Summary
OBJECTIVE
To systematically evaluate descriptive measures of spatial access to medical treatment, as part of the millennium development goals to reduce the burden of HIV/AIDS, tuberculosis and malaria.
METHODS
We obtained high-resolution spatial and epidemiological data on health services, population, transport network, topography, land cover and paediatric fever treatment in four Kenyan districts to develop access and use models for government health services in Kenya. Community survey data were used to model use of government health services by febrile children. A model based on the transport network was then implemented and adjusted for actual use patterns. We compared the predictive accuracy of this refined model to that of Euclidean distance metrics.
RESULTS
Higher-order facilities were more attractive to patients (54%, 58% and 60% in three scenarios) than lower-order ones. The transport network model, adjusted for competition between facilities, was most accurate and selected as the best-fit model. It estimated that 63% of the population of the study districts were within the 1 h national access benchmark, against 82% estimated by the Euclidean model.
CONCLUSIONS
Extrapolating the results from the best-fit model in study districts to the national level shows that approximately six million people are currently incorrectly estimated to have access to government health services within 1 h. Simple Euclidean distance assumptions, which underpin needs assessments and against which millennium development goals are evaluated, thus require reconsideration.
Keywords: millennium development goals, health services, access, use, distance models, kenya
Introduction
The international development agenda is now driven largely by eight millennium development goals (UN 2000; Sachs & McArthur 2005). Three are health related: the reduction of child mortality; improvements of maternal health; and combating HIV/AIDS, malaria and other major diseases. The failure of health systems in low-income countries to deliver equitably appropriate preventive and curative health interventions continues to be a major constraint to achieving these three millennium development goals (WHO 2001; Black et al. 2003; Bryce et al. 2003; Claeson et al. 2003; Jones et al. 2003; TFHSR 2004; Victora et al. 2004). An important indicator of whether a health system is equitable is the level of the population's access to and use of health services (Daniels et al. 2000; WHO 2000; Macinko & Starfield 2002).
Geographic factors play an important role in access to and use of health services (Shannon et al. 1969; Khan & Bhardwaj 1994; Snow et al. 1994). In sub-Saharan Africa (SSA) and other low-income countries, distance contributes to the time required to access health services (Garner & Giddings 1985; Bailey & Phillips 1990; Müller et al. 1998; Hjorstberg & Mwikisa 2002), delays in decisions to seek treatment (Schwartz et al. 1993; Amooti-Kaguna & Nuwaha 2000) and increases in household expenditure on treatment and opportunity costs as a result of time spent away from income generating activities (Akin & Hutchinson 1999; Khan et al. 2002; Ensor & Cooper 2004). For international comparisons and national disparity assessment the common yardstick used is the 1-hour to health services criteria of spatial access (World Bank 2001). The reliability of these estimates of health service coverage depends critically on how accurately distance is measured and how this predicts actual use of health services. These measures assume that people always use the nearest health service, with little regard for patients' actual use characteristics (Gething et al. 2004; Guargliardo et al. 2004) and almost exclusively use Euclidean or straight-line definitions of distance. In this paper, geographic information systems (GIS) are used to develop new approaches to combine high resolution spatial and epidemiological data to create more realistic spatial models that highlight the limitations of current access measures related to health development goals.
Methods
Study area
The study was undertaken in four districts of Kenya chosen to encompass the range of ecological, epidemiological, population and health service conditions nationwide. These were: Greater Kisii, located in the densely populated, high-intensity agricultural western highlands; Bondo, located along the shores of Lake Victoria; Kwale, located in the south-eastern corner of Kenya on the border with Tanzania and along the Indian Ocean; and Makueni, comprising an extensive semi-arid area south of Nairobi.
