Abstract
Background
Previous studies found higher cardiovascular risk with increased lipoprotein(a) [Lp(a)]. Whether Lp(a) concentration is related to type 2 diabetes is unclear.
Methods
In 26,746 healthy US women (mean age 54.6 years), we prospectively examined baseline Lp(a) concentrations and incident type 2 diabetes (N=1,670) over 13-year follow-up. We confirmed our findings in 9,652 Danish men and women with prevalent diabetes (N=419). Ananlyses were adjusted for risk factors including age, race, smoking, hormone use, family history, blood pressure, body mass index, hemoglobin A1c, C-reactive protein, and lipids.
Results
Lp(a) was inversely associated with incident diabetes, with fully-adjusted hazard ratios (HRs) and 95% CIs for quintiles 2–5 versus quintile 1: 0.87 (0.75–1.01), 0.80 (0.68–0.93), 0.88 (0.76–1.02), and 0.78 (0.67–0.91); P for trend 0.002. The association was stronger in nonfasting women, respective HRs 0.79 (0.58–1.09), 0.78 (0.57–1.08), 0.66 (0.46–0.93) and 0.56 (0.40–0.80); P for trend 0.001, P for interaction with fasting status 0.002. Using Lp(a) ≥1 mg/dL and HbA1c<5% as reference, the adjusted HR was 1.62 (0.91–2.89) for Lp(a) <1 mg/dL and HbA1c <5%; 3.50 (3.06–4.01) for Lp(a)≥1 mg/dL and HbA1c 5 to <6.5%; and 5.36 (4.00–7.19) for Lp(a)<1 mg/dL and HbA1c 5 to <6.5%. Results were similar in nonfasting Danish men and women: adjusted odds ratios 0.75 (0.55–1.03), 0.64 (0.46–0.88), 0.74 (0.54–1.01), and 0.58 (0.42–0.79) for Lp(a) quintiles 2–5 versus quintile 1; P for trend 0.002.
Conclusion
Lp(a) was associated inversely with risk of type 2 diabetes independent of risk factors, in contrast to prior positive associations of Lp(a) with cardiovascular risk.
Lipoprotein(a) [Lp(a)], a subtype of low-density-lipoprotein (LDL) that carries apolipoprotein(a), has been associated with risk of cardiovascular disease (CVD) (1), but its role in type 2 diabetes is unclear. Since its discovery by Berg in 1963, most studies have found higher risk of CVD with increased Lp(a) concentrations (2). Also, increased concentrations of Lp(a) have been associated with higher risk of CVD in diabetic patients (3–5). However, it is unclear if Lp(a) concentrations relate to risk of type 2 diabetes or insulin resistance (6). It has been suggested that hyperinsulinemia lowers Lp(a) concentrations (7,8), but prior case-control studies have been inconclusive (6). Some of these studies found no change in Lp(a) concentration in patients with type 2 diabetes (9), while others found either higher or lower Lp(a) concentrations (10,11).
While case-control studies are susceptible to bias, since the disease (e.g. diabetes) may alter lipoprotein levels, prospective studies are better for determining risk factor associations. Therefore, we conducted the first prospective study of Lp(a) concentration and risk of type 2 diabetes in a cohort of healthy US women. Based on prior work from this cohort suggesting that nonfasting concentrations of certain lipids may be superior to fasting concentrations for risk prediction (12,13), we also examined whether fasting status modified the association of Lp(a) with type 2 diabetes. Finally, we replicated our findings in a general population of men and women from Denmark (14).
MATERIALS AND METHODS
Study populations
The Women’s Health Study (WHS) is a completed randomized, double-blinded, placebo-controlled clinical trial of low-dose aspirin and vitamin E in US female healthcare professionals (15). Eligible participants were apparently healthy women, ages 45 years or older, who were free of self-reported cardiovascular disease or cancer at study entry (1992–1995). At the time of enrollment, participants gave written informed consent, completed questionnaires on demographics, medical history, medications, and lifestyle factors. They were also asked to provide a blood sample, if they were willing. Participants were requested, but not required, to have the sample drawn in the morning before eating, and reported the number of hours since their last meal before the blood draw. For the present analysis, we excluded women with prevalent diabetes (N=770), baseline hemoglobin A1c (HbA1c) ≥6.5% (N=270), or missing lipid measurements (N=237), resulting in 26,746 women for analysis. We also repeated the analyses after excluding the 164 women with HbA1c ≥6.0% and <6.5%. The study was approved by the institutional review boards of the Brigham and Women’s Hospital (Boston, Mass). We replicated our findings in a general population of 9,652 men and women (Copenhagen City Heart Study [CCHS])(14) in relation to prevalent type 2 diabetes (N=419).
Laboratory measurements
EDTA blood samples were obtained from WHS participants at the time of enrollment and stored in vapor phase liquid nitrogen (−170° C). Participants whose last meal was 8 hours or more prior to their blood draw comprised the fasting sample (N=19,292), and those who had eaten within 8 hours comprised the nonfasting sample (N=6,100). In a laboratory (N. Rifai) certified by the NHLBI/CDC Lipid Standardization program, baseline Lp(a) was measured using a commercially-available immunoturbidimetric assay that is not affected by the number of kringle IV type-2 repeats (16), with reagents and calibrators from Denka Seiken (Tokyo, Japan). There was no interference of this Lp(a) assay with triglycerides. The coefficients of variation (CVs) at Lp(a) concentrations of 17.6 and 58.1 mg/dL were 3.6 and 1.5%, respectively. Total, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) cholesterol were assayed directly. Hemoglobin A1c (HbA1c) was measured with turbidimetric immunoinhibition using hemolyzed whole blood or packed red cells (Roche Diagnostics). C-reactive protein (hsCRP) was measured using a high-sensitivity immunoturbidimetric assay using reagents and calibrators from Denka Seiken.
