Abstract
A decline in mitochondrial function plays a key role in the aging process and increases the incidence of age-related disorders. A deeper understanding of the intricate nature of mitochondrial dynamics, which is described as the balance between mitochondrial fusion and fission, has revealed that functional and structural alterations in mitochondrial morphology are important factors in several key pathologies associated with aging. Indeed, a recent wave of studies has demonstrated the pleiotropic role of fusion and fission proteins in numerous cellular processes, including mitochondrial metabolism, redox signaling, the maintenance of mitochondrial DNA and cell death. Additionally, mitochondrial fusion and fission, together with autophagy, have been proposed to form a quality-maintenance mechanism that facilitates the removal of damaged mitochondria from the cell, a process that is particularly important to forestall aging. Thus, dysfunctional regulation of mitochondrial dynamics might be one of the intrinsic causes of mitochondrial dysfunction, which contributes to oxidative stress and cell death during the aging process. In this Commentary, we discuss recent studies that have converged at a consensus regarding the involvement of mitochondrial dynamics in key cellular processes, and introduce a possible link between abnormal mitochondrial dynamics and aging.
Keywords: Mitochondria, Fusion, Fission, mtDNA, Apoptosis, Mitophagy, Mammalian aging
Introduction
The aging process results in a gradual and progressive structural and functional deterioration of biomolecules that is associated with many pathological conditions, including cancer, neurodegenerative diseases, sarcopenia (loss of muscle mass) and liver dysfunction (Chung et al., 2009; Chung et al., 2008; Seo et al., 2006). Although several theories have been proposed to explain the fundamental mechanisms mediating these age-related diseases and conditions, the free-radical theory of aging is by far the most popular. This theory proposes that cumulative damage to biological macromolecules by oxygen radicals (reactive oxygen species; ROS) leads to irreversible cell damage and an overall functional decline (Harman, 1956). The free-radical theory has also been extended to include mitochondria, as the accumulation of aging-associated mutations and deletions in mitochondrial DNA (mtDNA) can impair the function of the respiratory chain and enhance ROS production (Chomyn and Attardi, 2003; Harman, 1972). The increased ROS production can subsequently lead to a vicious cycle of exponentially increasing levels of mtDNA damage and oxidative stress in the cell (Bandy and Davison, 1990; Hiona and Leeuwenburgh, 2008; Kujoth et al., 2006; Seo et al., 2006; Seo et al., 2008) (Fig. 1). Although various genetic problems in mitochondria cause phenotypes that resemble premature aging (Wallace and Fan, 2009), additional support for this theory was provided by studies showing a direct link between mtDNA mutations and mammalian aging. In particular, mice with a proofreading-deficient version of PolgA, the catalytic subunit of mitochondrial DNA polymerase γ (POLG), accumulate mtDNA mutations that are associated with impaired respiratory-chain function and increased levels of apoptosis. These mtDNA-mutator mice, with accelerated levels of mutations, had a shorter life span and displayed age-related phenotypes [such as hair loss, kyphosis (curvature of the spine), osteoporosis and sarcopenia] at an early age (Kujoth et al., 2005; Trifunovic et al., 2004). Interestingly, these changes were not accompanied by increased levels of oxidative stress, a finding that has also been confirmed in humans (Hutter et al., 2007). This has resulted in much controversy regarding the idea that mtDNA mutations contribute to aging through increased ROS production and enhanced levels of oxidative stress in mitochondria. However, it is possible that the accumulation of mtDNA mutations that occur with age leads to alterations in cell-signaling pathways that can induce cell dysfunction and initiate apoptosis, irrespective of increased ROS production and oxidative stress in mitochondria. Whether mtDNA mutations play a causal role in the aging process is still an ongoing debate; however, the fact that a functional decline in mitochondria occurs with age and that properly functioning mitochondria are crucial for longevity and minimizing age-related diseases cannot be refuted.
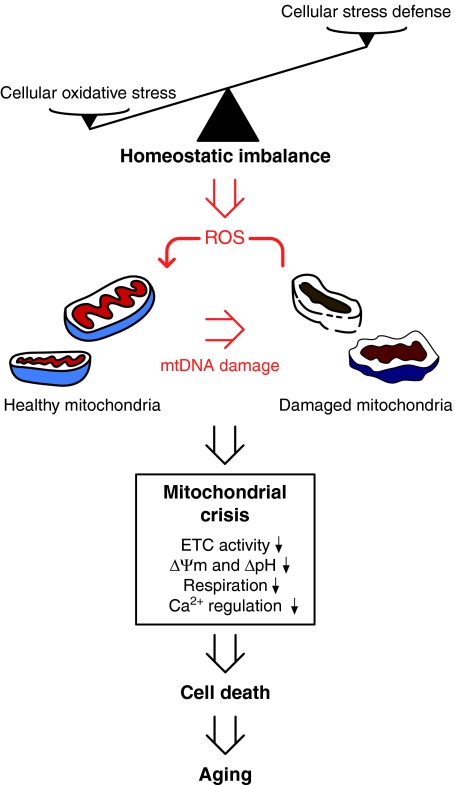
Fig. 1.
Proposed model of mitochondrial dysfunction in aging. Toxic ROS generated during normal biological activity gradually impair cellular homeostatic pathways that defend against cellular stress and damage mitochondrial constituents, including the electron transfer chain (ETC) and mtDNA. Oxidative insults to mitochondria, in turn, impair the life-sustaining functions of the organelle – such as energy transduction, biogenesis of metabolites, Ca2+ homeostasis and regulation of redox biology – with age, thereby contributing to a vicious cycle of accumulating mitochondrial damage that culminates in a mitochondrial functional crisis. This ultimately results in cell death and aging.
It is well established that mitochondria are highly motile and remarkably plastic organelles that continuously undergo fusion and fission events that actively alter their morphology. In addition, the dynamic regulation of mitochondrial fusion and fission has been shown to be an important mechanism of modulating cellular redox status, mtDNA integrity, organellar function and cell death (Liesa et al., 2009). Notably, genetic defects in the proteins involved in mitochondrial fusion and fission lead to severely altered mitochondrial shape, loss of mtDNA integrity, increased oxidative stress and apoptotic cell death; it has been shown that these alterations can subsequently cause developmental abnormality, neuromuscular degeneration and metabolic disorders in humans (Chen and Chan, 2009). However, the relevance of mitochondrial fusion and fission to underlying mechanisms of aging has not been fully appreciated, in part because the molecular events that underlie the aging process have not yet been completely elucidated. In this Commentary, we discuss current knowledge of mitochondrial dynamics, structure and function in relation to key cellular events, including mitochondrial biogenesis, mtDNA homeostasis, autophagy and cell death. By providing a basic overview of mitochondrial fusion and fission events and their general function in these crucial biological processes during normal stable environmental conditions, we hope to portray how alterations in mitochondrial dynamics can contribute to the mitochondrial dysfunction that is commonly associated with aging.
Mechanisms underlying mitochondrial dynamics
Mitochondria are highly complex and dynamic organelles that can alter their organization, shape and size, depending on intracellular and extracellular signals (Bereiter-Hahn and Voth, 1994; Rube and van der Bliek, 2004). Mitochondria undergo a continuous cycle of fusion and fission, and the balance between these opposing events determines the morphology of the organelle (Chen and Chan, 2004) (Fig. 2). Whereas decreased fusion can result in mitochondrial fragmentation because of excessive fission, decreased fission can generate long and highly interconnected mitochondria (Sesaki and Jensen, 1999).
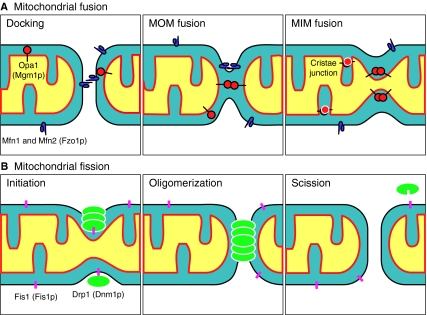
Fig. 2.
Schematic illustration depicting the core proteins of the molecular machinery that mediate mitochondrial fusion and fission in yeast and mammals. (A) Fusion proteins Mfn1 and Mfn2 (mammalian orthologs of yeast Fzo1p) contain four heptad repeats, one GTPase domain and two transmembrane domains. Opa1 (mammalian ortholog of yeast Mgm1p) is another fusion protein, located in the MIM and intermembrane space. The fusion process requires three steps: docking, MOM fusion and MIM fusion. Mfn1 and Mfn2 are thought to play an important role in docking and MOM fusion. Opa1 seems to be involved in the formation of cristae junctions as well as in MIM fusion, which occurs in a GTP-dependent manner. (B) The fission protein Drp1 (mammalian ortholog of yeast Dnm1p) contains one N-terminal GTPase domain, a C-terminal GED (GTPase effector domain) and a hydrophilic region in the middle. Drp1 self-oligomerizes and assembles a scission machine around the MOM. Fis1 (mammalian ortholog of yeast Fis1p) is a MOM protein and is thought to recruit Drp1 to the MOM by means of adaptor proteins.
During the past decade, various cellular components have been identified as key mediators of mitochondrial fusion and fission in yeast (Hoppins et al., 2007; Merz et al., 2007; Okamoto and Shaw, 2005). The fact that these components are structurally and functionally conserved in mammals indicates the ubiquitous importance of the fusion and fission pathway in mitochondrial biology (for reviews, see Liesa et al., 2009; Schafer and Reichert, 2009; Westermann, 2008) (Fig. 2A). In mammals, the best-studied proteins involved in mitochondrial fusion are the dynamin-related guanosine triphosphatases (GTPases), mitofusin 1 (Mfn1) and its paralog, mitofusin 2 (Mfn2). Mfn1 and Mfn2, which are the human orthologs of the Fuzzy onion (Fzo) protein found in Drosophila melanogaster and yeast, are each anchored to the mitochondrial outer membrane (MOM) through their C-terminal membrane-binding domain, whereas their N-terminal GTPase domain is present in the cytoplasm (Rojo et al., 2002). Both Mfn proteins mediate fusion through their active GTPase domains by tethering opposing mitochondrial membranes (Chen et al., 2003; Eura et al., 2003).
