Abstract
Arginase competitively inhibits nitric oxide synthase (NOS) via use of the common substrate L-arginine. Arginase II has recently reported as a novel therapeutic target for the treatment of cardiovascular diseases such as atherosclerosis. Here, we demonstrate that piceatannol-3'-O-β-D-glucopyranoside (PG), a potent component of stilbenes, inhibits the activity of arginase I and II prepared from mouse liver and kidney lysates, respectively, in a dose-dependent manner. In human umbilical vein endothelial cells, incubation of PG markedly blocked arginase activity and increased NOx production, as measured by Griess assay. The PG effect was associated with increase of eNOS dimer ratio, although the protein levels of arginase II or eNOS were not changed. Furthermore, isolated mice aortic rings treated with PG showed inhibited arginase activity that resulted in increased nitric oxide (NO) production upto 78%, as measured using 4-amino-5-methylamino-2',7'-difluorescein (DAF-FM) and a decreased superoxide anions up to 63%, as measured using dihydroethidine (DHE) in the intact endothelium. PG showed IC50 value of 11.22 µM and 11.06 µM against arginase I and II, respectively. PG as an arginase inhibitor, therefore, represents a novel molecule for the therapy of cardiovascular diseases derived from endothelial dysfunction and may be used for the design of pharmaceutical compounds.
Keywords: arginase; endothelium, vascular; nitric oxide synthase type III; superoxides; 3,3',4,5'-tetrahydroxystilbene
Introduction
The endothelium plays a central role in overall vascular homeostasis by regulating vasoreactivity, platelet activation, leukocyte adhesion, and smooth muscle cell proliferation and migration. Endothelial nitric oxide (NO), an important vasoprotective molecule, is a major modulator of these effects, and impaired NO signaling associated with endothelial function is considered an early marker of the atherogenic process. Arginase competitively inhibits nitric oxide synthase (NOS) via use of the common substrate L-arginine (Morris et al., 1998; Berkowitz et al., 2003; Simon et al., 2003; Holowatz et al., 2006; Steppan et al., 2006; Peyton et al., 2009). Arginase is present in 2 isoforms: arginase I, the hepatic isoform; and arginase II, the extrahepatic isoform; each of which is encoded by a distinct gene. The expression and function of arginase I in macrophages, hepatocytes, and vascular smooth muscle cells, is stimulated by lipopolysaccharide (LPS), IL-13, altered oxygen tension, and balloon dilatation of coronary arteries (Modolell et al., 1995; Louis et al., 1998; Que et al 1998; Klasen et al., 2001; Chicoine et al., 2004; Morris et al., 2004; Ryoo et al., 2006; Nelin et al., 2007). The activation and expression of endothelial arginase II can also be induced by a variety of vascular insults, including OxLDL, LPS, TNF-α, IFN-β, 8-bromo-cGMP, and hypoxia (Morris et al., 1998; Que et al., 1998; Chicoine et al., 2004; Ryoo et al., 2006; Nelin et al., 2007). Arginase inhibition actively augments NO production and reportedly has beneficial effects on normal cardiac function and on vascular dysfunction typical of atherogenesis, aging, and erectile dysfunction, and sickle cell disease (Bivalacqua et al., 2001; Berkowitz et al., 2003; Morris et al., 2004; Steppan et al., 2006; White et al., 2006; Bivalacqua et al., 2007; Hsu et al., 2007; Xu et al., 2007).
Rhubarb is an important medicinal original plant that has been used in traditional medicine as a remedy for the blood stagnation and a purgative agent. Rhubarb is the rhizome of Rheum undulatum L. commonly distributed in Asia and many components of rhizome has been demonstrated as anthraquinone derivatives or stilbenes such as emodin, chrysophanol, physcion, resveratrol, rhapontigenin, rhaponticin, and piceatannol (Xu et al., 2007; Ngoc et al., 2008). These compounds reportedly possess diverse biological activities such as anti-allergic (Matsuda et al., 2004), anti-diabetic (Choi et al., 2005), antioxidant (Matsuda et al., 2001), and vasorelaxant activities (Moon et al., 2006). Piceatannol is an important component in rhubarb extract and was recently found to possess various activities such as inhibition of C-jun N-terminal kinase activation (Jang et al., 2009), anti-carcinogenic activity (Vo et al., 2009), and inhibition of PDGF-BB-induced VSMC proliferation and migration (Choi et al., 2009). However, the biological activity and the molecular target for piceatannol-3'-O-β-D-glucopyranoside (PG) have not been investigated .
Thus, we tested whether piceatannol-3'-O-β-D-glucopyranoside( PG) has an inhibitory effect on arginase activity and whether this effect is associated with reciprocally regulation of NOx and ROS production in the endothelium.
