Abstract
Sexual reproduction is both extremely costly and widespread relative to asexual reproduction, meaning that it must also confer profound advantages in order to persist. One theorized benefit of sex is that it facilitates the clearance of harmful mutations, which would accumulate more rapidly in the absence of recombination. The extent to which ineffective purifying selection and mutation accumulation are direct consequences of asexuality and whether the accelerated buildup of harmful mutations in asexuals can occur rapidly enough to maintain sex within natural populations, however, remain as open questions. We addressed key components of these questions by estimating the rate of mutation accumulation in the mitochondrial genomes of multiple sexual and asexual representatives of Potamopyrgus antipodarum, a New Zealand snail characterized by mixed sexual/asexual populations. We found that increased mutation accumulation is associated with asexuality and occurs rapidly enough to be detected in recently derived asexual lineages of P. antipodarum. Our results demonstrate that increased mutation accumulation in asexuals can differentially affect coexisting and ecologically similar sexual and asexual lineages. The accelerated rate of mutation accumulation observed in asexual P. antipodarum provides some of the most direct evidence to date for a link between asexuality and mutation accumulation and implies that mutational buildup could be rapid enough to contribute to the short-term evolutionary mechanisms that favor sexual reproduction.
Keywords: sex, asexual, parthenogenetic, Muller's ratchet, mtDNA, Hill–Robertson, Potamopyrgus antipodarum
Introduction
Why sexual reproduction and recombination are so common in nature has puzzled biologists for over a century (e.g., Darwin 1862; Weismann 1889; Morgan 1914; Fisher 1930; reviewed in Meirmans 2009). The modern effort to understand the maintenance of sex in natural populations was largely inspired by Williams (1966, 1975), who emphasized the need to identify individual-level advantages of sex, and Maynard Smith (1971, 1978), who pointed out that the production of sons by sexual females creates a 2-fold cost of sex that should culminate in its rapid elimination. The predominance of sexual reproduction in spite of this “cost of males” means that sex must confer major advantages that have yet to be fully understood.
Theorized benefits of sex typically fall into one of two main classes (Kondrashov 1993): ecological advantages, where the generation of novel variants is favored by selection in a rapidly changing environment (e.g., the Red Queen; Jaenike 1978; Hamilton 1980), and mutational advantages, where sex aids in the clearance of deleterious mutations (e.g., Muller's ratchet; Muller 1964). The extent that either class of mechanism can explain the maintenance of sex in natural populations, however, remains unclear (Lynch and Gabriel 1990; Otto and Nuismer 2004; Peters and Lively 2007; Neiman et al. 2009; Otto 2009).
The power of the Red Queen, for example, is limited by the fact that it favors rare genotypes rather than sexually produced offspring, per se, meaning that its ability to maintain sex is greatly diminished when asexual competitors are genetically diverse enough for there to always be rare asexual genotypes (Lively and Howard 1994; Lythgoe 2000). Although mutation accumulation should inevitably cause asexual lineage extinction (Muller 1964; Lynch and Gabriel 1990; Charlesworth et al. 1993), it can only maintain sex if fitness loss due to mutational buildup is rapid enough to drive asexual lineages extinct before the sexuals are competitively excluded. Other models show that different selective mechanisms may interact synergistically to maintain sex (e.g., Howard and Lively 1994), but they depend on those mechanisms occurring simultaneously in natural populations.
Potamopyrgus antipodarum is a New Zealand freshwater snail which is characterized by mixed populations of obligately sexual and obligately asexual individuals and which is subject to infection by a sterilizing trematode parasite Microphallus sp. (Lively 1987). Several lines of evidence suggest that Red Queen dynamics in the P. antipodarum–Microphallus system are likely to play a central role in the persistence of sexual P. antipodarum (Jokela et al. 2009; King et al. 2009). However, the high within-population genetic diversity of asexual P. antipodarum (Dybdahl and Lively 1995; Jokela et al. 2003; Neiman and Lively 2004) means that parasite pressure alone is unlikely to explain why sex persists.
Here, we estimated the rate of accumulation of potentially harmful substitutions at nonsynonymous coding sites and functional noncoding sites in the mitochondrial genomes of a diverse array of sexual and asexual P. antipodarum as a means of evaluating whether asexual lineages experience increased mutation accumulation relative to sexual competitors. We also used these data to determine whether mutation accumulation occurs rapidly enough to be detected in asexual lineages that coexist with sexual P. antipodarum—that is, before sex is extirpated from populations.
Mitochondrial DNA (mtDNA) evolves rapidly enough in animals to serve as a marker of reduced efficacy of selection at an intraspecific scale (e.g., Paland and Lynch 2006; Johnson and Howard 2007). Moreover, though mitochondrial genomes are (usually) asexually transmitted, they are nevertheless affected by the transition to asexuality because they are thrust into complete linkage with the nuclear genome (Normark and Moran 2000). Mitochondrial–nuclear linkage is relevant in this context because tight linkage is expected to reduce effective population size and, hence, the ability of purifying selection to clear deleterious mutations (Hill and Robertson 1966; Ohta and Kimura 1971; Birky and Walsh 1988). This leads to the prediction that harmful substitutions should accumulate more rapidly in the mtDNA of asexuals because of selective interference from the linked nuclear genome (Normark and Moran 2000).
Comparisons of patterns of evolution at synonymous versus nonsynonymous sites in protein-coding DNA are frequently used to infer mutation accumulation (e.g., Charlesworth and Wright 2001; Funk et al. 2001; Glémin 2007). A common approach is to estimate and compare the ratio (dN/dS) of the number of nonsynonymous substitutions per nonsynonymous site (dN) to the number of synonymous substitutions per synonymous site (dS) among the lineages of interest. Because nonsynonymous mutations change amino acid sequence, they are often slightly deleterious (Nachman 1998), and it is these slightly deleterious mutations that are expected to accumulate at increasing rates in asexual lineages (Gabriel et al. 1993). In contrast, synonymous substitutions (which do not affect protein sequence and are presumed to have much smaller, if any, harmful effects) accumulate at rates much closer to the rate at which mutations occur.
This means that estimating dN/dS can provide a measure of the extent to which harmful mutations are accumulating while controlling for the underlying mutation rate of the lineage. In particular, higher values of dN/dS at loci that are presumed not to be the direct targets of positive selection imply a higher rate of accumulation of deleterious mutations. Based on this logic (Li et al. 1985), dN/dS estimates have been used successfully to detect evidence for an increased rate of fixation of harmful nonsynonymous mutations in the mitochondrial genomes of asexual lineages (e.g., Paland and Lynch 2006; Barraclough et al. 2007). We used this approach in our study of mutation accumulation in the 13 protein-coding genes in the mitochondrial genomes of sexual versus asexual P. antipodarum.
