Abstract
One of the most striking cases of sex chromosome reorganization in Drosophila occurred in the lineage ancestral to Drosophila pseudoobscura, where there was a translocation of Y-linked genes to an autosome. These genes went from being present only in males, never recombining, and having an effective population size of 0.5N to a state of autosomal linkage, where they are passed through both sexes, may recombine, and their effective population size has quadrupled. These genes appear to be functional, and they underwent a drastic reduction in intron size after the translocation. A Y-autosome translocation may pose problems in meiosis if the rDNA locus responsible for X–Y pairing had also moved to an autosome. In this study, we demonstrate that the Y-autosome translocation moved Y-linked genes onto the dot chromosome, a small, mainly heterochromatic autosome with some sex chromosome–like properties. The rDNA repeats occur exclusively on the X chromosome in D. pseudoobscura, but we found that the new Y chromosome of this species harbors four clusters bearing only the intergenic spacer region (IGS) of the rDNA repeats. This arrangement appears analogous to the situation in Drosophila simulans, where X-rDNA to Y-IGS pairing could be responsible for X–Y chromosome pairing. We postulate that the nascent D. pseudoobscura Y chromosome acquired and amplified copies of the IGS, suggesting a potential mechanism for X–Y pairing in D. pseudoobscura.
Keywords: Drosophila pseudoobscura, Y chromosome evolution, dot chromosome, rDNA
Introduction
The gene content of the Y chromosome is not conserved across the 12 sequenced Drosophila species (Koerich et al. 2008). Although the ancestral Drosophila Y likely contained at least five genes (kl-3, kl-2, ORY, PRY, and PPr-Y; Koerich et al. 2008), the Y chromosomes of two species, Drosophila pseudoobscura and Drosophila persimilis, are unique in that they contain none of the ancestral Drosophila Y-linked genes (Koerich et al. 2008). Instead, their Y chromosome, which is essential for male fertility (Morgan et al. 1930), may have originated from an X-autosome fusion event (Carvalho and Clark 2005) that occurred in the ancestor of these species (White 1973). Similar to a Y chromosome, after an X-autosome fusion event, the chromosome homologous to the fused autosome will segregate opposite the X-autosome fusion chromosome in males and thus is referred to as a neo-Y. It was hypothesized that the Y chromosome of D. pseudoobscura evolved from a neo-Y chromosome that formed in the X-autosome fusion event in an ancestor (Carvalho and Clark 2005).
Surprisingly, the ancestral Drosophila Y-linked genes are located on an autosome of D. pseudoobscura (Carvalho and Clark 2005; Koerich et al. 2008). These genes are also likely to be autosomal in D. persimilis, Drosophila miranda (pseudoobscura subgroup), Drosophila affinis, and Drosophila azteca (affinis subgroup) as they are not Y-linked in these species (Carvalho and Clark 2005).
The Y-autosome translocated genes are highly conserved, and are transcribed and correctly spliced, and all but one of these genes have retained their testis-restricted expression (Carvalho and Clark 2005). This suggests that these likely functional genes may perform the same functions as they do on the Drosophila melanogaster Y. Interestingly, these genes underwent a drastic reduction in size: The introns of some of these genes reach megabases in length on the Y of D. melanogaster and Drosophila hydei (Gatti and Pimpinelli 1983; Kurek et al. 2000), whereas on the D. pseudoobscura autosome, the introns are in the kilobase range (Carvalho and Clark 2005).
Mechanistically, a Y-autosome translocation could pose a problem with X–Y pairing in meiosis if the Y-linked elements that are responsible for X–Y pairing also moved to the autosome. In most Drosophila species, males do not undergo crossing over, and so the autosomes pair during male meiosis at several homologous regions (Vazquez et al. 2002; McKee 2004). However, the D. melanogaster sex chromosomes do not have homologous sequences outside the X-linked Stellate locus (with the Y-linked Suppressor of Stellate locus) and the rDNA repeats. In melanogaster subgroup species, the sex chromosomes pair at the intergenic spacer (IGS) region of the rDNA clusters (Mckee et al. 1992; Ault and Rieder 1994; McKee 1996; Lohe and Roberts 2000). A fusion of the ancestral Y to an autosome may mean that the ancestral Y chromosome rDNA cluster was also transferred, which raises a question about how the X and Y chromosomes pair in this species.
To preserve the mechanism of X–Y pairing, the current Y chromosome would need to either acquire copies of the rDNA cluster or IGS repeats or it could have evolved a novel mechanism for proper X–Y segregation. Previous results suggest X linkage of the rDNA in D. pseudoobscura, by mapping a “bobbed” mutation, whose phenotype includes scutellar bristle defects and delayed development caused by a deficit of rDNA (Sturtevant and Tan 1937; Sturtevant and Novitski 1941). Whether there are also copies of the rDNA and/or the IGS repeats on the D. pseudoobscura Y is a critical issue that is yet to be determined.
Here, we report the mapping of the formerly Y-linked genes in D. pseudoobscura to the dot chromosome, suggesting that the heterochromatic environment of the dot chromosome may be important for the success of the translocation. We discover that the current Y chromosome contains no detectable rDNA genes, yet it has at least four large blocks of IGS repeats. Because such repeats are not observed on the homologous autosome in other Drosophila species, the new Y chromosome of this species likely acquired and amplified the IGS, potentially to aid in X–Y pairing and normal disjunction.