Results
Arginase activity was decreased with treatment of piceatannol-3'-O-β-D-glucopyranoside (PG) treatment
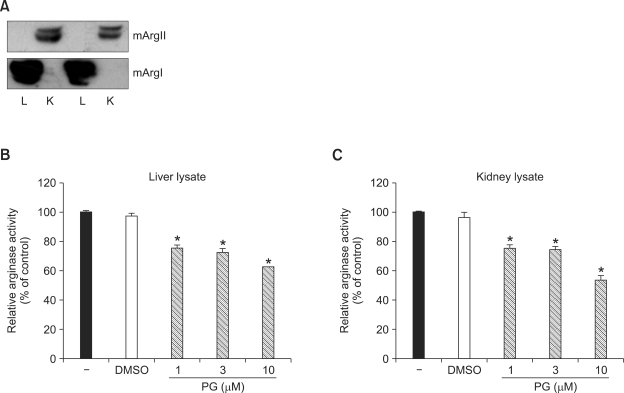
We first prepared arginase enzyme solutions for arginase I and arginase II from the lysates of mouse livers and kidneys, respectively, and the presence of specific arginase isoforms was detected by Western blot analysis. As shown in Figure 1A, arginase I was predominantly expressed in liver lysates while arginase II was in the kidney. In liver lysates, incubation of different concentrations of PG significantly decreased arginase I activity (Figure 2B, 75 ± 5% at 1 µM, 72 ± 7% at 3 µM, 62 ± 1% at 10 µM) compared to untreated control (100±9%). In kidney lysates, the residual arginase activities after incubation of 1, 3 and 10 µM PG were 75 ± 6, 74 ± 5, and 53 ± 8%, respectively (Figure 2C). These data indicated that PG has a strong and specific inhibitory effect on both arginase I and II even if PG has no selectivity for a specific arginase isoform.
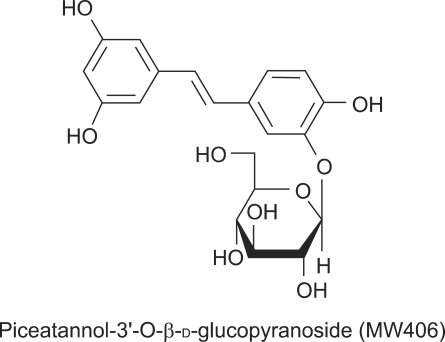
Figure 1.
Structure of piceatannol-3'-O-β-D-glucopyranoside (PG).
Figure 2.
PG decreases the activities of arginase I and II. Arginase solutions prepared from liver (L) and kidney (K) were confirmed to express arginase I or II by Western blot analysis (A). Arginase I was predominantly expressed in liver while arginase II was in kidney. Incubation of different concentrations PG significantly decreased arginase I (B, liver lysate) and II (C, kidney lysate) activities. DMSO (10 µM) was used as a control. * vs. untreated, P < 0.01, n = 12.
PG treatment reciprocally increased NOx production
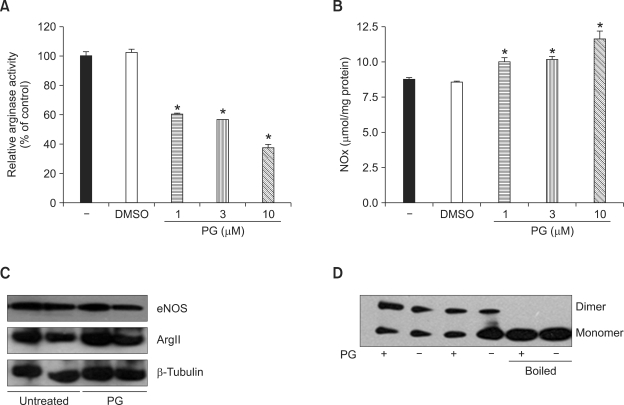
Given recent data suggesting that arginase reciprocally regulates NOS activity by limiting bioavailability of L-arginine (Ryoo et al 2006), we tested whether the PG-mediated decrease in arginase activity is associated with increased NOx production in HUVECs. As demonstrated in Figure 3, incubation of HUVECs with PG for 12 h markedly decreased arginase activity (A, PG vs. untreated, 60 ± 3% at 1 µM, 56 ± 1% at 3 µM, 37 ± 9% at 10 µM vs. 100 ± 9%, P < 0.01) and increased NOx production (B, PG vs. untreated, 9.99 ± 0.42% at 1 µM, 10.08 ± 0.43% at 3 µM, 11.55 ± 1.07 at 10 µM vs. 8.73 ± 0.3 µmol/mg protein, P < 0.01).
Figure 3.