For CCHS participants, Lp(a) concentrations were measured at the 1991–1994 examination with a well-characterized in-house immunoturbidimetric assay using a Technicon Axon autoanalyser (Miles Inc., Tarrytown, NY), rabbit anti-human lipoprotein(a) polyclonal antibodies (Q023, DAKO, Glostrup, Denmark), and a human serum lipoprotein(a) calibrator (DAKO) (14). There was no interference from triglycerides up to 8 mmol/L (708 mg/dL). Samples above a level of Lp(a) of 85 mg/dL or of triglycerides above 8 mmol/L were diluted 1:5. The CVs at Lp(a) levels of 9, 30, 42, 66, 100 and 127 mg/dL and at triglyceride levels of 1–2 mmol/L were 11%, 3%, 2%, 2%, 5% and 4%, respectively. Enzymatic assays were used to measure total and HDL cholesterol and triglycerides. LDL cholesterol was measured directly at triglycerides > 400 mg/dL and otherwise calculated according to Friedewald. hsCRP was measured using a high-sensitivity immunoturbidimetric assay (DAKO).
Ascertainment of type 2 diabetes
Incident type 2 diabetes in WHS participants was ascertained by self-report on annual follow-up questionnaires through March 2008 as previously described (17,18). Screening rates for diabetes were high (85–90%). All self-reported cases of type 2 diabetes were validated using a supplemental questionnaire based on diagnostic criteria recommended by the American Diabetes Association, additional information from the participants by telephone interview, or review of medical records, with a positive predictive value for incident type 2 diabetes validation of 91% (17). Only confirmed cases of incident type 2 diabetes were included in this analysis.
In the CCHS, prevalent type 2 diabetes was ascertained by self-report at the 1991–1994 examination, use of hypoglycemic drugs, or a nonfasting plasma glucose >200 mg/dL.
Statistical Analysis
Statistical analyses were performed using STATA version 10.1 (STATA Corporation, College Station, Texas).
WHS analyses
Statistical comparisons were obtained from student’s t-tests for continuous variables expressed as means, from Kruskal-Wallis tests for variables expressed as medians, and chi-square tests for categorical variables. We calculated Pearson’s correlation coefficients for Lp(a) with select covariates in fasting and nonfasting participants. Following guidelines from the Department of Health and Human Services, Lp(a) concentrations were divided into quintiles based on the distribution among women not taking hormone replacement. Quintile cut-points were defined separately in each of the fasting and nonfasting samples.
Next, cumulative probabilities of incident type 2 diabetes were calculated for WHS participants stratified by baseline Lp(a) quintiles and fasting status. Cox proportional hazard regression models were used to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) according to these quintiles. Incidence rates and regression models were examined for thresholds. To examine the extent to which Lp(a) was associated with incident events, we considered 3 levels of adjustment: 1) age, race, and randomized treatment assignment; 2) covariates in model 1 plus baseline smoking status, menopausal status, postmenopausal hormone use, family history of diabetes, blood pressure, body mass index, and baseline HbA1c; 3) covariates in model 2 plus hsCRP, LDL cholesterol, HDL cholesterol, and triglycerides. Further adjustment for exercise, alcohol use, and education level, as well as adjustment for time of blood draw resulted in almost identical findings. P value for trend was obtained using quantile number as a predictor. All P-values were two-tailed. Statistical tests for interaction between fasting status and Lp(a) concentration in relation to incident type 2 diabetes were obtained using likelihood ratio tests.
We repeated the analyses after excluding the 164 women with HbA1c ≥6.0% and <6.5%. Given prior reports of modification of Lp(a)-related CVD risk in the presence of high LDL cholesterol concentrations, we also examined this in relation to diabetes. We also repeated the analyses according to time of diabetes diagnosis (<6 and ≥6 years) to assess for potential confounding by participants who may have had subclinical pre-existing diabetes at baseline. We analyzed risk of type 2 diabetes in participants based on baseline concentrations of Lp(a) and HbA1c to examine additive effects of these two biomarkers. Finally, we repeated the analysis in women according to baseline hormones use.
CCHS analyses
Lp(a) concentrations were divided into quintiles and logistic regression models were used to calculate odds ratios (ORs) and 95% CIs according to these quintiles. Three levels of adjustment were considered as described for the WHS.
RESULTS
Table 1 shows the baseline characteristics of participants according to diabetes. Lp(a) concentrations in the WHS and CCHS were significantly lower in diabetes cases compared with non-cases, although the medians differed by study population (WHS 9.5 vs 10.7 mg/dL, P<0.001; CCHS 15.7 vs 17.4 mg/dL, P=0.006). As reflected in their risk factors, the WHS participants were generally healthier and lower risk than the CCHS population, since they were selected after excluding baseline CVD, cancer, and diabetes, unlike the CCHS population which was a general Danish population.
Table 1.
