016/j.bbrc.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 134.Pladzyk A, Ramana KV, Ansari NH, Srivastava SK. Exp Eye Res. 2006;83:408–416. doi: 10.1016/j.exer.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 135.Pavan-Langston D. Int Ophthalmol Clin. 1975;15:19–35. doi: 10.1097/00004397-197501540-00004. [DOI] [PubMed] [Google Scholar]
- 136.Kerr EC, Copland DA, Dick AD, Nicholson LB. Prog Retin Eye Res. 2008;27:527–535. doi: 10.1016/j.preteyeres.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 137.Skeie JM, Mullins RF. Eye. 2009;23:747–755. doi: 10.1038/eye.2008.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Romero J, Marak GE, Jr, Rao NA. Ophthalmic Res. 1989;21:112–117. doi: 10.1159/000266788. [DOI] [PubMed] [Google Scholar]
- 139.Suzuki Y, Ohgami K, Shiratori K, Jin XH, Ilieva I, Koyama Y, Yazawa K, Yoshida K, Kase S, Ohno S. Exp Eye Res. 2006;82:275–281. doi: 10.1016/j.exer.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 140.Jin XH, Ohgami K, Shiratori K, Suzuki Y, Hirano T, Koyama Y, Yoshida K, Ilieva I, Iseki K, Ohno S. Invest Ophthalmol Vis Sci. 2006;47:2562256–8. doi: 10.1167/iovs.05-1429. [DOI] [PubMed] [Google Scholar]
- 141.van Rooij J, Schwartzenberg SG, Mulder PG, Baarsma SG. Br J Ophthalmol. 1999;83:1277–1282. doi: 10.1136/bjo.83.11.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Eberlein-König B, Placzek M, Przybilla B. J Am Acad Dermatol. 1998;38:45–48. doi: 10.1016/s0190-9622(98)70537-7. [DOI] [PubMed] [Google Scholar]
- 143.Jung HA, Yoon NY, Kang SS, Kim YS, Choi JS. J Pharm Pharmacol. 2008;60:1227–1236. doi: 10.1211/jpp.60.9.0016. [DOI] [PubMed] [Google Scholar]
- 144.Kim YS, Kim NH, Jung DH, Jang DS, Lee YM, Kim JM, Kim JS. Eur J Pharmacol. 2008;594:18–25. doi: 10.1016/j.ejphar.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 145.Rathee P, Chaudhary H, Rathee S, Rathee D, Kumar V, Kohli K. Inflamm Allergy Drug Targets. 2009;8:229–235. doi: 10.2174/187152809788681029. [DOI] [PubMed] [Google Scholar]
- 146.Yokoi N, Niiya A, Komuro A, Yokogaki S, Naka H, Awata T, Honma Y, Yamada J, Tei M, Kinoshita S. Curr Eye Res. 1997;16:595–599. doi: 10.1076/ceyr.16.6.595.5076. [DOI] [PubMed] [Google Scholar]
- 147.Nakahara M, Miyata K, Otani S, Miyai T, Nejima R, Yamagami S, Amano S. British Journal of Ophthalmology. 2005;89:266–268. doi: 10.1136/bjo.2004.049841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ramana KV, Willis MS, White MD, Horton JW, DiMaio JM, Srivastava D, Bhatnagar A, Srivastava SK. Circulation. 2006;114:1838–1846. doi: 10.1161/CIRCULATIONAHA.106.630830. [DOI] [PubMed] [Google Scholar]
- 149.Petrovic MG, Peterlin B, Hawlina M, Petrovic D. J Diabetes Complications. 2005;19:70–73. doi: 10.1016/j.jdiacomp.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 150.Olmos P, Futers S, Acosta AM, Siegel S, Maiz A, Schiaffino R, Morales P, Díaz R, Arriagada P, Claro JC, Vega R, Vollrath V, Velasco S, Emmerich M. Diabetes Res Clin Pract. 2000;47:169–176. doi: 10.1016/s0168-8227(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 151.Demaine A, Cross D, Millward A. Invest Ophthalmol Vis Sci. 2000;41:4064–4068. [PubMed] [Google Scholar]
- 152.Kern TS. Exp Diabetes Res. 2007;2007:95103. doi: 10.1155/2007/95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Funatsu H, Noma H, Mimura T, Eguchi S, Hori S. Ophthalmology. 2009;116:73–79. doi: 10.1016/j.ophtha.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 154.Perrone L, Devi TS, Hosoya K, Terasaki T, Singh LP. J Cell Physiol. 2009;221:262–272. doi: 10.1002/jcp.21852. [DOI] [PubMed] [Google Scholar]