Inhibition of arginase activity is associated with a reciprocal increase in endothelial NOx production in HUVECs. HUVECs were incubated with different concentrations of PG for 12 h. PG inhibited arginase activity in HUVECs in a dose-dependent manner (A, * vs. untreated, P < 0.01, n = 4) and reciprocally increased NOx production (B, * vs. untreated, P < 0.01, n = 4). Protein levels of arginase II and eNOS were analyzed after overnight incubation with PG (10 µM). Arginase II and eNOS protein levels were not significantly changed by PG treatment (C, n = 4). PG incubation (10 µM, 6 h), however, induced eNOS dimerization in low-temperature SDS-PAGE and western blot analysis. Boiled samples were used as a control.
To further test the effect of PG on the protein levels of arginase II and eNOS, Western blot analysis was performed with PG-treated HUVECs. As demonstrated in Figure 3C, PG had no significant effect on the protein levels of arginase II or eNOS. Next, we tested eNOS dimerization to elucidate a mechanism associated with increased NOx production by PG treatment. Interestingly, PG treatment (6 h) resulted in increased ratio of eNOS dimer/monomer from 0.70 ± 0.15 to 1.02 ± 0.09 (Figure 3D). Therefore, these data indicate that increased NOx production upon PG treatment was dependent on the increased bioavailability of L-arginine resulting from the inhibition of arginase activity, which itself is associated with eNOS dimerization (Ryoo et al., 2008).
PG inhibits arginase activity, increases NO production, and decreases ROS production in mice aorta
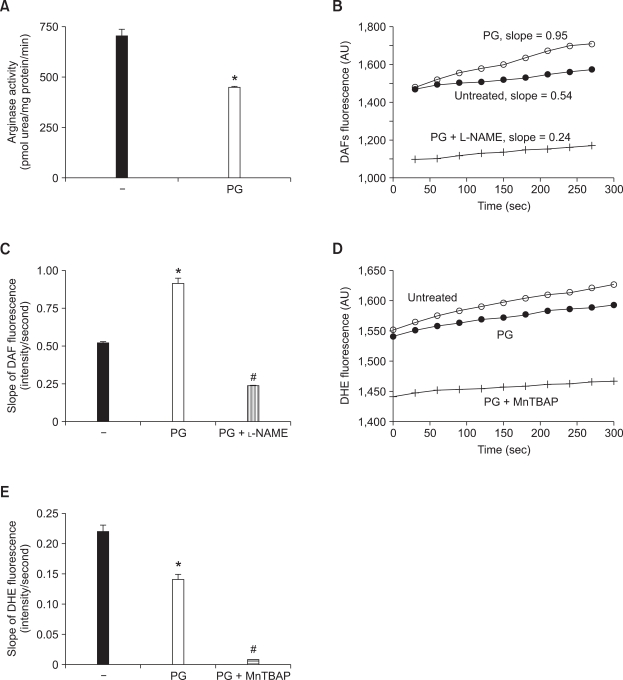
We next wished to determine whether or not increased NOx production in HUVECs translates into redox regulation in the endothelium of aortic tissue. Therefore, we measured the intensity of DAF-AM and DHE fluorescence at different time intervals. At first, PG treatment for overnight decreased arginase activity in aorta isolated from anethesized mice (Figure 4A, untreated vs. PG, 704 ±49 vs. 445 ±14 pmol Urea/mg protein/min, P < 0.01). We next tested whether PG-dependent arginase inhibition increased in NO production using an NO-sensitive fluorescence dye, DAF-FM diacetate (see materials and methods). Incubation of aorta with PG markedly increased the average slope of DAF fluorescence (Figure 4C; untreated vs. PG, 0.51 ± 0.02 vs. 0.91 ± 0.07). On the other hand, incubation of NG-Nitro-L-arginine methyl ester (L-NAME) acutely decreased the slope of DAF fluorescence (slope = 0.23 ± 0.01). This is consistent with previous observations in HUVECs. The representative traces of DAF fluorescence in the aortic endothelium were shown in Figure 4B.
Figure 4.
Arginase inhibition by PG increases NO production and decreases ROS generation in mouse aorta. Incubation of mice aortic rings with PG (40 µM, overnight≒16 h) resulted in significant decrease in arginase activity (A, * vs. untreated, P < 0.01, n = 4). (B) The pretreated aorta were loaded with DAF (5 µM) followed by measurement of fluorescence (endothelial side up). The graph shows representative traces of DAF fluorescence in PG- or PG plus L-NAME (10 µM)-treated aorta. (C) The slope of DAF fluorescence was monitored and then determined (* vs. untreated, P < 0.01; # vs. PG, P < 0.01; n = 4). (D) ROS production from the aorta was traced after preloading with DHE (5 µM). MnTBAP (10 µM) was used as a superoxide scavenger. (E) The slope of DHE fluorescence was determined based on cumulative data (* vs. untreated, P < 0.01; # vs. PG, P < 0.01; n = 4 mice).