Baseline Characteristics According to Incident (Women’s Health Study) or Prevalent (Copenhagen City Heart Study) Type 2 Diabetes
| Women’s Health Study | Copenhagen City Heart Study | |||||
|---|---|---|---|---|---|---|
| No Diabetes N=25,076 | Diabetes N=1,670 | P | No Diabetes N=9,233 | Diabetes N=419 | P | |
| Men, % | 0 | 0 | 42.9 | 60.4 | <0.001 | |
| Age, mean (SD), y | 54.6 (7.1) | 54.6 (6.5) | 0.93 | 58.0 (15.4) | 65.9 (10.5) | <0.001 |
| Current smoking, % | 11.5 | 13.0 | 0.06 | 49.2 | 43.5 | 0.02 |
| Hypertension, % | 22.4 | 47.1 | <0.001 | 54.5 | 76.3 | <0.001 |
| Postmenopausal status (women), % | 53.9 | 55.6 | <0.001 | 73.0 | 93.9 | <0.001 |
| Postmenopausal hormone use (women), % | 44.2 | 40.4 | 0.002 | 19.2 | 9.7 | 0.003 |
| Fasting, % | 75.8 | 78.6 | 0.01 | 0 | 0 | |
| Body mass index, mean (SD), kg/m2 | 25.4 (4.6) | 30.6 (6.0) | <0.001 | 25.5 (4.3) | 28.5 (5.3) | <0.001 |
| Family history of diabetes, % | 23.4 | 43.6 | <0.001 | 12.9 | 33.8 | <0.001 |
| Plasma concentrations, median (25th to 75th percentile) | ||||||
| Lp(a), mg/dL | 10.7 (4.5–33.1) | 9.5 (3.6–29.3) | <0.001 | 17.4 (5.7–39.9) | 15.7 (3.0–38.0) | 0.006 |
| Total cholesterol, mg/dL | 208 (183–235) | 213 (187–242) | <0.001 | 236 (205–271) | 240 (205–271) | 0.30 |
| Low-density lipoprotein cholesterol, mg/dL | 121 (100–144) | 126 (104–152) | <0.001 | 142 (115–173) | 139 (110–171) | 0.13 |
| High-density lipoprotein cholesterol, mg/dL | 53 (44–63) | 42 (36–50) | <0.001 | 58 (46–74) | 46 (39–58) | <0.001 |
| Triglycerides, mg/dL | 115 (82–167) | 174 (126–247) | <0.001 | 134 (96–193) | 204 (137–317) | <0.001 |
| Hemoglobin A1c,% | 4.98 (4.83–5.15) | 5.28 (5.07–5.53) | <0.001 | -- | -- | |
| High-sensitivity C-reactive protein, mg/L | 1.83 (0.74–3.98) | 4.22 (2.26–7.33) | <0.001 | 1.72 (1.25–2.92) | 2.88 (1.65–5.37) | <0.001 |
P values were obtained from Student’s t-tests for continuous variables expressed as means, Kruskal-Wallis tests for variables expressed as medians, and chi-square tests for categorical variables.
Pearson’s correlation coefficients showed low correlation of Lp(a) with other risk factors for diabetes in the WHS, including blood pressure, BMI, lipids, HbA1c and hsCRP (data not shown). Although some were statistically significant given the large sample size, the magnitude of the correlation was small (all coefficients <0.2).
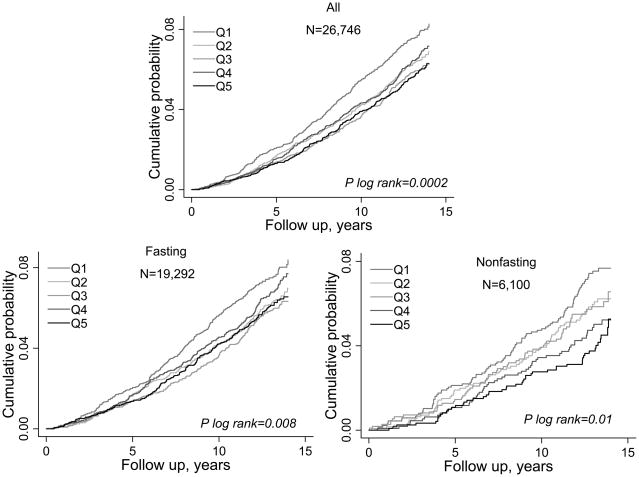
Cumulative event probabilities for incident diabetes in WHS during a median follow-up of 13.3 years (interquartile range 12.3 to 13.8 years) analyzed according to baseline Lp(a) quintiles demonstrated a significant inverse association overall as well as by fasting status (Fig. 1). Associations of Lp(a) concentrations with incident type 2 were examined by fasting status in WHS (Fig. 1 and Table 2). Incidence rates were significantly lower in quintiles 2–5 compared with quintile 1. In fasting participants, there was a threshold effect of ~ 20% lower relative risk in quintiles 2–5 compared to quintile 1. In nonfasting participants, there was a more linear effect, with up to 50% lower relative risk in quintile 5 compared with quintile 1. Notably, the inverse association of Lp(a) with diabetes remained significant and minimally attenuated after full adjustment for covariates, including LDL cholesterol, triglycerides, and HbA1c. Overall, nonfasting Lp(a) concentrations were more strongly associated with risk of incident diabetes (P for interaction 0.002) compared with fasting.
