Abstract
Background While dietary patterns that are both predictive of chronic disease and mortality have been identified, the confounding effects of cardiorespiratory fitness have not been properly addressed. The primary objective was to assess the relation between dietary patterns with all-cause mortality, while controlling for the potentially confounding effects of fitness.
Methods This was a prospective cohort study. Participants consisted of 13 621 men and women from the Aerobics Center Longitudinal Study (ACLS). Participants completed a clinical exam and 3-day diet record between 1987 and 1999. Participants were followed for mortality until 2003. Reduced rank regression (RRR) was used to identify dietary patterns that predicted unfavourable total and high-density lipoprotein-cholesterol, triglyceride, glucose, blood pressure, uric acid, white blood cell and body mass index values.
Results One primary dietary pattern emerged and was labelled the Unhealthy Eating Index. This pattern was characterized by elevated consumption of processed and red meat, white potato products, non-whole grains, added fat and reduced consumption of non-citrus fruits. The hazard ratio for all-cause mortality in the fifth vs the first quintile of the Unhealthy Eating Index was 1.40 (1.02–1.91). This risk estimate was reduced by 13.5 and 55.0% after controlling for self-reported physical activity and fitness, respectively.
Conclusion In this study the association between diet and overall mortality was, in large part, confounded by fitness.
Keywords: All-cause mortality, cardiorespiratory fitness, reduced rank regression
Background
A prudent diet is recognized by the scientific and medical communities and the general public as being a fundamental part of healthy living. Historically, studies in nutrition and health have examined chronic disease risk in relation to a single nutrient or food.1,2 However, because people eat meals comprised of a variety of nutrients and foods, studying individuals’ complete diets, through their overall dietary pattern, more closely approximates real-world eating conditions.
Recent literature reviews have summarized the evidence linking dietary patterns with chronic disease and mortality risk.3–6 Their findings suggest that dietary patterns characterized by a high consumption of processed, refined and fried foods and a low consumption of fresh fruit, vegetables and whole grains, are a modest to strong risk factor for chronic disease and mortality risk in both sexes.3–6
When studying the relationship between dietary patterns with chronic disease and mortality risk, it is important to realize that self-report biases in dietary measures can distort estimates of effect.7,8 It is also important to consider the potentially confounding effect of other lifestyle characteristics. Physical activity, for example, is related to both dietary patterns9 and chronic disease risk.10,11 While most studies assessing the relation between dietary patterns and chronic disease risk have controlled for physical activity, the physical activity measures employed have been, without exception, based on self-reported questionnaires. Such measures of physical activity are only modestly correlated with objective measures obtained using criterion methods.12–14 Thus, the potentially confounding effects of physical activity on the relation between dietary patterns and chronic disease risk may not have been fully captured in the existing literature. One approach to objectively determine a persons’ recent physical activity level is to assess their cardiorespiratory fitness (hereafter referred to as fitness).12,15
The primary objective of this study was to assess the relationship between dietary patterns with mortality risk from all-cause and cardiovascular disease (CVD) while controlling for the potentially confounding effects of fitness. The secondary objective was to examine the combined effects of dietary patterns and fitness on mortality risk.
Methods
Study overview
The study was divided into two parts, with the Aerobics Center Longitudinal Study (ACLS) cohort forming the subject pool for both. In Part A, data reduction techniques were employed to identify dietary patterns from 3-day food records, which were related to clinical markers of chronic disease and mortality risk. In Part B, the risk of CVD and all-cause mortality according to the dietary pattern score (identified in Part A) were determined. The potential confounding influence of physical activity and fitness on the relation between dietary patterns and mortality were considered.
Study population
The study sample consisted of 13 621 participants from the ACLS who completed a standardized medical examination and 3-day diet record between 1987 and 1999. The ACLS is a prospective, observational study of men and women who participated in a clinical examination at the Cooper Clinic in Dallas, TX, USA. ACLS participants are predominantly (>95%) non-Hispanic white, well educated, employed in professional or executive occupations and of middle to high socio-economic status. Participants who did not achieve ≥85% of their age-predicted maximal heart rate on the treadmill test were excluded.
The standardized data collection procedures employed in the ACLS are described in greater detail in previous studies.11,16 Briefly, data collected included a medical history questionnaire, a thorough medical and physical exam, fasting blood chemistry analyses, symptom-limited maximal exercise test for the measurement of fitness and a dietary assessment. The medical history questionnaire was self administered and included a demographic, family history and personal health habits questionnaire. Physical examination procedures were conducted in a standardized manner by trained personnel. All participants gave written informed consent. The study was reviewed and approved annually by the institutional review board at the Cooper Institute.10,11,17 Ethics approval for the completion of the secondary analyses for this study was granted by the Queen's University Health Sciences Ethics Review Board.
Part A: identification of dietary patterns
Several different approaches can be used to measure individuals’ dietary patterns, as reviewed elsewhere.18,19 Of the available methods, reduced rank regression (RRR) has been shown to be the best approach for developing dietary patterns that are predictive of chronic disease risk,20 and was, therefore, employed here. RRR is a statistical data reduction technique that determines linear functions of predictors (e.g. food groups from diet records) by explaining as much variation as possible in a group of response variables (e.g. biomarkers that are in the pathways between the foods and health outcomes).18,19
Dietary variables (predictor variables for RRR)
The dietary assessment consisted of a 3-day diet record that required respondents to keep detailed records of everything they ate over 2 pre-assigned weekdays and 1 weekend day. Participants were provided with written instructions on how to accurately describe foods and estimate portion sizes. Participants kept an on-going, real-time written record of foods consumed during and between meals, including assessing portion sizes in common household measures. Registered dieticians at the Cooper Clinic coded and analysed the diet records using the Cooper Clinic Nutrition and Exercise Evaluation system.17 This provided detailed dietary information on the overall diet such as the number of foods consumed from specific food groups, total caloric content, the volume of micronutrients (vitamins and minerals) and the volume and caloric content of macronutrients (fat, protein, carbohydrate and alcohol).
For the purpose of this study, food items from the diet records were initially divided into specific food groups. For example, nut varieties (peanuts, cashews and almonds) were combined into an overall nuts variable. These food groups were then used as the predictor variables in the RRR analysis. The following 24 food groups were considered: alcohol, added sugar, cheese, milk, yogurt, added fats, citrus fruit, non-citrus fruit, non-whole grains, whole grains, legumes, eggs, seafood, processed meat, red meat, nuts, poultry, soy, deep-yellow vegetables, green vegetables, other vegetables, potatoes, starchy vegetables and tomatoes. Food components included within each food group are listed in Appendix 1A (available as Supplementary data at IJE online).
Clinical variables (response variables for RRR)
Eight biomarkers that are related to diet, predictive of chronic disease and mortality risk, available within the ACLS database, and routinely measured within the clinical setting were used as response variables in the RRR analysis. The biomarkers consisted of the body mass index [BMI = weight (kg)/height (m)2] as a measure of adiposity, blood pressure, total cholesterol, high-density lipoprotein (HDL)-cholesterol, triglycerides, uric acid, white blood cell count and fasting glucose. The response variables represent the biological pathways by which dietary patterns may influence chronic disease and mortality risk.