To determine whether increased NO production upon arginase inhibition contributes to ROS reduction, we measured O2·- generation using the O2·--sensitive dye DHE in the endothelia of PG-treated aorta. The time-dependent intensity of DHE fluorescence was decreased by incubation of PG compared to untreated control (Figure 4E. slope of DHE fluorescence; * vs. untreated, 0.14 ± 0.01 vs. 0.22 ± 0.01). The DHE signal was completely quenched with MnTBAP (slope <0.01). The representative traces of DHE fluorescence are shown in Figure 4D.
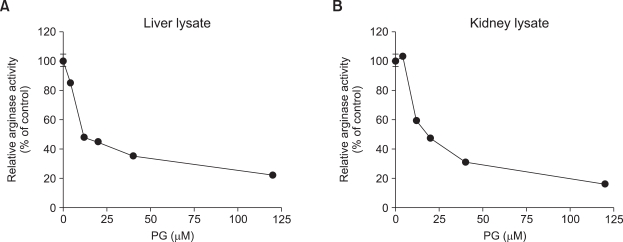
PG inhibits arginase activity in a dose-dependent manner
Given that PG inhibited the arginase activity of both isoforms, we wished to determine the efficacy of PG on arginase isoforms. Arginase activity was measured in the presence of different concentration of PG (from 0 to 120 µM) using liver lysate and kidney lysate. The 50% inhibitory concentrations (IC50) were 11.22 µM for the liver lysate and 11.06 µM for kidney lysate. The values were obtained using the software of Graphpad prizm 4.0 (Figure 5).
Figure 5.
PG inhibits the activities of arginase I and II in a dose-dependent manner. Arginase I (A) and II (B) activities were measured with increasing doses of PG. IC50 values of PG were 11.22 µM and 11.06 µM against arginase I and II, respectively. Relative enzyme activity is an average from 3 different experiments.
Discussion
With the idea that arginase modulates NOx production by NOS through limiting L-arginine substrate is emerging as a general mechanism for NOS regulation and appear to contribute to the pathobiology of a number of disease processes in which NO is dysregulated, we here demonstrate for the first time that PG, as an active component of Rhubarb, inhibits arginase activity and reciprocally increases NOx production.
PG-dependent inhibition of arginase activity contributed to increase of NO production in both HUVECs and endothelium of isolated mice aorta (Figures 3 and 4). These results are consistent with previous observations that arginase inhibition accentuates NO release in rat aortic endothelium (Berkowitz et al., 2003), bovine pulmonary endothelial cells (Chicoine et al., 2004), and a porcine coronary artery model (Zhang et al., 2001). The PG-mediated increase in NO production may result in eNOS coupling through increased L-arginine availability. During eNOS uncoupling, electrons flowing from the reductase domain in the heme to molecular oxygen rather than L-arginine, resulting in production of O2·- instead of NO. There are number of circumstances in which this may occur specifically tetrahydrobiopterin cofactor deficiency and relative L-arginine deficiency. As shown in Figures 3 and 4, the availability of substrate, cofactors as well as local eNOS microdomain concentration of L-arginine, rather than the expression level and abundance of the eNOS enzyme were critical to NO production. Regarding the physiological role of arginase in reciprocal NO regulation, arginase isoforms play important roles in regulating the synthesis of polyamines and proline (Li et al., 2001, 2002) and arginase inhibition blocked HUVECs proliferation, which is an emerging phenomenon associated with angiogenesis (Faffe et al., 2005). Furthermore, reciprocal regulation of NOS by arginase has been demonstrated in cells and organs in which NO is an important signaling molecule including the endothelium, cardiac myocytes, penis, airway, skin, and inflammatory cells(Bivalacqua et al., 2001; Berkowitz et al., 2003; Morris et al., 2004; Steppan et al., 2006; White et al., 2006; Bivalacqua et al., 2007; Hsu et al., 2007; Xu et al., 2007; Kim et al., 2008). It was demonstrated that arginase II activity is upregulated in atherosclerosis-prone mice and is associated with impaired endothelial NO production, endothelial dysfunction, vascular stiffness, and ultimately, aortic plaque development. Conversely, inhibition of endothelial arginase or deletion of the arginase II gene enhances NO production, restores endothelial function and aortic compliance, and reduces plaque burden. Therefore, arginase II representes a novel target for the prevention and treatment of atherosclerotic vascular disease (Ryoo et al., 2008). Furthermore, upregulation of arginase activity contributes to endothelial dysfunction in systematic and pulmonary hypertension, aging, diabetes and erectile dysfunction and to bronchodilatory dysfunction in asthma.
With together arginase inhibitory activity as presented in this report, Ngoc et al. (2008) also showed other biological activities of PG. PG inhibited lipoxygenase activity upto 66% at the concentration of 100 µM and IC50 value was 69 µM, although resveratrol had much potent activity, such that 92% inhibition at the same concentration and 12 µM at IC50 value. Furthermore, they showed that PG had a potency as a radical scavenger in ABTS·- radical scavenging assay in which resveratrol exhibited the most potent scavenging activity.