Fig. 1.
Cumulative probability of incident type 2 diabetes according to quintiles of Lp(a) concentration in the Women’s Health Study, stratified by nonfasting or fasting state at the time of the blood draw.
Table 2.
Association of Lp(a) with Incident Type 2 Diabetes in the Women’s Health Study According to Fasting Status
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P for Trend | PInteraction for fasting status | ||
|---|---|---|---|---|---|---|---|---|
| Range, mg/dL | ||||||||
| Fasting | N=19,292 | <3.9 | 3.9–8.2 | 8.3–16.5 | 16.6–46.6 | >46.6 | ||
| Nonfasting | N=6,100 | <3.9 | 3.9–8.5 | 8.6–16.9 | 17.0–43.3 | >43.3 | ||
| All | N=26,746 | <3.9 | 3.9–8.4 | 8.5–16.6 | 16.7–45.3 | >45.3 | ||
| Incidence rate (95% CI) per 1000 p-y | ||||||||
| Fasting | 6.02 (5.39–6.72) | 4.88 (4.30–5.53) | 4.55 (3.98–5.20) | 5.36 (4.73–6.08) | 4.75 (4.16–5.43) | 0.008 | ||
| Nonfasting | 5.69 (4.67–6.94) | 4.59 (3.65–5.77) | 4.57 (3.58–5.84) | 3.74 (2.86–4.90) | 3.24 (2.45–4.27) | 0.01 | ||
| All | 5.97 (5.43–6.55) | 4.87 (4.39–5.42) | 4.50 (4.01–5.06) | 5.03 (4.50–5.61) | 4.43 (3.94–4.97) | <0.001 | ||
| Model 1, HR (95% CI) | ||||||||
| Fasting | Ref. | 0.80 (0.68–0.95) | 0.73 (0.61–0.87) | 0.86 (0.72–1.01) | 0.77 (0.65–0.92) | 0.01 | ||
| Nonfasting | Ref. | 0.81 (0.59–1.09) | 0.78 (0.57–1.07) | 0.64 (0.46–0.90) | 0.56 (0.40–0.79) | <0.001 | 0.004 | |
| All | Ref. | 0.81 (0.70–0.94) | 0.73 (0.63–0.85) | 0.82 (0.71–0.95) | 0.73 (0.63–0.85) | <0.001 | ||
| Model 2, HR (95% CI) | ||||||||
| Fasting | Ref. | 0.88 (0.74–1.05) | 0.76 (0.64–0.92) | 0.86 (0.72–1.02) | 0.80 (0.66–0.95) | 0.01 | ||
| Nonfasting | Ref. | 0.82 (0.60–1.13) | 0.81 (0.58–1.11) | 0.66 (0.47–0.94) | 0.54 (0.38–0.76) | <0.001 | 0.003 | |
| All | Ref. | 0.89 (0.77–1.03) | 0.78 (0.67–0.91) | 0.86 (0.74–1.00) | 0.75 (0.65–0.88) | <0.001 | ||
| Model 3, HR (95% CI) | ||||||||
| Fasting | Ref. | 0.86 (0.72–1.02) | 0.78 (0.65–0.94) | 0.87 (0.73–1.04) | 0.84 (0.70–1.01) | 0.09 | ||
| Nonfasting | Ref. | 0.79 (0.58–1.09) | 0.78 (0.57–1.08) | 0.66 (0.46–0.93) | 0.56 (0.40–0.80) | 0.001 | 0.002 | |
| All | Ref. | 0.87 (0.75–1.01) | 0.80 (0.68–0.93) | 0.88 (0.76–1.02) | 0.78 (0.67–0.91) | 0.005 | ||
P for trend for event rates obtained from log-rank tests for equality of survivor functions.
P for trend for hazard ratios were obtained from Cox regression models using quintile number as predictor.
P for interaction with fasting status was obtained from likelihood ratio tests for interaction with fasting/nonfasting status and the Lp(a) concentration as a continuous variable, in relation to incident type 2 diabetes.
Model 1: Adjusted for age, race, and randomized treatment assignment.
Model 2: Adjusted for model 1 variables plus smoking status, menopausal status, postmenopausal hormone use, family history of diabetes, blood pressure, body mass index, and HbA1c.
Model 3: Adjusted for model 2 variables plus hsCRP, LDL cholesterol, HDL cholesterol, and triglycerides.
We repeated our analyses in Table 3 adjusting additionally for time of blood draw, with no change in results. Almost identical results were also obtained when we added exercise, alcohol use, and education to the models. We also examined the association of Lp(a) with diabetes in the WHS participants who were not taking hormones at baseline (N= 14,825). Similar results were obtained in non-hormone users. For example, the fully-adjusted HRs and 95% CIs for quintiles 2–5 versus quintile 1 in non-hormone users were: 0.90 (0.74–1.10), 0.86 (0.70–1.05), 0.91 (0.75–1.11), and 0.76 (0.61–0.93); P for trend 0.02.
Table 3.