Materials and Methods
Male Parent Backcrosses
The genic content of chromosome arms tends to be conserved in Drosophila; these conserved chromosome arms are called Muller elements A–F. D. pseudoobscura has three acrocentric autosomes (Muller B, C, and E), a metacentric X chromosome (Muller A and D), a heterochromatic Y chromosome, and a dot chromosome (Muller F). We used two reciprocal male parent backcrosses between parental strains of D. pseudoobscura: y;gl;or;inc × Mather10 and Baja1 to map the D. melanogaster Y-linked genes in D. pseudoobscura. Mather10 was an isofemale line collected in Mather, CA, in 1997 by M.A.F. Noor and Baja1 was an isofemale line collected in Baja, Mexico in 2001 by William Etges. The mutant line, y;gl;or;inc was constructed by H. Allen Orr (Orr 1987). These D. pseudoobscura strains are available upon request to the authors. All results presented in this paper were from flies grown on standard yeast medium at 20 °C. We used the following visible or molecular markers in the backcrosses: glass (gl) on the second chromosome (Muller E), orange (or) on the third chromosome (Muller C), eyeless (ey) on the fifth chromosome (Muller F or the dot chromosome), and an indel and a single nucleotide polymorphism (SNP) detected in the formerly Y-linked genes ORY and kl-3. The fourth chromosome marker, inc, was not used because it is an unreliable marker with incomplete penetrance. We instead used microsatellites DPS4032 and DPS4033 on the fourth chromosome (Muller B; Ortiz-Barrientos et al. 2006). We scored 40 progeny of the male F1 heterozygotes crossed to y;gl;or;inc. The genotype at gl and or was determined by scoring phenotypes and in some cases confirmed using microsatellite markers on the same chromosome. The genotypes at ey, ORY, and kl-3 were determined by PCR resequencing both strands of products containing a single base-pair deletion in ey (forward 5′ ACTTCACAGGTTGTACAGTAATGTGTACC; reverse 5′ GTAGGTCGAGGCTATGAGGTCG; Noor et al. 2001), a 5-bp deletion in ORY (forward primer 5′ ATCGACTCGGCTATTGATGC and reverse primer 5′ ACCATGAGCGTCTTTTTGCT), and an A/G SNP in kl-3 (forward primer 5′ TTTGGCGCTAGTAGCTGGTT and reverse primer 5′ GGTCCCTTACCACGATCAGA). The Baja1 line contained a 97-bp deletion in the 5′ upstream region of ORY (forward primer 5′ CACCGACTCTACGTCGATGA and reverse primer 5′ TTTTAGCCGAATCCCACATC) that was genotyped by visualizing PCR products on a gel. All PCR resequencing was done using BigDye chemistry and run on an ABI 3700 or ABI 3730XL DNA sequencer.
Female Parent Backcrosses
In an attempt to use recombination mapping to identify the location of the translocated genes on the dot chromosome, we scored the progeny of female parent backcrosses. We set up crosses using Baja1 and y;gl;or;inc flies to generate the F1 female heterozygotes that were then backcrossed to Baja1 males to get progeny for mapping. We scored a 5-bp deletion found in ORY and a single base-pair deletion in the intron of ey in 296 progeny using PCR resequencing methods described above.
Probes
In order to determine the location of the rDNA genes and their IGS repeats, we performed fluorescent in situ hybridization using larval brain squashes. Probes were designed to 1,212 bp of the 18S rDNA gene (forward primer 5′ TATCCGAGGCCCTGTAATTG and reverse primer 5′ AATCCCAAGCATGAAAGTGG), 863 bp of the 28S rDNA gene (forward primer 5′ GGGGAAAGAAGACCCTTTTG and reverse primer 5′ AACGGACGTAGCGTCATACC). Probes were designed to two of the IGS subrepeats (Stage and Eickbush 2007; 226-bp IGS repeat forward primer 5′ GTGGTCGTTTGTGGAACTTG and reverse primer 5′ CTGTATTCATAATCAAATCATGCTCA; 267-bp IGS repeat forward primer 5′ GAAAAGAAACTATTGTTAAGAGGCACT and reverse primer 5′ AAATACACAGACATTGTCGGCTAA) using nick translation and biotinylated nucleotides (BioNick Labeling System, Invitrogen). The probes for both IGS subrepeats (267- and 226-bp IGS) and the probes for both 18S and 28S were combined before hybridization (figs. 2–4), unless stated otherwise (supplementary figs. 1 and 2, Supplementary Material online). The probes used for D. persimilis (226-bp IGS, 267-bp IGS, and 226- and 267-bp IGS combined; fig. 3, supplementary fig. 2, Supplementary Material online), D. affinis (226-bp IGS, 267-bp IGS, and 226 and 267-bp IGS combined; fig. 3), and Drosophila guanche (18S and 28S combined and 267-bp IGS combined; fig. 4) are the same probes designed in and used for D. pseudoobscura.
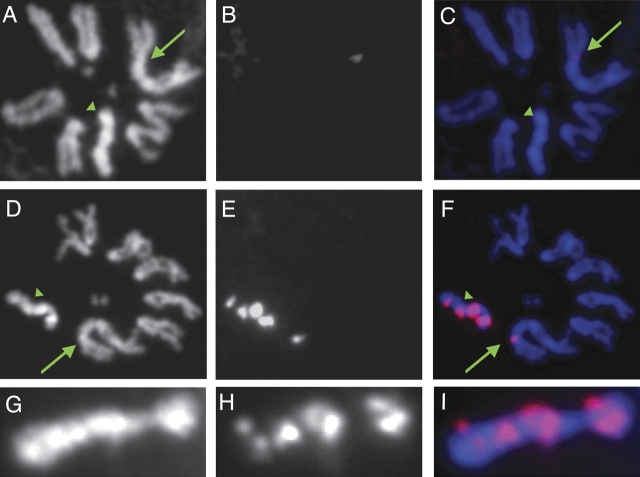
FIG. 2.
Hybridization of the rDNA probes 18S and 28S and IGS probes to Drosophila pseudoobscura mitotic chromosomes from larval brains suggest that the rDNA repeats are exclusively X-linked in D. pseudoobscura and that the rDNA IGS spacer region is found on the X and in multiple clusters on the Y. The green arrow points to the X chromosome, and the green arrowhead points to the Y chromosome. (A) DAPI staining of D. pseudoobscura mitotic chromosomes. (B) Signal for the 18S and 28S probes. (C) Overlay of A and B with DAPI staining colored in blue and the probe staining colored in red. (D,E,F) DAPI DNA staining, probe hybridization, and overlay for the IGS probes (for the 226- and 267-bp subrepeats, combined), respectively. (G,H,I) DAPI DNA staining, IGS probe hybridization, and overlay for just the Y chromosome, respectively. The rDNA genes 18S and 28S map exclusively to the X chromosome (shown as green arrow in C); no signal is seen on the Y chromosome, supporting the mapping of bobbed (Sturtevant and Tan 1937; Sturtevant and Novitski 1941). The IGS subrepeats are present in at least four clusters on the Y chromosome (shown as green arrowhead in F) and in one cluster on the X chromosome (shown as green arrow in F). There is no signal from the dot chromosome.