In summary, we present a novel molecule, piceatannol-3'-O-β-D-glucopyranoside (PG), that inhibited arginase activity and increased NO production in HUVECs. PG showed an IC50 of about 11 µM against arginase I and II. Furthermore, PG increased NO production and decreased ROS production in isolated mice aorta. Although the inhibitory potency of PG against arginase activity was a little higher compared to boronic acid analogues, identification of a new moiety that inhibits arginase activity would be very useful for the development of a new pharmaceutical compound. The continued development of the derivatives with increased specificity and selectivity against arginase isoforms may lead to novel therapies for the treatment of various diseases from NO dysregulation.
Methods
Materials
Arginase lysates were prepared from livers and kidneys of anesthetized C57BL/6 mice. MnTBAP (Mn(III) Tetra (4-benzoic acid) porphyrin chloride) and L-NAME (NG-nitro-L-arginine methyl ester) were obtained from Calbiochem. All reagents were purchased from Sigma unless otherwise stated.
PG preparation
PG was prepared as previously described (Ngoc et al., 2008). Briefly, the dried and milled rhizomes of rhubarb were extracted with ethanol. The extract was dried and then resolved again in ethylacetate. The ethylacetate-soluble fraction was diluted with acetone and subjected to silica gel column chromatography. The active fraction eluted with the mixture of chloroform and methanol was further purified with ODS column, eluting with methanol and water (1:2 to 3:1) to afford PG.
Cell culture
HUVECs were purchased from Cascade Biologics and maintained as supplier's protocol in Medium230 plus low-serum growth supplement (LSGS) at 37℃ in 5% CO2.
Arginase activity assay
Tissue lysates were prepared using lysis buffer (50 mM Tris-HCl, pH7.5, 0.1 mM EDTA and protease inhibitors) by homogenization at 4℃ followed by centrifugation for 20 min at 14,000 × g at 4℃. The supernatants were used to assay for arginase activity as previously described (Ryoo et al., 2006).
NOx measurement
NO was estimated by Griess reaction based upon the concentration of nitrate/nitrite (NOx) after conversion of nitrate to nitrite by nitrate reductase using the Nitric oxide assay kit (Calbiochem). The concentration of NOx in HUVECs was expressed as µmol/mg protein.
Western blot analysis
The livers or kidneys from C57BL/6 mice (10 weeks) were homogenized in the buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Nonidet P-40, 1 mM EDTA, 1 µg/ml of leupeptin, 1 µg/ml of pepstatin, 1 µg/ml of aprotinin, 1 mM phenylmethylsulfonylflouride, 1 mM sodium orthovanadate, and 1 mM NaF) and centrifuged for 30 min at 14,000 × g. The protein amount of the supernatant was analyzed by the Bradford method. Protein (100 µg) were separated in a 10% SDS-PAGE and then transferred to a nitrocellulose membrane (Bio-Rad). The blots were incubated with a monoclonal anti-arginase I (Santa Cruz), anti-arginase II (Santa Cruz), anti-endothelial nitric oxide synthase(eNOS, BD Bioscience), or anti-β-tubulin (BD bioscience) antibodies followed by the secondary antibody (Amersham). The signals were detected using an enhanced chemiluminescence detection reagent with X-ray films.
Determination of eNOS dimerization
Dimers and monomers of eNOS were separated using low-temperature SDS-PAGE as previously described (Takimoto et al., 2005). Band intensities were analyzed using NIH ImageJ Software.
Estimation of NO or ROS generation in isolated mice aorta using DAF-FM or DHE
Mice aortic rings were isolated and incubated overnight at 37℃, 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) medium containing 2% FBS and antibiotics (1×) in the presence of picaetannol-3'-O-β-D-glucopyranoside (50 µmol/L) (White et al., 2006). The aorta cut longitudinally and pinned to the bottom of a silgard-coated chamber (endothelial layer on top) filled with HEPES buffer (NaCl 120 mM, KH2PO4 2.6 mM, KCl 4 mM, CaCl2 2 mM, MgCl2 0.6 mM, HEPES 25 mM, glucose 14 mM, pH7.4). The chamber was allowed to equilibrate into the heating stage for 30 min at 37℃. The chamber allowed for static bath conditions during fluorescence measurements. Tissue background along with DAF-FM (4-Amino-5-methylamino-2',7'-difluorofluorescein) diacetate or DHE (dihydroethidine) fluorescence were measured using an Olympus 10× objective with optimized excitation and emission wavelength (DAF-FM, 470/525 nm; or DHE, 470/580), an intensified camera (Luca 658M-TL), and a custom image acquisition program (Cell software, Olympus). Following initial equilibrium, background fluorescence was recorded and aorta was allowed to return to room temperature for 15 min. The aorta were then loaded with 5 µM DAF-FM or 5 µM DHE (molecular Probes) in HEPES buffer for 45 min followed by washout of DAF-FM or DHE and a 20 min equilibrium period at 37℃. Fluorescence intensity was averaged (5 frames, 2 × 2 binning) from the entire field of view and recorded by the acquisition program. Changes in DAF-FM or DHE fluorescence were recorded once after washout of DAF-FM or DHE in order to establish baseline changes in intensity and then again following treatment with L-NAME (10-5 mol/L) or MnTBAP (10-5 mol/L). The slopes of changes in fluorescence over time were determined by linear regression in Origin (version 7.5, OrignLab Corp, Northampton, MA) and used for statistical comparison.