Height and weight were measured using a stadiometre and balance beam scale, respectively, and were used to calculate the BMI. Resting systolic and diastolic blood pressure were measured with a mercury sphygmomanometer following standard procedures.16 Mean arterial blood pressure was calculated by adding two-third of diastolic to one-third of systolic blood pressure.21 Fasting plasma levels of total cholesterol, HDL-cholesterol, triglycerides, white blood cell count and glucose were determined by automated techniques in the Cooper Clinic laboratory, which meets quality control standards of the Centres for Disease Control and Prevention Lipid Standardization Program.16 Finally, serum uric acid was measured by the URCA method using a Dimension clinical chemistry system.22
Part B: relation between dietary patterns with mortality
The purpose of this section was to determine whether the dietary patterns identified in Part A were related to CVD mortality and all-cause mortality, and whether these associations were confounded by physical activity and fitness.
Cardiovascular disease and all-cause mortality
After their baseline exam, participants were followed until they died, or until 31 December 2003. Possible decedents were identified from the National Death Index and verified with official death certificates from their home states. The National Death Index has a sensitivity of 96% and specificity of 100% in cohort studies.23 A nosologist coded the death certificates for the underlying and up to four contributing causes of death. CVD mortality was defined by International Classification of Diseases, Ninth Revision (ICD-9) codes 390−449.9 before 1999 and Tenth Revision (ICD-10) codes I00−I78 from 1999 to 2003.
Covariates
Covariates included age, sex, year of examination, parental history of premature CVD (0 = no, 1 = either parent had stroke or myocardial infarction before age 50), history of cancer (0 = no, 1 = any form of cancer prior to baseline), prevalent CVD (0 = no, 1 = stroke or myocardial infarction prior to baseline), smoking (never, former and current), alcohol, physical activity and fitness. Alcohol was coded as heavy drinker (5 drinks or more/week) or light or non-drinker (less than 5 drinks/week). One unit of alcohol was defined as 12 ounces (3.41 dl) of beer, 5 ounces (1.42 dl) of wine or 1.5 ounces (0.43 dl) of liquor. Physical activity was measured through a self-report questionnaire, which assessed participant's physical activity patterns over the last 3 months.24 If the participant reported partaking in an activity, they were asked to provide additional information about the frequency and distance or time spent in the activity. This information was then used to divide participants into three categories: (i) inactive (no activity); (ii) moderately active (sporting or leisure-time physical activity other than walking or jogging; or walk or jog up to 16 km/week); and (iii) highly active (walk or jog ≥16 km/week).24 Cardiorespiratory fitness was assessed by a symptom-limited maximal exercise treadmill test using a modified Balke protocol.25 Participants began walking on the treadmill with no elevation at 5.3 km/h. At the end of the first minute the elevation was increased to 2%. Thereafter, the elevation was increased 1% /min until the 25th minute. For the few participants who were able to continue >25 min, elevation remained constant and speed was increased each minute by 0.32 km/h. Participants continued the test to the limits of volitional fatigue. We defined low, moderate and high fitness according to the lowest 20%, the middle 40% and the upper 40%, respectively, of the age- and sex-specific distribution of maximal exercise duration in the overall ACLS population. These cut-points are from previous reports on the relation between fitness and all-cause mortality in the ACLS.10 Because there is no widely accepted categorization of fitness, and because we wanted to maintain consistency in our study methods, we continue to use the above approach. Note that total energy intake was not included as a covariate in any of the regression analyses as we felt that this was, at least in part, within the causal pathway by which dietary patterns influence chronic disease and mortality risk. Furthermore, caloric intake is positively influenced by physical activity.26 Regardless, inclusion of absolute (kcal/day) or relative (kcal/kg body weight/day) energy intake values as an additional covariate in the regression models had a negligible effect on the risk estimates that are presented (data not shown).
Statistical analysis
Part A: identification of dietary patterns
RRR was used to identify dietary patterns that are predictive of chronic disease risk. RRR is a true multivariate statistical test in that several predictor and several response variables are included within a single analysis. The goal of RRR is to identify linear functions of the predictor variables (e.g. 24 food groups) by explaining as much variation as possible in the group of response variables (e.g. eight biomarkers indicative of chronic disease risk).18,19 In order to choose the number of dietary patterns, a random sample cross-validation procedure was performed27,28 in which 50 random subsets (each had 2% of the data randomly selected) were used as test sets. For each test set, the complete dataset, excluding observations in the test set, formed a training set. RRR was performed 50 times on the 50 different training sets. Their predictions to the observations in the corresponding test sets were summarized [predicted residual sum of squares (PRESS)] and compared with several different dietary patterns. The choice of the number of dietary patterns for use was based on the comparison of these PRESSes. Once the appropriate number of patterns was determined, those food groups with loadings of ≥0.2 within each dietary pattern were retained.20,29 RRR was then repeated using only those food groups retained.
Part B: relation between dietary patterns with mortality
Quintiles of the dietary pattern scores developed in Part A were created. Significant differences across quintiles for the covariates were assessed using a χ2 test for categorical variables and general linear model for continuous variables. Linear regression for continuous covariates and polytomous regression for categorical variables were further applied to identify trends across quintiles.30,31
Next, quintiles of the dietary pattern scores were used in a series of Cox proportional hazard regression analyses to predict the presence of CVD and all-cause mortality over the follow-up period. For each outcome, four models were run. The first model included age, sex and year of examination as covariates. In addition to these covariates, the second model included smoking, alcohol, parental history of CVD, prevalent CVD and history of cancer. The third model included the aforementioned covariates as well as self-reported physical activity categories. The fourth model differed from the third in that physical activity was replaced by fitness categories. To examine the extent to which physical activity and fitness confounded the effect of dietary patterns on chronic disease and mortality risk, we examined the magnitude of change in the hazard ratios when physical activity or fitness were excluded (Model 2) and included (Models 3 and 4) in the models. The proportion of the risk of a poor dietary pattern explained by the confounding effects of physical activity (or fitness) were then calculated as: (HRModel2–HRModel3(or Model4))/(HRModel2 –1) × 100%.
Cox regression analyses also were used to examine the relationship between fitness categories, and combination of dietary and fitness categories, with the study outcomes.
Data management and statistical analyses were performed with SAS version 9.1 (SAS Institute, Cary, NC, USA).
Results
Part A: identification of dietary patterns
Baseline characteristics of the participants are summarized in Table 1. Participants were primarily male (75.7%) with an average age at baseline of 47 years (range 20−84). Based on self-reported physical activity, 49.1% of participants were moderately active, while 25.0% were highly active. Sixty percent, however, had a high cardiorespiratory fitness. The average caloric intake was 2151 kcal/day. Participants had an average BMI of 25.7 kg/m2, with 39.7% falling in the overweight range and 13.0% falling in the obese range. Within the cohort, 190 participants had a history of CVD and 792 had a history of cancer. Over the follow-up period there were 445 deaths within the cohort, 136 of which were attributed to CVD.