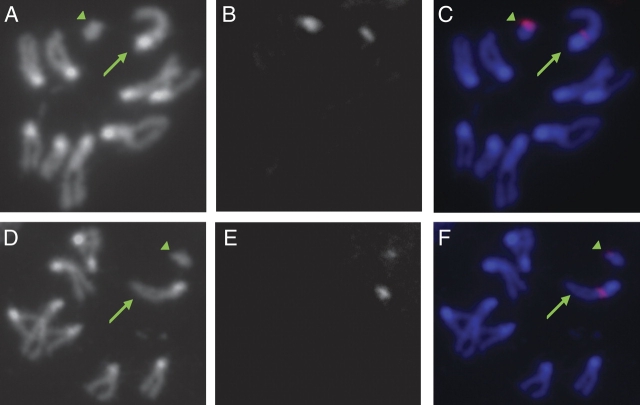
FIG. 3.

FISH in Drosophila affinis and D. persimilis using D. pseudoobscura probe shows that the current Y chromosomes acquired rDNA genes and spacers. The green arrow points to the X chromosome, and the green arrowhead points to the Y chromosome. (A) DAPI staining of D. affinis mitotic chromosomes. (B) Signal for the 18S and 28S probes. (C) Overlay of B and C with DAPI staining colored in blue and the probe staining colored in red. (D,E,F) DAPI DNA staining of D. affinis mitotic chromosomes, probe hybridization, and the overlay for the IGS probes, respectively. Both the rDNA genes 18S and 28S and the IGS repeats map to the X and Y chromosomes in D. affinis (shown as a green arrow and a green arrowhead for the X and Y, respectively, in C and F). The IGS repeats are not tandemly repeated on the Y chromosome in D. affinis. (G) DAPI staining of D. persimilis mitotic chromosomes. (H) Signal for the 18S and 28S probes. (I) Overlay of G and H with DAPI staining colored in blue and the probe staining colored in red. Signal amplification (described in Materials and Methods) was required to detect the signal in D. persimilis panels H and I. (J,K,L) DAPI DNA staining of D. persimilis mitotic chromosomes, probe hybridization, and the overlay for the IGS probes, respectively. The rDNA genes 18S and 28S map to both the X chromosome and the current Y chromosome in D. persimilis (shown as a green arrow and a green arrowhead for the X and Y, respectively, in I). The IGS repeats occur in at least four clusters on the current D. persimilis Y chromosome, similar to D. pseudoobscura (L).
FIG. 4.
FISH in D. guanche using Drosophila pseudoobcura probes suggests that the ancestral locations of the rDNA for D. pseudoobscura were likely on the X and Y chromosomes. The green arrow points to the X chromosome and the green arrowhead points to the Y chromosome. (A) DAPI staining of D. guanche mitotic chromosomes. (B) Signal for the 18S and 28S probes. (C) Overlay of B and C with DAPI staining colored in blue and the probe staining colored in red. (D,E,F) DAPI DNA staining of D. guanche mitotic chromosomes, probe hybridization, and the overlay for the IGS probes, respectively. Both the rDNA genes 18S and 28S and the IGS repeats map to the X and Y chromosomes in D. guanche (shown as a green arrow and a green arrowhead for the X and Y, respectively, in C and F). The IGS repeats are not tandemly repeated on the Y chromosome in this species, indicating that the current Y-linked IGS arrays in D. pseudoobscura are derived.
Chromosome Preparation
Brain squashes were carried out according to Pimpinelli et al. (2000) with some modification. Brains were dissected from third instar larvae; transferred to a hypotonic solution of 0.5% sodium citrate for 10 min; then were fixed in a solution of 1.8% formaldehyde, 45% acetic; and then squashed. The slides were frozen in liquid nitrogen, dehydrated in absolute ethanol, and kept dehydrated until use.
Fluorescence In Situ Hybridization (FISH)
FISH was carried out according to Pimpinelli et al. (2000) with some modification. Denaturation was achieved by placing slides on a heat block at 95 °C for 6 min. Hybridization was performed at 30 °C overnight. The slides were blocked in a 3% bovine serum albumin (BSA) solution and then treated with Avidin–Rhodamine (Roche) for 30 min at 37 °C and washed in 4× SSC/0.1% Tween. Signal amplification was necessary in some cases to confirm the presence/absence of a weak signal. In these cases, chromosomes were fixed in a solution of 2.5% formaldehyde (rather than 1.8%) and 45% acetic acid. Amplification was achieved with an additional treatment with Avidin–Rhodamine after blocking in 10% normal goat serum in 1× PBS at 37 °C, then washed (4× SSC/0.1% Tween) and treated with biotinylated antiavidin (Vector laboratories; for 30 min at 37 °C). The slides were then washed again in 4× SSC/0.1% Tween, blocked in 3% BSA, and treated with Avidin–Rhodamine again for 30 min both at 37 °C and washed before mounting. The slides were mounted in Vectashield with DAPI (Vector laboratories) and visualized on an Olympus BX50 epifluorescence microscope. Images were taken with a QImaging camera (Retiga Exi Fast 1394) at 200× with Metamorph imaging software, and then pseudocolored and overlaid in Adobe Photoshop 7.0. FISH was done with larval brains pooled from several D. pseudoobscura lines collected from Mesa Verde National Park, CO by Steve Schaeffer and larval brains pooled from several D. persimilis lines collected from Santa Cruz Island, Channel Islands, CA by Luciano Matzkin. The D. guanche and D. affinis lines used for FISH were obtained through the Tucson Drosophila stock center: stock numbers 14011-0095.00 and 14012-0141.03, respectively.