Development of spatial geographic information systems data
The development of the population and health service data is described in detail elsewhere (Noor et al. 2003). In brief, all health facilities in the study districts were mapped using handheld global positioning system (GPS) (Garmin etrex, Garmin Ltd., Kansas, USA). Population totals from the 1999 national census recorded at the enumeration area level (the smallest census unit) were obtained for the four districts (CBS 2001). Each enumeration area comprises part of a village, a whole village or group of villages that are usually not more than 100 households (circa 500 people). The enumeration area population maps were transformed to raster-based population distribution maps at 100 m spatial resolution using ArcView GIS (version 3.2 1992–1999, ESRI Inc). The most recent national road network map (0.040 km/km2 for Kenya) was obtained from Africover (Hay et al. 2005; URL: http://www.africover.org). This road map was used as a template, updated by digitising additional roads, at the footpath level, from 1:50 000 scale topographic and enumeration area maps for the four study districts. To account for the influence of slope on accessibility to health services, a Kenya digital elevation model developed from contours at 20 m intervals digitised from 1:50 000 scale topographic maps (International Livestock Research Institute, Nairobi, unpublished) and raterised into a 100 × 100 m spatial resolution was used. Data on rivers, other water features and forests were also abstracted from an Africover database for Kenya. These were of limited coverage and coarse spatial resolution for the four study districts hence enumeration area maps, which included details of rivers, swamps, lakes and forests, were used to augment the Africover maps. A digital map of all parks, game reserves and other sanctuaries in Kenya was obtained from the Kenya Wildlife Service (Wycliffe Mutero, Personal Communication).
Development of empirical data of fever treatment sources
In 2001 a community household survey was conducted in the study districts using a stratified random sample of approximately 230 EAs across the four districts covering between 25 040 and 25 928 people within each district (Amin et al. 2003; Guyatt et al. 2004). All homesteads in each of the randomly selected enumeration area were mapped using GPS (Garmin etrex) and assigned a unique identification number. Informed consent was sought from each homestead head before the household members were enumerated under protocol #659 approved by the Kenyan National IRB. Guardians of children aged less than 5 years were questioned about the presence of fever in the last 14 days and sources and types of treatment obtained (Amin et al. 2003). Each source of treatment was matched to codes of facilities and outlets created for each district. Data were double entered and checked using Microsoft® Access 2000 (Microsoft Corp., USA).
Modelling actual use of government health services
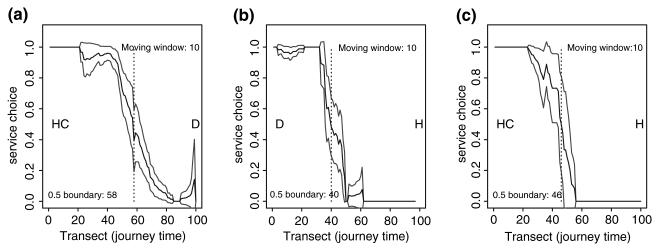
Only children who were treated at government health facilities were selected for this analysis. The government health sector is the main channel through which health reforms aimed at disease and poverty reduction are implemented. A health coverage target of ensuring Kenya's population live within 1 h of effective health services by 2010 has been set by the government (MoH 1999). As such, there is an immediate need to measure the population's access and use of these services. To assess the pattern of use of government health services the transect algorithm was developed (S-PLUS version 6.1, Insightful Corporation, UK) to quantify competition for patients between hospitals (H), health centres (HC) and dispensaries (D). This algorithm considered patients to have made a three-way choice between a H, HC and D. This choice was recorded at a point on each of three transects, representing the patient's relative location between the facility type chosen and either of the two not chosen. The three transects, each divided into 100 equal and discrete sections were HC–D, D– H and HC–H. At each section a ‘patient choice’ value was calculated, which was simply the proportion of total patients recorded in each section who chose the first facility in the pair (e.g. D–H), such that a value of one indicates that all patients attended facility D and a value of zero that all attended H. Plots of patient choice were then constructed along each transect. The position along the aggregate transect where the probability of choosing either of the facilities in a pair was the same (0.5) was taken to represent the location of the catchment boundary between the two facilities. The limits of the 95% confidence interval around the line of the facility choice were also generated and considered to represent the overlap area between the two facilities and accounted for cross-border use. The resulting boundary displacement factors were then applied to the model based on proximity to health services on the transport network described below.