One cannot so easily discriminate between relatively “neutral” versus relatively “deleterious” mutations within noncoding functional DNA sequence. Mutations in noncoding functional loci such as transfer RNAs (tRNAs) and ribosomal RNAs (rRNAs) that lower fitness, however, should still be subject to removal by natural selection (following Moran 1996; Lynch 2007). Given the inverse relationship between the efficacy of selection and tightness of genetic linkage and because most noncoding functional mtDNA mutations are probably deleterious (Lynch 2007; also see fig. 2), asexual lineages should experience more rapid divergence in these loci than sexual lineages. These predictions are supported by evidence for increased rRNA (Moran 1996; Woolfit and Bromham 2003) and tRNA (Lynch 1997) substitution rates in genomes that experience relatively small effective population size. Thus, we used the rate of evolution of mitochondrial tRNAs and rRNAs to estimate and compare mutation accumulation in the noncoding functional mtDNA of sexual versus asexual P. antipodarum.
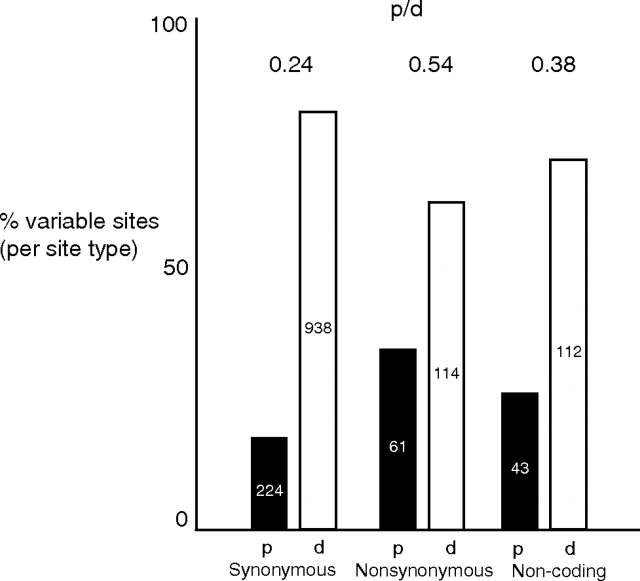
FIG. 2.
Percent polymorphic versus divergent sites across all variable sites in the mitochondrial genome of the four sexual P. antipodarum lineages; fixed differences (divergent sites) are relative to Potamopyrgus estuarinus. For each site type, polymorphism (p) and divergence (d) are indicated by black and white bars, respectively; numbers inside each bar indicate the total number of each type of site. There was a significantly higher ratio of polymorphism in nonsynonymous/synonymous sites than divergence in nonsynonymous/synonymous sites (Fisher's exact test, P < 0.0001). There was a significantly higher ratio of polymorphism/divergence in nonsynonymous versus synonymous sites (Fisher's exact test, P < 0.0001) and in functional noncoding versus synonymous sites (Fisher's exact test, P < 0.0001) but no significant difference in the ratio of polymorphisms to divergence in nonsynonymous sites versus noncoding sites (Fisher's exact test, P = 0.192).
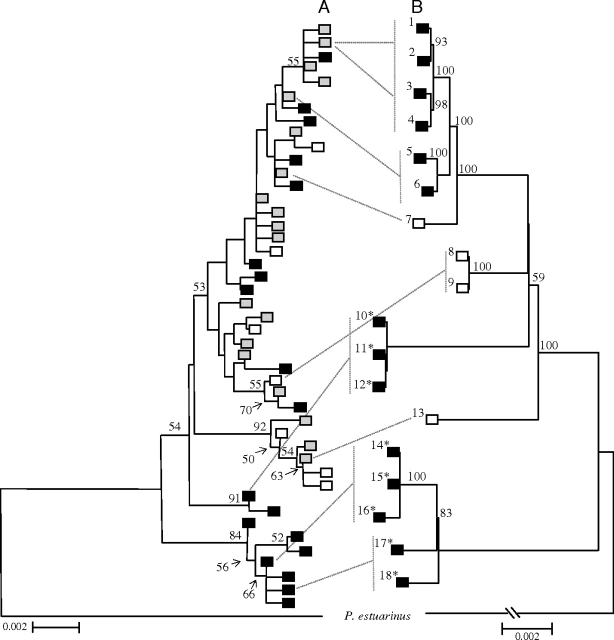
We used previously established phylogeographic data (Neiman and Lively 2004) and evidence for the bimodal distribution of asexual lineage age in P. antipodarum (Neiman et al. 2005) to select 18 P. antipodarum lineages for mitochondrial genome sequencing that captured most of the genetic diversity and asexual lineage age variation within the species (fig. 1). The wide variation in asexual lineage age allowed us to assess whether increased mutation accumulation was evident in recently derived asexual lineages and whether old asexual lineages were characterized by especially high mutation loads.
FIG. 1.
(A) The maximum likelihood phylogeny determined from mitochondrial cytochrome b DNA sequence (“cytochrome b tree”; adapted from Neiman et al. 2005) used to assign lineage sexuality and age for the mitochondrial genomic data (B). Maximum likelihood bootstrap values >50% are given (500 replicates). The square at the end of each branch indicates whether the haplotype is represented by only sexual individuals (white), both sexual and asexual individuals (gray), or only asexual individuals (black). The two entirely asexual clades at the bottom of the cytochrome b tree appear to have been diverging from sexuals for at least 500 000 years (“old asexual clades” and “old asexual lineages”); all other asexual lineages are <40 000 years old and likely to be considerably younger (“young asexual lineages”). The lines from tree A to tree B indicate where the snails used for the genome trees were represented on the cytochrome b tree. Asexual individuals from “mixed” sexual/asexual cytochrome b haplotypes (haplotypes 1 and 18, Neiman and Lively 2004) were used to represent young asexual lineages for the genomic data set. Because asexual lineages 1–4 and 5 and 6 do not share a most recent sexual common ancestor, they represent two independently derived sets of young asexual lineages. The cytochrome b tree was generated from an extensive species-wide sample (Neiman et al. 2005), allowing us to assign asexual lineage ages to the 14 asexual and 4 sexual Potamopyrgus antipodarum lineages represented in the genome tree. (B) Maximum likelihood tree constructed from the entire protein-coding (B, ∼11 kb) mitochondrial genome DNA sequence of 14 asexual (black) and 4 sexual (white) P. antipodarum lineages. “*” Indicates old asexual lineages; all other asexual lineages are young. The numbers to the left of the boxes on each tree represent lineage identity across the genomic trees. Bootstrap values >50% are given. Branch lengths from a maximum likelihood baseml analysis with no clock constraint were used to provide a representation of branch length before correction for differences in evolutionary rate among different branch types due to sexuality or branch position. A tree generated with ∼4 kb of functional noncoding mtDNA sequence is topologically identical and is not shown here.