Results
Mapping the Y Translocation
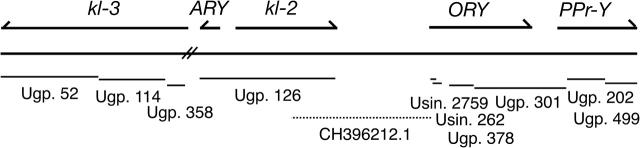
The orthologs of the D. melanogaster Y-linked genes kl-3, ARY, kl-2, ORY, and PPr-Y are located on 10 unmapped scaffolds from the Comparative Assembly Freeze 1 (CAF1) release of the D. pseudoobscura genome (fig. 1; kl-3: Unknown group 52, Unknown group 114, Unknown group 358; ARY and kl-2: Unknown group 126; ORY: Unknown singleton 2759, Unknown singleton 626, Unknown group 378, Unknown group 301; and PPr-Y: Unknown group 301, Unknown group 499 and Unknown group 202; Drosophila 12 Genomes Consortium 2007), confirming the results of Carvalho and Clark (2005), where an older assembly was used. In the closely related species D. persimilis, a single unmapped scaffold (Super 64) contains all five genes, with at least four in the same order as they occur in D. pseudoobscura (Drosophila 12 Genomes Consortium 2007). An improved assembly of the Y-to-autosome translocated region was obtained by using an additional assembly from TIGR made with the Celera assembler in 2004 (CABA assembly; accession AAFS01000000). Scaffold 92961 (CH396212.1) links kl-2 to ORY.
FIG. 1.
The five genes that were translocated from the Y chromosome to the dot chromosome are found on 10 scaffolds from the CAF1 assembly of the D. pseudoobscura genome (Drosophila 12 Genomes Consortium 2007). The scaffolds are represented by lines, and the length of the lines drawn are proportional to the length of the scaffolds. These scaffolds cover at least 158 kb, including estimated gap lengths, but this is likely to be a gross underestimate because it does not account for gaps between scaffolds. In Drosophila persimilis, this region spans approximately 312 kb including estimated gaps within the single scaffold in which these genes are found. The scaffolds are all unmapped (Ugp. = “unknown group” and Usin. = “unknown singleton”). The scaffold containing kl-3 does not overlap with the rest of the Y-to-dot genes in D. pseudoobscura, although in D. persimilis all of the genes, including kl-3, are contained on a single scaffold and are in the same order. ARY and kl-2 can be linked to the first exons of ORY using a scaffold (CH396212.1) from the CABA assembly (shown as dotted line).
The formerly Y-linked genes ARY, kl-2, ORY, and PPr-Y remain physically linked in D. pseudoobscura and can be ordered based on their locations in the CAF1 scaffolds (fig. 1; Carvalho and Clark 2005). We, therefore, only scored markers in ORY and kl-3 to map the translocated region using two male parent backcrosses. ORY and ey markers cosegregated in all 26 progeny able to be scored at ORY for the cross y;gl;or;inc × Mather10. ORY, kl-3, and ey markers cosegregated in all 21 progeny that were able to be scored at both ORY and kl-3 for the cross y;gl;or;inc × Baja1. In contrast, alleles on the other three autosomes were inherited independently of the markers on the ancestral Y and ey. These crosses indicate that the D. pseudoobscura orthologs of the D. melanogaster Y-linked genes are linked to the dot chromosome (chromosome 5) in D. pseudoobscura. These genes could not be mapped within the dot chromosome: We found no recombinant genotypes out of 296 progeny scored in our female parent backcrosses. This is consistent with the dot chromosome of D. pseudoobscura, experiencing little if any recombination. However, as yet, the physical distance between the Y-to-dot translocated genes and ey is unknown. Bacterial artificial chromosome end sequence reads from D. persimilis indicate that the five D. melanogaster Y-linked genes are located on a scaffold that is linked to a known dot chromosome scaffold from the CAF1 assembly (Scaffold 51, Drosophila 12 Genomes Consortium 2007; Wing R, Goicoechea JL, personal communication; Supplementary fig. 3, Supplementary Material online), suggesting that this species possesses the same Y-to-dot translocation as D. pseudoobscura. Efforts to map these genes in D. persimilis and a closely related species, D. affinis, using FISH have so far been unsuccessful (see Supplementary Material online).
Identifying rDNA Locations Using In Situ Hybridizations
In situ hybridization of the 18S and 28S probes indicates that the rDNA array is on the left arm of the X chromosome, supporting the mapping of bobbed in D. pseudoobscura (Sturtevant and Tan 1937; Sturtevant and Novitski 1941). Our results suggest that few, if any, copies of 18S and 28S rDNA exist on the Y chromosome, as no signal is detected (fig. 2). In situ hybridizations of the 226- and 267-bp IGS subrepeat probes map the IGS to the X chromosome, at a position that appears coincident to the location of the rDNA. We also find at least four additional blocks of bright staining on the Y chromosome using the IGS probes (fig. 2). Interestingly, only one subrepeat, the 267-bp IGS, is distributed widely across the current Y chromosome (supplementary fig. 1, Supplementary Material online); the 226-bp IGS subrepeat has few, if any copies on this chromosome (supplementary fig. 1, Supplementary Material online). We see a very similar pattern on the D. persimilis Y: four clusters of IGS (mostly consisting of 267-bp repeats) are on the Y chromosome and one small band on the X chromosome (supplementary fig. 2, Supplementary Material online). However, the 226-bp subrepeat occurs in two small clusters on the Y chromosome of D. persimilis (supplementary fig. 2, Supplementary Material online). Drosophila persimilis also differs from D. pseudoobscura in rDNA repeat distribution; two small clusters of rDNA genes (18S and 28S) appear on the current Y chromosome in addition to the rDNA repeats on the X chromosome (fig. 3). Drosophilia affinis, an obscura-group species that has the X–D fusion and a Y translocation presumed to be the same as in D. pseudoobscura, has rDNA genes both on the ancestral X and current Y chromosomes as evidenced by FISH signals (fig. 3). Unlike D. pseudoobscura, D. affinis only has one block of IGS repeats on the X and Y chromosomes, each that appear coincident with the rDNA loci. In D. guanche, a related obscura-group species that does not have the Y–A translocation or X–D fusion, FISH suggests that the rDNA and IGS repeats occur on both the X and the Y chromosomes (fig. 4).
Discussion
Our findings suggest that most of the genic content of the ancestral Y chromosome translocated to the dot chromosome in D. pseudoobscura. The only ancestral Y-linked gene that is not on the dot chromosome in D. pseudoobscura is PRY, a gene that is now X-linked in this species (Koerich et al. 2008), and was transferred to the X independently of the Y-to-dot translocation.