The transport network model adjusted for competition
The distance to health services measured on the actual transport network accounting for the influence of topography and other natural barriers was modelled. Only pedestrian movement was modelled, since in the community survey >80% of the patients reported no transport costs in travelling to health facilities and were, therefore, assumed to have walked to health services. The Naismith–Langmuir rule (Langmuir 1984) was used to define travel speed and develop a shortest-path algorithm, coded in C++, to implement the model (Table 1). This algorithm required an input raster (grid) map for each district, which listed a travel speed value for every pixel. This speed depended on the nature of the pixel (e.g. road, non-road, river etc) and represented the time in minutes taken to traverse it. Barriers such as rivers, forests and parks were masked as impassable. Where a road traversed a river or other water features, however, the road speed was assigned to the pixel(s) of intersection. The algorithm used an iterative region-growing approach in which each pixel containing a health facility was taken as a ‘seed’ pixel around which regions of assigned pixels were grown. The first iteration reached all un-assigned pixels that were contiguous neighbours of the seed pixels. As soon as a pixel was traversed it was allocated a travel time value derived from the number of iterations (converted into a value in minutes) that had been completed at that point. This ensured that only the fastest route to a given pixel was used to calculate travel time to the health facility.
Table 1.
Travel on different terrains based on a modification of Naismith–Langmuir rule (Langmuir 1984)
| Naismith/Langmuir rule | |
|---|---|
| Flat (Road) | 1.2 min per 100 m (5 km/h) |
| Flat (Off-Road) | 2.4 min per 100 m (2.5 km/h) |
| Ascents | +0.1 min per 1 m ascent (+1 hr per 600 m) |
| Moderate descents (−5°–−12°) | −0.03 min per 1 m descent (−10 min per 300 m) |
| Steep descents (steeper than −12°) | +0.03 min per 1 m descent (+10 min per 300 m) |
The transport network model was then adjusted using the boundary displacement factors using an allocation algorithm. The allocation algorithm was raster-based and its principle inputs were three grids consisting of the per-pixel travel time to the nearest hospital, health centre and dispensary and the three corresponding grids of facility codes. The longitudes and latitudes of all facilities were also required, along with the between-facility boundary displacement factors (for D–H, D–HC and HC–H). The algorithm simply allocated each pixel to a hospital, health centre or dispensary based on the adjustment factors. A constraint of contiguity was imposed, such that, no segments of a catchment were isolated from the main section by another catchment. The first input was the grid where every pixel was allocated to its nearest dispensary. The algorithm then took the location of every health centre as the seed in an iterative region-growing function. In each iteration, the travel-time-to-health-centre value was checked against the travel-time-to-dispensary value for each pixel with reference to the D–HC displacement factor. If the value was below the displacement threshold, the allocation remained the same (to dispensary), if it was above, then it was re-allocated to the health centre until every pixel was allocated to the appropriate facility. The process was then repeated using this new dispensary and health centre grid as input and the locations of all hospitals as the seed pixels.
Model output and accuracy assessment
The transport network model, before and after adjustment for competition, was compared to the traditional Euclidean model for the four study districts. The Euclidean model was simply implemented in ArcView GIS using the Find Distance function with government health services as origin and every other 100 × 100 m pixel on the map as destination. The Kappa (κ) statistic was used to measure the agreement between predicted and observed use of government health services for the models. The κ statistic adjusts the overall accuracy (OA) of the model for chance agreement between categories (Congalton & Green 1999). The ArcView 3.2 Kappa Analysis extension (URL: http//http://www.jennessent.com) was used to provide a packaged approach for accuracy assessment, using the κ statistic as well as several additional metrics, to gauge model performance (see Table 2 for details). Based on these accuracy statistics the best-fit model was selected. To visually compare agreement around a perfect fit between the poorest performing and best-fit model, a scatter plot of the travel time to government health services for both models attributed to the children who used government facilities was constructed. For all models, the proportion of people within 1 h of government health facilities was extracted from the EA population map using ArcView GIS.
Table 2.