Table 1.
Baseline characteristics of participants,a ACLS 1987–99
| Variable | Mean or prevalence |
|---|---|
| General characteristics | |
| N | 13 621 |
| Age (years) | 47.0 (10.2) |
| Men (%) | 75.7 |
| Women (%) | 24.3 |
| Physical activity (%) | |
| Inactive | 25.9 |
| Moderately active | 49.1 |
| Highly active | 25.0 |
| Cardiorespiratory fitness (%) | |
| Low | 9.0 |
| Moderate | 30.8 |
| High | 60.2 |
| Smoking status (%) | |
| Never smoker | 55.9 |
| Former smoker | 33.2 |
| Current smoker | 10.9 |
| Alcohol intake (%) | |
| Light or non-drinker (less than 5 drinks/week) | 86.8 |
| Heavy drinker (5 or more drinks/week) | 13.2 |
| Energy intake | |
| Absolute (kcal/day) | 2151 (711) |
| Relative to weight (kcal/kg/day) | 12.6 (4.4) |
| Clinical variables | |
| BMI (kg/m2) | 25.7 (4.2) |
| Mean arterial pressure (mm Hg) | 94 (10) |
| Total cholesterol (mg/dl) | 207.4 (40.3) |
| HDL-cholesterol (mg/dl) | 51.6 (15.6) |
| Triglycerides (mg/dl) | 123.3 (90.9) |
| Fasting glucose (mg/dl) | 99.2 (17.1) |
| Uric acid (mg/dl) | 5.5 (1.5) |
| White blood cell count (cells/mm3) | 5903 (1444) |
aData presented as mean (SD) for continuous variables or prevalence for categorical variables.
The correlation matrix between the biomarkers is shown in Table 2. The correlations were weak to modest in strength, with the strongest found between BMI and uric acid (r = 0.47) and the weakest between total cholesterol and HDL-cholesterol (r = 0.06).
Table 2.
Pearson correlation coefficientsa (r-values) between the biomarker response variables, ACLS 1987–99
| Mean arterial pressure | Total cholesterol | HDL- cholesterol | Triglycerides | Fasting glucose | Uric acid | White blood cell count | |
|---|---|---|---|---|---|---|---|
| BMI | 0.35 | 0.19 | −0.39 | 0.35 | 0.24 | 0.47 | 0.23 |
| Mean arterial pressure | 0.20 | −0.17 | 0.24 | 0.20 | 0.30 | 0.09 | |
| Cholesterol | 0.06 | 0.36 | 0.12 | 0.21 | 0.09 | ||
| HDL-cholesterol | −0.42 | −0.15 | −0.40 | −0.18 | |||
| Triglycerides | 0.22 | 0.34 | 0.20 | ||||
| Fasting glucose | 0.14 | 0.09 | |||||
| Uric acid | 0.16 |
aAll correlations had P < 0.0001.
Results from the RRR suggested that five dietary patterns existed, with 18 of the 24 food groups having loadings of ≥0.2 for at least one of these patterns. Table 3 shows the percent variation in the multivariate biomarker index (labelled ‘Total’) and the eight individual biomarkers that were explained by the five identified dietary patterns. Together, the five patterns explained 5.66% of the variation within the total biomarker index. Pattern 1 explained 4.33% of the overall variation with the other four patterns only explaining an additional 1.33% between them.
Table 3.
Percent variation in clinical biomarkers explained by the five identified dietary patterns, ACLS 1987–99
|
Dietary pattern |
||||||
|---|---|---|---|---|---|---|
| Clinical biomarker | 1 (Unhealthy Eating Index) | 2 | 3 | 4 | 5 | Totala |
| Total biomarker indexb | 4.33 | 0.86 | 0.29 | 0.11 | 0.07 | 5.66 |
| Uric acid | 10.17 | 0.57 | 0.54 | 0.04 | 0.09 | 11.39 |
| BMI | 9.81 | 0.03 | 0.16 | 0.16 | 0.1 | 10.26 |
| HDL-cholesterol | 5.51 | 3.67 | 0.03 | 0.17 | 0.01 | 9.39 |
| Triglycerides | 3.56 | 0.07 | 0.01 | 0.06 | 0.04 | 3.74 |
| White blood cell count | 2.14 | 0.03 | 1.24 | 0.01 | 0.02 | 3.44 |
| Mean arterial pressure | 1.69 | 0.16 | 0.24 | 0.03 | 0.26 | 2.38 |
| Fasting glucose | 1.08 | 0.16 | 0.01 | 0.08 | 0.06 | 1.39 |
| Total cholesterol | 0.70 | 2.22 | 0.13 | 0.34 | 0.02 | 3.28 |
a‘Total’ column represents the total percentage of variation in biomarkers explained by the five dietary patterns combined.
b‘Total biomarker index’ row represents the total percentage of variation for all eight biomarkers combined explained by the identified dietary patterns.
It is important to note that the patterns that are derived from RRR are mutually independent. Thus, because the amount of variation explained by Pattern 1 accounted for 76.50% of the total variation explained, this pattern is focused on in the rest of the article. As is shown in Table 3, Pattern 1 explained 10.17% of the variation in uric acid, 9.81% in BMI, 5.51% in HDL-cholesterol, 3.56% in triglycerides, 2.14% in white blood cell count, 1.69% in mean arterial pressure, 1.08% in glucose, and 0.70% in total cholesterol.
The six key food groups that contributed to dietary Pattern 1 (e.g. factor loadings ≥0.2 or more for Pattern 1) were added fats, non-whole grains, processed meat, red meat, white potato products and non-citrus fruits. Their respective loadings and correlation with the pattern score are shown in Table 4. When considered amongst all 18 food groups, the six key food groups explained 76.4% of the variation in the pattern score; the most important contributors were meat and non-whole grains. More details about the other four derived dietary patterns are contained within Appendix 1B (available as Supplementary data at IJE online).
Table 4.