Statistics
All data are represented as mean ± S.D. of at least four independent experiments. An unpaired Student's t-test or 1-way ANOVA was used to assess significant differences. A value of P < 0.05 was accepted as significant.
Acknowledgements
This work was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0072449 and 2009-0084675) and the Korea Research Foundation Grand founded by the Korea government (MEST) (Regional Research Universities Program/Medical & Bio-materials Research Center).
Abbreviations
- ArgI
arginase I
- ArgII
arginase II
- DAF-FM
4-amino-5-methylamino-2',7'-difluorescein
- DHE
dihydroethidine
- eNOS
endothelial nitric oxide synthase
- HUVEC
human umbilical vein endothelial cells
- IC50
half maximal inhibitory concentration
- L-NAME
nitro-L-arginine methyl ester
- NO
nitric oxide
- NOx
nitrite and nitrate
- PG
piceatannol-3'-O-β-D-glucopyranoside
References
- 1.Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, Burke S, Shoukas AA, Nyhan D, Champion HC, Hare JM. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003;108:2000–2006. doi: 10.1161/01.CIR.0000092948.04444.C7. [DOI] [PubMed] [Google Scholar]
- 2.Bivalacqua TJ, Hellstrom WJ, Kadowitz PJ, Champion HC. Increased expression of arginase II in human diabetic corpus cavernosum: in diabetic-associated erectile dysfunction. Biochem Biophys Res Commun. 2001;283:923–927. doi: 10.1006/bbrc.2001.4874. [DOI] [PubMed] [Google Scholar]
- 3.Bivalacqua TJ, Liu T, Musicki B, Champion HC, Burnett AL. Endothelial nitric oxide synthase keeps erection regulatory function balance in the penis. Eur Urol. 2007;51:1732–1740. doi: 10.1016/j.eururo.2006.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chicoine LG, Paffett ML, Young TL, Nelin LD. Arginase inhibition increases nitric oxide production in bovine pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L60–L68. doi: 10.1152/ajplung.00194.2003. [DOI] [PubMed] [Google Scholar]
- 5.Choi SZ, Lee SO, Jang KU, Chung SH, Park SH, Kang HC, Yang EY, Cho HJ, Lee KR. Antidiabetic stilbene and anthraquinone derivatives from Rheum undulatum. Arch Pharm Res. 2005;28:1027–1030. doi: 10.1007/BF02977396. [DOI] [PubMed] [Google Scholar]
- 6.Choi KH, Kim JE, Song NR, Son JE, Hwang MK, Byun S, Kim JH, Lee KW, Lee HJ. Phosphoinositide-3-kinase is a novel target of piceatannol for inhibiting PDGF-BB-induced proliferation and migration in human aortic smooth muscle cells. Cardiovasc Res. 2009;85:836–844. doi: 10.1093/cvr/cvp359. [DOI] [PubMed] [Google Scholar]
- 7.Christianson DW. Arginase: structure, mechanism, and physiological role in male and female sexual arousal. Acc Chem Res. 2005;38:191–201. doi: 10.1021/ar040183k. [DOI] [PubMed] [Google Scholar]
- 8.Faffe DS, Flynt L, Mellema M, Whitehead TR, Bourgeois K, Panettieri RA, Jr, Silverman ES, Shore SA. Oncostatin M causes VEGF release from human airway smooth muscle: synergy with IL-1beta. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1040–L1048. doi: 10.1152/ajplung.00333.2004. [DOI] [PubMed] [Google Scholar]
- 9.Holowatz LA, Thompson CS, Kenney WL. L-Arginine supplementation or arginase inhibition augments reflex cutaneous vasodilatation in aged human skin. J Physiol. 2006;574:573–581. doi: 10.1113/jphysiol.2006.108993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu LL, Champion HC, Campbell-Lee SA, Bivalacqua TJ, Manci EA, Diwan BA, Schimel DM, Cochard AE, Wang X, Schechter AN, Noguchi CT, Gladwin MT. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood. 2007;109:3088–3098. doi: 10.1182/blood-2006-08-039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jang YJ, Kim JE, Kang NJ, Lee KW, Lee HJ. Piceatannol attenuates 4-hydroxynonenal-induced apoptosis of PC12 cells by blocking activation of c-Jun N-terminal kinase. Ann N Y Acad Sci. 2009;1171:176–182. doi: 10.1111/j.1749-6632.2009.04727.