Mammalian Mfn1 and Mfn2 proteins have greater than 70% sequence similarity and share much of the same functional domain organization (Zhang and Chan, 2007). Despite some redundancies in their function, the two proteins possess several distinct properties, suggesting that they fulfill different roles in fusion (de Brito and Scorrano, 2008; Eura et al., 2003; Ishihara et al., 2004). Although both proteins are widely expressed, Mfn2 is highly abundant in heart and skeletal muscle and is reportedly expressed at low levels in numerous other human tissues (Bach et al., 2003; Santel et al., 2003). Moreover, the fact that mutations in Mfn2 disrupt several cellular processes regulating oxidative metabolism, cell-cycle progression and cell death, and cause Charcot-Marie-Tooth neuropathy type 2A (CMT2A), has increased interest in determining its physiological role and contribution to the pathology of human disease.
Mitochondrial fusion involves multiple steps, including mitochondrial tethering and fusion of MOMs, docking and fusion of the mitochondrial inner membranes (MIMs) and mixing of intramitochondrial components (Ishihara et al., 2004). MIM fusion is controlled by another dynamin-related GTPase, Opa1 (known as Mgm1p in yeast), which was first identified through its association with a neurodegenerative disease known as autosomal dominant optic atrophy (ADOA). Opa1 is targeted to and imported into mitochondria by a cleavable N-terminal pre-sequence and exists as both a full-length integral MIM protein and a soluble intermembrane space peptide (Alexander et al., 2000). Post-transcriptional modification of Opa1 by alternative splicing generates several isoforms that are differentially expressed in a wide variety of species (Delettre et al., 2001). Further adding to the complexity of Opa1 function, levels of Opa1 activity are also controlled by proteolytic cleavage; a decrease in the amount of long or short isoforms, for example, has been shown to be important in the regulation of cell death mediated by fragmented mitochondria (Arnoult et al., 2005a; Duvezin-Caubet et al., 2006). Although the precise function of the different isoforms is not known, Opa1 is believed to play a constitutive role in MIM fusion and remodeling of mitochondrial cristae structure (Frezza et al., 2006).
The regulation of mitochondrial fission in mammalian cells is controlled by two key proteins: dynamin-related protein 1 (Drp1; Dnm1p in yeast) and fission protein 1 (Fis1) (Fig. 2B). Drp1, a member of the dynamin family of large GTPases, is predominantely located in the cytosol and is recruited to mitochondrial surfaces, where it associates with Fis1 and assembles into foci that serve as potential scission sites for future fission events (Smirnova et al., 2001). Early studies revealed that, when Drp1 activity is inhibited, wild-type mitochondria are transformed into long and interconnected organelles (Pitts et al., 1999) and, conversely, overexpression of Drp1 in cells results in mitochondrial fragmentation (Arnoult et al., 2005b). The finding that Drp1 activity is post-translationally modified (e.g. by phosphorylation, ubiquitylation and sumoylation) revealed another level of cellular control whereby complex intracellular signaling pathways can regulate mitochondrial morphology during altered states of mitochondrial function by modulating the activity of Drp1 (Wasilewski and Scorrano, 2009).
In contrast to Drp1, mammalian Fis1 does not contain a GTPase domain and is primarily localized to the MOM by a transmembrane domain located in its C-terminal region (Suzuki et al., 2003). Fis1 overexpression in cultured cells results in mitochondrial fragmentation and depletion of Fis1 leads to elongated mitochondria (Yoon et al., 2003). The role of Fis1 in mitochondrial fission is primarily to act as an anchor protein for Drp1 (Wasilewski and Scorrano, 2009). In mammalian cells, fission also requires several accessory proteins, including mitochondrial protein of 18 kDa (MTP18) (Tondera et al., 2005), endophilin B1 (also known as Bif-1) (Karbowski et al., 2004; Takahashi et al., 2005), ganglioside-induced differentiation-associated protein 1 (GDAP1) (Niemann et al., 2005) and death-associated protein 3 (DAP3) (Mukamel and Kimchi, 2004).
Extensive ongoing research is being carried out to understand the cellular and molecular mechanisms that regulate mitochondrial dynamics in mammalian cells. However, more work is required to identify additional proteins involved in regulating mitochondrial dynamics and, more importantly, the upstream events that trigger and control mitochondrial dynamics.
The importance of mitochondrial dynamics for mitochondrial function
Mitochondrial biogenesis
The constant renewal of mitochondria is crucial for maintaining healthy mitochondria with age. Accurate organellar turnover requires the coordination of two key cellular processes: mitochondrial biogenesis and selective degradation (autophagy). Although a clear understanding of the molecular details controlling mitochondrial turnover is lacking, it is known that increased mitochondrial number and mass results from the growth and division of pre-existing organelles. The capacity for mitochondrial biogenesis diminishes with age and this is an important parameter in the mitochondrial dysfunction associated with aging (Fannin et al., 1999; Sugiyama et al., 1993).
The induction of organellar biogenesis can occur in response to several physiological stimuli, including muscle myogenesis, exercise, cold exposure and calorie restriction (Civitarese et al., 2007; Holloszy, 1967; Holloszy and Booth, 1976; Klingenspor, 2003; Moyes et al., 1997). Interestingly, tissues that have high rates of aerobic metabolism, such as skeletal muscle and heart, have pronounced mitochondrial networks with a high capacity for mitochondrial biogenesis (Bakeeva et al., 1978; Hood, 2001; Kirkwood et al., 1986). Peroxisome proliferative activated receptor-γ coactivator 1α (PGC-1α) is the best-known intracellular mediator of organellar biogenesis (Scarpulla, 2008). PGC-1α is a transcriptional coactivator that enhances the activity of specific transcription factors, in turn coordinating the expression of key nuclear-encoded mitochondrial genes that are required for the proper functioning of the organelle (Mootha et al., 2004; Wu et al., 1999). PGC-1α and its downstream target gene product, estrogen-related receptor-α (ERRα), stimulate the expression of Mfn2 (Cartoni et al., 2005; Soriano et al., 2006). Repression of Mfn2 in cells decreases oxygen consumption, glucose oxidation, mitochondrial membrane potential and the expression of oxidative phosphorylation proteins (Bach et al., 2003; Chen et al., 2005; Pich et al., 2005). Notably, decreased levels of PGC-1α and Mfn2 have been reported in the skeletal muscle of obese and type-2 diabetic subjects (Bach et al., 2005; Kelley et al., 2002). In addition, weight loss and exercise increase insulin sensitivity and the expression levels of these gene products in healthy, obese and type-2 diabetic individuals (Cartoni et al., 2005; Mingrone et al., 2005). Furthermore, calorie-restriction-induced mitochondrial biogenesis in mice increases PGC-1α, Mfn1 and Mfn2 expression levels in several tissues (Nisoli et al., 2005). Among the many activators of PGC-1α, AMP-activated kinase (AMPK) seems to be the most crucial for regulating mitochondrial metabolism and biogenesis. Reduced AMPK activity has been reported in aged animals and is directly linked to age-related insulin resistance and impaired fatty-acid oxidation (Qiang et al., 2007; Reznick et al., 2007).
Collectively, these studies indicate that alterations in mitochondrial dynamics are a key component of the mitochondrial adaptations that occur in response to mitochondrial biogenesis. These mitochondrial adaptations seem to be driven by a regulatory pathway that involves PGC-1α, ERRα and Mfn2, and can be activated by several physiological stimuli, including exercise and calorie restriction. However, these studies are not sufficient to reveal the functional relationship between mitochondrial dynamics and biogenesis, or to explain how fusion and fission events contribute to mitochondrial turnover. Further characterization of the mechanisms linking mitochondrial dynamics, biogenesis and degradation is needed to obtain clearer insight into the relationship between mitochondrial homeostasis and aging (Fig. 3).
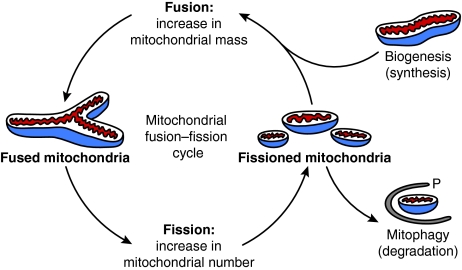
Fig. 3.