Food group components of the Unhealthy Eating Index (the first dietary pattern) and corresponding loadings, ACLS 1987–99
|
RRR using 18 food groups |
RRR using 6 key food groups only |
||||||
|---|---|---|---|---|---|---|---|
| Food group | Food group contents | Standardized parameter score (loading) | Pearson correlation with dietary pattern score | Explained proportion of score variationa (%) | Standardized parameter score (loading) | Pearson correlation with dietary pattern score | Explained proportion of score variationa (%) |
| Red meat | Cooked meat from beef, pork, veal, lamb and game | 0.50 | 0.69 | 25.00 | 0.57 | 0.77 | 32.74 |
| Added fat | Added butter, margarine, oils and dressings | 0.37 | 0.51 | 13.69 | 0.42 | 0.57 | 17.86 |
| White potato products | White potatoes including such things as french fries and mashed potatoes | 0.35 | 0.48 | 12.25 | 0.40 | 0.54 | 16.33 |
| Non-whole grains | All grain products that are enriched, unenriched, or fortified (e.g. white bread, white pasta, cereals) | 0.31 | 0.42 | 9.61 | 0.35 | 0.47 | 12.50 |
| Processed meat | Frankfurters, sausages and luncheon meats | 0.25 | 0.34 | 6.25 | 0.28 | 0.38 | 7.99 |
| Non-citrus fruit | All fruit except for citrus (lemon, lime, orange, grapefruit) | −0.31 | −0.43 | 9.61 | −0.35 | −0.48 | 12.58 |
| Total | 76.41 | 100 | |||||
aCalculated as the square of the standardized parameter score multiplied by 100%.
The RRR analysis was repeated after only including the six key food groups for dietary Pattern 1. The loadings and correlations for that analysis are also shown in Table 4. Given the foods that contributed to this pattern and the direction of their factor loadings, this food pattern will be referred to as the ‘Unhealthy Eating Index’ for the remainder of the manuscript. The Unhealthy Eating Index score was based on the RRR analysis that was limited to the six key food groups in Table 4.
Sensitivity analyses
Two different sensitivity analyses were conducted to test the robustness of the RRR findings. First, the RRR analysis was repeated eight times to a reduced set of response variables. In each instance one of the eight biomarkers was removed from the complete set. The first dietary pattern that was identified in these eight repeated RRR analyses was comparable with the first pattern that was identified using all eight response variables (e.g. the Unhealthy Eating Index). There were some minor exceptions. In particular, when BMI, HDL-cholesterol and white blood cell count were removed, yogurt, alcohol and poultry, respectively, also contributed to the dietary pattern.
Next, the sample was randomly split into half and separate RRR analyses were run in the two subsamples. The dietary pattern developed in the first subsample was identical to the Unhealthy Eating Index. The dietary pattern developed in the second subsample contained yogurt in addition to the six food groups found in the Unhealthy Eating Index.
Part B: relation between dietary pattern with mortality
The cohort was divided into quintiles based on the Unhealthy Eating Index developed in Part A. The characteristics of participants across Unhealthy Eating Index quintiles are shown in Table 5. The quintiles were different from each other for each of the variables listed in the table. The mean age of participants decreased from quintiles 1 to 5 suggesting that younger participants had poorer dietary habits. Total caloric intake increased steadily across quintiles, with quintile 5 having absolute (kcal/day) caloric intake values that were 31.3% higher than quintile 1. Those in quintile 1 also had a lower BMI when compared with those in quintile 5 (24.3 vs 27.6 kg/m2). The values of the remaining clinical variables also increased steadily from quintiles 1 to 5. Finally, there were gradients in physical activity and fitness across the Unhealthy Eating Index quintiles. The percentage of those in the inactive category increased from 15.2% in quintile 1 to 39.5% in quintile 5. A similar trend was seen for fitness where the prevalence of those in the low-fitness category increased from 4.6% in quintile 1 to 16.8% in quintile 5.
Table 5.
Baseline characteristicsa across quintiles of the Unhealthy Eating Index, ACLS 1987–99
|
Quintiles of the Unhealthy Eating Indexb |
|||||
|---|---|---|---|---|---|
| Q1 (Healthiest) | Q2 | Q3 | Q4 | Q5 (Least Healthy) | |
| General characteristics | |||||
| N | 2724 | 2724 | 2725 | 2724 | 2724 |
| Age (years) | 50.0 (10.7) | 48.1 (10.0) | 47.3 (10.2) | 45.2 (9.6) | 43.7 (9.3) |
| Men (%) | 60.7 | 65.5 | 74.6 | 83.6 | 94.1 |
| Women (%) | 39.3 | 34.5 | 25.4 | 16.4 | 5.9 |
| Physical activity (%) | |||||
| Inactive | 15.2 | 19.9 | 25.8 | 29.0 | 39.5 |
| Moderately active | 49.7 | 51.6 | 50.0 | 49.5 | 44.7 |
| Highly active | 35.1 | 28.5 | 24.2 | 21.5 | 15.8 |
| Cardiorespiratory fitness (%) | |||||
| Low | 4.6 | 5.4 | 7.6 | 10.6 | 16.8 |
| Moderate | 21.6 | 27.0 | 30.6 | 34.5 | 40.3 |
| High | 73.8 | 67.6 | 61.8 | 54.9 | 42.9 |
| Smoking status (%) | |||||
| Never smoker | 60.1 | 57.9 | 56.0 | 55.3 | 50.3 |
| Former smoker | 35.0 | 24.7 | 34.1 | 31.4 | 31.1 |
| Current smoker | 5.0 | 7.4 | 9.9 | 13.4 | 18.6 |
| Alcohol intake (%) | |||||
| Light or non-drinker (less than 5 drinks/week) | 92.9 | 90.3 | 87.8 | 83.3 | 79.6 |
| Heavy drinker (5 drinks or more/week) | 7.1 | 9.7 | 12.2 | 16.7 | 20.5 |
| Energy intake | |||||
| Absolute (kcal/day) | 1834 (576) | 1922 (558) | 2064 (553) | 2265 (585) | 2668 (894) |
| Relative to weight (kcal/kg/day) | 11.9 (4.2) | 11.9 (3.8) | 12.3 (3.9) | 12.8 (3.9) | 14.1 (5.5) |
| Clinical variables | |||||
| BMI (kg/m2) | 24.3 (3.9) | 24.9 (3.6) | 25.5 (3.9) | 26.3 (4.3) | 27.6 (4.5) |
| Mean arterial pressure (mm Hg) | 92 (11) | 93 (10) | 93 (10) | 94 (10) | 96 (10) |
| Total cholesterol (mg/dl) | 203.6 (39.2) | 206.2 (40.7) | 207.6 (40.0) | 208.3 (40.8) | 211.3 (40.5) |
| HDL-cholesterol (mg/dl) | 55.9 (16.7) | 54.0 (16.3) | 52.3 (15.6) | 49.5 (14.4) | 46.3 (13.0) |
| Triglycerides (mg/dl) | 101.2 (65.1) | 113.0 (86.2) | 119.8 (78.6) | 133.1 (97.0) | 149.2 (112.6) |
| Fasting glucose (mg/dl) | 97.1 (14.2) | 98.5 (16.5) | 98.8 (14.4) | 100.1 (19.8) | 101.6 (19.3) |
| Uric acid (mg/dl) | 5.0 (1.4) | 5.2 (1.4) | 5.5 (1.4) | 5.8 (1.4) | 6.1 (1.4) |
| White blood cell count (cells/mm3) | 5641 (1411) | 5764 (1418) | 5906 (1427) | 5964 (1394) | 6239 (1505) |
aData presented as mean (SD) for continuous variables or prevalence for categorical variables.
bP < 0.05 between quintiles for all variables.