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim KO, Park SY, Han CW, Chung HK, Yoo DH, Han JS. Effect of sildenafil citrate on interleukin-1beta-induced nitric oxide synthesis and iNOS expression in SW982 cells. Exp Mol Med. 2008;40:286–293. doi: 10.3858/emm.2008.40.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klasen S, Hammermann R, Fuhrmann M, Lindemann D, Beck KF, Pfeilschifter J, Racke K. Glucocorticoids inhibit lipopolysaccharide-induced up-regulation of arginase in rat alveolar macrophages. Br J Pharmacol. 2001;132:1349–1357. doi: 10.1038/sj.bjp.0703951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Meininger CJ, Hawker JR, Jr, Haynes TE, Kepka-Lenhart D, Mistry SK, Morris SM, Jr, Wu G. Regulatory role of arginase I and II in nitric oxide, polyamine, and proline syntheses in endothelial cells. Am J Physiol Endocrinol Metab. 2001;280:E75–E82. doi: 10.1152/ajpendo.2001.280.1.E75. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Meininger CJ, Kelly KA, Hawker JR, Jr, Morris SM, Jr, Wu G. Activities of arginase I and II are limiting for endothelial cell proliferation. Am J Physiol Regul Integr Comp Physiol. 2002;282:R64–R69. doi: 10.1152/ajpregu.2002.282.1.R64. [DOI] [PubMed] [Google Scholar]
- 16.Louis CA, Reichner JS, Henry WL, Jr, Mastrofrancesco B, Gotoh T, Mori M, Albina JE. Distinct arginase isoforms expressed in primary and transformed macrophages: regulation by oxygen tension. Am J Physiol. 1998;274:R775–R782. doi: 10.1152/ajpregu.1998.274.3.R775. [DOI] [PubMed] [Google Scholar]
- 17.Matsuda H, Morikawa T, Toguchida I, Park JY, Harima S, Yoshikawa M. Antioxidant constituents from rhubarb: structural requirements of stilbenes for the activity and structures of two new anthraquinone glucosides. Bioorg Med Chem. 2001;9:41–50. doi: 10.1016/s0968-0896(00)00215-7. [DOI] [PubMed] [Google Scholar]
- 18.Matsuda H, Tewtrakul S, Morikawa T, Yoshikawa M. Anti-allergic activity of stilbenes from Korean rhubarb (Rheum undulatum L.): structure requirements for inhibition of antigen-induced degranulation and their effects on the release of TNF-alpha and IL-4 in RBL-2H3 cells. Bioorg Med Chem. 2004;12:4871–4876. doi: 10.1016/j.bmc.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Modolell M, Corraliza IM, Link F, Soler G, Eichmann K. Reciprocal regulation of the nitric oxide synthase/arginase balance in mouse bone marrow-derived macrophages by TH1 and TH2 cytokines. Eur J Immunol. 1995;25:1101–1104. doi: 10.1002/eji.1830250436. [DOI] [PubMed] [Google Scholar]
- 20.Moon MK, Kang DG, Lee JK, Kim JS, Lee HS. Vasodilatory and anti-inflammatory effects of the aqueous extract of rhubarb via a NO-cGMP pathway. Life Sci. 2006;78:1550–1557. doi: 10.1016/j.lfs.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 21.Morris SM, Jr, Kepka-Lenhart D, Chen LC. Differential regulation of arginases and inducible nitric oxide synthase in murine macrophage cells. Am J Physiol. 1998;275:E740–E747. doi: 10.1152/ajpendo.1998.275.5.E740. [DOI] [PubMed] [Google Scholar]
- 22.Morris CR, Poljakovic M, Lavrisha L, Machado L, Kuypers FA, Morris SM., Jr Decreased arginine bioavailability and increased serum arginase activity in asthma. Am J Respir Crit Care Med. 2004;170:148–153. doi: 10.1164/rccm.200309-1304OC. [DOI] [PubMed] [Google Scholar]
- 23.Nelin LD, Wang X, Zhao Q, Chicoine LG, Young TL, Hatch DM, English BK, Liu Y. MKP-1 switches arginine metabolism from nitric oxide synthase to arginase following endotoxin challenge. Am J Physiol Cell Physiol. 2007;293:C632–C640. doi: 10.1152/ajpcell.00137.2006. [DOI] [PubMed] [Google Scholar]