Possible relationship between mitochondrial fusion, fission, biogenesis and degradation. An ongoing mitochondrial fusion–fission cycle allows mitochondrial functional and genetic complementation, and the proper distribution of newly synthesized mitochondria during cell division. However, an imbalance in fusion and fission events – for example, more frequent fission than fusion – might increase the total number of small mitochondria per cell if extra mitochondria are not eliminated by mitophagy. Conversely, more frequent fusion could result in large tubular networks of mitochondria. Mitochondrial biogenesis is required to compensate for decreased mitochondrial biomass resulting from mitochondrial degradation (Berman et al., 2009). Therefore, an imbalance between mitochondrial fusion, fission, biogenesis and degradation events could cause substantial changes in mitochondrial number, biomass, shape and function. P indicates a phagophore by which targeted mitochondria are engulfed during the sequestering process required for mitophagy.
mtDNA maintenance and integrity
Mitochondria are unique in that they contain their own genome, which is organized into nucleoprotein complexes known as nucleoids that are distributed throughout mitochondrial matrix subcompartments (Bibb et al., 1981; Legros et al., 2004). The maintenance of mtDNA is crucial for normal cellular function, as it encodes proteins required for oxidative phosphorylation and ATP synthesis (Wallace and Fan, 2009). A unique feature of mitochondrial genetics is mtDNA heteroplasmy – the coexistence of both mutant and wild-type mtDNA in a single cell. Mutations in mtDNA accumulate over time and must reach a crucial threshold (~70-90% of total mtDNA) before associated clinical symptoms manifest (Wallace and Fan, 2009). Proper mitochondrial dynamics are important for the accurate segregation and transmission of mtDNA during mitosis, and numerous studies have shown that there is a relationship between mitochondrial dynamics and genomic stability. This was originally reported in yeast studies, in which defects in Fzo1p and Mgm1p (orthologs of mammalian Mfns and Opa1, respectively) were observed to result in slow growth on rich medium and a loss of mtDNA (Hermann et al., 1998; Rapaport et al., 1998). The results of subsequent studies in mammalian cells agreed with these findings and confirmed the roles of Mfn1, Mfn2 and Opa1 in mtDNA maintenance (Chen et al., 2007). It has been suggested that the exchange of mitochondrial contents by mitochondrial fusion enables the complementation of pathogenic (mutant) mtDNA in heteroplasmic cells, as introducing wild-type mtDNA dilutes the mutant mtDNA molecules and prevents them from reaching a crucial threshold in the cell (Chen et al., 2007; Sato et al., 2006; Sato et al., 2009). In a recent study conducted by Chen et al., muscle-specific Mfn1- and Mfn2-knockout mice were reported to have impaired mitochondrial function, abnormal mitochondrial proliferation and muscle loss (Chen et al., 2010). Furthermore, loss of mitochondrial fusion in skeletal muscle resulted in increased mtDNA point mutations and deletions as well as severe mtDNA depletion, which preceded the phenotypic changes observed in these mutant mice. This study supports the hypothesis that mitochondrial fusion through intramitochondrial exchange increases the tolerance of a cell to mutant mtDNA and protects the integrity of the mitochondrial genome. Interestingly, depletion of Drp1 in HeLa cells also results in mitochondrial dysfunction owing to a loss of mtDNA, suggesting that Drp1 is also important for mitochondrial inheritance during cell division, as well as for mitochondrial function (Parone et al., 2008). Silencing of Drp1 and Fis1 genes in a human cell line heteroplasmic for a pathological mtDNA mutation increased the levels of mutant mtDNA compared with wild-type mtDNA (Malena et al., 2009). Therefore, alterations in the expression levels of both fusion and fission proteins can influence the mitotic segregation of mutant and wild-type mtDNA, and might be an important factor in the proliferation of dysfunctional mitochondria that is observed with aging and mitochondrial disease.
Recently, it has also been postulated that the size and complexity of mtDNA nucleoid structure can influence mtDNA damage accumulation through mitochondrial fusion and fission pathways during aging of many somatic tissues (Bogenhagen, 2009). Specifically, mitochondrial fusion-mediated genetic complementation in large nucleoids that contain multiple mtDNA copies impedes the removal of mtDNA deletions and/or point mutations. By contrast, in cells that contain simpler mtDNA nucleoid structures with fewer mtDNA copies (e.g. oocytes), mitochondrial fission promotes the removal of mtDNA insults by targeting dysfunctional mitochondria for autophagic degradation. Although significant advances have been made in understanding the role of mitochondrial dynamics in mtDNA transmission, segregation and stability, additional studies are required to directly link the aging process with the organization of mtDNA nucleoids and the proteins that regulate mitochondrial dynamics. Moreover, the possibility that fusion and fission proteins affect the levels of other regulatory factors involved in mtDNA maintenance (such as POLG and mitochondrial transcription factor A) cannot be ruled out and requires further analysis.
Autophagy
The cell contains a number of intracellular repair and renewal mechanisms, one of the most prominent being autophagy, which sequesters and degrades intracellular components through delivery to the lysosomal machinery (Yorimitsu and Klionsky, 2005). In healthy cells, autophagy is essential for the removal of damaged or energy-deficient mitochondria that would otherwise accumulate and induce mitochondrion-mediated cell death (Wohlgemuth et al., 2010). The selective degradation of damaged mitochondria (also known as mitophagy) has been shown in fibroblasts from patients harboring mtDNA mutations. A greater than normal autophagic response has been observed in these patient cells compared with control cells (James et al., 1996).
Regulated degradation of mitochondria is intimately linked to mitochondrial fission (Narendra et al., 2008) and is particularly important for long-lived post-mitotic cells that possess a low regenerative capacity and experience high levels of oxidative damage. Indeed, reduced lysosomal degradation capacity and autophagy have been reported in aged hepatocytes (Donati et al., 2001; Terman, 1995) and, more recently, have been observed in skeletal muscle from aged Fischer 344 rats (Wohlgemuth et al., 2010). Consistent with a relationship between mitochondrial dynamics and aging, numerous studies have reported the accumulation of enlarged (often referred to as ‘giant’) or highly interconnected mitochondria in aging cells (Murakoshi et al., 1985; Tandler and Hoppel, 1986). These mitochondria are typically characterized as having low ATP production, loss of cristae structure and a swollen morphology (Terman and Brunk, 2005). Although the role of these giant mitochondria remains unclear, it has been postulated that they form because of dysregulation in mitochondrial dynamics and impaired autophagy. This is corroborated by studies conducted both in models of replicative senescence and in aging animals, in which the presence of abnormal mitochondria was associated with an overall shift towards more fusion events, which resulted mainly from the downregulation of fission proteins and reduced clearance of mitochondria by autophagy (Lee et al., 2007; Yoon et al., 2006). Repression of Fis1 activity has been shown to cause prolonged mitochondrial elongation (fusion) and senescence-related phenotypes [i.e. reduced mitochondrial membrane potential (Δψm) and increased levels of ROS], whereas overexpressed Fis1 inhibits senescence (Lee et al., 2007; Yoon et al., 2006). Intriguingly, in response to oxidant-induced damage, mitochondrial fission generates daughter mitochondria with different Δψm, whereby mitochondria with a lower Δψm can be targeted for degradation by autophagy, whereas parent mitochondria remain intact (Gomes and Scorrano, 2008; Twig et al., 2008). These depolarized mitochondria no longer have the capacity to fuse with other mitochondria, which is consistent with the lower levels of Opa1 observed in these mitochondria. The fragmentation of mitochondria is a prerequisite for autophagy (Twig et al., 2008), which might be physiologically favorable: smaller mitochondria might be autophagocytosed more easily than larger ones and require less energy. Therefore, in healthy cells, fission might prevent the sustained elongation of mitochondria that can induce cellular senescence. It does this in part through autophagy and the selective clearance of damaged organelles.
Apoptosis
It is well known that mitochondria play a crucial role in mediating apoptosis (Green, 1998). A key event in this process involves mitochondrial outer membrane permeabilization (MOMP) and the remodeling of mitochondrial membranes, permitting the release of apoptogenic factors such as cytochrome c (Liu et al., 1996) and apoptosis-inducing factor (AIF) (Susin et al., 1999) from the intermembrane space into the cytosol (Kroemer et al., 2007). These apoptogenic factors can then initiate apoptosis through caspase-dependent or -independent pathways (Green and Kroemer, 2004). In addition to requiring various physiological and electrochemical factors, including oxidative stress and Ca2+ signals, MOMP is regulated by the relative abundance of various pro-apoptotic (e.g. Bax and Bak) and anti-apoptotic proteins of the Bcl-2 family, as well as their subcellular localization and oligomerization states (Kroemer et al., 2007).
It is well established that apoptosis is elevated during aging of cells and tissues, and significantly contributes to cell loss and the pathogenesis of several age-related diseases (Marzetti et al., 2008). It was first described less than a decade ago that mitochondria in cells undergoing apoptosis are drastically altered and converted from long reticular tubules to small puncta-like organelles (Frank et al., 2001). Since then, mitochondrial fusion and fission proteins have been shown to have a central role in apoptosis, regulating not only mitochondrial dynamics but also mitochondrion-dependent cell death (Yamaguchi and Perkins, 2009). The role of mitochondrial dynamics in regulating apoptosis is mainly based on the finding that overexpression of the dominant-negative mutant Drp1K38A induces mitochondrial fusion and confers resistance to apoptosis by preventing a loss of Δψm and inhibiting cytochrome c release (Breckenridge et al., 2003; Brooks et al., 2009; Frank et al., 2001; Karbowski et al., 2002). Moreover, these studies revealed that silencing of Drp1 expression was not associated with reduced translocation of the pro-apoptotic protein Bax to mitochondria, suggesting that Drp1 functions downstream of Bax during apoptosis. Drp1-mediated release of cytochrome c is selective, as Drp1-induced cristae remodeling does not affect the release of SMAC (second mitochondrion-derived activator of caspases; also known as Diablo), a pro-apoptotic protein located in the intermembrane space (Arnoult et al., 2005b; Germain et al., 2005; Parone et al., 2006; Scorrano et al., 2002). The role of Drp1 as a pro-fission and pro-apoptotic protein is dependent on its stable association with the MOM and is governed by Bax and/or Bak sumoylation (Wasiak et al., 2007) and other post-translational modifications (Chang and Blackstone, 2007; Cribbs and Strack, 2007). Similarly to Drp1, downregulation of Fis1 expression significantly enhances fusion and inhibits cell death (Lee et al., 2004). However, in some cell types, mitochondrial fragmentation has been shown to occur following MOMP and cytochrome c release (Dinsdale et al., 1999; Gao et al., 2001; Parone et al., 2006; Zhuang et al., 1998) and also in the absence of any detectable cell death (Alirol et al., 2006). Once again, this illustrates that, although the roles of fission proteins in mitochondrial dynamics and cell death are closely associated, they are clearly distinct from one another.