Table 6 lists the results of the regression analyses in which quintiles of the Unhealthy Eating Index were associated with CVD and all-cause mortality over the follow-up period. After adjusting for age, sex and year of examination in Model 1, none of the hazard ratios for CVD mortality in quintiles 2−5 was appreciably different from quintile 1. For all-cause mortality, the risks were not elevated in quintiles 2−4 relative to quintile 1. However, quintile 5 had a modestly increased hazard ratio of 1.39 [95% confidence interval (CI) 1.02−1.90]. For each of the two outcome measures, further adjustment for family history of CVD, prevalent CVD, history of cancer, smoking and alcohol in Model 2 had a minimal impact on the risk estimates that were observed in Model 1.
Table 6.
Risk of cardiovascular disease mortality, and all-cause mortality across quintiles of the Unhealthy Eating Index; ACLS 1987–99
| Quintiles of Unhealthy Eating Index |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 (Healthiest) |
Q2 |
Q3 |
Q4 |
Q5 (Least Healthy) |
||||||
| Referent | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | P for trend | |
| CVD mortality | ||||||||||
| Model 1a | 1.00 | 1.19 | 0.74–1.93 | 0.93 | 0.55–1.57 | 1.26 | 0.74–2.15 | 1.36 | 0.77–2.42 | 0.36 |
| Model 2b | 1.00 | 1.25 | 0.77–2.02 | 0.98 | 0.58–1.67 | 1.29 | 0.75–2.23 | 1.38 | 0.77–2.47 | 0.34 |
| Model 3c | 1.00 | 1.25 | 0.77–2.03 | 0.99 | 0.58–1.68 | 1.30 | 0.75–2.25 | 1.39 | 0.77–2.53 | 0.33 |
| Model 4d | 1.00 | 1.23 | 0.76–2.00 | 0.92 | 0.54–1.57 | 1.16 | 0.67–2.01 | 1.10 | 0.61–2.01 | 0.86 |
| Deaths (total 136) | 35 | 32 | 24 | 24 | 21 | |||||
| All-cause mortality | ||||||||||
| Model 1a | 1.00 | 1.05 | 0.80–1.37 | 1.06 | 0.80–1.39 | 1.03 | 0.76–1.41 | 1.39 | 1.02–1.90 | 0.10 |
| Model 2b | 1.00 | 1.06 | 0.81–1.39 | 1.09 | 0.82–1.43 | 1.04 | 0.76–1.43 | 1.40 | 1.02–1.91 | 0.10 |
| Model 3c | 1.00 | 1.05 | 0.80–1.38 | 1.07 | 0.80–1.41 | 1.03 | 0.75–1.41 | 1.36 | 0.99–1.87 | 0.15 |
| Model 4d | 1.00 | 1.05 | 0.80–1.37 | 1.03 | 0.78–1.36 | 0.96 | 0.70–1.31 | 1.18 | 0.86–1.64 | 0.55 |
| Deaths (total 445) | 118 | 95 | 91 | 67 | 74 | |||||
aAdjusted for age, gender and year of examination.
bAdjusted for age, gender, year of examination, parental history of cardiovascular disease, history of cardiovascular disease, history of cancer, smoking and alcohol.
cAdjusted for age, gender, year of examination, parental history of cardiovascular disease, history of cardiovascular disease, history of cancer, smoking, alcohol and physical activity.
dHR: hazard ratio. Adjusted for age, gender, year of examination, parental history of cardiovascular disease, history of cardiovascular disease, history of cancer, smoking, alcohol and fitness.
We next considered whether the relation between the Unhealthy Eating Index and all-cause mortality was confounded by physical activity (Table 6, Model 3) and fitness (Table 6, Model 4). The risk estimates for all-cause mortality did not change considerably after further controlling for physical activity (Model 2 vs Model 3); however, the risk estimates changed considerably after further controlling for fitness (Model 2 vs Model 4). Only 10.0% of the increased risk for all-cause mortality for participants within quintile 5 of the Unhealthy Eating Index was due to the confounding effect of physical activity. Conversely, 55.0% of the increased risk for all-cause mortality for participants in quintile 5 of the Unhealthy Eating Index was due to the confounding effect of fitness.
The differences in risk across high, moderate and low physical activity categories for model 3 in Table 6 were as follows: 1.00, 0.70 (95% CI 0.48−1.04) and 1.01 (95% CI 0.64−1.60) for CVD mortality; and 1.00, 0.89 (95% CI 0.71−1.11) and 1.16 (95% CI 0.89−1.49) for all-cause mortality. When looking at Model 4, the differences in risk across high, moderate and low fitness categories were: 1.00, 1.26 (95% CI 0.85−1.88) and 3.16 (95% CI 1.99−5.12) for CVD mortality; and 1.00, 1.32 (95% CI 1.06−1.62) and 2.41 (95% CI 1.80−3.22) for all-cause mortality. Removal of the Unhealthy Eating Index quintile from the models had a minimal impact on the risk estimates for both physical activity and fitness (data not shown).
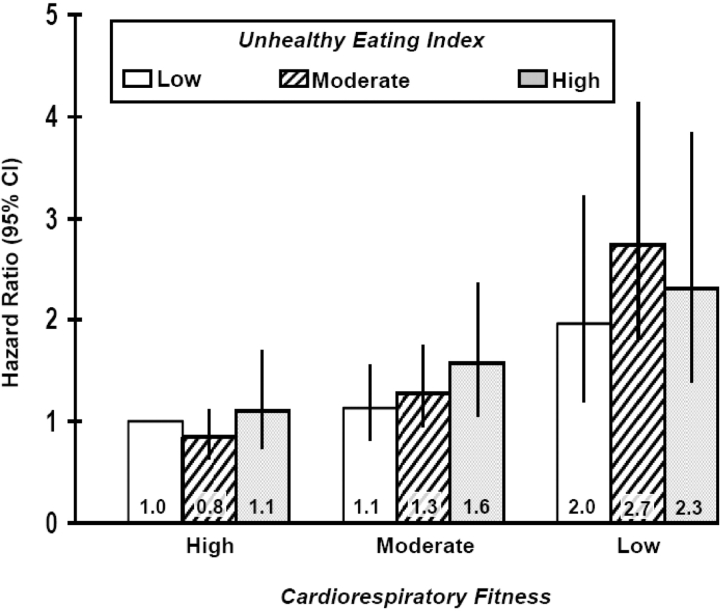
To further illustrate the independent effects of diet and fitness, participants were divided into groups based on their fitness and Unhealthy Eating Index. For this analysis, the Unhealthy Eating Index scores were grouped into three categories: low (quintiles 1−2), medium (quintiles 3−4) and high (quintile 5) in a similar but inverted manner to which the three fitness groups were created (e.g. the lowest quintile for fitness vs the highest quintile for the Unhealthy Eating Index indicate the highest-risk participants). The fitness and dietary pattern groups were then cross-tabulated to create nine total groups (three fitness × three dietary pattern groups). As shown in Figure 1, fitness was a strong predictor of all-cause mortality after consideration of the Unhealthy Eating Index (Ptrend <0.0001). Conversely, the Unhealthy Eating Index was only modestly related to all-cause mortality after consideration of fitness (Ptrend = 0.071). Thus, for any given level of fitness the risk of all-cause mortality was only modestly different across the Unhealthy Eating Index categories. No interactions between the fitness and unhealthy eating categories were found for all-cause mortality (P = 0.26).