Predictive accuracy metrics for the adjusted and unadjusted models of access and utilisation of GoK–MoH health services for the treatment of paediatric fevers
| Model | Overall Accuracy† | Kappa κ‡ | P-value | 95% Confidence Interval |
|---|---|---|---|---|
| Euclidean | 0.72 | 0.71 | 0.00 | 0.70–0.72 |
| Transport network-not adjusted for competition | 0.74 | 0.73 | 0.00 | 0.73–0.74 |
| Transport network-adjusted for competition | 0.84 | 0.83 | 0.00 | 0.79–0.87 |
Overall accuracy: this is simply the number of correctly classified sample points divided by the total number of sample points
Kappa statistic (κ): is the chance-corrected measure of model accuracy, based on the actual agreement between predicted and observed values and the chance agreement between the row and column totals for each classification (Congalton & Green 1999). P-values reflect the probability that a model performs better than random chance at predicting the choice of a health facility
Results
Summary of the community survey data
The four districts had a total of 173 government health facilities (12 H, 45 HC and 116 D) and population of 2 459 183 spread across 5236 enumeration areas according to the 1999 national census (CBS 2001). A total of 6287 children <5 years of age were interviewed during the community survey and 2655 were reported to have had a fever during the preceding 14 days. A total of 668 febrile children used government health services as the first source of treatment and these were used in developing the spatial access and utilisation models. Limiting the analysis to febrile children only purposively excluded those who used the clinics for routine health checks or vaccination.
Competition between health facilities of different types
The transect algorithm produced three plots showing patterns of patients' choice of service between HC–D, D–H and HC–H, respectively (Figure 1). The boundary between any pair of facilities was the point on the x-axis which corresponded to a probability value of 0.5 on the y-axis. The plots showed that boundaries between HCs and Ds in the study districts were shared 58/42% in favour of the HC. For the D–H and HC–H relationships, these were 40/60% and 46/54%, respectively, all in favour of the higher order facility, i.e. the hospital. Where the facilities were of the same type, the boundary patients drawing' capacity were generally equal. The 95% CI for the HC–D, D–H and HC–H boundary displacement factors were 56–70%, 38–48% and 42–56%, respectively.
Figure 1.
Plots showing the pattern of patients' choice of health services in a HC–D, D–H and HC–H relationship. The black line plots the patient's choice between any pair of relationship. The grey lines represent the limits of the 95% confidence interval, which give the extents of the overlap area. The position on the y-axis corresponding to a value of 0.5 on the x-axis provides the boundary displacement factor.
Output of models and selection of ‘best-fit’ model
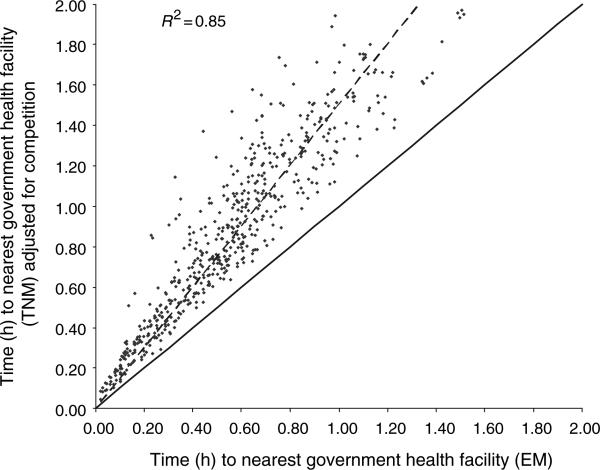
The Euclidean model had an OA of 0.72 and Kappa (κ) of 0.71; the unadjusted transport network model had OA = 0.74 and κ = 0.73; while the adjusted transport network model had an OA = 0.84 and κ = 0.83; a difference of more than 10% to the Euclidean model (Table 2). The adjusted transport network model was therefore the best fit to the actual patient data derived from the community survey. A scatterplot of time taken by febrile children to access health services as defined by the best-fit and Euclidean models is shown in Figure 2. As expected the correlation between the models was high (R2 = 0.85) but the difference between the trendline and the line of perfect fit indicates that the Euclidean distance model consistently underestimates travel time. Moreover, this plot shows that as distance from health services increased, the difference in travel time assigned to the patients by the two models widened (Figure 2).