Materials and Methods
Cloning the Initial Genomic mtDNA Sequence
Total DNA was isolated with the Qiagen DNeasy Plant Kit following the standard miniprep protocol. The complete mtDNA of a single asexual female P. antipodarum was amplified from total DNA in four overlapping fragments. Primer pair 1a (F-ACTAGGATCCGTTGATTTAGCT; R-GCTGGTTTACATAGGATCCAT) amplified a 7.6-kb fragment; primer pair 2a (F-TACAACTGCAGCAGAAGATAAC; R-GCACTATACAGCACACGTAGA) amplified a 7.6-kb fragment; primer pair 3a (F-GCTGGTTTACATAGGATCCAT; R-CATACAAAGCTTCCATCACAGT) amplified a 2.3-kb fragment; and primer pair 4a (F-GGAGTGAACGGAAATCA; R-AGACGTAAAGATGGCAAAG) amplified a 3.3-kb fragment. Reactions contained 3.75 U Expand Long Polymerase (Roche, Indianapolis, IN), 1× Expand buffer 3, 0.5 mM each dNTP, 0.3 μM each primer, and 50 ng genomic DNA. Following denaturation of samples at 94 °C for 2 min, amplification was performed in two stages: 10 cycles of 94 °C for 10 s, 51 °C for 30 s, and 68 °C for 12 min, and 25 cycles of 94 °C for 15 s, 51 °C for 30 s, and 68 °C for 12 min with a 20-s extension in the polymerization step for each additional cycle. Reactions were then incubated at 68 °C for 7 min. PCR fragments were gel purified using QIAquick Gel Extraction Kit (Qiagen, Inc., Valencia, CA), inserted into pCR2.1 XL TOPO (Invitrogen Corp., Carlsbad, CA), and transformed into TOP10 cells (Invitrogen Corp.). Both strands of the inserts were sequenced using vector primers and by sequence walking.
Amplification of other mtDNAs
Complete mtDNAs from four P. antipodarum individuals (both sexual and asexual females) were amplified from total DNA in six overlapping fragments. Primer pair 1b (F-ACTAGGATCCGTTGATTTAGCT; R-CTCTTGAGTATGCTGAGTACA) amplified a 3.2-kb fragment; primer pair 2b (F-CTTTGCCAGGGAGAACT; R-CAAGCCTCAGAGGTTGA) amplified a 4.0-kb fragment; primer pair 3b (F-CCATTCGACTTTGCAGAG; R-GCAGCCAGAACTATTGAC) amplified a 3.2-kb fragment; primer pair 4b (F-CAAGCTAGGTGGTGTTATTG; R-CCTCTCAGAAAGTAGCAGA) amplified a 3.6-kb fragment; primer pair 5b (F-GCATTCTTCTACTCTTGTCA; R-GCCTCAAAGTGTTAAAGC) amplified a 3.6-kb fragment; and primer pair 6b (F-GGAGTGAACGGAAATCA; R-AGACGTAAAGATGGCAAAG) amplified a 3.3-kb fragment. Reactions contained 1 U Phusion Polymerase (New England BioLabs, Ipswich, MA), 1× HF buffer, 0.2 mM each dNTP, 0.5 μM each primer, and 50 ng genomic DNA. Following denaturation of samples at 98 °C for 30 s, amplification was performed for 35 cycles of 98 °C for 20 s, 60 °C for 30 s, and 72 °C for 2 min 30 s. Reactions were then incubated at 72 °C for 10 min.
Complete mtDNAs from 14 individual P. antipodarum (both sexual and asexual females) were amplified from total DNA in four overlapping fragments. Primer pair 1c (F-GAGGTAGGAGACTGTAGT; R-GAGTCCTAAGCCCAATGCA) amplified a 4.3-kb fragment; primer pair 2c (F-GCTAGTATGAATGGTTTGACG; R-CACTAGAGCTGAAACTGGT) amplified a 5.8-kb fragment; primer pair 3c (F-TCAGCTTGTGGATCTGA; R-GCCTAATCAGTATGAGGAAG) amplified a 3.5-kb fragment; and primer pair 4c (F-GGAGTGAACGGAAATCA; R-CTCTTGAGTATGCTGAGTACA) amplified a 5.1-kb fragment. Reaction reagents and conditions were as described above, except primer pair 1c and 2c used 64 °C for annealing and 2 min 30 s for extension.
The complete mtDNA extracted from a single Potamopyrgus estuarinus individual was amplified from total DNA in three overlapping fragments. Primer pair 1d (F-GAGGTAGGAGACTGTAGT; R-GAGTCCTAAGCCCAATGCA) amplified a 9.2-kb fragment. Primer pairs 3c and 4c were as described above. Reaction reagents and conditions were as described above, except primer pair 1d used 58 °C for annealing and 4 min 30 s for extension.
DNA Sequencing
PCR fragments were purified by QIAquick PCR Purification (Qiagen, Inc.). Both strands were directly sequenced using primers (as described above) and, where necessary, by sequence walking. Sequencing was performed on an ABI Prism 3730 (AB Applied Biosystems, Foster City, CA), and the profiles were analyzed and edited with ChromasPro (Technelysium Pty Ltd, Tewantin, Australia).
Statistical and Phylogenetic Analyses
DNA sequences from the 19 Potamopyrgus genomes were aligned using Clustal within Mega 4.0 and corrected manually. We then used a contingency table-based variation of the McDonald–Kreitman test (MK test) and Fisher's exact test to test the baseline assumption that most nonsynonymous mutations are deleterious (e.g., Paland and Lynch 2006). Specifically, we evaluated whether the ratio of nonsynonymous to synonymous polymorphisms apparent within sexual P. antipodarum was higher than the ratio of non-synonymous to synonymous substitutions separating P. antipodarum from P. estuarinus. We also used a modified MK approach to compare the ratio of functional noncoding polymorphism/divergence in sexual P. antipodarum to the ratio of synonymous polymorphism/divergence (e.g., Andolfatto 2005). We used only sexual P. antipodarum for these comparisons in order to assess whether nonsynonymous mutations and mutations in noncoding functional regions are generally deleterious in lineages where purifying selection is expected to be effective (at least relative to asexuals).
To generate the phylogenies used in this study, we used the “FindModel” application of Modeltest 3.7 (Posada and Crandall 1998) to select the model of nucleotide substitution that best fit each of the two (coding and functional noncoding) P. antipodarum data sets. For both data sets, the best-fitting model was general time reversible + gamma. We used this model to generate a maximum likelihood tree with the DNAml function of PHYLIP 3.68 (Felsenstein 1989) for each data set. We then used Seqboot (500 replicates), DNAml, and Consense (as implemented within PHYLIP 3.68) to perform a maximum likelihood bootstrapping analysis on each tree.
Next, we used PAML (Yang 2007) to determine whether nonsynonymous and functional noncoding substitutions accumulated at different rates in sexual versus asexual lineages (following Paland and Lynch 2006; Johnson and Howard 2007). This software works by calculating the goodness of fit of a particular, user-specified model of evolution to a phylogenetic data set. Here, we used PAML to compare the fit of simple models where the entire P. antipodarum phylogeny experienced the same rate of evolution to more complex models where, for example, sexual and asexual lineages were allowed to evolve at different rates. Following Paland and Lynch (2006), the sexual outgroup P. estuarinus was excluded from the trees used in these analyses because it cannot be classified as either a sexual or asexual lineage of P. antipodarum.