Figure 1.
All-cause mortality risk according to categories of cardiorespiratory fitness and the unhealthy eating index, ACLS 1987–99
Discussion
In this large sample of men and women, one dominant dietary pattern emerged. This pattern, labelled the Unhealthy Eating Index, was characterized by a higher intake of red meat, added fat and simple carbohydrates, and a lower intake of non-citrus fruits. The Unhealthy Eating Index was associated with all-cause mortality risk; however, these relations were highly confounded by cardiorespiratory fitness.
Part A: identification of dietary patterns
Principal component and cluster analyses have traditionally been used in nutrition research to derive dietary patterns. In principal component analysis, factor scores are derived by combining food items/groups based on the degree to which they are correlated with one another. In cluster analysis, groups of individuals with similar dietary habits are identified. Conversely, the RRR employed in the present study determined dietary patterns by identifying food groups that were predictive of chronic disease risk factors in a multivariate model. RRR is a mix of an exploratory and a hypothesis-oriented approach that is considered to be more methodologically sound than traditional approaches.6
The Unhealthy Eating Index derived using RRR in the present study is similar to dietary patterns that have previously been derived using the same statistical approach. For example, within a large cohort of European adults a dietary pattern characterized by high consumption of red meat, processed meat, poultry, non-whole grains, legumes, beer and sugary soft drinks, and a low consumption of fresh fruit was identified using glycosylated haemoglobin (HbA1c), HDL-cholesterol, C-reactive protein and adiponectin as the response variables.32 Within the Coronary Risk Factors for Atherosclerosis in Women Study, a dietary pattern high in meat, margarine, poultry and sauce; and low in vegetarian dishes, wine, vegetables and whole-grain cereals was identified using HDL-cholesterol, low-density lipoprotein (LDL)-cholesterol, lipoprotein(a), C-peptide and C-reactive protein.33 Within these two studies the food patterns explained 7.432 and 7.8%33 of the variation in the response variables, which is slightly higher than the variation of 4.3% that was observed in the present study. This difference is likely due to the increased number of biomarker response variables (n = 8) included in the current RRR analysis. That similar dietary patterns to the Unhealthy Eating Index were identified in different cohorts, despite the fact that different dietary assessment tools and different biomarker response variables were considered, demonstrates the robustness of these findings.
Part B: relation between dietary pattern with mortality
Within our study, higher Unhealthy Eating Index scores were not associated with a greater risk of CVD mortality. This may be a result of the low number of cardiovascular deaths that occurred in the cohort. It could further be explained by the fact that BMI and uric acid were the two biomarkers that were the most strongly related to the Unhealthy Eating Index, and that these biomarkers are stronger risk factors for chronic diseases such as diabetes34,35 than they are for CVD.36–39
Several recent studies have reported, increased risks of morbidity and mortality in relation to dietary patterns identified using RRR.20,33,40–42 For example, Hoffmann and colleagues reported that individuals with unhealthy diets were 61% more likely to die of all causes than those with healthier diets.20 Several studies using other methods to derive dietary patterns, such as principal component analysis, also have found moderate to strong relationships between dietary patterns with CVD43,44 and all-cause mortality.45,46
Although previous studies controlled for physical activity when examining the relation between dietary patterns with chronic disease and mortality risk, without exception these studies relied on self-reported measures of physical activity. For instance, McNaughton and colleagues examined dietary patterns that were predictive of diabetes within the Whitehall II study.42 The physical activity questionnaire employed in that study asked participants how often they participated in sports or activates that were mildly energetic, moderately energetic or vigorous. Participants had four response options ranging from ‘three times a week or more’ to ‘never/hardly ever’. Participants were then asked to give the average number of hours per week spent in each intensity.47 Measuring physical activity as such is problematic as it is well established that both the volume and intensity of physical activity is likely to be over reported.12 Moreover, questionnaire measures of physical activity are only modestly correlated with objective measures.12,13,48 Thus, the confounding effects of physical activity on the relation between dietary patterns with chronic disease and mortality risk may not have been fully controlled for in the existing literature.
Cardiorespiratory fitness was used as a marker of physical activity in our study, and our findings concur that by employing an objective measure, we were better able to control for the confounding influence of physical activity. Although fitness has a genetic component,49 fitness is in large measure a reflection of an individual's physical activity habits over recent months and weeks.12,17 The ACLS 6-month physical activity records obtained before an exercise test showed correlations ranging from 0.66 to 0.83 for the activity records and treadmill test results, indicating that fitness is primarily determined by recent exercise habits.50 Within the present study, fitness was strongly related to the Unhealthy Eating Index and was a strong independent risk factor for the mortality outcomes examined, confirming that it was an important confounder to consider. Indeed, after controlling for fitness, the relation between the Unhealthy Eating Index and all-cause mortality was attenuated to the point that it was no longer significant. Conversely, after controlling for physical activity, the relation between the Unhealthy Eating Index and all-cause mortality was only minimally attenuated. These findings confirm the a priori research hypothesis, and suggest that future studies need to employ objective measures of physical activity.
As with any study, this one had several limitations. Self-reported dietary data are prone to a variety of unintentional measurement errors. One such bias that has become well established is a response set reflecting a tendency to present a diet that adheres more closely to social norms and public health recommendations.7,8,51 The ACLS participants who completed 3-day diet records did so voluntarily with the knowledge that they would be reviewing their diet records with a dietician. Therefore, additional selection and reporting biases may have been introduced. Furthermore, 3 days of dietary intake may not be an accurate representation of habitual intake. Validation studies have suggested that obtaining diet records for a minimum of 7 days would be more appropriate for establishing long-term intake.52,53 Despite the limitations of the methods used to assess dietary intake, 3-day diet records have been shown to be a more accurate representation of actual food intake than food frequency questionnaires,54,55 which have been routinely used in similar studies.32,33,40 In addition to the limitations inherent to the measurement of dietary intake, it is important to recognize that the generalizability of the results is restricted, given that the ACLS cohort is predominantly white and from middle and upper socio-economic strata. Nevertheless, the homogenous nature of the sample ensures internal control over factors such as ethnicity and socio-economic status.
In conclusion, a dietary pattern high in processed meat, red meat, added fats, non-whole grains and white potato products and low in fresh fruit was a risk factor for all-cause mortality. However, the diet-disease relationship was largely confounded by fitness. Future research in nutritional epidemiology needs to develop improved methods for both measuring and controlling physical activity as well as diet.