Mfn2 colocalizes with Drp1 and Bax in mitochondrial foci (Karbowski et al., 2002) and directly associates with anti-apoptotic family members (Delivani et al., 2006) and Bak (Brooks et al., 2007). It has been shown that mitochondrial fusion is inhibited following MOMP (Brooks et al., 2007), primarily due to repression of Mfn2 activity by Bax (Karbowski et al., 2002; Karbowski et al., 2006). Moreover, overexpression of Mfn2, or the inhibition of its presence in mitochondrial foci, prevents the translocation of Bax to mitochondria and/or delays MOMP (Karbowski et al., 2006; Neuspiel et al., 2005). A decrease in mitochondrial fusion caused by reduced levels of Opa1 can also influence the progression of cell-death pathways (Lee et al., 2004; Olichon et al., 2003). Knockdown of Opa1 expression with small interfering RNA (siRNA) results in increased mitochondrial fission and decreased Δψm in HeLa cells, leading to abnormal mitochondrial cristae, mitochondrial fragmentation and the release of cytochrome c (Olichon et al., 2003).
In summary, these studies support a model whereby crosstalk between the mitochondrial fusion and fission machinery and members of the Bcl-2 family enables fine control of MOMP and the release of apoptogenic factors from mitochondria. Moreover, these studies collectively highlight the strong relationship between mitochondrial dynamics and the susceptibility to apoptosis. However, the real challenge has been to draw a clear distinction between mitochondrial fission and fusion events and the induction of cell death, which is particularly relevant in the context of aging tissues. This is because aged tissues are highly susceptible to increased oxidative stress and have a high incidence of apoptosis (Dirks and Leeuwenburgh, 2005; Dupont-Versteegden, 2005; Seo et al., 2008). Given that increased fusion and/or decreased fission seem to confer resistance to cell death, manipulating the levels of the proteins that make up the mitochondrial fusion and fission machinery might be a potential way to ameliorate unwanted cell death and delay the onset of age-related conditions, such as sarcopenia, metabolic diseases and neurodegenerative disorders.
Implications for mitochondrial dynamics in neurodegenerative disease
Neuronal cells have a high metabolic demand, making them particularly dependent on mitochondrial function. Although the mechanisms of neuronal-cell degeneration are uncertain, perturbations in mitochondrial dynamics have been implicated not only in CMT2A and ADOA, but also in the progression of age-associated neurodegenerative diseases such as Alzheimer's disease (AD), Parkinson's disease (PD) and Huntington's disease (HD).
Accumulation of β-amyloid (Aβ)-containing plaques derived from the amyloid precursor protein (APP) causes cellular toxicity, mitochondrial dysfunction and aberrant mitochondrial structure, which contribute to the pathogenesis of AD. Overexpression of APP in neuronal cells results in mitochondrial fragmentation by altering the levels of mitochondrial fusion and fission proteins (Wang et al., 2008). Aβ-induced mitochondrial fission occurs in part because of the presence of Drp1 that has been S-nitrosylated (a redox-related modification of cysteine thiol groups mediated by nitric oxide), which enhances its fission activity. Interestingly, blocking S-nitrosylation of Drp1 attenuates mitochondrial fission and reduces neuronal-cell damage (Cho et al., 2009). Similar findings for S-nitrosylated Drp1 were reported in the brain tissue of AD patients, further supporting the involvement of increased mitochondrial fission events in the pathogenesis of AD (Cho et al., 2009).
HD is caused by a mutation in the huntingtin gene (HTT). Expression of mutant huntingtin (mtHtt) leads to impaired energy metabolism, abnormal Ca2+ signaling and mitochondrial membrane potential, and extensive changes in mitochondrial structure (Benchoua et al., 2006; Panov et al., 2002; Squitieri et al., 2006). It was recently suggested that mtHtt affects mitochondrial fusion and fission events by colocalizing with Drp1 at specific fission sites on the MOM (Bossy-Wetzel et al., 2008).
Mitochondrial dynamics have also been linked to inherited PD and, specifically, to defects in two genes, Parkin (PARK) and PINK1 (Dodson and Guo, 2007). Although there are conflicting data regarding the specific effects of these proteins on mitochondrial morphology in mammalian cells, extensive studies in Drosophila revealed that knocking out either gene caused muscle degeneration that was associated with enlarged mitochondria (Clark et al., 2006; Greene et al., 2003). In Drosophila, ablation of the genes encoding Parkin and Pink1 seems to promote mitochondrial fusion or inhibit fission, as this phenotype can be rescued by overexpressing the fission protein Drp1 or downregulating the expression of the fusion proteins Mfn2 and Opa1 (Deng et al., 2008; Poole et al., 2008). Consistent with the role of Parkin and Pink1 in Drosophila, knocking down Pink1 in mouse neuronal cells leads to mitochondria with a swollen morphology (Wood-Kaczmar et al., 2008). By contrast, human fibroblasts from PD patients exhibit elevated levels of fragmented mitochondria, which suggests that enhanced fission events are involved in the pathogenesis of human PD (Exner et al., 2007). Adding to the complexity of the role of Parkin and Pink1 in PD pathogenesis, these proteins also seem to be involved in mitochondrial quality control by promoting the removal of damaged mitochondria by autophagy in human cells (Dagda et al., 2009; Narendra et al., 2008).
Although these studies indicate that mitochondrial fusion and fission pathways are involved in neurodegenerative diseases, the precise roles of these proteins have remained elusive owing to the contradictory findings that have been reported in studies of different cell lines and animal models, and with varying pathologies. Therefore, future work will help to understand the role of mitochondrial dynamics in the complex interactions underlying these age-related diseases and the potential for manipulation of mitochondrial dynamics to treat neurodegenerative diseases (for reviews, see Chen and Chan, 2009; Lu, 2009).
A synthesis: linking mitochondrial dynamics and aging
The process of aging involves a multitude of complex biological phenomena; a decline in mitochondrial turnover caused by reduced mitochondrial biogenesis and/or inefficient mitochondrial degradation seems to be a particularly crucial factor (Terman et al., 2010). In healthy cells, mitochondrial fusion provides a synchronized internal cable for translocating metabolites and intramitochondrial mixing during biogenesis, whereas mitochondrial division facilitates the equal distribution of mitochondria into daughter cells during mitosis and allows the selective degradation of damaged mitochondria through autophagy (Chen and Chan, 2009; Skulachev, 2001). However, it has become increasingly clear that these protective mechanisms are markedly impaired in aging and that faulty mitochondrial dynamics might be involved in the aging process.
As discussed above, mitochondria are highly dynamic structures that can adapt their morphology and function in response to a wide range of intracellular and extracellular stimuli. The plasticity of these organelles decreases with age: their capacity for mitochondrial biogenesis is reduced owing to a decline in PGC-1α activity, which might be instigated by an age-related increase in ROS production (Qiang et al., 2007; Reznick et al., 2007). An example that supports this concept is provided by recent studies of mitochondria in muscle. The subcellular localization of mitochondria and the overall mitochondrial network in multinucleated muscle fibers are tightly controlled by mitochondrion-shaping proteins, and an imbalance in mitochondrial fusion and fission dynamics probably impairs their function and contributes to the age-related loss of muscle. Indeed, it has recently been shown that the expression of Mfn2 and Drp1 genes is reduced in the skeletal muscle of aging individuals (Crane et al., 2010). Furthermore, the role of mitochondrion-shaping proteins has recently been investigated in mice with a muscle-specific deficiency in Fis1 and Drp: diminished mitochondrial fission was associated with reduced muscle atrophy and attenuated activation of atrophy-related genes during fasting (Romanello et al., 2010). Interestingly, the level of PGC-1α is decreased in various models of muscle atrophy (Adhihetty et al., 2007; Sandri et al., 2006), whereas overexpression of PGC-1α protects skeletal muscle from atrophy, mainly by inhibiting the induction of genes that are crucial for the atrophy process (Sandri et al., 2006). The finding that PGC-1α regulates Mfn2 expression supports the idea that there is a direct link between mitochondrial biogenesis and mitochondrial morphology (Cartoni et al., 2005). Collectively, these findings support the existence of a newly identified pathway that regulates muscle atrophy involving transcriptional changes in PGC-1α and in fission- and atrophy-specific genes. In addition, it opens up the possibility that muscle mass and function could be preserved during aging and other pathological conditions through physiological stimuli (e.g. exercise) and/or pharmacological intervention.
Mitochondrial dynamics are also important for proper organellar turnover in that they affect mitochondrial degradation pathways. Although there is conflicting evidence regarding the effect of aging on various proteolytic pathways (for reviews, see Attaix et al., 2005; Combaret et al., 2009), it has been reported that the lysosomal-autophagy system declines in a variety of tissues with age (Cuervo and Dice, 2000; Donati et al., 2001; Wohlgemuth et al., 2010). The appearance of giant mitochondria in aging cells suggests that these cells possess dysregulated degradation pathways. This is consistent with investigations of senescent cells: Fis1 expression was found to be reduced in abnormal mitochondria, whereas overexpression of this protein blocked the senescence-related phenotype and maintained cells in a proliferating state (Lee et al., 2007; Yoon et al., 2006). The link between fission proteins and mitochondrial turnover has also been illustrated in neuronal cells, in which Fis1 was shown to activate autophagy and the selective degradation of depolarized mitochondria (Twig et al., 2008).
Cell-death pathways are also induced in postmitotic aging cells, leading to irreversible damage to mitochondrial proteins and DNA, and the loss of nuclei. Mitochondrial fragmentation due to elevated fission events has been reported to both precede and follow the release of apoptogenic factors from mitochondria in many cell types. Interestingly, it has also been described that autophagy of mitochondria occurs following opening of the mitochondrial permeability transition pore and the depolarization of the mitochondrial membrane, suggesting that autophagy plays a protective role by preventing the cellular damage caused by activation of pro-apoptotic pathways (Elmore et al., 2001; Kim et al., 2007). This idea is further corroborated by results of a recent study demonstrating that autophagy is negatively correlated with oxidative damage and apoptosis in skeletal muscle from aged rodents (Wohlgemuth et al., 2010). In yeast, autophagy is required for chronological longevity (Alvers et al., 2009) and for preventing damage to mitochondria in chronologically old cells (A.Y.S., Ashley Alvers, Jennifer Westcott, Michael Wood, Roy Ferraiuolo, Michelle Marraffini et al., unpublished data). It is possible that the engulfment of dysfunctional mitochondria by autophagosomes blocks the release of pro-apoptotic proteins from mitochondria into the cytosol, thereby inhibiting DNA fragmentation and irreversible cell death. However, an open question is what determines the fate of a mitochondrion – that is, whether it will be selectively degraded by autophagy or undergo MOMP and trigger irreversible cell death. One possibility is that, during aging, the homeostatic regulation of mitochondrial biogenesis, dynamics and turnover by autophagy no longer efficiently maintains functional mitochondria, resulting in cellular senescence. As a result, oxidative damage might surpass a crucial threshold above which apoptosis is triggered, leading to substantial changes in mitochondrial morphology and irreversible cell death (Fig. 4). To address this issue conclusively, detailed studies that examine the specific effects of aging on mitochondrial fusion and fission proteins, mitochondrial morphology and mitochondrial turnover processes in a wide range of cells and tissues are required.