- 24.Ngoc TM, Minh PT, Hung TM, Thuong PT, Lee I, Min BS, Bae K. Lipoxygenase inhibitory constituents from rhubarb. Arch Pharm Res. 2008;31:598–605. doi: 10.1007/s12272-001-1199-0. [DOI] [PubMed] [Google Scholar]
- 25.Peyton KJ, Ensenat D, Azam MA, Keswani AN, Kannan S, Liu XM, Wang H, Tulis DA, Durante W. Arginase promotes neointima formation in rat injured carotid arteries. Arterioscler Thromb Vasc Biol. 2009;29:488–494. doi: 10.1161/ATVBAHA.108.183392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Que LG, Kantrow SP, Jenkinson CP, Piantadosi CA, Huang YC. Induction of arginase isoforms in the lung during hyperoxia. Am J Physiol. 1998;275:L96–L102. doi: 10.1152/ajplung.1998.275.1.L96. [DOI] [PubMed] [Google Scholar]
- 27.Ryoo S, Lemmon CA, Soucy KG, Gupta G, White AR, Nyhan D, Shoukas A, Romer LH, Berkowitz DE. Oxidized low-density lipoprotein-dependent endothelial arginase II activation contributes to impaired nitric oxide signaling. Circ Res. 2006;99:951–960. doi: 10.1161/01.RES.0000247034.24662.b4. [DOI] [PubMed] [Google Scholar]
- 28.Ryoo S, Gupta G, Benjo A, Lim HK, Camara A, Sikka G, Sohi J, Santhanam L, Soucy K, Tuday E, Baraban E, Ilies M, Gerstenblith G, Nyhan D, Shoukas A, Christianson DW, Alp NJ, Champion HC, Huso D, Berkowitz DE. Endothelial arginase II: a novel target for the treatment of atherosclerosis. Circ Res. 2008;102:923–932. doi: 10.1161/CIRCRESAHA.107.169573. [DOI] [PubMed] [Google Scholar]
- 29.Simon A, Plies L, Habermeier A, Martine U, Reining M, Closs EI. Role of neutral amino acid transport and protein breakdown for substrate supply of nitric oxide synthase in human endothelial cells. Circ Res. 2003;93:813–820. doi: 10.1161/01.RES.0000097761.19223.0D. [DOI] [PubMed] [Google Scholar]
- 30.Steppan J, Ryoo S, Schuleri KH, Gregg C, Hasan RK, White AR, Bugaj LJ, Khan M, Santhanam L, Nyhan D, Shoukas AA, Hare JM, Berkowitz DE. Arginase modulates myocardial contractility by a nitric oxide synthase 1-dependent mechanism. Proc Natl Acad Sci USA. 2006;103:4759–4764. doi: 10.1073/pnas.0506589103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, Lazzarino G, Paolocci N, Gabrielson KL, Wang Y, Kass DA. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest. 2005;115:1221–1231. doi: 10.1172/JCI21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vo TP, Madlener S, Bago-Horvath Z, Herbacek I, Stark N, Gridling M, Probst P, Giessrigl B, Bauer S, Vonach C, Saiko P, Grusch M, Szekeres T, Fritzer-Szekeres M, Jager W, Krupitza G, Soleiman A. Pro- and anti-carcinogenic mechanisms of piceatannol are activated dose-dependently in MCF-7 breast cancer cells. Carcinogenesis. 2009 doi: 10.1093/carcin/bgp199. [Epub Ahead of Print] [DOI] [PubMed] [Google Scholar]
- 33.White AR, Ryoo S, Li D, Champion HC, Steppan J, Wang D, Nyhan D, Shoukas AA, Hare JM, Berkowitz DE. Knockdown of arginase I restores NO signaling in the vasculature of old rats. Hypertension. 2006;47:245–251. doi: 10.1161/01.HYP.0000198543.34502.d7. [DOI] [PubMed] [Google Scholar]
- 34.Xu X, Gao X, Potter BJ, Cao JM, Zhang C. Anti-LOX-1 rescues endothelial function in coronary arterioles in atherosclerotic ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2007;27:871–877. doi: 10.1161/01.ATV.0000259358.31234.37. [DOI] [PubMed] [Google Scholar]
- 35.Yoo MY, Oh KS, Lee JW, Seo HW, Yon GH, Kwon DY, Kim YS, Ryu SY, Lee BH. Vasorelaxant effect of stilbenes from rhizome extract of rhubarb (Rheum undulatum) on the contractility of rat aorta. Phytother Res. 2007;21:186–189. doi: 10.1002/ptr.2042. [DOI] [PubMed] [Google Scholar]
- 36.Zhang C, Hein TW, Wang W, Chang CI, Kuo L. Constitutive expression of arginase in microvascular endothelial cells counteracts nitric oxide-mediated vasodilatory function. Faseb J. 2001;15:1264–1266. doi: 10.1096/fj.00-0681fje. [DOI] [PubMed] [Google Scholar]