Incidence Rates and Hazard Ratios in the Women’s Health Study According to Lp(a) Thresholds
| Lp(a), mg/dL | N | N incident diabetes | Incidence rate per 1,000 p-y (95% CI) | Adjusted hazard ratio* (95% CI) | P |
|---|---|---|---|---|---|
| ≥1 | 26,063 | 1,599 | 4.90 (4.66–5.14) | Ref. | |
| <1 | 683 | 71 | 8.40 (6.66–10.61) | 1.57 (1.23–2.01) | <0.001 |
| ≥2 | 23,968 | 1,456 | 4.85 (4.60–5.10) | Ref. | |
| <2 | 2,778 | 214 | 6.17 (5.40–7.06) | 1.19 (1.02–1.38) | 0.02 |
| ≥3 | 22,173 | 1,329 | 4.78 (4.53–5.04) | Ref. | |
| <3 | 4,573 | 341 | 5.97 (5.37–6.64) | 1.17 (1.03–1.32) | 0.01 |
| ≥4 | 20,767 | 1,230 | 4.72 (4.47–4.99) | Ref. | |
| <4 | 5,979 | 440 | 5.90 (5.37–6.48) | 1.18 (1.05–1.32) | 0.004 |
Adjusted for age, race, randomized treatment assignment, smoking status, menopausal status, postmenopausal hormone use, blood pressure, body mass index, HbA1c, family history of diabetes, hsCRP, LDL cholesterol, HDL cholesterol, and triglycerides.
The association of Lp(a) with diabetes was also similar in participants stratified by LDL cholesterol (below or above median, 121 mg/dL) or by year of study follow-up (less or greater than 6 years). The results were essentially unchanged when we additionally excluded the 164 women with baseline HbA1c ≥6.0%.
We further investigated the threshold effect of Lp(a) on incident diabetes that was suggested for the lowest quintile concentration (<4 mg/dL), using lower cut-points of 3, 2, and 1 mg/dL (Table 3). The incidence rates increased by approximately 1.5- to 2-fold for Lp(a) concentrations <1 mg/dL compared with higher cut-points. Using Lp(a) ≥1 mg/dL as the reference, the age- and treatment-adjusted hazard ratio for Lp(a) <1 mg/dL was 1.74 (95% CI 1.37–2.21), and the fully-adjusted hazard ratio was 1.57 (95% CI 1.23–2.01), P<0.001 for both.
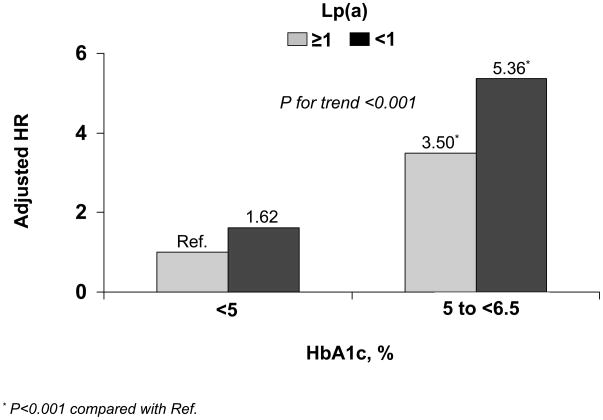
Next, we examined whether baseline Lp(a) concentrations < and ≥1 mg/dL provide additive risk information to HbA1c concentrations within the normal range (<5% and 5 to <6.5%, Fig. 2). Using the reference group of Lp(a) ≥1 mg/dL and HbA1c <5%, participants with Lp(a) <1 mg/dL and HbA1c <5% had a fully-adjusted HR (95% CI) of 1.62 (0.91–2.89), while those with Lp(a) ≥1 mg/dL and HbA1c 5 to <6.5% had an adjusted HR of 3.50 (3.06–4.01), and those with Lp(a) <1 mg/dL and HbA1c 5 to <6.5% had an adjusted HR of 5.36 (4.00–7.19), P for trend <0.001.
Fig. 2.
Additive association of Lp(a) (mg/dL) and HbA1c (%) concentrations with incident type 2 diabetes in the Women’s Health Study. Hazard ratios were adjusted for age, race, randomized treatment assignment, smoking status, menopausal status, postmenopausal hormone use, blood pressure, body mass index, family history of diabetes, high-sensitivity C-reactive protein, LDL cholesterol, HDL cholesterol, and triglycerides.
Finally, we replicated our findings in two settings. First, we internally validated the findings in a case-control analysis of 797 WHS women with baseline prevalent diabetes or HbA1c≥6.5% (cases) who were excluded from the prospective WHS study. We used as controls the 25,076 women who remained free of diabetes over the 13-year follow-up. Using Lp(a) quintile 1 as reference, the adjusted ORs (95% CIs) for quintiles 2–5 were 0.75 (0.59–0.94), 0.67 (0.52–0.85), 0.72 (0.56–0.91), and 0.74 (0.58–0.93), P for trend 0.01. Lp(a) <1 versus ≥1 mg/dL was associated with an adjusted OR of 2.29 (1.59–3.28), P<0.001.
Second, we externally validated the findings in 9,652 nonfasting men and women enrolled in the CCHS in relation to prevalent type 2 diabetes (Table 4) with adjusted ORs of 0.75(0.55–1.03), 0.64(0.46–0.88), 0.74(0.54–1.01), and 0.58(0.42–0.79) for quintiles 2–5 versus 1, P for trend 0.002. Lp(a) <1 mg/dL (versus ≥1 mg/dL) was associated with an adjusted OR of 1.54 (1.14–2.08), P=0.005. When stratified by sex, risk estimates appeared stronger in men. However, tests for interaction of sex and Lp(a) concentration in relation to diabetes were non-significant.