We can use the location of the rDNA and IGS to shed light on this sex chromosome rearrangement. The conspicuous absence of the rDNA from the current D. pseudoobscura Y chromosome appears to be derived. Although the location and copy number of the rDNA evolve very rapidly in short periods of time (Lohe and Roberts 2000; Eickbush TH and Eickbush DG 2007), our results in obscura-group species D. guanche coupled with the known locations of the rDNA in other Drosophila species (Hennig et al. 1975; Lohe and Roberts 1990; Roy et al. 2005; Brianti et al. 2009) suggests that the rDNA repeats are ancestrally X and Y linked (fig. 5 and 6A). It is possible that the absence/presence of rDNA may be segregating within D. pseudoobscura and/or D. persimilis, and it is absent in the populations we investigated. The Y chromosomes of these species are known to vary in size and morphology between populations (Dobzhansky 1935, 1937). We are currently exploring this possibility.
FIG. 5.
The location of the rDNA in the melanogaster group, obscura-group and Drosophila hydei in the Drosophila subgenus suggest that the ancestral locations of the rDNA are on the X and Y chromosomes. D. simulans presents an exception where the ancestral Y-linked rDNA locus was lost after the amplification of acquired IGS repeats on the Y chromosome (Lohe and Roberts 1990). D. hydei has two clusters of rDNA repeats on the Y chromosome in addition to the X (Hennig et al. 1975). The three species that have the X-Muller D fusion (X–D) and the Y–A translocation (Y–A), Drosophila pseudoobscura, Drosophila persimilis, and Drosophila affinis, appear to have a more recent acquisition, followed by the amplification of the IGS repeats on the current Y chromosomes of D. pseudoobscura and D. persimilis (figs. 2 and 4). D. pseudoobscura appears to have lost its ancestral Y-linked rDNA locus (fig. 2), whereas D. persimilis and D. affinis retained rDNA on their current Y chromosomes.
FIG. 6.
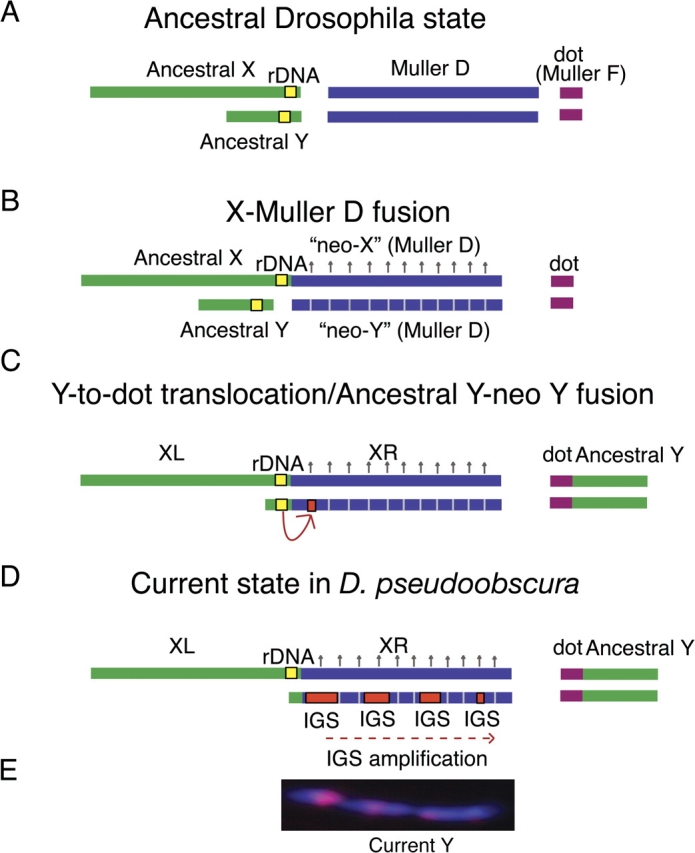
We propose that there was a Y-to-dot translocation in D. pseudoobscura and that the current Y chromosome originated from an X–D fusion, followed by acquisition of IGS sequences. (A) The Drosophila ancestral state with respect to the sex chromosomes and rDNA repeats. (B) An X-Muller D fusion occurred between 11 and 18 Ma. The homolog of the fused element (neo-Y) was transmitted as a Y. The neo-Y eventually degenerates (represented by hatched lines) and, in response, the neo-X becomes dosage compensated (represented by upward arrows). (C) The IGS spacer is transferred to the neo-Y chromosome either from the ancestral X or as diagrammed: from the ancestral Y chromosome. Some fraction of the ancestral Y containing the rDNA may have remained free and fused with the current Y. (D) The ancestral Y chromosome translocated to the dot chromosome, and the current Y chromosome is a degenerated neo-Y chromosome that originated from the X–D fusion event. The IGS spacer acquired by the current Y chromosome is amplified, producing at least four clusters, and the ancestral Y-linked rDNA locus is lost. We illustrate this as a translocation of most of the ancestral Y to the dot with subsequent fusion of the remaining ancestral Y with the current Y rather than a complete fusion, although both scenarios are possible. The left arm of the X (XL) in D. pseudoobscura corresponds to the ancestral X chromosome and the right arm (XR) is the Muller D element. (E) The IGS spacer repeats (in red) occur in at least four large clusters on the Y chromosome (DAPI staining).
Model for the Y-Dot Translocation
Several evolutionary scenarios are consistent with the current state in D. pseudoobscura. We propose a model to explain this sex chromosome rearrangement. After the well-documented X–D fusion (fig. 6B), the ancestor of D. pseudoobscura (also D. persimilis, D. affinis, and D. azteca) went through the transitional phase in which both the ancestral Y and the former Muller D autosome pair with the X–D fusion (fig. 6B). The fused Muller D arm is referred to as a “neo-X,” and the male-restricted homolog of the fused Muller D is referred to as a neo-Y chromosome. Because the neo-Y is transmitted like a Y chromosome, it eventually degenerated (reviewed in Charlesworth B and Charlesworth D 2000).