Supplementary Data
Supplementary data are available at IJE online.
Funding
National Institutes of Health (AG06945 and HL62508 to S.B.); New Investigator Award from the Canadian Institutes of Health Research (to I.J.); an Early Researcher Award from the Ontario Ministry of Research and Innovation (to I.J.).
Supplementary Material
Acknowledgements
We thank the Cooper Clinic physicians and technicians for collecting the baseline data, and staff at the Cooper Institute for data entry and data management.
Conflict of interest: None declared.
KEY MESSAGES.
A dietary pattern high in processed and red meat, white potato products, non-whole grains, added fat and a smaller consumption of non-citrus fruits is a modest risk factor for all-cause mortality.
Fitness is an important confounder in the diet–mortality relationship.
References
- 1.Committee on Diet and Health NRC. Diet and Health: Implications for Reducing Chronic Disease Risk. Washington, DC: National Academy Press; 1989. [PubMed] [Google Scholar]
- 2.Lopez-Garcia E, Schulze MB, Fung TT, et al. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2004;80:1029–35. doi: 10.1093/ajcn/80.4.1029. [DOI] [PubMed] [Google Scholar]
- 3.Baxter AJ, Coyne T, McClintock C. Dietary patterns and metabolic syndrome–a review of epidemiologic evidence. Asia Pac J Clin Nutr. 2006;15:134–42. [PubMed] [Google Scholar]
- 4.Kant AK. Dietary patterns and health outcomes. J Am Diet Assoc. 2004;104:615–35. doi: 10.1016/j.jada.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Newby PK, Tucker KL. Empirically derived eating patterns using factor or cluster analysis: a review. Nutr Rev. 2004;62:177–203. doi: 10.1301/nr.2004.may.177-203. [DOI] [PubMed] [Google Scholar]
- 6.Schulze MB, Hoffmann K. Methodological approaches to study dietary patterns in relation to risk of coronary heart disease and stroke. Br J Nutr. 2006;95:860–69. doi: 10.1079/bjn20061731. [DOI] [PubMed] [Google Scholar]
- 7.Hebert JR, Clemow L, Pbert L, Ockene IS, Ockene JK. Social desirability bias in dietary self-report may compromise the validity of dietary intake measures. Int J Epidemiol. 1995;24:389–98. doi: 10.1093/ije/24.2.389. [DOI] [PubMed] [Google Scholar]
- 8.Hebert JR, Hurley TG, Peterson KE, et al. Social desirability trait influences on self-reported dietary measures among diverse participants in a multicenter multiple risk factor trial. J Nutr. 2008;138:226S–34S. doi: 10.1093/jn/138.1.226S. [DOI] [PubMed] [Google Scholar]
- 9.Gillman MW, Pinto BM, Tennstedt S, Glanz K, Marcus B, Friedman RH. Relationships of physical activity with dietary behaviors among adults. Prev Med. 2001;32:295–301. doi: 10.1006/pmed.2000.0812. [DOI] [PubMed] [Google Scholar]
- 10.Blair SN, Kampert JB, Kohl HW, 3rd, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276:205–10. [PubMed] [Google Scholar]
- 11.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 12.Aadahl M, Kjaer M, Kristensen JH, Mollerup B, Jorgensen T. Self-reported physical activity compared with maximal oxygen uptake in adults. Eur J Cardiovasc Prev Rehabil. 2007;14:422–28. doi: 10.1097/HJR.0b013e3280128d00. [DOI] [PubMed] [Google Scholar]
- 13.Tudor-Locke CE, Myers AM. Challenges and opportunities for measuring physical activity in sedentary adults. Sports Med. 2001;31:91–100. doi: 10.2165/00007256-200131020-00002. [DOI] [PubMed] [Google Scholar]
- 14.Siconolfi SF, Lasater TM, Snow RC, Carleton RA. Self-reported physical activity compared with maximal oxygen uptake. Am J Epidemiol. 1985;122:101–5. doi: 10.1093/oxfordjournals.aje.a114068. [DOI] [PubMed] [Google Scholar]
- 15.American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30:975–91. doi: 10.1097/00005768-199806000-00032. [DOI] [PubMed] [Google Scholar]
- 16.Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nutr. 1999;69:373–80. doi: 10.1093/ajcn/69.3.373. [DOI] [PubMed] [Google Scholar]
- 17.Finley CE, LaMonte MJ, Waslien CI, Barlow CE, Blair SN, Nichaman MZ. Cardiorespiratory fitness, macronutrient intake, and the metabolic syndrome: the Aerobics Center Longitudinal Study. J Am Diet Assoc. 2006;106:673–79. doi: 10.1016/j.jada.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Moeller SM, Reedy J, Millen AE, et al. Dietary patterns: challenges and opportunities in dietary patterns research an Experimental Biology workshop, April 1, 2006. J Am Diet Assoc. 2007;107:1233–39. doi: 10.1016/j.jada.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Jinlin F, Binyou W, Terry C. A new approach to the study of diet and risk of type 2 diabetes. J Postgrad Med. 2007;53:139–43. doi: 10.4103/0022-3859.32219. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann K, Boeing H, Boffetta P, et al. Comparison of two statistical approaches to predict all-cause mortality by dietary patterns in German elderly subjects.[see comment] Br J Nutr. 2005;93:709–16. doi: 10.1079/bjn20051399. [DOI] [PubMed] [Google Scholar]
- 21.McArdle W, Katch F, Katch V. Exercise Physiology: Energy, Nutrition and Human Performance. 5th. Maryland: Lippincott Williams & Wilkins; 2001. p. 312. [Google Scholar]
- 22.Church TS, Finley CE, Earnest CP, Kampert JB, Gibbons LW, Blair SN. Relative associations of fitness and fatness to fibrinogen, white blood cell count, uric acid and metabolic syndrome. Int J Obes Relat Metab Disord. 2002;26:805–13. doi: 10.1038/sj.ijo.0802001. [DOI] [PubMed] [Google Scholar]
- 23.National Death Index. 2003. National Center for Health Statistics. [Google Scholar]
- 24.Kampert JB, Blair SN, Barlow CE, Kohl HW., 3rd Physical activity, physical fitness, and all-cause and cancer mortality: a prospective study of men and women. Ann Epidemiol. 1996;6:452–57. doi: 10.1016/s1047-2797(96)00059-2. [DOI] [PubMed] [Google Scholar]
- 25.Balke B, Ware RW. An experimental study of physical fitness of Air Force personnel. US Armed Forces Med J. 1959;10:675–88. [PubMed] [Google Scholar]
- 26.Brodney S, McPherson RS, Carpenter RS, Welten D, Blair SN. Nutrient intake of physically fit and unfit men and women. Med Sci Sports Exerc. 2001;33:459–67. doi: 10.1097/00005768-200103000-00020. [DOI] [PubMed] [Google Scholar]