For the protein-coding sequence, we used the “codeml” implementation of PAML to apply a one-ratio maximum likelihood model to the data that estimated a single dN/dS value for the (unrooted) P. antipodarum phylogeny. We then ran a two-ratio model where sexual and asexual lineages were allowed to have different dN/dS (sex vs. asex model) and used a likelihood ratio test to assess whether the two-ratio versus one-ratio model provided a better fit to the data. This test evaluated whether asexual lineages had a different rate of mutation accumulation relative to sexual lineages. A higher estimated dN/dS value for the asexual lineages and a significantly better fit of the two-ratio model to the data would indicate that asexual P. antipodarum experience accelerated accumulation of harmful mutations.
Because the time lag between the appearance of a new mutation and its clearance via purifying selection means that mildly deleterious mutations are expected to be at higher frequency in terminal versus internal branches (Paland and Lynch 2006), apparent differences between sexual and asexual dN/dS could be confounded by differences in branch length between sexual and asexual lineages. We dealt with this issue by using PAML to evaluate the fit of two additional models to the data: where terminal versus internal branches were allowed to have different dN/dS (terminal vs. internal model) and where terminal sexual, terminal asexual, internal sexual, and internal asexual branches were allowed to have different dN/dS (four-ratio model). We then used likelihood ratio tests to compare the fit of both of the two-ratio models (sex vs. asex and terminal vs. internal) to the one-ratio model and to compare the four-ratio model to both two-ratio models and to the one-ratio model.
We used an identical approach for the functional noncoding sequence but with a noncodon-based approach, baseml. Here, we used likelihood ratio tests to compare the fit of a one-rate model to a two-rate model where sexuals and asexuals were allowed to evolve at different rates (sex vs. asex model), a two-rate model where terminal and internal branches could evolve at different rates (terminal vs. internal model), and a four-ratio model where terminal versus internal sexual branches and terminal versus internal asexual branches were all allowed to evolve at different rates (four-rate model). Because these analyses require the implementation of a molecular clock at various scales (global for one-rate models, local for >1 rate models), they also require the use of rooted trees.
Finally, we used one-ratio versus two-ratio (sex vs. asex) tests and one-rate versus two-rate (sex vs. asex) likelihood ratio tests to assess whether the overall finding of significantly higher rates of mutation accumulation in asexual lineages held up when only young asexual lineages were included in the phylogeny. We conducted these comparisons in order to evaluate whether mutation accumulation occurs rapidly enough to be detected in very recently derived asexual lineages.
Results and Discussion
We sequenced the mitochondrial genomes of four sexual individuals, an individual from each of six “young” asexual lineages that are known to be of recent derivation from sexual ancestors (and of at least two independent transitions to asexuality; fig. 1), an individual from each of eight “old” asexual lineages from two independently derived asexual clades, and one P. estuarinus individual. The data set comprised 11 196 bp of coding mtDNA from 13 protein-coding genes and 3,896 bp of noncoding functional DNA (22 tRNAs and 2 rRNA loci). All 19 mitochondrial genome sequences were deposited in GenBank (accession nos GQ996415–GQ996433).
A contingency table-based variation of the MK test showed that the ratio of nonsynonymous/synonymous polymorphisms within sexual P. antipodarum (0.27) was significantly higher than the ratio of nonsynonymous/synonymous substitutions separating sexual P. antipodarum from P. estuarinus (0.12; see fig. 2). This result indicates that when nonsynonymous mtDNA mutations occur in P. antipodarum, they are less likely to proceed to fixation and, hence, contribute less to interspecific divergence (Hasegawa et al. 1998; Meiklejohn et al. 2007; Marshall et al. 2008; Rand 2008). The implication is that these mutations are usually deleterious and are, thus, removed by natural selection. A similar conclusion follows from the significantly higher dN/dS in terminal versus internal branches (table 1), suggesting that nonsynonymous mutations in terminal branches are often cleared by purifying selection before they contribute to divergence in internal branches (Paland and Lynch 2006).
Table 1.
Summary of PAML Analyses of Comparisons of dN/dS Ratios in Coding mtDNA and the Rate of Evolution of Functional Noncoding mtDNA in Sexual versus Asexual Potamopyrgus antipodarum.
| Model | No. Free Parameters | lnL | χ2 (df) | dN/dS or Ratea |
| Coding | ||||
| One ratio | 1 | −19680.08 | NA | 0.101 |
| Two ratio (sex vs. asex) | 2 | −19674.046 | vs. one ratio: 12.068 (1)*** | Sex: 0.084 Asex: 0.161 |
| Two ratio (T, I)b | 2 | −19675.866 | vs. one ratio: 8.428 (1)** | T: 0.143 I: 0.0851 |
| Four ratio (SexT, AsexT, SexI, AsexI) | 4 | −19671.78 | vs. one ratio: 16.6 (3)*** | SexT: 0.103 AsexT: 0.204 SexI:0.079 |
| vs. two ratio (sex/asex): 4.532 (2) | AsexI: 0.123 | |||
| vs. two ratio (T vs. I): 8.172 (2)** | ||||
| One ratio (pruned)c | 1 | −16807.664 | NA | 0.098 |
| Two ratio (pruned, sex vs. asex) | 2 | −16805.709 | vs. one ratio: 3.91 (1)* | Sex: 0.086 Young asex: 0.162 |
| Functional noncoding | ||||
| One rate | 1 | −6114.496 | NA | NA |
| Two rate (sex vs. asex) | 2 | −6112.204 | vs. one rate: 4.584 (1)* | Sex: 1.00 Asex: 1.598 |
| Two rate (T vs. I) | 2 | −6113.924 | vs. one rate: 2.144 (1) | T: 1.283 I: 1.00 |
| Four rate (SexT, AsexT, SexI, AsexI) | 4 | −6109.782 | vs. one rate: 9.428 (3)** | SexT: 1.526 AsexT: 5.973 SexI: 1.00 |
| vs. two rate (sex/asex): 4.844 (2) | AsexI: 1.962 | |||
| vs. two rate (T vs. I): 8.284 (2)** | ||||
| One rate (pruned) | 1 | −5554.967 | NA | NA |
| Two rate (pruned, sex vs. asex) | 2 | −5554.812 | vs. one rate: 0.155 (1) | Sex: 1.00 Asex: 1.367 |
NOTE.—df, degrees of freedom; NA, not applicable.
First branch type entered in the control file automatically set to 1 by baseml; all other rates are estimated relative to this value.
“T” indicates terminal branches and “I” indicates internal branches.
“Pruned” represents analyses from pruned trees containing only sexuals and young asexual lineages.
*Indicates significance at P < 0.05, **Indicates significance at P < 0.025, and ***Indicates significance at P < 0.001.