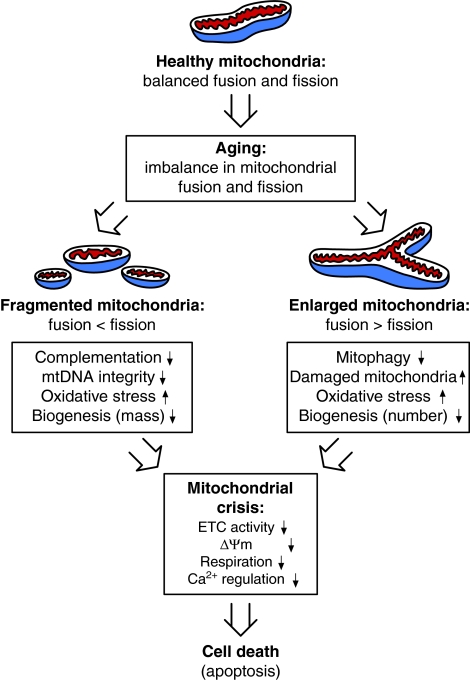
Fig. 4.
Model of the influence of mitochondrial dynamics on aging. Aging compromises the plasticity of mitochondria by disrupting the homeostatic regulation of mitochondrial fusion and fission pathways, resulting in abnormal mitochondrial morphology. The presence of fragmented mitochondria owing to a decline in fusion and/or an increase in fission events can compromise mtDNA integrity, mitochondrial structural and functional complementation, and mitochondrial biogenesis, all of which can lead to mitochondrial dysfunction. Conversely, the formation of enlarged mitochondria as a result of decreased fission and/or increased fusion events can diminish mitochondrial turnover by impairing mitophagy and biogenesis, leading to the accumulation of damaged mitochondria in aged cells. In both cases, the abnormal mitochondria are unable to fulfill their life-sustaining roles. Therefore, age-associated alterations in mitochondrial fusion and fission dynamics might play a causative role in mitochondrial dysfunction, and increase susceptibility to cell death in response to various types of stress during progressive aging.
Perspectives
It is well established that alterations in mitochondrial function are preceded by or occur concurrently with changes in mitochondrial architecture. This is now apparent in age-related pathologies, wherein energy-challenged mitochondria have disparate levels of fusion and fission proteins and, subsequently, altered mitochondrial morphology. Major advances have recently been made in understanding the association between mitochondrial dynamics and mitochondrial function. However, given the dynamic nature of mitochondria and the difficulty in visualizing fusion and fission events in in vivo models of mammalian aging, progress in the field has been slow. Recent advances in live-cell and tissue imaging techniques, such as fluorescent labeling of proteins and three-dimensional reconstruction of mitochondrial electron micrographic images in tissues, have facilitated the study of mitochondrial dynamics in animal models.
Extensive research has led to the consensus that regulation of mitochondrial dynamics is vital to basic biological processes and for proper mitochondrial function. However, a full appreciation of the multifaceted role of fusion and fission proteins has not been ascertained. For example, what are the upstream signaling molecules that regulate these proteins and their specific binding partners? An even more intriguing question is where in the aging process are these different factors integrated? The development of genetic tools to manipulate the levels of mitochondrial morphology proteins, in addition to the application of imaging techniques, will be crucial for understanding the physiological relevance of these proteins and their potential use in delaying the aging process and preventing the onset of age-related diseases.
Acknowledgments
This work was supported by grants to J.P.A. (AG023719), C.L. (NIA R01 AG17994), the University of Florida Institute on Aging and the Claude D. Pepper Older Americans Independence Center (1 P30 AG028740).
References
- Adhihetty P. J., O'Leary M. F., Chabi B., Wicks K. L., Hood D. A. (2007). Effect of denervation on mitochondrially mediated apoptosis in skeletal muscle. J. Appl. Physiol. 102, 1143-1151 [DOI] [PubMed] [Google Scholar]
- Alexander C., Votruba M., Pesch U. E., Thiselton D. L., Mayer S., Moore A., Rodriguez M., Kellner U., Leo-Kottler B., Auburger G., et al. (2000). OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat. Genet. 26, 211-215 [DOI] [PubMed] [Google Scholar]
- Alirol E., James D., Huber D., Marchetto A., Vergani L., Martinou J. C., Scorrano L. (2006). The mitochondrial fission protein hFis1 requires the endoplasmic reticulum gateway to induce apoptosis. Mol. Biol. Cell 17, 4593-4605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvers A. L., Fishwick L. K., Wood M. S., Hu D., Chung H. S., Dunn W. A., Jr, Aris J. P. (2009). Autophagy and amino acid homeostasis are required for chronological longevity in Saccharomyces cerevisiae. Aging Cell 8, 353-369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoult D., Grodet A., Lee Y. J., Estaquier J., Blackstone C. (2005a). Release of OPA1 during apoptosis participates in the rapid and complete release of cytochrome c and subsequent mitochondrial fragmentation. J. Biol. Chem. 280, 35742-35750 [DOI] [PubMed] [Google Scholar]
- Arnoult D., Rismanchi N., Grodet A., Roberts R. G., Seeburg D. P., Estaquier J., Sheng M., Blackstone C. (2005b). Bax/Bak-dependent release of DDP/TIMM8a promotes Drp1-mediated mitochondrial fission and mitoptosis during programmed cell death. Curr. Biol. 15, 2112-2118 [DOI] [PubMed] [Google Scholar]
- Attaix D., Mosoni L., Dardevet D., Combaret L., Mirand P. P., Grizard J. (2005). Altered responses in skeletal muscle protein turnover during aging in anabolic and catabolic periods. Int. J. Biochem. Cell Biol. 37, 1962-1973 [DOI] [PubMed] [Google Scholar]
- Bach D., Pich S., Soriano F. X., Vega N., Baumgartner B., Oriola J., Daugaard J. R., Lloberas J., Camps M., Zierath J. R., et al. (2003). Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J. Biol. Chem. 278, 17190-17197 [DOI] [PubMed] [Google Scholar]
- Bach D., Naon D., Pich S., Soriano F. X., Vega N., Rieusset J., Laville M., Guillet C., Boirie Y., Wallberg-Henriksson H., et al. (2005). Expression of Mfn2, the Charcot-Marie-Tooth neuropathy type 2A gene, in human skeletal muscle: effects of type 2 diabetes, obesity, weight loss, and the regulatory role of tumor necrosis factor alpha and interleukin-6. Diabetes 54, 2685-2693 [DOI] [PubMed] [Google Scholar]
- Bakeeva L. E., Chentsov, Yu S., Skulachev V. P. (1978). Mitochondrial framework (reticulum mitochondriale) in rat diaphragm muscle. Biochim. Biophys. Acta 501, 349-369 [DOI] [PubMed] [Google Scholar]
- Bandy B., Davison A. J. (1990). Mitochondrial mutations may increase oxidative stress: implications for carcinogenesis and aging? Free Radic. Biol. Med. 8, 523-539 [DOI] [PubMed] [Google Scholar]
- Benchoua A., Trioulier Y., Zala D., Gaillard M. C., Lefort N., Dufour N., Saudou F., Elalouf J. M., Hirsch E., Hantraye P., et al. (2006). Involvement of mitochondrial complex II defects in neuronal death produced by N-terminus fragment of mutated huntingtin. Mol. Biol. Cell 17, 1652-1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereiter-Hahn J., Voth M. (1994). Dynamics of mitochondria in living cells: shape changes, dislocations, fusion, and fission of mitochondria. Microsc. Res. Tech. 27, 198-219 [DOI] [PubMed] [Google Scholar]
- Berman S. B., Chen Y. B., Qi B., McCaffery J. M., Rucker E. B., 3rd, Goebbels S., Nave K. A., Arnold B. A., Jonas E. A., Pineda F. J., et al. (2009). Bcl-x L increases mitochondrial fission, fusion, and biomass in neurons. J. Cell Biol. 184, 707-719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. (1981). Sequence and gene organization of mouse mitochondrial DNA. Cell 26, 167-180 [DOI] [PubMed] [Google Scholar]
- Bogenhagen D. F. (2010). Does mtDNA nucleoid organization impact aging? Exp. Gerontol. 45, 473-477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossy-Wetzel E., Petrilli A., Knott A. B. (2008). Mutant huntingtin and mitochondrial dysfunction. Trends Neurosci. 31, 609-616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge D. G., Stojanovic M., Marcellus R. C., Shore G. C. (2003). Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J. Cell Biol. 160, 1115-1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks C., Wei Q., Feng L., Dong G., Tao Y., Mei L., Xie Z. J., Dong Z. (2007). Bak regulates mitochondrial morphology and pathology during apoptosis by interacting with mitofusins. Proc. Natl. Acad. Sci. USA 104, 11649-11654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks C., Wei Q., Cho S. G., Dong Z. (2009). Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J. Clin. Invest. 119, 1275-1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartoni R., Leger B., Hock M. B., Praz M., Crettenand A., Pich S., Ziltener J. L., Luthi F., Deriaz O., Zorzano A., et al. (2005). Mitofusins 1/2 and ERRalpha expression are increased in human skeletal muscle after physical exercise. J. Physiol. 567, 349-358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. R., Blackstone C. (2007). Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J. Biol. Chem. 282, 21583-21587 [DOI] [PubMed] [Google Scholar]
- Chen H., Chan D. C. (2004). Mitochondrial dynamics in mammals. Curr. Top. Dev. Biol. 59, 119-144 [DOI] [PubMed] [Google Scholar]
- Chen H., Chan D. C. (2009). Mitochondrial dynamics-fusion, fission, movement, and mitophagy-in neurodegenerative diseases. Hum. Mol. Genet. 18, R169-R176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Detmer S. A., Ewald A. J., Griffin E. E., Fraser S. E., Chan D. C. (2003). Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 160, 189-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Chomyn A., Chan D. C. (2005). Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J. Biol. Chem. 280, 26185-26192 [DOI] [PubMed] [Google Scholar]