Rather than the neo-Y chromosome being lost, as was previously thought to have happened in D. pseudoobscura (White 1973), or the fusion of the neo-Y and current Y, which has happened in other Drosophila species including D. albomicans (Yu et al. 1999), the ancestral Y translocated to the dot chromosome. This may have been an ancestral Y-dot fusion; however, it is more likely that the majority of the ancestral Y translocated to the dot chromosome, but some fraction, including the rDNA, remained free and later fused with the neo-Y. It is possible that the neo-Y chromosome acquired copies of the rDNA IGS from the X chromosome, possibly through extrachromosomal circular chromosomes containing copies of the IGS repeats (Pont et al. 1987; Cohen et al. 2003), as has been hypothesized in D. simulans (Lohe and Roberts 1990). Alternatively, the neo-Y could have acquired IGS repeats from the ancestral Y after the two chromosomes fused (fig. 6C and D). The IGS was amplified to form what appear to be tandem arrays (fig. 6E), and the ancestral Y-linked rDNA locus was lost. This hypothesis gains plausibility because D. affinis and D. persimilis have Y-linked rDNA genes. Moreover, if the rDNA were never transferred to the dot, this would avoid problems with dot chromosome spuriously pairing with the X in meiosis.
This model suggests that the current Y chromosome is the degenerated neo-Y that originated from the Muller D element in the X–D fusion. This is supported by the observation that the closest homologs of 10 of the 15 identified genes and pseudogenes on the D. pseudoobscura Y chromosome are on the Muller D chromosome of D. melanogaster, although it is currently unknown whether these represent more recent duplications (Carvalho and Clark 2005; Carvalho et al. 2009). D. affinis and D. persimilis may represent intermediate steps in the evolution of rDNA in D. pseudoobscura. D. affinis may represent the state following the acquisition of the rDNA by the neo-Y (fig. 6C), and D. persimilis may represent the state after the amplification of the IGS on the neo-Y, but this species has not lost its Y-linked rDNA.
It is also tempting to speculate that X–Y pairing in D. pseudoobscura is mediated by the IGS repeats. A large tandem array of Y-linked IGS repeats is found in D. simulans, yet the Y has no detectable rDNA repeats (Lohe and Roberts 1990). It is hypothesized that the Y-linked IGS maintains X–Y pairing in the absence of the rDNA (Ault and Rieder 1994; Lohe and Roberts 2000). It is unknown whether one of the ancestral functions of the IGS subrepeats is in X–Y pairing; however, the Y-specific IGS amplification in D. pseudoobscura seems to parallel D. simulans, suggesting that its function is conserved. No Y chromosomes have been found that impact the phenotype of X-linked bobbed mutations in D. affinis (Sturtevant 1940), suggesting that the rDNA on the D. affinis Y chromosome, and so perhaps the ancestor of D. affinis, D. pseudoobscura, and D. persimilis, may not be transcribed. After amplification of the IGS on the D. pseudoobscura current Y, there may not have been a need for the rDNA in the ancestral Y location, and so these repeats could be lost and the pairing function could rest with the Y-linked IGS array.
Factors Potentially Contributing to the Success of the Translocation
Similar to the Y chromosome, the dot chromosome is highly heterochromatic and has many features that distinguish it from the major autosomes (reviewed in Larsson and Meller 2006; Riddle and Elgin 2006). Most notably, it associates with “Painting of Fourth” (POF), a dot-specific protein reminiscent of the MSL dosage compensation complex, which regulates the expression of dot-linked genes in D. melanogaster (Johansson, Stenberg, Bernhardsson, and Larsson 2007; Johansson, Stenberg, Pettersson, and Larsson 2007). Because POF also binds the dot of D. pseudoobscura, it could influence the expression of the Y-to-dot genes (Larsson et al. 2004).
Initially, the translocation of male-related Y-linked genes to the dot chromosome may have offered some fitness advantage to males bearing the translocation. The nature of this advantage is unknown; however, it could be related to the doubling in dosage of the genes following translocation and/or the mechanics of gene expression on the dot chromosome. The heterochromatic nature of the dot may contribute to the success of the translocation, because translocation to the dot chromosome would still require the ability to function when in heterochromatin.
If these genes possessed cis-regulatory sequences that directed testes-specific expression on the Y chromosome, there should be no adverse effects of moving to the dot chromosome from an expression standpoint. However, the expression of genes on the Y chromosome of D. melanogaster seems to be controlled at the level of chromosome-wide decondensation during spermatogenesis. If the genes lost their male-restricted expression on transfer to the dot, they would be exposed to female counterselection; therefore, these loci may face selection pressures from preventing misexpression in females.
In the long term, a Y-autosome translocation would enjoy an increased efficacy of selection due to a quadrupled effective population size, resulting in lower frequencies of slightly deleterious variation in mutation–selection balance as well as augmenting the selective response to weakly beneficial mutations. Similar to other Drosophila species (Bridges 1935; Ashburner 1989; Wang et al. 2002, 2004), the dot chromosome of D. pseudoobscura may experience little to no meiotic crossing over. We found no recombinant genotypes in our screen, although the small sample (n = 296) only allows one to infer that recombination is at most rare. But even a small amount of recombination, as perhaps by gene conversion, could increase the efficacy of natural selection (Fisher 1930). In conjunction with the larger effective population size, and in the presence of a specific male advantage, this may be the reason this translocation became fixed and that the drastic reduction in intron size was evolutionarily possible.
Y-autosome translocations are frequently deleterious, as evidenced by the variety of human syndromes and cases of male infertility caused by such chromosomal rearrangements (Weber et al. 1987; Delobel et al. 1998; Chen et al. 2008 and references therein). However, translocations like these have been documented in many species outside Drosophila and are implicated in the evolution of sex chromosomes and speciation (White 1973; Ma et al. 1976; Veyrunes et al. 2004; Deuve et al. 2008). In most cases, the autosome segregates with the Y chromosome in males, creating a neo-sex chromosome. We are unaware of any other species in which the Y chromosome, or a large part of the Y chromosome, segregates in both sexes with an autosome, as in D. pseudoobscura.
Questions about the mechanistic details of this rearrangement remain unanswered: What comprises the centromere of the X–D fusion and how, immediately following the X–D fusion, did the X–D fusion pair with the unfused Muller D element? These aspects of genomic rearrangements are largely unknown. The discovery that the ancestral Y chromosome moved to the dot chromosome in D. pseudoobscura is an exciting addition to the parallels between the dot and the sex chromosomes.