We used another variation of the MK approach to evaluate whether mutations in functional noncoding mtDNA are deleterious as compared with relatively “neutral” synonymous mutations. We found a significantly higher ratio of functional noncoding polymorphism/divergence (0.38) than synonymous polymorphism/divergence (0.24; see fig. 2) in sexual P. antipodarum versus P. estuarinus. The fact that these noncoding mutations are less likely to contribute to divergence between species than synonymous mutations suggests that they are generally deleterious. The somewhat higher rate of evolution of functional noncoding mtDNA in terminal versus internal branches table 1) is also consistent with this conclusion. Other studies of substitution and polymorphism in both coding and noncoding functional mtDNA have reached similar conclusions (reviewed in Lynch 2007; Rand 2008), pointing toward the likelihood that mtDNA evolution in P. antipodarum is generally governed by the balance between purifying selection and genetic drift.
The PAML analyses indicated that asexual lineages experienced a substantially higher rate of accumulation of deleterious nonsynonymous substitutions than sexual lineages, as evidenced by the significantly better fit of the two-ratio sex versus asex model to the protein-coding data and the ∼2× higher point estimate of dN/dS in asexual (0.161) versus sexual lineages (0.084; table 1). These dN/dS values for sexuals versus asexuals are similar to those reported in Paland and Lynch (2006) and Johnson and Howard (2007), both in terms of the value of dN/dS and the magnitude of discrepancy between sexual and asexual lineages. The four-ratio model (contrasting sexual, asexual, internal, and external branches) provided a significantly better fit than the two-ratio model contrasting terminal versus internal branches but not the two-ratio model contrasting sexual versus asexual branches. This, and the fact that asexual branches had higher dN/dS regardless of whether they were internal or external, demonstrates that the sex/asex differences in dN/dS are not due to discrepancies in branch length.
We obtained a result similar to the outcome of the analyses described above using a pruned tree containing only young asexual and sexual lineages. Here, we found that the two-ratio model where sexual and asexual branches could have different dN/dS was a significantly better fit to the data than the one-ratio model and that the young asexual dN/dS was ∼2× higher than that of the sexuals (table 1). This result indicates that young asexual lineages have experienced a discernable increase in the rate of accumulation of deleterious nonsynonymous substitutions when compared with close sexual relatives.
We addressed the possibility that high dN/dS in asexuals could be due to low dS rather than elevated dN by comparing a tree with branch lengths generated only by variation at synonymous sites to a tree with branch lengths determined only by variation at nonsynonymous sites. This comparison shows that although dS varies among lineages, it is not systematically decreased in asexuals relative to sexuals. By contrast, the tree based upon only nonsynonymous sites showed that all the asexual lineages had longer branches than their closest sexual relative. This difference indicates that the increased dN/dS in asexual P. antipodarum is due to increased dN in asexual relative to sexual lineages (fig. 3), rather than variation in the underlying mutation rate (Sloan et al. 2009) or decreased mutation rate in asexual lineages (Schön et al. 1998).
FIG. 3.
A–C) A comparison of trees with (A) branch lengths determined by the number of synonymous substitutions/synonymous site (dS), (B) the number of nonsynonymous substitutions/nonsynonymous site (dN), and (C), the number of functional noncoding substitutions/functional noncoding site (noncoding). We used the dS and dN branch lengths generated by the best-fitting (four ratio) codeml model for the coding mtDNA. Because the best-fitting baseml model, the four-rate model, requires the implementation of a molecular clock, all branches are constrained to end at the same point. Thus, in order to provide a representation of branch lengths for the functional noncoding mtDNA that could be compared with the dS and dN trees, we used branch lengths from a baseml analysis applied to the noncoding data set in the absence of a clock. Although these trees are shown unrooted, rooted trees are required for analyses involving a molecular clock and, accordingly, were used for all baseml analyses of evolutionary rate. Box colors and numbering follow figure 1B. Branch lengths are shown at the same scale of 0.01 substitutions/site, as indicated in the scale bar in the middle of the figure.
In noncoding functional sequence (e.g., tRNAs and rRNAs), any mutations that lower fitness can be removed from a lineage by purifying selection. Therefore, we used comparisons of the substitution rate in these loci as an additional assay of the efficacy of selection, where accelerated rates are assumed to reflect less effective purifying selection (e.g., Lynch 2007). As above, we compared the fit of a one-rate model to two types of two-rate models (sex vs. asex, terminal vs. internal) and a four-rate model to the one-rate and two-rate models. These analyses suggested that asexual P. antipodarum experienced a two to four times higher substitution rate in their functional noncoding mtDNA than sexual counterparts, even when branch length was taken into account (table 1). The rate acceleration experienced in the functional noncoding mtDNA of asexual P. antipodarum is evident in a tree generated using only functional noncoding sites, where asexual lineages generally have longer branches than their closest sexual relatives (fig. 3).
As with the coding sequence, we also ran PAML analyses with pruned trees containing only young asexual and sexual lineages. Although the direction of differences between sexuals and asexuals was similar to the analyses using the full data set in that asexual branches had higher rates of substitution than sexual branches, the two-rate sex versus asex model did not provide a significantly better fit to the data than the single-rate model. This difference in outcome between the PAML analyses with coding versus noncoding mtDNA is, perhaps, not surprising given that there was a somewhat higher rate of fixation of functional noncoding mutations than nonsynonymous mutations (fig. 2), suggesting that, on average, mutations in noncoding functional mtDNA are subject to weaker purifying selection than nonsynonymous mutations.
Although variation in the rate of accumulation of apparently harmful mutations between asexual and sexual lineages is likely due to differences in the efficacy of selection, we cannot exclude a role for other factors associated with asexual P. antipodarum. For example, the triploidy of asexuals may influence the rate of mutation accumulation within asexual lineages, either directly by affecting the rate and spectrum of mutation or, indirectly, by altering gene expression or phenotype (reviewed in Otto 2007). We also cannot rule out the possibility that asexual P. antipodarum experience less effective and/or relaxed purifying selection due to factors such as smaller census population size or lowered selective constraint. However, sexual and young asexual lineages of P. antipodarum coexist within many lakes (Lively 1987, 1992) and in habitats within lakes (Jokela and Lively 1995; Fox et al. 1996) and often make up a sizeable fraction of these sympatric populations (Lively and Jokela 2002; Neiman et al. 2005). It is therefore unlikely that asexual P. antipodarum experience a systematically lower census population size.