- Chen H., McCaffery J. M., Chan D. C. (2007). Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell 130, 548-562 [DOI] [PubMed] [Google Scholar]
- Chen H., Vermulst M., Wang Y. E., Chomyn A., Prolla T. A., McCaffery J. M., Chan D. C. (2010). Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 141, 280-289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho D. H., Nakamura T., Fang J., Cieplak P., Godzik A., Gu Z., Lipton S. A. (2009). S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science 324, 102-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomyn A., Attardi G. (2003). MtDNA mutations in aging and apoptosis. Biochem. Biophys. Res. Commun. 304, 519-529 [DOI] [PubMed] [Google Scholar]
- Chung H. Y., Cesari M., Anton S., Marzetti E., Giovannini S., Seo A. Y., Carter C., Yu B. P., Leeuwenburgh C. (2009). Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res. Rev. 8, 18-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J. H., Seo A. Y., Chung S. W., Kim M. K., Leeuwenburgh C., Yu B. P., Chung H. Y. (2008). Molecular mechanism of PPAR in the regulation of age-related inflammation. Ageing Res. Rev. 7, 126-136 [DOI] [PubMed] [Google Scholar]
- Civitarese A. E., Carling S., Heilbronn L. K., Hulver M. H., Ukropcova B., Deutsch W. A., Smith S. R., Ravussin E. (2007). Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 4, e76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. E., Dodson M. W., Jiang C., Cao J. H., Huh J. R., Seol J. H., Yoo S. J., Hay B. A., Guo M. (2006). Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 441, 1162-1166 [DOI] [PubMed] [Google Scholar]
- Combaret L., Dardevet D., Bechet D., Taillandier D., Mosoni L., Attaix D. (2009). Skeletal muscle proteolysis in aging. Curr. Opin. Clin. Nutr. Metab. Care 12, 37-41 [DOI] [PubMed] [Google Scholar]
- Crane J. D., Devries M. C., Safdar A., Hamadeh M. J., Tarnopolsky M. A. (2010). The effect of aging on human skeletal muscle mitochondrial and intramyocellular lipid ultrastructure. J. Gerontol. A Biol. Sci. Med. Sci. 65, 119-128 [DOI] [PubMed] [Google Scholar]
- Cribbs J. T., Strack S. (2007). Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 8, 939-944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo A. M., Dice J. F. (2000). Regulation of lamp2a levels in the lysosomal membrane. Traffic 1, 570-583 [DOI] [PubMed] [Google Scholar]
- Dagda R. K., Cherra S. J., 3rd, Kulich S. M., Tandon A., Park D., Chu C. T. (2009). Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J. Biol. Chem. 284, 13843-13855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito O. M., Scorrano L. (2008). Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456, 605-610 [DOI] [PubMed] [Google Scholar]
- Delettre C., Griffoin J. M., Kaplan J., Dollfus H., Lorenz B., Faivre L., Lenaers G., Belenguer P., Hamel C. P. (2001). Mutation spectrum and splicing variants in the OPA1 gene. Hum. Genet. 109, 584-591 [DOI] [PubMed] [Google Scholar]
- Delivani P., Adrain C., Taylor R. C., Duriez P. J., Martin S. J. (2006). Role for CED-9 and Egl-1 as regulators of mitochondrial fission and fusion dynamics. Mol. Cell 21, 761-773 [DOI] [PubMed] [Google Scholar]
- Deng H., Dodson M. W., Huang H., Guo M. (2008). The Parkinson's disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc. Natl. Acad. Sci. USA 105, 14503-14508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinsdale D., Zhuang J., Cohen G. M. (1999). Redistribution of cytochrome c precedes the caspase-dependent formation of ultracondensed mitochondria, with a reduced inner membrane potential, in apoptotic monocytes. Am. J. Pathol. 155, 607-618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirks A. J., Leeuwenburgh C. (2005). The role of apoptosis in age-related skeletal muscle atrophy. Sports Med. 35, 473-483 [DOI] [PubMed] [Google Scholar]
- Dodson M. W., Guo M. (2007). Pink1, Parkin, DJ-1 and mitochondrial dysfunction in Parkinson's disease. Curr. Opin. Neurobiol. 17, 331-337 [DOI] [PubMed] [Google Scholar]
- Donati A., Cavallini G., Paradiso C., Vittorini S., Pollera M., Gori Z., Bergamini E. (2001). Age-related changes in the regulation of autophagic proteolysis in rat isolated hepatocytes. J. Gerontol. A Biol. Sci. Med. Sci. 56, B288-B293 [DOI] [PubMed] [Google Scholar]
- Dupont-Versteegden E. E. (2005). Apoptosis in muscle atrophy: relevance to sarcopenia. Exp. Gerontol. 40, 473-481 [DOI] [PubMed] [Google Scholar]
- Duvezin-Caubet S., Jagasia R., Wagener J., Hofmann S., Trifunovic A., Hansson A., Chomyn A., Bauer M. F., Attardi G., Larsson N. G., et al. (2006). Proteolytic processing of OPA1 links mitochondrial dysfunction to alterations in mitochondrial morphology. J. Biol. Chem. 281, 37972-37979 [DOI] [PubMed] [Google Scholar]
- Elmore S. P., Qian T., Grissom S. F., Lemasters J. J. (2001). The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J. 15, 2286-2287 [DOI] [PubMed] [Google Scholar]
- Eura Y., Ishihara N., Yokota S., Mihara K. (2003). Two mitofusin proteins, mammalian homologues of FZO, with distinct functions are both required for mitochondrial fusion. J. Biochem. 134, 333-344 [DOI] [PubMed] [Google Scholar]
- Exner N., Treske B., Paquet D., Holmstrom K., Schiesling C., Gispert S., Carballo-Carbajal I., Berg D., Hoepken H. H., Gasser T., et al. (2007). Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J. Neurosci. 27, 12413-12418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fannin S. W., Lesnefsky E. J., Slabe T. J., Hassan M. O., Hoppel C. L. (1999). Aging selectively decreases oxidative capacity in rat heart interfibrillar mitochondria. Arch. Biochem. Biophys. 372, 399-407 [DOI] [PubMed] [Google Scholar]
- Frank S., Gaume B., Bergmann-Leitner E. S., Leitner W. W., Robert E. G., Catez F., Smith C. L., Youle R. J. (2001). The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev. Cell 1, 515-525 [DOI] [PubMed] [Google Scholar]
- Frezza C., Cipolat S., Martins de Brito O., Micaroni M., Beznoussenko G. V., Rudka T., Bartoli D., Polishuck R. S., Danial N. N., De Strooper B., et al. (2006). OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 126, 177-189 [DOI] [PubMed] [Google Scholar]
- Gao W., Pu Y., Luo K. Q., Chang D. C. (2001). Temporal relationship between cytochrome c release and mitochondrial swelling during UV-induced apoptosis in living HeLa cells. J. Cell Sci. 114, 2855-2862 [DOI] [PubMed] [Google Scholar]
- Germain M., Mathai J. P., McBride H. M., Shore G. C. (2005). Endoplasmic reticulum BIK initiates DRP1-regulated remodelling of mitochondrial cristae during apoptosis. EMBO J. 24, 1546-1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes L. C., Scorrano L. (2008). High levels of Fis1, a pro-fission mitochondrial protein, trigger autophagy. Biochim. Biophys. Acta 1777, 860-866 [DOI] [PubMed] [Google Scholar]
- Green D. R. (1998). Apoptosis. Death deceiver. Nature 396, 629-630 [DOI] [PubMed] [Google Scholar]
- Green D. R., Kroemer G. (2004). The pathophysiology of mitochondrial cell death. Science 305, 626-629 [DOI] [PubMed] [Google Scholar]
- Greene J. C., Whitworth A. J., Kuo I., Andrews L. A., Feany M. B., Pallanck L. J. (2003). Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc. Natl. Acad. Sci. USA 100, 4078-4083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. (1956). Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 11, 298-300 [DOI] [PubMed] [Google Scholar]
- Harman D. (1972). The biologic clock: the mitochondria? J. Am. Geriatr. Soc. 20, 145-147 [DOI] [PubMed] [Google Scholar]
- Hermann G. J., Thatcher J. W., Mills J. P., Hales K. G., Fuller M. T., Nunnari J., Shaw J. M. (1998). Mitochondrial fusion in yeast requires the transmembrane GTPase Fzo1p. J. Cell Biol. 143, 359-373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiona A., Leeuwenburgh C. (2008). The role of mitochondrial DNA mutations in aging and sarcopenia: implications for the mitochondrial vicious cycle theory of aging. Exp. Gerontol. 43, 24-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloszy J. O. (1967). Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J. Biol. Chem. 242, 2278-2282 [PubMed] [Google Scholar]
- Holloszy J. O., Booth F. W. (1976). Biochemical adaptations to endurance exercise in muscle. Annu. Rev. Physiol. 38, 273-291 [DOI] [PubMed] [Google Scholar]
- Hood D. A. (2001). Invited Review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. J. Appl. Physiol. 90, 1137-1157 [DOI] [PubMed] [Google Scholar]
- Hoppins S., Lackner L., Nunnari J. (2007). The machines that divide and fuse mitochondria. Annu. Rev. Biochem. 76, 751-780 [DOI] [PubMed] [Google Scholar]
- Hutter E., Skovbro M., Lener B., Prats C., Rabol R., Dela F., Jansen-Durr P. (2007). Oxidative stress and mitochondrial impairment can be separated from lipofuscin accumulation in aged human skeletal muscle. Aging Cell 6, 245-256 [DOI] [PubMed] [Google Scholar]