Supplementary Material
Supplementary figures 1–3 are available at Molecular Biology and Evolution online (http://www.mbe.oxfordjournals.org/).
Supplementary Material
Acknowledgments
We thank Nadia Singh, Dan Barbash, Chip Aquadro, and A. Bernardo Carvalho for helpful comments that improved the manuscript and Patrick Ferree for help with FISH. We also thank Steve Schaeffer for the generous donation of D. pseudoobscura and D. persimilis strains. This work was supported by an NSF Dissertation Improvement Grant 0708584 to A.G.C. and A.M.L, NIH grant GM64590 to A.G.C. and A. Bernardo Carvalho and an NSF grant 0715484 and NIH grant GM076051 to M.A.F.N.
References
- Ashburner M. Drosophila. a laboratory handbook. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Ault JG, Rieder CL. Meiosis in Drosophila males. I. The question of separate conjunctive mechanisms for the XY and autosomal bivalents. Chromosoma. 1994;103:352–356. doi: 10.1007/BF00417883. [DOI] [PubMed] [Google Scholar]
- Brianti MT, Ananina G, Recco-Pimentel SM, Klaczko LB. Comparative analysis of the chromosomal positions of rDNA genes in species of the tripunctata radiation of Drosophila. Cytogenet Genome Res. 2009;125:149–157. doi: 10.1159/000227840. [DOI] [PubMed] [Google Scholar]
- Bridges CB. The mutants and linkage data of chromosome four of Drosophila melanogaster. Biol Zh. 1935;4:401–420. [Google Scholar]
- Carvalho AB, Clark AG. Y chromosome of D. pseudoobscura is not homologous to the ancestral Drosophila Y. Science. 2005;307:108–110. doi: 10.1126/science.1101675. [DOI] [PubMed] [Google Scholar]
- Carvalho AB, Koerich LB, Clark AG. Origin and evolution of Y chromosomes: Drosophila tales. Trends Genet. 2009;25:270–277. doi: 10.1016/j.tig.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth B, Charlesworth D. The degeneration of Y chromosomes. Philos Trans R Soc Lond B Biol Sci. 2000;355:1563–1572. doi: 10.1098/rstb.2000.0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CP, Lin SP, Tsai FJ, Wang TH, Chern SR, Wang W. Characterization of a de novo unbalanced Y;autosome translocation in a 45,X mentally retarded male and literature review. Fertil Steril. 2008;90(1198):e1111–e1198. doi: 10.1016/j.fertnstert.2007.11.065. [DOI] [PubMed] [Google Scholar]
- Cohen S, Yacobi K, Segal D. Extrachromosomal circular DNA of tandemly repeated genomic sequences in Drosophila. Genome Res. 2003;13:1133–1145. doi: 10.1101/gr.907603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delobel B, Djlelati R, Gabriel-Robez O, Croquette MF, Rousseaux-Prevost R, Rousseaux J, Rigot JM, Rumpler Y. Y-autosome translocation and infertility: usefulness of molecular, cytogenetic and meiotic studies. Hum Genet. 1998;102:98–102. doi: 10.1007/s004390050660. [DOI] [PubMed] [Google Scholar]
- Deuve JL, Bennett NC, Ruiz-Herrera A, Waters PD, Britton-Davidian J, Robinson TJ. Dissection of a Y-autosome translocation in Cryptomys hottentotus (Rodentia, Bathyergidae) and implications for the evolution of a meiotic sex chromosome chain. Chromosoma. 2008;117:211–217. doi: 10.1007/s00412-007-0140-6. [DOI] [PubMed] [Google Scholar]
- Dobzhansky T. The y chromosome of Drosophila pseudoobscura. Genetics. 1935;20:355–376. doi: 10.1093/genetics/20.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky T. Further data on the variation of the y chromosome of Drosophila pseudoobscura. Genetics. 1937;22:340–346. doi: 10.1093/genetics/22.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosophila 12 Genomes Consortium. Evolution of genes and genomes on the Drosophila phylogeny. Nature. 2007;450:203–218. doi: 10.1038/nature06341. [DOI] [PubMed] [Google Scholar]
- Eickbush TH, Eickbush DG. Finely orchestrated movements: evolution of the ribosomal RNA genes. Genetics. 2007;175:477–485. doi: 10.1534/genetics.107.071399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA. The genetical theory of natural selection. Oxford: Oxford University Press; 1930. [Google Scholar]
- Gatti M, Pimpinelli S. Cytological and genetic analysis of the Y chromosome of Drosophila melanogaster. I. Organization of the fertility factors. Chromosoma. 1983;88:349–373. [Google Scholar]
- Hennig W, Link B, Leoncini O. The location of the nucleolus organizer regions in Drosophila hydei. Chromosoma. 1975;51:57–63. doi: 10.1007/BF00285808. [DOI] [PubMed] [Google Scholar]
- Johansson AM, Stenberg P, Bernhardsson C, Larsson J. Painting of fourth and chromosome-wide regulation of the 4th chromosome in Drosophila melanogaster. Embo J. 2007;26:2307–2316. doi: 10.1038/sj.emboj.7601604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson AM, Stenberg P, Pettersson F, Larsson J. POF and HP1 bind expressed exons, suggesting a balancing mechanism for gene regulation. PLoS Genet. 2007;3:e209. doi: 10.1371/journal.pgen.0030209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerich LB, Wang X, Clark AG, Carvalho AB. Low conservation of gene content in the Drosophila Y chromosome. Nature. 2008;456:949–951. doi: 10.1038/nature07463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurek R, Reugels AM, Lammermann U, Bunemann H. Molecular aspects of intron evolution in dynein encoding mega-genes on the heterochromatic Y chromosome of Drosophila sp. Genetica. 2000;109:113–123. doi: 10.1023/a:1026552604229. [DOI] [PubMed] [Google Scholar]
- Larsson J, Meller VH. Dosage compensation, the origin and the afterlife of sex chromosomes. Chromosome Res. 2006;14:417–431. doi: 10.1007/s10577-006-1064-3. [DOI] [PubMed] [Google Scholar]