Table 4.
Association of Lp(a) with Prevalent Type 2 Diabetes in the Copenhagen City Heart Study (Nonfasting Samples)
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P for Trend | PInteraction for sex | ||
|---|---|---|---|---|---|---|---|---|
| Range, mg/dL | ||||||||
| Women | N=5,441 | <4.4 | 4.4–13.0 | 13.1–25.5 | 25.6–54.0 | >54.0 | ||
| Men | N=4,211 | <3.3 | 3.3–10.5 | 10.6–22.7 | 22.8–46.4 | >46.4 | ||
| All | N=9,652 | <3.9 | 3.9–11.7 | 11.8–24.1 | 24.2–49.8 | >49.8 | ||
| Model 1, OR (95% CI) | ||||||||
| Women | Ref. | 0.69(0.41–1.17) | 0.97(0.61–1.55) | 0.75(0.46–1.23) | 0.94(0.59–1.50) | 0.90 | ||
| Men | Ref. | 0.65(0.44–0.94) | 0.43(0.28–0.66) | 0.65(0.45–0.94) | 0.49(0.33–0.74) | 0.001 | 0.16 | |
| All | Ref. | 0.65(0.48–0.88) | 0.59(0.44–0.81) | 0.68(0.50–0.91) | 0.63(0.46–0.85) | 0.005 | ||
| Model 2, OR (95% CI) | ||||||||
| Women | Ref. | 0.71(0.42–1.20) | 0.89(0.55–1.44) | 0.74(0.45–1.22) | 0.87(0.54–1.40) | 0.64 | ||
| Men | Ref. | 0.67(0.46–0.99) | 0.43(0.28–0.65) | 0.66(0.45–0.97) | 0.53(0.35–0.80) | 0.003 | 0.38 | |
| All | Ref. | 0.66(0.48–0.90) | 0.57(0.42–0.78) | 0.68(0.50–0.92) | 0.63(0.46–0.86) | 0.007 | ||
| Model 3, OR (95% CI) | ||||||||
| Women | Ref. | 0.89(0.51–1.54) | 1.03(0.62–1.71) | 0.94(0.55–1.59) | 0.85(0.51–1.41) | 0.61 | ||
| Men | Ref. | 0.75(0.51–1.12) | 0.48(0.31–0.75) | 0.70(0.47–1.04) | 0.49(0.32–0.74) | 0.001 | 0.28 | |
| All | Ref. | 0.75(0.55–1.03) | 0.64(0.46–0.88) | 0.74(0.54–1.01) | 0.58(0.42–0.79) | 0.002 | ||
Quintiles were defined based on the distribution among women not taking hormone replacement, among men, or among the entire cohort, respectively.
P for trend for odds ratios (OR) were obtained from logistic regression models using quintile number as predictor.
P for interaction with sex was obtained from likelihood ratio tests for interaction with sex and the Lp(a) concentration as a continuous variable, in relation to prevalent type 2 diabetes.
Model 1: Adjusted for age and sex.
Model 2: Adjusted for model 1 variable plus smoking status, menopausal status (women only), postmenopausal hormone use (women only), family history of diabetes, hypertension, and body mass index.
Model 3: Adjusted for model 2 variables plus hsCRP, LDL cholesterol (corrected for the Lp[a] contribution(14)), HDL cholesterol, and triglycerides.
DISCUSSION
In this prospective study of 26,746 initially healthy US women with 13-year follow-up, we found an inverse association of Lp(a) with risk of type 2 diabetes with approximately 20–50% lower relative risk in quintiles 2–5 compared with quintile 1. Lp(a) concentrations showed low correlation with other risk factors, and full adjustment for these risk factors resulted in almost no attenuation of the association. We externally validated these findings in a general population of 9,652 Danish men and women with prevalent diabetes, confirming the inverse association of Lp(a) with diabetes with nearly identical results.
To our knowledge, this is the first prospective study that examines the association of Lp(a) with type 2 diabetes. Predicting risk of diabetes has focused largely on glycemic factors (19), with fewer studies on lipoprotein factors. Our finding an inverse association between Lp(a) concentration and risk of incident diabetes stands in marked contrast to prior studies that have shown a positive association of Lp(a) with CVD (2), including prior findings from the same two cohorts in this study (14,20). Risk factors for diabetes may differ from those for CVD (21). For example, LDL cholesterol is a strong risk factor for CVD and atherosclerosis, but most prior studies have shown no independent association for LDL cholesterol with diabetes (21).
While increased concentrations of Lp(a), in particular >30 mg/dL, have been associated with higher risk of CVD (20), little is known about the association of Lp(a) with diabetes in the absence of CVD. Small case-control studies examining Lp(a) with type 2 diabetes have shown mixed results (6). In one small case-control study of Mexican-Americans, lower concentrations of Lp(a) were found in diabetic subjects compared with controls (11). The present finding of an inverse association of Lp(a) with diabetes after adjustment for other risk factors deserves investigation in other prospective study populations for repolication of findings and determining potential clinical utility.