- Ishihara N., Eura Y., Mihara K. (2004). Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J. Cell Sci. 117, 6535-6546 [DOI] [PubMed] [Google Scholar]
- James A. M., Wei Y. H., Pang C. Y., Murphy M. P. (1996). Altered mitochondrial function in fibroblasts containing MELAS or MERRF mitochondrial DNA mutations. Biochem. J. 318, 401-407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M., Lee Y. J., Gaume B., Jeong S. Y., Frank S., Nechushtan A., Santel A., Fuller M., Smith C. L., Youle R. J. (2002). Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J. Cell Biol. 159, 931-938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M., Jeong S. Y., Youle R. J. (2004). Endophilin B1 is required for the maintenance of mitochondrial morphology. J. Cell Biol. 166, 1027-1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M., Norris K. L., Cleland M. M., Jeong S. Y., Youle R. J. (2006). Role of Bax and Bak in mitochondrial morphogenesis. Nature 443, 658-662 [DOI] [PubMed] [Google Scholar]
- Kelley D. E., He J., Menshikova E. V., Ritov V. B. (2002). Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51, 2944-2950 [DOI] [PubMed] [Google Scholar]
- Kim I., Rodriguez-Enriquez S., Lemasters J. J. (2007). Selective degradation of mitochondria by mitophagy. Arch Biochem. Biophys. 462, 245-253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood S. P., Munn E. A., Brooks G. A. (1986). Mitochondrial reticulum in limb skeletal muscle. Am. J. Physiol. 251, C395-C402 [DOI] [PubMed] [Google Scholar]
- Klingenspor M. (2003). Cold-induced recruitment of brown adipose tissue thermogenesis. Exp. Physiol. 88, 141-148 [DOI] [PubMed] [Google Scholar]
- Kroemer G., Galluzzi L., Brenner C. (2007). Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 87, 99-163 [DOI] [PubMed] [Google Scholar]
- Kujoth G. C., Hiona A., Pugh T. D., Someya S., Panzer K., Wohlgemuth S. E., Hofer T., Seo A. Y., Sullivan R., Jobling W. A., et al. (2005). Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309, 481-484 [DOI] [PubMed] [Google Scholar]
- Kujoth G. C., Leeuwenburgh C., Prolla T. A. (2006). Mitochondrial DNA mutations and apoptosis in mammalian aging. Cancer Res. 66, 7386-7389 [DOI] [PubMed] [Google Scholar]
- Lee S., Jeong S. Y., Lim W. C., Kim S., Park Y. Y., Sun X., Youle R. J., Cho H. (2007). Mitochondrial fission and fusion mediators, hFis1 and OPA1, modulate cellular senescence. J. Biol. Chem. 282, 22977-22983 [DOI] [PubMed] [Google Scholar]
- Lee Y. J., Jeong S. Y., Karbowski M., Smith C. L., Youle R. J. (2004). Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol. Biol. Cell 15, 5001-5011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legros F., Malka F., Frachon P., Lombes A., Rojo M. (2004). Organization and dynamics of human mitochondrial DNA. J. Cell Sci. 117, 2653-2662 [DOI] [PubMed] [Google Scholar]
- Liesa M., Palacin M., Zorzano A. (2009). Mitochondrial dynamics in mammalian health and disease. Physiol. Rev. 89, 799-845 [DOI] [PubMed] [Google Scholar]
- Liu X., Kim C. N., Yang J., Jemmerson R., Wang X. (1996). Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86, 147-157 [DOI] [PubMed] [Google Scholar]
- Lu B. (2009). Mitochondrial dynamics and neurodegeneration. Curr. Neurol. Neurosci. Rep. 9, 212-219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malena A., Loro E., Di Re M., Holt I. J., Vergani L. (2009). Inhibition of mitochondrial fission favours mutant over wild-type mitochondrial DNA. Hum. Mol. Genet. 18, 3407-3416 [DOI] [PubMed] [Google Scholar]
- Marzetti E., Lawler J. M., Hiona A., Manini T., Seo A. Y., Leeuwenburgh C. (2008). Modulation of age-induced apoptotic signaling and cellular remodeling by exercise and calorie restriction in skeletal muscle. Free Radic. Biol. Med. 44, 160-168 [DOI] [PubMed] [Google Scholar]
- Merz S., Hammermeister M., Altmann K., Durr M., Westermann B. (2007). Molecular machinery of mitochondrial dynamics in yeast. Biol. Chem. 388, 917-926 [DOI] [PubMed] [Google Scholar]
- Mingrone G., Manco M., Calvani M., Castagneto M., Naon D., Zorzano A. (2005). Could the low level of expression of the gene encoding skeletal muscle mitofusin-2 account for the metabolic inflexibility of obesity? Diabetologia 48, 2108-2114 [DOI] [PubMed] [Google Scholar]
- Mootha V. K., Handschin C., Arlow D., Xie X., St Pierre J., Sihag S., Yang W., Altshuler D., Puigserver P., Patterson N., et al. (2004). Erralpha and Gabpa/b specify PGC-1alpha-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle. Proc. Natl. Acad. Sci. USA 101, 6570-6575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyes C. D., Mathieu-Costello O. A., Tsuchiya N., Filburn C., Hansford R. G. (1997). Mitochondrial biogenesis during cellular differentiation. Am. J. Physiol. 272, C1345-C1351 [DOI] [PubMed] [Google Scholar]
- Mukamel Z., Kimchi A. (2004). Death-associated protein 3 localizes to the mitochondria and is involved in the process of mitochondrial fragmentation during cell death. J. Biol. Chem. 279, 36732-36738 [DOI] [PubMed] [Google Scholar]
- Murakoshi M., Osamura Y., Watanabe K. (1985). Mitochondrial alterations in aged rat adrenal cortical cells. Tokai J. Exp. Clin. Med. 10, 531-536 [PubMed] [Google Scholar]
- Narendra D., Tanaka A., Suen D. F., Youle R. J. (2008). Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 183, 795-803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuspiel M., Zunino R., Gangaraju S., Rippstein P., McBride H. (2005). Activated mitofusin 2 signals mitochondrial fusion, interferes with Bax activation, and reduces susceptibility to radical induced depolarization. J. Biol. Chem. 280, 25060-25070 [DOI] [PubMed] [Google Scholar]
- Niemann A., Ruegg M., La Padula V., Schenone A., Suter U. (2005). Ganglioside-induced differentiation associated protein 1 is a regulator of the mitochondrial network: new implications for Charcot-Marie-Tooth disease. J. Cell Biol. 170, 1067-1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisoli E., Tonello C., Cardile A., Cozzi V., Bracale R., Tedesco L., Falcone S., Valerio A., Cantoni O., Clementi E., et al. (2005). Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 310, 314-317 [DOI] [PubMed] [Google Scholar]
- Okamoto K., Shaw J. M. (2005). Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu. Rev. Genet. 39, 503-536 [DOI] [PubMed] [Google Scholar]
- Olichon A., Baricault L., Gas N., Guillou E., Valette A., Belenguer P., Lenaers G. (2003). Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J. Biol. Chem. 278, 7743-7746 [DOI] [PubMed] [Google Scholar]
- Panov A. V., Gutekunst C. A., Leavitt B. R., Hayden M. R., Burke J. R., Strittmatter W. J., Greenamyre J. T. (2002). Early mitochondrial calcium defects in Huntington's disease are a direct effect of polyglutamines. Nat. Neurosci. 5, 731-736 [DOI] [PubMed] [Google Scholar]
- Parone P. A., James D. I., Da Cruz S., Mattenberger Y., Donze O., Barja F., Martinou J. C. (2006). Inhibiting the mitochondrial fission machinery does not prevent Bax/Bak-dependent apoptosis. Mol. Cell. Biol. 26, 7397-7408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parone P. A., Da Cruz S., Tondera D., Mattenberger Y., James D. I., Maechler P., Barja F., Martinou J. C. (2008). Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS One 3, e3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pich S., Bach D., Briones P., Liesa M., Camps M., Testar X., Palacin M., Zorzano A. (2005). The Charcot-Marie-Tooth type 2A gene product, Mfn2, up-regulates fuel oxidation through expression of OXPHOS system. Hum. Mol. Genet. 14, 1405-1415 [DOI] [PubMed] [Google Scholar]
- Pitts K. R., Yoon Y., Krueger E. W., McNiven M. A. (1999). The dynamin-like protein DLP1 is essential for normal distribution and morphology of the endoplasmic reticulum and mitochondria in mammalian cells. Mol. Biol. Cell 10, 4403-4417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole A. C., Thomas R. E., Andrews L. A., McBride H. M., Whitworth A. J., Pallanck L. J. (2008). The PINK1/Parkin pathway regulates mitochondrial morphology. Proc. Natl. Acad. Sci. USA 105, 1638-1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang W., Weiqiang K., Qing Z., Pengju Z., Yi L. (2007). Aging impairs insulin-stimulated glucose uptake in rat skeletal muscle via suppressing AMPKalpha. Exp. Mol. Med. 39, 535-543 [DOI] [PubMed] [Google Scholar]
- Rapaport D., Brunner M., Neupert W., Westermann B. (1998). Fzo1p is a mitochondrial outer membrane protein essential for the biogenesis of functional mitochondria in Saccharomyces cerevisiae. J. Biol. Chem. 273, 20150-20155 [DOI] [PubMed] [Google Scholar]
- Reznick R. M., Zong H., Li J., Morino K., Moore I. K., Yu H. J., Liu Z. X., Dong J., Mustard K. J., Hawley S. A., et al. (2007). Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab. 5, 151-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo M., Legros F., Chateau D., Lombes A. (2002). Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J. Cell Sci. 115, 1663-1674 [DOI] [PubMed] [Google Scholar]