Figure 2.
A scatter plot of travel time comparing the Euclidean model (EM) and the competition-adjusted transport network model (TNM) for 668 children who were treated at government health services. The dashed line represents the trend-line of the scatter plot. The solid line represents the theoretical line of complete agreement between the two models.
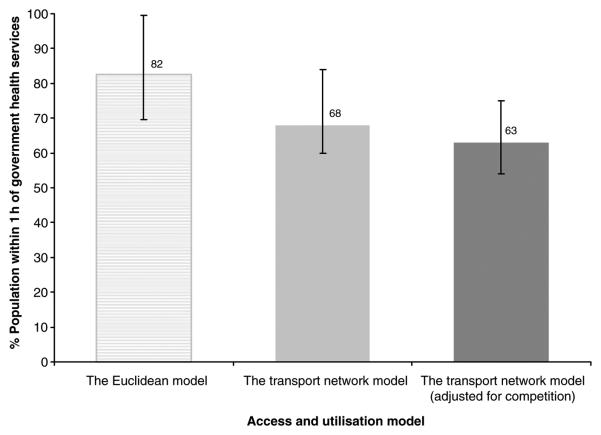
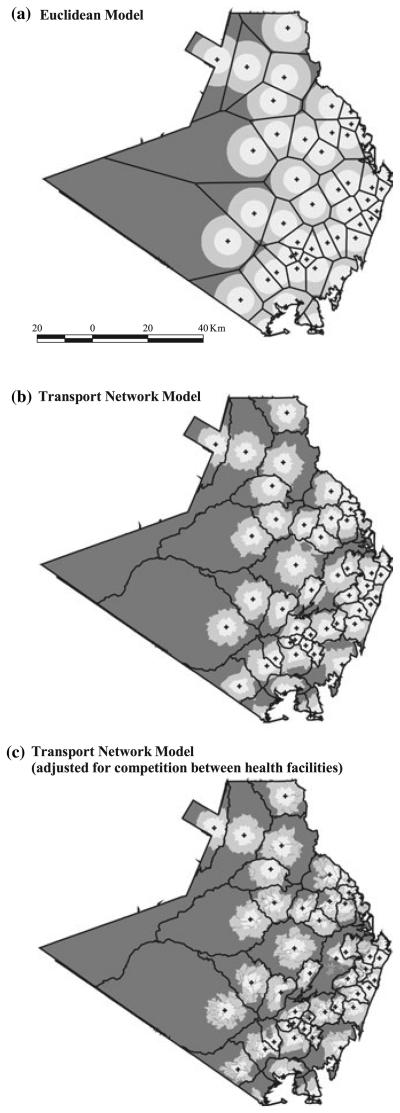
The traditional Euclidean model estimated that 82% of the population of the four districts had access to government health services within 1 h. The unadjusted transport network model reduced this to 68%. The best-fit model revised this estimate to 63% of the population (Figure 3). An example of how these model assumptions affect distance and use metrics spatially is shown for Kwale district (Figure 4).
Figure 3.
Graph showing the percentage of population within 1 h to the nearest government health facilities for both the Euclidean and the transport network model before and after adjustment for competition between facilities. The interval bars represent the lowest and highest proportion of population within 1 h of government health services attributed to each model across the four districts.
Figure 4.
(a–c) Maps of Kwale showing access to government health services based on travel time (hours) for the Euclidean, the transport network and the adjusted transport network models.  = government health facility;
= government health facility;  = catchment area;
= catchment area;  = 0-0.5 hours;
= 0-0.5 hours;  = >0.5–1 h;
= >0.5–1 h;  = >1 hour. All maps are at the same scale as shown on Figure 4a. The North direction is towards the top of the page.
= >1 hour. All maps are at the same scale as shown on Figure 4a. The North direction is towards the top of the page.
Discussion
The major limitation in describing physical access to health services are the assumptions that people use the nearest health service and that they travel to it in a straight line. The assumptions of this Euclidean distance model are inherent in the widely used access metrics such as the proportion of population within 1 h of effective health service (MoH 1999; World Bank 2001).