To estimate how these accelerated substitution rates translate into increased nonsynonymous substitutions within particular classes of P. antipodarum lineages, we used DnaSP v5.10 (Librado and Rozas 2009) to estimate the number of nonsynonymous substitutions experienced by each of the 18 P. antipodarum lineages relative to P. estuarinus. Across the 13 protein-coding genes (∼8330 nonsynonymous sites) in the P. antipodarum mitochondrial genome, an average of 65.5 (±5.74 standard deviation [SD]) nonsynonymous substitutions separated sexual lineages of P. antipodarum from P. estuarinus. Asexual lineages accumulated about five additional nonsynonymous substitutions during this divergence (mean = 71.25 ± 4.67 SD). When young versus old asexual lineages were considered separately, old asexual lineages (mean = 72.81 ± 5.54 SD) had accumulated about four more nonsynonymous substitutions than young asexual lineages (mean = 69.0 ± 2.13 SD). The elevated mutation accumulation in asexual taxa may represent a substantial mutation burden, especially if this phenomenon also occurs in the nuclear genome. If we assume that the nuclear genome has approximately 2000 times more genes than the mitochondrial genome, even accounting for the nuclear genome having roughly one-tenth the mutation rate (e.g., Haag-Liutard et al. 2008), the rate acceleration we observed would burden asexual nuclear genomes with hundreds of nonsynonymous substitutions. Indeed, mutation accumulation is often cited as one of the most likely reasons for why most asexual lineages seem to be short lived and for why the success of ancient asexual lineages such as the bdelloid rotifers is at least superficially an evolutionary paradox (e.g., Maynard Smith 1986). Our data indicate that this apparent paradox can be extended to much shorter lived asexual taxa, including asexual lineages within species.
Implications for the Maintenance of Sex
All the “young” asexual P. antipodarum lineages that we sampled (lineages no. 1–6, fig. 1B) share cytochrome b haplotypes with sexual individuals (haplotypes 1 and 18; Neiman and Lively 2004), meaning that they are likely to have been derived from sexual ancestors within several thousand years (Dybdahl and Lively 1995; Neiman et al. 2005). These lineages also are often found coexisting with sexual P. antipodarum (Neiman and Lively 2004; Neiman et al. 2005). The accelerated rate of accumulation of nonsynonymous substitutions in these recently derived asexual lineages relative to sexuals demonstrates that this process can occur rapidly enough to be detectable before the sexuals have been competitively excluded. This may be especially likely in a system like P. antipodarum where Red Queen dynamics are likely to be operating because they should prolong the persistence of sexuals in mixed populations (Howard and Lively 1994; Neiman et al. 2009).
Elevated rates of mutation accumulation in recently derived asexual lineages have also been reported in Daphnia (Paland and Lynch 2006) and Campeloma (Johnson and Howard 2007). However, because the geographic distributions of sexual versus asexual Daphnia and Campeloma are almost entirely nonoverlapping (Paland et al. 2005; Johnson 2006), it is not clear that accelerated mutation accumulation can be relevant to the maintenance of sex or even that sex requires an explanation in those systems. Our study involves a system where sexuals and asexuals are frequently sympatric and ecologically similar, meaning that the accelerated mutation accumulation that we documented in asexual lineages is more likely to be a direct consequence of the absence of sex. The fact that there is increased mutation accumulation in recently derived asexual lineages in a system where sexuals and asexuals frequently coexist demonstrates that these mutational processes could be rapid enough to contribute to the maintenance of sex in natural populations.
Several lines of evidence suggest that the mutation accumulation observed in asexual P. antipodarum could have phenotypic consequences that might influence the persistence of sex in this system. First, mutation accumulation experiments in genetic model systems show that accelerated mutational buildup resulting from a decreased efficacy of purifying selection can cause marked declines in fitness in just a few dozen to a few hundred generations (e.g., Vassilieva et al. 2000; Denver et al. 2004). Second, the results of our MK tests strongly suggest that nonsynonymous and functional noncoding mutations are, on average, deleterious. Third, empirical studies have demonstrated negative phenotypic effects of mtDNA mutations (reviewed in Ballard and Rand 2005). Finally, the reduced efficacy of selection experienced by mtDNA in asexual P. antipodarum should—by extension—also affect the nuclear genome, perhaps, even more severely because the nuclear genome experiences the direct loss of physical recombination in a newly asexual lineage (as predicted by Normark and Moran 2000). Thus, the rapid increase in mutation accumulation in mtDNA may therefore reflect only a small fraction of the increased mutational load experienced by asexual lineages in P. antipodarum.
A role for mutation accumulation in the maintenance of sex requires that the mutations that build up at a higher rate in asexuals have harmful phenotypic consequences. Although earlier studies comparing morphology and life history (Jokela et al. 1997) and response to starvation (Lively et al. 1998) in sexual versus asexual P. antipodarum found no persistent effect of mating system, these earlier studies were limited to snails from Lake Alexandrina, where the asexual lineages are known to be recently derived (Dybdahl and Lively 1995; Neiman et al. 2005). Thus, the evidence for wide variation in asexual lineage age in P. antipodarum (Neiman et al. 2005) sets the stage for comparisons of traits associated with fitness in sexual versus asexual and young versus old asexual lineages. If mutation accumulation is relevant to the maintenance of sex, we would expect that representatives of old asexual lineages might suffer in comparison to younger asexual lineages (and sexuals) with regard to important traits such as mitochondrial function and the rate of offspring production and population growth. Because harmful effects of mutation accumulation might be heightened under stressful conditions (e.g., Vassilieva et al. 2000; Young et al. 2009), experiments utilizing some form of stressor (e.g., disease, starvation, heat, cold) might be particularly useful. Studies evaluating these hypotheses would provide an important step forward in evaluating the extent to which mutation accumulation might contribute to the predominance and persistence of sex in natural populations.
Acknowledgments
We would like to thank J. Jokela, A. Kay, B. Koskella, C. Lively, M. Mayry, and K. Theisen for snail collection and maintenance, J. Jokela, C. Lively, and T. Schwander for helpful discussions, K. Theisen for DNA extraction, and D. Sloan for help with tree drawing. We are grateful to A. F. Agrawal, J. Busch, J. Hey, J. Jokela, C. Lively, E. Savelkoul, D. Sloan, K. Sterner, N. Ting, and anonymous reviewers for comments on earlier versions of the manuscript. This work was supported by the College of Liberal Arts and Sciences at the University of Iowa and the Carver Trust to M.N.