The physiological function and exact mechanisms that may underlie the role of Lp(a) in CVD remain unclear, and even less is known about the role of Lp(a) in diabetes. The independence of the association of Lp(a) with diabetes from known risk factors suggests another mechanism. It is possible that low Lp(a) concentrations may be a marker of insulin resistance. However, we adjusted for correlates of insulin resistance, such as triglycerides, HDL cholesterol, HbA1c and hsCRP, as well as BMI and family history of diabetes, although we did not have insulin concentrations or more sophisticated measures of insulin resistance. Lp(a) has been associated inversely with insulin and 2-hour glucose (7), consistent with our finding an inverse association with diabetes. Insulin suppressed apolipoprotein(a) function in hepatocytes at the post-transcriptional level of apolipoprotein(a) (8), which may account for lower concentrations of Lp(a) found in type 2 diabetes (hyperinsulinemia and insulin resistance) and higher concentrations in type 1 diabetes (insulin deficiency). In addition, there is hormonal regulation of Lp(a), including lowering of Lp(a) by testosterone (22) and insulin-like growth factor-1 (IGF-1) (23), and a stimulatory effect of growth hormone (24), all of which may be implicated in glucose and lipid metabolism (25). The role of Lp(a) in insulin resistance and glucose metabolism deserves further investigation for clarification of mechanisms.
It is unclear whether the relative deficiency of Lp(a) promotes the development of diabetes or whether increased concentrations of Lp(a) may be protective. The increase in incidence rates at an Lp(a) concentration cut-point of <1 mg/dL compared to higher cut-points suggests that the relative deficiency of Lp(a) may be involved in risk. Although Lp(a) concentrations <1 mg/dL had the highest relative risk, concentrations <4 mg/dL were associated with approximately 20% to 50% higher relative risk in both study populations. The WHS and CCHS participants differed with respect to Lp(a) concentrations (higher in CCHS) and other risk factors, but the association of Lp(a) with diabetes was consistent and similar in magnitude in the two studies. Moreover, Lp(a) concentrations <4 mg/dL represented the bottom quintile of both populations. Lp(a) was better for predicting incident diabetes when measured on nonfasting samples, consistent with prior data from the WHS in relation to certain lipids, in particular triglycerides, and incident cardiovascular events (12,13).
A potential limitation of this study is that ascertainment of type 2 diabetes was by self-report. However, all self-reports were confirmed, and this approach has been demonstrated to be valid in these female healthcare professionals (17). Lp(a) measurements were only available once at baseline and results could not be corrected for potential regression dilution bias. While we measured standard and emerging risk factors for diabetes, including BMI, triglycerides, HbA1c and hsCRP, we did not have a more specific measure of insulin resistance. Strengths of the present study include the large sample size of both cohorts, the prospective design of the WHS cohort, confirmation of the WHS findings in a second population of Danish men and women, and replication of the findings using two different Lp(a) assays, including a previously validated immunoassay in WHS which is independent of kringle IV type-2 repeats (16). Finally, the finding that Lp(a) concentration predicted incident diabetes similarly both early (years <6) and late (years ≥6) in follow-up makes reverse causality seem an unlikely explanation for our findings.
In summary, we found an inverse association for Lp(a) with risk of incident type 2 diabetes in women, with external confirmation of this finding in a general population of Danish men and women with respect to prevalent diabetes. Lp(a) was associated with diabetes independent of other risk factors, including BMI, HbA1c, or triglycerides, a finding that deserves further investigation.
Acknowledgments
The research for this article was supported by a research grant to Dr. Mora from the National Heart, Lung, and Blood Institute (K08 HL094375). The Women’s Health Study is supported by grants HL 43851, HL 080467 and CA 47988 from the National Heart, Lung, and Blood Institute and the National Cancer Insitute, the Donald W. Reynolds Foundation (Las Vegas, NV), and Leducq Foundation (Paris, France). The Copenhagen City Heart Study is supported by grants from The Danish Heart Foundation. The funding agencies played no role in the design, conduct, data management, analysis, or manuscript preparation related to this manuscript.
Footnotes
Disclosure
The authors report no conflicts.