These assumptions have been tested in four intensively studied districts in Kenya where community surveys of actual patient use were conducted. First, a transect algorithm was developed to quantify how patients respond to the competing array of health service providers. Not surprisingly, it revealed that where pairs of adjacent government health facilities were of different types, the higher order facility attracted more patients and the boundary between the facilities was displaced in its favour. Second, an approach for measuring the overlap area between adjacent health facilities was developed. This defined the 95% confidence interval around the position of the discrete boundary between adjacent health facilities. Third, the transport network, elevation and other natural barriers were used to define more accurately real distances that people travelled. Finally, combining these advances resulted in a best-fit model with 10% more predictive power than the widely used Euclidean model.
The Euclidean distance model overestimates population within 1 h of a health facility by 19% in the four districts (Figure 2). Extrapolating this result to Kenya reveals 19 million people (based on a projected population of 31 million nationwide for 2004) are within 1 h of government health services, rather than the 25 million estimated by the Euclidean distance model. Current estimates of access to health services thus grossly overestimate health coverage. In addition, since the differences between models become larger with distance from health services (Figure 3), those with least access are under-represented to a proportionately greater extent. Although the unadjusted transport network model does perform better than the Euclidean model, it still puts 5% more people to within 1 h of government health services than the best-fit model.
There have been recent efforts to improve spatial access models by international agencies. The Pan-American Health Organization (PAHO) group have developed ‘SIGEpi’, which incorporates vector data on population, land cover, transport network, elevation, administrative boundaries and health facility location in defining distance (http://ais.paho.org/SIGEpi). A further model developed by the World Health Organization's Evidence and Information for Policy group, ‘AccessMod’ is similar in the data used but is raster based (http://gis.esri.com/library/userconf/health04/papers/pap3023.pdf., accessed 28 December 2005). Both models therefore overcome the straight-line travel assumption but continue to assign populations to the nearest health services. Although AccessMod has a function to incorporate a facility's capacity by using the average number of patients seen in a day or number of visits per capita, no attempt is made to model catchment areas based on the variation of actual use with distance from health facilities. Furthermore, neither model has had their accuracy evaluated formally against actual use data.
Current research effort is directed to continuing refinements of the best-fit model. Simple improvements relate to accommodating vehicular transport and resting time in journeys to health services and incorporating more detailed information on movement barriers such as farmland and settlements. More complex adjustments need to be made for the socio-economic determinants of access to health services, which were not investigated here.
The more sophisticated access models outlined here cannot be scaled-up to the national level in low-income countries such as Kenya with precision due to paucity of relevant GIS data. International agencies and national governments with a mandate to monitor and evaluate health targets towards the MDGs may therefore be wise to invest in improving the spatial GIS infrastructure. Without these investments international and national plans to address health systems inequities will continue to underestimate the proportion of the population without adequate access to health services, the resources required to tackle these issues and thus be in danger of missing their targets. As is so often the case these underestimates disproportionately affect the poor.
Acknowledgements
This study received financial support from The Wellcome Trust, UK (#058992), the Roll Back Malaria Initiative, AFRO (AFRO/WHO/RBM# AF/ICP/CPC/400/XA/00) and the Kenya Medical Research Institute. The authors are grateful to Dr. Sam Ochola, Head of the Division of Malaria Control for providing a policy framework within which the study was conducted, Dr Andy Tatem for comments on the analysis and manuscript. The authors are also grateful to Priscilla Wairimu, Lydiah Mwangi and Lucy Muhunyo for assistance with data handling and linkage. SIH is a Research Career Development Wellcome Trust Fellow (#056642) and RWS a Senior Wellcome Trust Fellow (#058992). This paper is published with the permission of the director, KEMRI.