- 27.Krzanowski W, Kline P. Cross-Validation for choosing the number of important components in principal component analysis. Multivariate Behav Res. 1995;30:6. doi: 10.1207/s15327906mbr3002_2. [DOI] [PubMed] [Google Scholar]
- 28.Giancarlo D, Tommasi C. Cross-validation methods in principal component analysis: a comparison. Stat Methods Appl. 2002;11:11. [Google Scholar]
- 29.Raubenheimer J. An item selection procedure to maximize scale reliability and validity. S Afr J Ind Psychol. 2004;30:5. [Google Scholar]
- 30.Hosmer D, Lemeshow S. Applied Logisitc Regression. New York: Wiley; 2000. [Google Scholar]
- 31.Selvin S. Statistical Analysis of Epidemiologic Data. 2nd. Oxford: Oxford Univeristy Press; 1996. p. 227. [Google Scholar]
- 32.Heidemann C, Hoffmann K, Spranger J, et al. A dietary pattern protective against type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)–Potsdam Study cohort. Diabetologia. 2005;48:1126–34. doi: 10.1007/s00125-005-1743-1. [DOI] [PubMed] [Google Scholar]
- 33.Hoffmann K, Zyriax BC, Boeing H, Windler E. A dietary pattern derived to explain biomarker variation is strongly associated with the risk of coronary artery disease. Am J Clin Nutr. 2004;80:633–40. doi: 10.1093/ajcn/80.3.633. [DOI] [PubMed] [Google Scholar]
- 34.Katzmarzyk PT, Janssen I. The economic costs associated with physical inactivity and obesity in Canada: an update. Can J Appl Physiol. 2004;29:90–115. doi: 10.1139/h04-008. [DOI] [PubMed] [Google Scholar]
- 35.Dehghan A, van Hoek M, Sijbrands EJ, Hofman A, Witteman JC. High serum uric acid as a novel risk factor for type 2 diabetes. Diabetes Care. 2008;31:361–62. doi: 10.2337/dc07-1276. [DOI] [PubMed] [Google Scholar]
- 36.Montalcini T, Gorgone G, Gazzaruso C, Sesti G, Perticone F, Pujia A. Relation between serum uric acid and carotid intima-media thickness in healthy postmenopausal women. Intern Emerg Med. 2007;2:19–23. doi: 10.1007/s11739-007-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. 1999;131:7–13. doi: 10.7326/0003-4819-131-1-199907060-00003. [DOI] [PubMed] [Google Scholar]
- 38.Manzato E. Uric acid: an old actor for a new role. Intern Emerg Med. 2007;2:1–2. doi: 10.1007/s11739-007-0001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toschi V. Elevated uric acid and cardiovascular disease. How strong is the evidence of a pathogenetic link? Intern Emerg Med. 2007;2:320–21. doi: 10.1007/s11739-007-0087-x. [DOI] [PubMed] [Google Scholar]
- 40.Hoffmann K, Schulze MB, Schienkiewitz A, Nothlings U, Boeing H. Application of a new statistical method to derive dietary patterns in nutritional epidemiology. Am J Epidemiol. 2004;159:935–44. doi: 10.1093/aje/kwh134. [DOI] [PubMed] [Google Scholar]
- 41.Schulze MB, Hoffmann K, Manson JE, et al. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr. 2005;82:675–84. doi: 10.1093/ajcn.82.3.675. ; quiz 714–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McNaughton SA, Mishra GD, Brunner EJ. Dietary patterns, insulin resistance, and incidence of type 2 diabetes in the Whitehall II Study. Diabetes Care. 2008;31:1343–48. doi: 10.2337/dc07-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fung TT, Willett WC, Stampfer MJ, Manson JE, Hu FB. Dietary patterns and the risk of coronary heart disease in women. Arch Intern Med. 2001;161:1857–62. doi: 10.1001/archinte.161.15.1857. [DOI] [PubMed] [Google Scholar]
- 44.Hu FB, Rimm EB, Stampfer MJ, Ascherio A, Spiegelman D, Willett WC. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr. 2000;72:912–21. doi: 10.1093/ajcn/72.4.912. [DOI] [PubMed] [Google Scholar]
- 45.Heidemann C, Schulze MB, Franco OH, van Dam RM, Mantzoros CS, Hu FB. Dietary patterns and risk of mortality from cardiovascular disease, cancer, and all causes in a prospective cohort of women. Circulation. 2008;118:230–37. doi: 10.1161/CIRCULATIONAHA.108.771881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Osler M, Heitmann BL, Gerdes LU, Jorgensen LM, Schroll M. Dietary patterns and mortality in Danish men and women: a prospective observational study. Br J Nutr. 2001;85:219–25. doi: 10.1079/bjn2000240. [DOI] [PubMed] [Google Scholar]
- 47.1993. Civil Service Occupational Health Service. Health Survey: Whitehall II Study. [Google Scholar]
- 48.LaPorte RE, Montoye HJ, Caspersen CJ. Assessment of physical activity in epidemiologic research: problems and prospects. Public Health Rep. 1985;100:131–46. [PMC free article] [PubMed] [Google Scholar]
- 49.Bouchard C, Malina R, Perusse L. Genetics of Fitness and Physical Performance. 1st. Champaign: Human Kinetics; 1997. [Google Scholar]
- 50.Paffenbarger RS, Jr, Blair SN, Lee IM, Hyde RT. Measurement of physical activity to assess health effects in free-living populations. Med Sci Sports Exerc. 1993;25:60–70. doi: 10.1249/00005768-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 51.Hebert JR, Ma Y, Clemow L, et al. Gender differences in social desirability and social approval bias in dietary self-report. Am J Epidemiol. 1997;146:1046–55. doi: 10.1093/oxfordjournals.aje.a009233. [DOI] [PubMed] [Google Scholar]
- 52.Basiotis PP, Welsh SO, Cronin FJ, Kelsay JL, Mertz W. Number of days of food intake records required to estimate individual and group nutrient intakes with defined confidence. J Nutr. 1987;117:1638–41. doi: 10.1093/jn/117.9.1638. [DOI] [PubMed] [Google Scholar]
- 53.Acheson KJ, Campbell IT, Edholm OG, Miller DS, Stock MJ. The measurement of food and energy intake in man-an evaluation of some techniques. Am J Clin Nutr. 1980;33:1147–54. doi: 10.1093/ajcn/33.5.1147. [DOI] [PubMed] [Google Scholar]
- 54.Tokudome Y, Goto C, Imaeda N, et al. Relative validity of a short food frequency questionnaire for assessing nutrient intake versus three-day weighed diet records in middle-aged Japanese. J Epidemiol. 2005;15:135–45. doi: 10.2188/jea.15.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Day N, McKeown N, Wong M, Welch A, Bingham S. Epidemiological assessment of diet: a comparison of a 7-day diary with a food frequency questionnaire using urinary markers of nitrogen, potassium and sodium. Int J Epidemiol. 2001;30:309–17. doi: 10.1093/ije/30.2.309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.