References
- Andolfatto P. Adaptive evolution of non-coding DNA in Drosophila. Nature. 2005;437:1149–1952. doi: 10.1038/nature04107. [DOI] [PubMed] [Google Scholar]
- Ballard JWO, Rand DM. The population biology of mitochondrial DNA and its phylogenetic interpretations. Annu Rev Ecol Syst. 2005;36:621–642. [Google Scholar]
- Barraclough TG, Fontaneto D, Ricci C, Herniou EA. Evidence for inefficient selection against deleterious mutations in cytochrome oxidase I of asexual bdelloid rotifers. Mol Biol Evol. 2007;24:1952–1962. doi: 10.1093/molbev/msm123. [DOI] [PubMed] [Google Scholar]
- Birky B, Walsh JB. Effects of linkage on rates of molecular evolution. Proc Natl Acad Sci USA. 1988;85:6414–6418. doi: 10.1073/pnas.85.17.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth D, Morgan MT, Charlesworth B. Mutation accumulation in finite outbreeding and inbreeding populations. Genet Res. 1993;61:39–56. [Google Scholar]
- Charlesworth D, Wright SI. Mating systems and genome evolution. Curr Opin Genet Dev. 2001;11:685–690. doi: 10.1016/s0959-437x(00)00254-9. [DOI] [PubMed] [Google Scholar]
- Darwin CR. On the two forms, or dimorphic condition, in the species of Primula, and on their remarkable sexual relations. J Proc Linn Soc Lond (Botany) 1862;6:77–96. [Google Scholar]
- Denver DR, Morris K, Lynch M, Vassilieva LL, Thomas WK. High mutation rate and predominance of insertions in the Caenorhabditis nuclear genome. Nature. 2004;430:679–682. doi: 10.1038/nature02697. [DOI] [PubMed] [Google Scholar]
- Dybdahl MF, Lively CM. Diverse, endemic and polyphyletic clones in mixed populations of a freshwater snail. J Evol Biol. 1995;8:385–398. [Google Scholar]
- Felsenstein J. PHYLIP - Phylogeny Inference Package (Version 3.2) 1989. Cladistics 5:164–166. [Google Scholar]
- Fisher RA. The genetical theory of natural selection. Oxford: Clarendon Press; 1930. [Google Scholar]
- Fox JA, Dybdahl MF, Jokela J, Lively CM. Genetic structure of coexisting sexual and clonal subpopulations in a freshwater snail (Potamopyrgus antipodarum) Evolution. 1996;50:1541–1548. doi: 10.1111/j.1558-5646.1996.tb03926.x. [DOI] [PubMed] [Google Scholar]
- Funk DJ, Wernegreen JJ, Moran NA. Intraspecific variation in symbiont genomes: bottlenecks and the aphid-Buchnera association. Genetics. 2001;157:477–489. doi: 10.1093/genetics/157.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel W, Lynch M, Bürger R. Muller's ratchet and mutational meltdowns. Evolution. 1993;47:1744–1757. doi: 10.1111/j.1558-5646.1993.tb01266.x. [DOI] [PubMed] [Google Scholar]
- Glémin S. Mating systems and the efficacy of selection at the molecular level. Genetics. 2007;177:905–916. doi: 10.1534/genetics.107.073601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag-Liutard C, Coffey N, Houle D, Lynch M, Charlesworth B, Keightley PD. Direct estimation of the mitochondrial DNA mutation rate in Drosophila melanogaster. PLoS Biol. 2008;6:e204. doi: 10.1371/journal.pbio.0060204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton WD. Sex vs. non-sex vs. parasite. Oikos. 1980;35:282–290. [Google Scholar]
- Hasegawa M, Cao Y, Yang Z. Preponderance of slightly deleterious polymorphism in mitochondrial DNA: nonsynonymous/synonymous rate ratio is much higher within species than between species. Mol Biol Evol. 1998;15:1499–1505. doi: 10.1093/oxfordjournals.molbev.a025877. [DOI] [PubMed] [Google Scholar]
- Hill WG, Robertson A. The effect of linkage on limits to artificial selection. Genet Res. 1966;8:269–294. [PubMed] [Google Scholar]
- Howard RS, Lively CM. Parasitism, mutation accumulation, and the maintenance of sex. Nature. 1994;367:554–557. doi: 10.1038/367554a0. [DOI] [PubMed] [Google Scholar]
- Jaenike J. An hypothesis to account for the maintenance of sex within populations. Evol Theor. 1978;3:191–194. [Google Scholar]
- Johnson SG. Geographic ranges, population structure, and ages of sexual and parthenogenetic snail lineages. Evolution. 2006;60:1417–1426. [PubMed] [Google Scholar]
- Johnson SG, Howard RS. Contrasting patterns of synonymous and nonsynonymous sequence evolution in asexual and sexual freshwater snail lineages. Evolution. 2007;61:2728–2735. doi: 10.1111/j.1558-5646.2007.00233.x. [DOI] [PubMed] [Google Scholar]
- Jokela J, Dybdahl MF, Lively CM. The maintenance of sex, clonal dynamics, and host- parasite coevolution in a mixed population of sexual and asexual snails. Am Nat. 2009;174:S43–S53. doi: 10.1086/599080. [DOI] [PubMed] [Google Scholar]
- Jokela J, Lively CM. Spatial variation in infection by digenetic trematodes in a population of freshwater snails (Potamopyrgus antipodarum) Oecologia. 1995;103:509–517. doi: 10.1007/BF00328690. [DOI] [PubMed] [Google Scholar]
- Jokela J, Lively CM, Dybdahl MF, Fox JA. Evidence for a cost of sex in the freshwater snail Potamopyrgus antipodarum. Ecology. 1997;78:452–460. [Google Scholar]
- Jokela J, Lively CM, Dybdahl MF, Fox JA. Genetic variation in sexual and clonal lineages of a freshwater snail. Biol J Linn Soc. 2003;79:165–181. [Google Scholar]
- King KC, Delph LF, Jokela J, Lively CM. The geographic mosaic of sex and the Red Queen. Curr Biol. 2009;19:1–4. doi: 10.1016/j.cub.2009.06.062. [DOI] [PubMed] [Google Scholar]
- Kondrashov AS. Classification of hypotheses on the advantage of amphimixis. J Hered. 1993;84:372–387. doi: 10.1093/oxfordjournals.jhered.a111358. [DOI] [PubMed] [Google Scholar]
- Li W-H, Wu C-I, Luo C-C. A new method for estimating synonymous and nonsynonymous rates of nucleotide substitution considering the relative likelihood and nucleotide and codon changes. Mol Biol Evol. 1985;2:150–174. doi: 10.1093/oxfordjournals.molbev.a040343. [DOI] [PubMed] [Google Scholar]
- Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- Lively CM. Evidence from a New Zealand snail for the maintenance of sex by parasitism. Nature. 1987;328:519–521. [Google Scholar]
- Lively CM. Parthenogenesis in a freshwater snail: reproductive assurance versus parasitic release. Evolution. 1992;46:907–913. doi: 10.1111/j.1558-5646.1992.tb00608.x. [DOI] [PubMed] [Google Scholar]
- Lively CM, Howard RS. Selection by parasites for clonal diversity and mixed mating. Philos Trans R Soc Lond B Biol Sci. 1994;346:271–281. doi: 10.1098/rstb.1994.0144. [DOI] [PubMed] [Google Scholar]
- Lively CM, Jokela J. Temporal and spatial distributions of parasites and sex in a freshwater snail. Evol Ecol Res. 2002;4:219–226. [Google Scholar]