- Romanello V., Guadagnin E., Gomes L., Roder I., Sandri C., Petersen Y., Milan G., Masiero E., Del Piccolo P., Foretz M., et al. (2010). Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J. 29, 1774-1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rube D. A., van der Bliek A. M. (2004). Mitochondrial morphology is dynamic and varied. Mol. Cell Biochem. 256-257, 331-339 [DOI] [PubMed] [Google Scholar]
- Sandri M., Lin J., Handschin C., Yang W., Arany Z. P., Lecker S. H., Goldberg A. L., Spiegelman B. M. (2006). PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc. Natl. Acad. Sci. USA 103, 16260-16265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santel A., Frank S., Gaume B., Herrler M., Youle R. J., Fuller M. T. (2003). Mitofusin-1 protein is a generally expressed mediator of mitochondrial fusion in mammalian cells. J. Cell Sci. 116, 2763-2774 [DOI] [PubMed] [Google Scholar]
- Sato A., Nakada K., Hayashi J. (2006). Mitochondrial dynamics and aging: mitochondrial interaction preventing individuals from expression of respiratory deficiency caused by mutant mtDNA. Biochim. Biophys. Acta 1763, 473-481 [DOI] [PubMed] [Google Scholar]
- Sato A., Nakada K., Hayashi J. (2009). Mitochondrial complementation preventing respiratory dysfunction caused by mutant mtDNA. Biofactors 35, 130-137 [DOI] [PubMed] [Google Scholar]
- Scarpulla R. C. (2008). Nuclear control of respiratory chain expression by nuclear respiratory factors and PGC-1-related coactivator. Ann. NY Acad. Sci. 1147, 321-334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer A., Reichert A. S. (2009). Emerging roles of mitochondrial membrane dynamics in health and disease. Biol. Chem. 390, 707-715 [DOI] [PubMed] [Google Scholar]
- Scorrano L., Ashiya M., Buttle K., Weiler S., Oakes S. A., Mannella C. A., Korsmeyer S. J. (2002). A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev. Cell 2, 55-67 [DOI] [PubMed] [Google Scholar]
- Seo A. Y., Hofer T., Sung B., Judge S., Chung H. Y., Leeuwenburgh C. (2006). Hepatic oxidative stress during aging: effects of 8% long-term calorie restriction and lifelong exercise. Antioxid. Redox. Signal. 8, 529-538 [DOI] [PubMed] [Google Scholar]
- Seo A. Y., Xu J., Servais S., Hofer T., Marzetti E., Wohlgemuth S. E., Knutson M. D., Chung H. Y., Leeuwenburgh C. (2008). Mitochondrial iron accumulation with age and functional consequences. Aging Cell 7, 706-716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesaki H., Jensen R. E. (1999). Division versus fusion: Dnm1p and Fzo1p antagonistically regulate mitochondrial shape. J. Cell Biol. 147, 699-706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skulachev V. P. (2001). Mitochondrial filaments and clusters as intracellular power-transmitting cables. Trends Biochem. Sci. 26, 23-29 [DOI] [PubMed] [Google Scholar]
- Smirnova E., Griparic L., Shurland D. L., van der Bliek A. M. (2001). Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol. Biol. Cell 12, 2245-2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano F. X., Liesa M., Bach D., Chan D. C., Palacin M., Zorzano A. (2006). Evidence for a mitochondrial regulatory pathway defined by peroxisome proliferator-activated receptor-gamma coactivator-1 alpha, estrogen-related receptor-alpha, and mitofusin 2. Diabetes 55, 1783-1791 [DOI] [PubMed] [Google Scholar]
- Squitieri F., Cannella M., Sgarbi G., Maglione V., Falleni A., Lenzi P., Baracca A., Cislaghi G., Saft C., Ragona G., et al. (2006). Severe ultrastructural mitochondrial changes in lymphoblasts homozygous for Huntington disease mutation. Mech. Ageing Dev. 127, 217-220 [DOI] [PubMed] [Google Scholar]
- Sugiyama S., Takasawa M., Hayakawa M., Ozawa T. (1993). Changes in skeletal muscle, heart and liver mitochondrial electron transport activities in rats and dogs of various ages. Biochem. Mol. Biol. Int. 30, 937-944 [PubMed] [Google Scholar]
- Susin S. A., Lorenzo H. K., Zamzami N., Marzo I., Snow B. E., Brothers G. M., Mangion J., Jacotot E., Costantini P., Loeffler M., et al. (1999). Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397, 441-446 [DOI] [PubMed] [Google Scholar]
- Suzuki M., Jeong S. Y., Karbowski M., Youle R. J., Tjandra N. (2003). The solution structure of human mitochondria fission protein Fis1 reveals a novel TPR-like helix bundle. J. Mol. Biol. 334, 445-458 [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Karbowski M., Yamaguchi H., Kazi A., Wu J., Sebti S. M., Youle R. J., Wang H. G. (2005). Loss of Bif-1 suppresses Bax/Bak conformational change and mitochondrial apoptosis. Mol. Cell. Biol. 25, 9369-9382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandler B., Hoppel C. L. (1986). Studies on giant mitochondria. Ann. NY Acad. Sci. 488, 65-81 [DOI] [PubMed] [Google Scholar]
- Terman A. (1995). The effect of age on formation and elimination of autophagic vacuoles in mouse hepatocytes. Gerontology 41, 319-326 [DOI] [PubMed] [Google Scholar]
- Terman A., Brunk U. T. (2005). The aging myocardium: roles of mitochondrial damage and lysosomal degradation. Heart Lung Circ. 14, 107-114 [DOI] [PubMed] [Google Scholar]
- Terman A., Kurz T., Navratil M., Arriaga E. A., Brunk U. T. (2010). Mitochondrial turnover and aging of long-lived postmitotic cells: the mitochondrial-lysosomal axis theory of aging. Antioxid. Redox Signal. 12, 503-535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondera D., Czauderna F., Paulick K., Schwarzer R., Kaufmann J., Santel A. (2005). The mitochondrial protein MTP18 contributes to mitochondrial fission in mammalian cells. J. Cell Sci. 118, 3049-3059 [DOI] [PubMed] [Google Scholar]
- Trifunovic A., Wredenberg A., Falkenberg M., Spelbrink J. N., Rovio A. T., Bruder C. E., Bohlooly Y. M., Gidlof S., Oldfors A., Wibom R., et al. (2004). Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429, 417-423 [DOI] [PubMed] [Google Scholar]
- Twig G., Elorza A., Molina A. J., Mohamed H., Wikstrom J. D., Walzer G., Stiles L., Haigh S. E., Katz S., Las G., et al. (2008). Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 27, 433-446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace D. C., Fan W. (2009). The pathophysiology of mitochondrial disease as modeled in the mouse. Genes Dev. 23, 1714-1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Su B., Siedlak S. L., Moreira P. I., Fujioka H., Wang Y., Casadesus G., Zhu X. (2008). Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc. Natl. Acad. Sci. USA 105, 19318-19323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasiak S., Zunino R., McBride H. M. (2007). Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J. Cell Biol. 177, 439-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasilewski M., Scorrano L. (2009). The changing shape of mitochondrial apoptosis. Trends Endocrinol. Metab. 20, 287-294 [DOI] [PubMed] [Google Scholar]
- Westermann B. (2008). Molecular machinery of mitochondrial fusion and fission. J. Biol. Chem. 283, 13501-13505 [DOI] [PubMed] [Google Scholar]
- Wohlgemuth S. E., Seo A. Y., Marzetti E., Lees H. A., Leeuwenburgh C. (2010). Skeletal muscle autophagy and apoptosis during aging: Effects of calorie restriction and life-long exercise. Exp. Gerontol. 45, 138-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood-Kaczmar A., Gandhi S., Yao Z., Abramov A. Y., Miljan E. A., Keen G., Stanyer L., Hargreaves I., Klupsch K., Deas E., et al. (2008). PINK1 is necessary for long term survival and mitochondrial function in human dopaminergic neurons. PLoS One 3, e2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Puigserver P., Andersson U., Zhang C., Adelmant G., Mootha V., Troy A., Cinti S., Lowell B., Scarpulla R. C., et al. (1999). Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98, 115-124 [DOI] [PubMed] [Google Scholar]
- Yamaguchi R., Perkins G. (2009). Dynamics of mitochondrial structure during apoptosis and the enigma of Opa1. Biochim. Biophys. Acta 1787, 963-972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y., Krueger E. W., Oswald B. J., McNiven M. A. (2003). The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol. Cell. Biol. 23, 5409-5420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y. S., Yoon D. S., Lim I. K., Yoon S. H., Chung H. Y., Rojo M., Malka F., Jou M. J., Martinou J. C., Yoon G. (2006). Formation of elongated giant mitochondria in DFO-induced cellular senescence: involvement of enhanced fusion process through modulation of Fis1. J. Cell Physiol. 209, 468-480 [DOI] [PubMed] [Google Scholar]
- Yorimitsu T., Klionsky D. J. (2005). Autophagy: molecular machinery for self-eating. Cell Death Differ. 12, 1542-1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chan D. C. (2007). New insights into mitochondrial fusion. FEBS Lett. 581, 2168-2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang J., Dinsdale D., Cohen G. M. (1998). Apoptosis, in human monocytic THP.1 cells, results in the release of cytochrome c from mitochondria prior to their ultracondensation, formation of outer membrane discontinuities and reduction in inner membrane potential. Cell Death Differ. 5, 953-962 [DOI] [PubMed] [Google Scholar]