References
- Akin JS, Hutchinson P. Health-care facility choice and the phenomena of by-passing. Health Policy and Planning. 1999;14:135–151. doi: 10.1093/heapol/14.2.135. [DOI] [PubMed] [Google Scholar]
- Amooti-Kaguna B, Nuwaha F. Factors influencing choice of delivery sites in Rakai district of Uganda. Social Science and Medicine. 2000;50:2033. doi: 10.1016/s0277-9536(99)00275-0. [DOI] [PubMed] [Google Scholar]
- Amin AA, Marsh V, Noor AM, Ochola SA, Snow RW. The use of formal and informal curative services in the management of paediatric fever in four districts in Kenya. Tropical Medicine and International Health. 2003;8:1143–1152. doi: 10.1046/j.1360-2276.2003.01140.x. [DOI] [PubMed] [Google Scholar]
- Bailey W, Phillips DR. Spatial patterns of use of health services in the Kingston metropolitan area, Jamaica. Social Science and Medicine. 1990;30:1–12. doi: 10.1016/0277-9536(90)90324-l. [DOI] [PubMed] [Google Scholar]
- Black R, Morris S, Bryce J. Where and why are 10 million children dying every year? Lancet. 2003;361:2226–2234. doi: 10.1016/S0140-6736(03)13779-8. [DOI] [PubMed] [Google Scholar]
- Bryce J, el Arifeen S, Pariyo G, Lanata C, Gwatkin D, Habicht JP, Multi-Country Evaluation of IMCI Study Group Reducing child mortality: can public health deliver? Lancet. 2003;362:159–164. doi: 10.1016/s0140-6736(03)13870-6. [DOI] [PubMed] [Google Scholar]
- Central Bureau of Statistics . 1999 population and housing census: counting our people for development. Volume I: Population distribution by administrative areas and urban centres. Central Bureau of Statistics, Ministry of Finance & Planning; GoK: Jan, 2001. (2001) [Google Scholar]
- Claeson M, Gillespie D, Mshinda H, Troedsson H, Victoria CG. Knowledge into action for child survival. Lancet. 2003;362:323–327. doi: 10.1016/s0140-6736(03)13977-3. [DOI] [PubMed] [Google Scholar]
- Congalton RG, Green K. Assessing the Accuracy of Remotely Sensed Data: Principles and Practices. Lewis Publishers; New York: 1999. p. 137. [Google Scholar]
- Daniels N, Bryant J, Castano R, Dantes O, Khan K, Pannarunothai S. Benchmarks of fairness for health care reform: a policy tool for developing countries. Bulletin of the World Health Organization. 2000;78:740–750. [PMC free article] [PubMed] [Google Scholar]
- Ensor T, Cooper S. Overcoming barriers to health service access: influencing the demand side. Health Policy and Planning. 2004;19:69–79. doi: 10.1093/heapol/czh009. [DOI] [PubMed] [Google Scholar]
- Garner P, Giddings P. Rural health centre use: variations with distance and disease. Papua New Guinea Medical Journal. 1985;30:105–108. [PubMed] [Google Scholar]
- Gething PW, Noor AM, Zurovac D, et al. Empirical modeling of Government health service use by children with fevers in Kenya. Acta Tropica. 2004;91:227–237. doi: 10.1016/j.actatropica.2004.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guargliardo MF, Ronzio CR, Cheung I, Chacko E, Joseph JG. Physician accessibility: an urban case study of pediatric providers. Health and Place. 2004;10:273–283. doi: 10.1016/j.healthplace.2003.01.001. [DOI] [PubMed] [Google Scholar]
- Guyatt HL, Noor AM, Ochola SA, Snow RW. Use of intermittent presumptive treatment and insecticide treated bed nets by pregnant women in four Kenyan districts. Tropical Medicine and International Health. 2004;9:255–261. doi: 10.1046/j.1365-3156.2003.01193.x. [DOI] [PubMed] [Google Scholar]
- Hay SI, Noor AM, Nelson A, Tatem AJ. Demography for epidemiology: the precision of large-area human population maps. Tropical Medicine and International Health. 2005;10:1–14. doi: 10.1111/j.1365-3156.2005.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorstberg CA, Mwikisa CN. Cost of access to health services in Zambia. Health Policy and Planning. 2002;17:71–77. doi: 10.1093/heapol/17.1.71. [DOI] [PubMed] [Google Scholar]