- Lively CM, Lyons EJ, Peters AD, Jokela J. Environment stress and the maintenance of sex in a freshwater snail. Evolution. 1998;52:1482–11186. doi: 10.1111/j.1558-5646.1998.tb02029.x. [DOI] [PubMed] [Google Scholar]
- Lynch M. Mutation accumulation in nuclear, organelle, and prokaryotic transfer RNA genes. Mol Biol Evol. 1997;14:914–925. doi: 10.1093/oxfordjournals.molbev.a025834. [DOI] [PubMed] [Google Scholar]
- Lynch M. The origins of genome architecture. Sunderland (MA): Sinauer Associates; 2007. [Google Scholar]
- Lynch M, Gabriel W. Mutation load and the survival of small populations. Evolution. 1990;44:1725–1737. doi: 10.1111/j.1558-5646.1990.tb05244.x. [DOI] [PubMed] [Google Scholar]
- Lythgoe KA. The coevolution of parasites with host-acquired immunity and the evolution of sex. Evolution. 2000;54:1142–1156. doi: 10.1111/j.0014-3820.2000.tb00550.x. [DOI] [PubMed] [Google Scholar]
- Marshall HD, Coulson MW, Carr SM. Near neutrality, rate heterogeneity, and linkage govern mitochondrial genome evolution in Atlantic cod (Gadus morhua) and other gadine fish. Mol Biol Evol. 2009;26:579–589. doi: 10.1093/molbev/msn279. [DOI] [PubMed] [Google Scholar]
- Maynard Smith J. The origin and maintenance of sex. In: Williams GC, editor. Group selection. Chicago: Aldine-Atherton; 1971. pp. 164–175. [Google Scholar]
- Maynard Smith J. The evolution of sex. Cambridge: Cambridge University Press; 1978. [Google Scholar]
- Maynard Smith J. Contemplating life without sex. Nature. 1986;324:300–301. doi: 10.1038/324300a0. [DOI] [PubMed] [Google Scholar]
- Meiklejohn CD, Montooth KL, Rand DM. Positive and negative selection on the mitochondrial genome. Trends Genet. 2007;23:259–263. doi: 10.1016/j.tig.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Meirmans S. The evolution of the problem of sex. In: Schön I, Martens K, van Dijk P, editors. Lost sex: The evolutionary biology of parthenogenesis. Amsterdam: Springer; 2009. pp. 21–46. [Google Scholar]
- Moran NA. Accelerated evolution and Muller's ratchet in endosymbiotic bacteria. Proc Natl Acad Sci USA. 1996;93:2873–2878. doi: 10.1073/pnas.93.7.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan TH. Heredity and sex. New York: Columbia University Press; 1914. [Google Scholar]
- Muller HJ. The relation of recombination to mutational advance. Mutat Res. 1964;1:2–9. doi: 10.1016/0027-5107(64)90047-8. [DOI] [PubMed] [Google Scholar]
- Nachman MW. Deleterious mutations in animal mitochondrial DNA. Genetica. 1998;102:61–69. [PubMed] [Google Scholar]
- Neiman M, Jokela J, Lively CM. Variation in asexual lineage age in Potamopyrgus antipodarum, a New Zealand snail. Evolution. 2005;59:1945–1952. [PubMed] [Google Scholar]
- Neiman M, Lively CM. Pleistocene glaciation is implicated in the phylogeographical structure of Potamopyrgus antipodarum, a New Zealand snail. Mol Ecol. 2004;13:3085–3098. doi: 10.1111/j.1365-294X.2004.02292.x. [DOI] [PubMed] [Google Scholar]
- Neiman M, Meirmans S, Meirmans PG. What can asexual lineage age tell us about the maintenance of sex? Ann N Y Acad Sci. 2009;1168:185–200. doi: 10.1111/j.1749-6632.2009.04572.x. [DOI] [PubMed] [Google Scholar]
- Normark BB, Moran NA. Testing for the accumulation of deleterious mutations in asexual eukaryotic genomes using molecular sequences. J Nat Hist. 2000;34:1719–1729. [Google Scholar]
- Ohta T, Kimura M. On the constancy of the evolutionary rate of cistrons. J Mol Evol. 1971;1:18–25. doi: 10.1007/BF01659391. [DOI] [PubMed] [Google Scholar]
- Otto SP. The evolutionary consequences of polyploidy. Cell. 2007;131:452–462. doi: 10.1016/j.cell.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Otto SP. The evolutionary enigma of sex. Am Nat. 2009;174:S1–S14. doi: 10.1086/599084. [DOI] [PubMed] [Google Scholar]
- Otto SP, Nuismer SL. Species interactions and the evolution of sex. Science. 2004;304:1018–1020. doi: 10.1126/science.1094072. [DOI] [PubMed] [Google Scholar]
- Paland S, Colbourne J, Lynch M. Evolutionary history of contagious asexuality in Daphnia pulex. Evolution. 2005;59:800–813. [PubMed] [Google Scholar]
- Paland S, Lynch M. Transitions to asexuality result in excess amino acid substitutions. Science. 2006;311:990–992. doi: 10.1126/science.1118152. [DOI] [PubMed] [Google Scholar]
- Peters AD, Lively CM. Short- and long-term benefits and detriments to recombination under antagonistic coevolution. J Evol Biol. 2007;20:1206–1217. doi: 10.1111/j.1420-9101.2006.01283.x. [DOI] [PubMed] [Google Scholar]
- Posada D, Crandall KA. Modeltest: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Rand DM. Mitigating mutational meltdown in mammalian mitochondria. PLoS Biol. 2008;6:0229–0232. doi: 10.1371/journal.pbio.0060035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schön I, Butlin RK, Griffiths HI, Martens K. Slow molecular evolution in an ancient asexual ostracod. Proc R Soc Lond B Biol Sci. 1998;265:235–242. [Google Scholar]
- Sloan DB, Oxelman B, Rautenberg A, Taylor DR. Phylogenetic analysis of mitochondrial substitution rate variation in the angiosperm tribe Sileneae (Caryophyllaceae) BMC Evol Biol. 2009;9:260. doi: 10.1186/1471-2148-9-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilieva LL, Hook AM, Lynch M. The fitness effects of spontaneous mutations in Caenorhabditis elegans. Evolution. 2000;54:1234–1246. doi: 10.1111/j.0014-3820.2000.tb00557.x. [DOI] [PubMed] [Google Scholar]
- Weismann A. The significance of sexual reproduction in the theory of natural selection. In: Poulton EB, Schönland S, Shipley AE, editors. Essays upon heredity and kindred biological problems. Oxford: Clarendon Press; 1889. pp. 255–1232. [Google Scholar]
- Williams GC. Adaptation and natural selection: a critique of some current evolutionary thought. Princeton (NJ): Princeton University Press; 1966. [Google Scholar]
- Williams GC. Sex and evolution. Princeton (NJ): Princeton University Press; 1975. [Google Scholar]
- Woolfit M, Bromham L. Increased rates of sequence evolution in endosymbiotic bacteria and fungi with small effective population sizes. Mol Biol Evol. 2003;20:1545–1555. doi: 10.1093/molbev/msg167. [DOI] [PubMed] [Google Scholar]
- Yang Z. PAML 4: a program package for phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007;24:1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- Young JA, Yourth CP, Agrawal AF. The effects of pathogens on selection against deleterious mutations in Drosophila melanogaster. J Evol Biol. 2009;22:2125–2129. doi: 10.1111/j.1420-9101.2009.01830.x. [DOI] [PubMed] [Google Scholar]