- Larsson J, Svensson MJ, Stenberg P, Makitalo M. Painting of fourth in genus Drosophila suggests autosome-specific gene regulation. Proc Natl Acad Sci U S A. 2004;101:9728–9733. doi: 10.1073/pnas.0400978101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohe AR, Roberts PA. An unusual Y chromosome of Drosophila simulans carrying amplified rDNA spacer without ribosomal-RNA genes. Genetics. 1990;125:399–406. doi: 10.1093/genetics/125.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohe AR, Roberts PA. Evolution of DNA in heterochromatin: the Drosophila melanogaster sibling species subgroup as a resource. Genetica. 2000;109:125–130. doi: 10.1023/a:1026588217432. [DOI] [PubMed] [Google Scholar]
- Ma NS, Elliott MW, Morgan L, Miller A, Jones TC. Translocation of Y chromosome to an autosome in the Bolivian owl monkey, Aotus. Am J Phys Anthropol. 1976;45:191–202. doi: 10.1002/ajpa.1330450205. [DOI] [PubMed] [Google Scholar]
- McKee BD. The license to pair: identification of meiotic pairing sites in Drosophila. Chromosoma. 1996;105:135–141. doi: 10.1007/BF02509494. [DOI] [PubMed] [Google Scholar]
- McKee BD. Homologous pairing and chromosome dynamics in meiosis and mitosis. Biochim Biophys Acta. 2004;1677:165–180. doi: 10.1016/j.bbaexp.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Mckee BD, Habera L, Vrana JA. Evidence that intergenic spacer repeats of Drosophila melanogaster ribosomal-RNA genes function as X–Y pairing sites in male meiosis, and a general model for achiasmatic pairing. Genetics. 1992;132:529–544. doi: 10.1093/genetics/132.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan TH, Bridges CB, Schultz J. The constitution of the germinal material in relation to heredity. Carnegie Inst Washington Publ. 1930;29:352–360. [Google Scholar]
- Noor MAF, Grams KL, Bertucci LA, Almendarez Y, Reiland J, Smith KR. The genetics of reproductive isolation and the potential for gene exchange between Drosophila pseudoobscura and D. persimilis via backcross hybrid males. Evolution. 2001;55:512–521. doi: 10.1554/0014-3820(2001)055[0512:tgoria]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Orr HA. Genetics of male and female sterility in hybrids of Drosophila pseudoobscura and D. persimilis. Genetics. 1987;116:555–563. doi: 10.1093/genetics/116.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Barrientos D, Chang AS, Noor MAF. A recombinational portrait of the Drosophila pseudoobscura genome. Genet Res. 2006;87:23–31. doi: 10.1017/S0016672306007932. [DOI] [PubMed] [Google Scholar]
- Pimpinelli S, Bonaccorsi S, Fanti L, Gatti M. Preparation and analysis of Drosophila mitotic chromosomes in Drosophila Protocol. In: Sullivan W, Ashburner M, Hawley RS, editors. Drosophila: a laboratory manual. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- Pont G, Degroote F, Picard G. Some extrachromosomal circular DNAs from Drosophila embryos are homologous to tandemly repeated genes. J Mol Biol. 1987;195:447–451. doi: 10.1016/0022-2836(87)90665-6. [DOI] [PubMed] [Google Scholar]
- Riddle NC, Elgin SC. The dot chromosome of Drosophila: insights into chromatin states and their change over evolutionary time. Chromosome Res. 2006;14:405–416. doi: 10.1007/s10577-006-1061-6. [DOI] [PubMed] [Google Scholar]
- Roy V, Monti-Dedieu L, Chaminade N, Siljak-Yakovlev S, Aulard S, Lemeunier F, Montchamp-Moreau C. Evolution of the chromosomal location of rDNA genes in two Drosophila species subgroups: ananassae and melanogaster. Heredity. 2005;94:388–395. doi: 10.1038/sj.hdy.6800612. [DOI] [PubMed] [Google Scholar]
- Stage DE, Eickbush TH. Sequence variation within the rRNA gene loci of 12 Drosophila species. Genome Res. 2007;17:1888–1897. doi: 10.1101/gr.6376807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant AH. Genetic data on Drosophila affinis, with a discussion of the relationships in the subgenus Sophophora. Genetics. 1940;25:337–353. doi: 10.1093/genetics/25.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant AH, Novitski E. The homologies of the chromosome elements in the genus Drosophila. Genetics. 1941;26:517–541. doi: 10.1093/genetics/26.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant AH, Tan CC. The comparative genetics of Drosophila pseudoobscura and D. melanogaster. J Genet. 1937;34:415–437. [Google Scholar]
- Vazquez J, Belmont AS, Sedat JW. The dynamics of homologous chromosome pairing during male Drosophila meiosis. Curr Biol. 2002;12:1473–1483. doi: 10.1016/s0960-9822(02)01090-4. [DOI] [PubMed] [Google Scholar]
- Veyrunes F, Catalan J, Sicard B, Robinson TJ, Duplantier JM, Granjon L, Dobigny G, Britton-Davidian J. Autosome and sex chromosome diversity among the African pygmy mice, subgenus Nannomys (Murinae; Mus) Chromosome Res. 2004;12:369–382. doi: 10.1023/B:CHRO.0000034098.09885.e6. [DOI] [PubMed] [Google Scholar]
- Wang W, Thornton K, Berry A, Long M. Nucleotide variation along the Drosophila melanogaster fourth chromosome. Science. 2002;295:134–137. doi: 10.1126/science.1064521. [DOI] [PubMed] [Google Scholar]
- Wang W, Thornton K, Emerson JJ, Long M. Nucleotide variation and recombination along the fourth chromosome in Drosophila simulans. Genetics. 2004;166:1783–1794. doi: 10.1534/genetics.166.4.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber B, Schempp W, Orth U, Seidel H, Gal A. A Y/5 translocation in a 45,X male with cri du chat syndrome. Hum Genet. 1987;77:145–150. doi: 10.1007/BF00272382. [DOI] [PubMed] [Google Scholar]
- White MJD. Animal cytology and evolution. Cambridge: Cambridge University Press; 1973. [Google Scholar]
- Yu YC, Lin FJ, Chang HY. Stepwise chromosome evolution in Drosophila albomicans. Heredity. 1999;83(Pt 1):39–45. doi: 10.1038/sj.hdy.6885470. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.