References
- 1.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.The Emerging Risk Factors Collaboration. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–23. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruiz J, Thillet J, Huby T, James RW, Erlich D, Flandre P, Froguel P, Chapman J, Passa P. Association of elevated lipoprotein(a) levels and coronary heart disease in NIDDM patients. Relationship with apolipoprotein(a) phenotypes. Diabetologia. 1994;37:585–91. doi: 10.1007/BF00403377. [DOI] [PubMed] [Google Scholar]
- 4.Hiraga T, Kobayashi T, Okubo M, Nakanishi K, Sugimoto T, Ohashi Y, Murase T. Prospective study of lipoprotein(a) as a risk factor for atherosclerotic cardiovascular disease in patients with diabetes. Diabetes Care. 1995;18:241–4. doi: 10.2337/diacare.18.2.241. [DOI] [PubMed] [Google Scholar]
- 5.James RW, Boemi M, Sirolla C, Amadio L, Fumelli P, Pometta D. Lipoprotein (a) and vascular disease in diabetic patients. Diabetologia. 1995;38:711–4. doi: 10.1007/BF00401844. [DOI] [PubMed] [Google Scholar]
- 6.Koschinsky ML, Marcovina SM. The relationship between lipoprotein(a) and the complications of diabetes mellitus. Acta Diabetol. 2003;40:65–76. doi: 10.1007/s005920300007. [DOI] [PubMed] [Google Scholar]
- 7.Rainwater DL, Haffner SM. Insulin and 2-hour glucose levels are inversely related to Lp(a) concentrations controlled for LPA genotype. Arterioscler Thromb Vasc Biol. 1998;18:1335–41. doi: 10.1161/01.atv.18.8.1335. [DOI] [PubMed] [Google Scholar]
- 8.Neele DM, de Wit EC, Princen HM. Insulin suppresses apolipoprotein(a) synthesis by primary cultures of cynomolgus monkey hepatocytes. Diabetologia. 1999;42:41–4. doi: 10.1007/s001250051110. [DOI] [PubMed] [Google Scholar]
- 9.Haffner SM, Morales PA, Stern MP, Gruber MK. Lp(a) concentrations in NIDDM. Diabetes. 1992;41:1267–72. doi: 10.2337/diab.41.10.1267. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins AJ, Steele JS, Janus ED, Santamaria JD, Best JD. Plasma apolipoprotein (a) is increased in type 2 (non-insulin-dependent) diabetic patients with microalbuminuria. Diabetologia. 1992;35:1055–9. doi: 10.1007/BF02221681. [DOI] [PubMed] [Google Scholar]
- 11.Rainwater DL, MacCluer JW, Stern MP, VandeBerg JL, Haffner SM. Effects of NIDDM on lipoprotein(a) concentration and apolipoprotein(a) size. Diabetes. 1994;43:942–6. doi: 10.2337/diab.43.7.942. [DOI] [PubMed] [Google Scholar]
- 12.Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298:309–16. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 13.Mora S, Rifai N, Buring JE, Ridker PM. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 2008;118:993–1001. doi: 10.1161/CIRCULATIONAHA.108.777334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamstrup PR, Benn M, Tybjaerg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population: the Copenhagen City Heart Study. Circulation. 2008;117:176–84. doi: 10.1161/CIRCULATIONAHA.107.715698. [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A Randomized Trial of Low-Dose Aspirin in the Primary Prevention of Cardiovascular Disease in Women. N Engl J Med. 2005;352:1293–304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 16.Marcovina SM, Albers JJ, Scanu AM, Kennedy H, Giaculli F, Berg K, Couderc R, Dati F, Rifai N, Sakurabayashi I, Tate JR, Steinmetz A. Use of a reference material proposed by the International Federation of Clinical Chemistry and Laboratory Medicine to evaluate analytical methods for the determination of plasma lipoprotein(a) Clin Chem. 2000;46:1956–67. [PubMed] [Google Scholar]
- 17.Liu S, Lee IM, Song Y, Van Denburgh M, Cook NR, Manson JE, Buring JE. Vitamin E and risk of type 2 diabetes in the women’s health study randomized controlled trial. Diabetes. 2006;55:2856–62. doi: 10.2337/db06-0456. [DOI] [PubMed] [Google Scholar]
- 18.Pradhan AD, Cook NR, Manson JE, Ridker PM, Buring JE. A Randomized Trial of Low-Dose Aspirin in the Prevention of Clinical Type 2 Diabetes in Women. Diabetes Care. 2009;32:3–8. doi: 10.2337/dc08-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Screening for type 2 diabetes mellitus in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;148:846–54. doi: 10.7326/0003-4819-148-11-200806030-00007. [DOI] [PubMed] [Google Scholar]
- 20.Suk Danik J, Rifai N, Buring JE, Ridker PM. Lipoprotein(a), measured with an assay independent of apolipoprotein(a) isoform size, and risk of future cardiovascular events among initially healthy women. JAMA. 2006;296:1363–70. doi: 10.1001/jama.296.11.1363. [DOI] [PubMed] [Google Scholar]
- 21.Wilson PWF, Meigs J. Risk of type 2 diabetes mellitus and coronary heart disease: a pivotal role for metabolic factors. European Heart Journal Supplements. 2008;10:B11–5. [Google Scholar]
- 22.von Eckardstein A, Kliesch S, Nieschlag E, Chirazi A, Assmann G, Behre HM. Suppression of endogenous testosterone in young men increases serum levels of high density lipoprotein subclass lipoprotein A-I and lipoprotein(a) J Clin Endocrinol Metab. 1997;82:3367–72. doi: 10.1210/jcem.82.10.4267. [DOI] [PubMed] [Google Scholar]
- 23.Olivecrona H, Johansson AG, Lindh E, Ljunghall S, Berglund L, Angelin B. Hormonal regulation of serum lipoprotein(a) levels. Contrasting effects of growth hormone and insulin-like growth factor-I. Arterioscler Thromb Vasc Biol. 1995;15:847–9. doi: 10.1161/01.atv.15.7.847. [DOI] [PubMed] [Google Scholar]
- 24.Eden S, Wiklund O, Oscarsson J, Rosen T, Bengtsson BA. Growth hormone treatment of growth hormone-deficient adults results in a marked increase in Lp(a) and HDL cholesterol concentrations. Arterioscler Thromb. 1993;13:296–301. doi: 10.1161/01.atv.13.2.296. [DOI] [PubMed] [Google Scholar]
- 25.Zenobi PD, Jaeggi-Groisman SE, Riesen WF, Roder ME, Froesch ER. Insulin-like growth factor-I improves glucose and lipid metabolism in type 2 diabetes mellitus. J Clin Invest. 1992;90:2234–41. doi: 10.1172/JCI116109. [DOI] [PMC free article] [PubMed] [Google Scholar]