- Jones G, Steketee RW, Black RE, Bhutta ZA, Morris SS, Bellagio Child Survival Study Group How many child deaths can we prevent this year? Lancet. 2003;362:65–71. doi: 10.1016/S0140-6736(03)13811-1. [DOI] [PubMed] [Google Scholar]
- Khan A, Bhardwaj SM. Access to health care. A conceptual framework and its relevance to health care planning. Evaluation and the Health Professions. 1994;17:60–76. doi: 10.1177/016327879401700104. [DOI] [PubMed] [Google Scholar]
- Khan MA, Walley JD, Witter SN, Imran A, Safdar N. Cost and cost-effectiveness of different DOTS strategies for treatment of tuberculosis in Pakistan. Health Policy and Planning. 2002;17:178–186. doi: 10.1093/heapol/17.2.178. [DOI] [PubMed] [Google Scholar]
- Langmuir E. Mountain craft and Leadership. The Scottish Sport Council/MLTB; Cordee, Leicester: 1984. [Google Scholar]
- Macinko JA, Starfield B. Annotated bibliography on equity in health 1980–2001. Internal Journal for Equity in Health. 2002;1:1. doi: 10.1186/1475-9276-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MoH . National Health Sector Strategy Plan. Government of Kenya; Nairobi, Kenya: 1999. [Google Scholar]
- Müller I, Smith T, Mellor S, Rare L, Genton B. The effect of distance from home on attendance at a small rural health centre in Papua New Guinea. International Journal of Epidemiology. 1998;27:878–884. doi: 10.1093/ije/27.5.878. [DOI] [PubMed] [Google Scholar]
- Noor AM, Zurovac D, Hay SI, Ochola SA, Snow RW. Defining equity in physical access to clinical services using geographical information systems as part of malaria planning and monitoring in Kenya. Tropical Medicine and International Health. 2003;8:917–926. doi: 10.1046/j.1365-3156.2003.01112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs JD, McArthur JW. The Millennium Project: a plan for meeting the Millennium Development Goals. Lancet. 2005;365:347–353. doi: 10.1016/S0140-6736(05)17791-5. [DOI] [PubMed] [Google Scholar]
- Shannon GW, Bashshur RL, Metzner CA. The concept of distance as a factor in accessibility and utilisation of health care. Medical Care Review. 1969;26:143–161. [Google Scholar]
- Schwartz JB, Akin JS, Popkin BM. Economic determinants of demand for modern infant-delivery in low-income countries: the case of the Phillipines. In: Mills A, Lee K, editors. Health Economic Research in Developing Countries. Oxford Medical Publications; Oxford and New York: 1993. [Google Scholar]
- Snow RW, Schellenberg JRMA, Forster D, Mungala VO, Marsh K. Factors influencing admission to hospital during terminal childhood illnesses in Kenya. International Journal of Epidemiology. 1994;23:1013–1019. doi: 10.1093/ije/23.5.1013. [DOI] [PubMed] [Google Scholar]
- Task Force for Health Systems Research Informed choices for attaining the millennium development goals: toward an international cooperative agenda for health systems research. Lancet. 2004;364:997–1003. doi: 10.1016/S0140-6736(04)17026-8. [DOI] [PubMed] [Google Scholar]
- UN . United Nations Millennium Declaration. United Nations General Assembly; 2000. A/RES/55/2 ( http://www.un.org) [Google Scholar]
- Victora CG, Hanson K, Bryce J, Vaughn JP. Achieving universal coverage with health interventions. Lancet. 2004;364:1541–1548. doi: 10.1016/S0140-6736(04)17279-6. [DOI] [PubMed] [Google Scholar]
- WHO . World Health Report: Health Systems: Improving Performance. World Health Organization; Geneva, Switzerland: 2000. p. 206. [Google Scholar]
- WHO . Macroeconomics and Health: Investing in Health for Economic Development. World Health Organization; Geneva, Switzerland: 2001. p. 202. Report of the commission on macroeconomics and health. [Google Scholar]
- World Bank . African Development Indicators. Washington DC: 2001. p. 366. http://publications.worldbank.org/ [Google Scholar]