Abstract
Tests for pleural tuberculosis are insensitive and expensive. We compared nonproprietary microscopic-observation drug-susceptibility (MODS) culture with Löwenstein-Jensen culture for evaluation of pleural specimens. MODS culture was associated with greatly increased diagnostic sensitivity and shorter time to diagnosis, compared with Löwenstein-Jensen culture (sensitivity of culture of biopsy specimens, 81% vs. 51%; time to diagnosis, 11 days vs. 24 days; P < .001). The MODS technique is inexpensive, allows drug-susceptibility testing, and is a considerably improved diagnostic method for pleural tuberculosis.
Tuberculosis is the leading curable cause of death due to an infectious disease. Increasing prevalence of HIV coinfection increases the incidence of paucibacillary and extrapulmonary tuberculosis, making more-sensitive tests important for tuberculosis control. Diagnosis of pleural tuberculosis relies on examination of pleural fluid and/or biopsy specimens using acid-fast microscopic examination, culture, PCR, evaluation of pleural fluid characteristics, and/or histopathologic examination. However, these methodologies are associated with unsatisfactory sensitivity, specificity, time to diagnosis, and/or cost [1–5]. Microscopic-observation drug-susceptibility (MODS) culture is a recently developed nonproprietary technique for diagnosing tuberculosis that, for sputum specimens, has demonstrated more-rapid and more-sensitive detection than existing gold-standard methods [6]. We compared MODS culture with standard culture for patients with suspected pleural tuberculosis and hypothesized that MODS culture would improve diagnostic sensitivity and reduce the time to diagnosis.
Patients, materials, and methods
Participants were recruited from the Hospital Nacional Dos de Mayo (Lima, Peru). Inclusion criteria were age >16 years and radiographic evidence of pleural effusion that led the physician to perform thoracentesis and pleural biopsy. We excluded patients with positive results of Ziehl-Neelsen microscopic examination of a sputum specimen, because they did not require thoracentesis or biopsy for the diagnosis of pleural tuberculosis, and therefore, their inclusion might have biased the analysis of validity. All subjects gave written informed consent, and the study received local and international ethical approval.
Thoracentesis and pleural biopsy were performed using standard methods by the collaborating physicians, who decided the specimen volume and number. Samples were transported on ice for analysis. Each patient had both pleural fluid and pleural biopsy specimens cultured by each of the following techniques: (1) direct Löwenstein-Jensen culture, (2) decontaminated Löwenstein-Jensen culture, (3) direct MODS culture, and (4) decontaminated MODS culture. Therefore, every patient had a total of 8 diagnostic cultures performed.
Pleural fluid was concentrated by centrifugation, and supernatants were discarded, leaving 4 mL of concentrated sample, which was divided into halves for (1) direct culture and (2) decontamination, auramine-staining, and culture. Pleural biopsy specimens were divided into halves for (1) fixation in 10% formalin, embedding in paraffin wax (for Ziehl-Neelsen testing), and hematoxylin and eosin staining and (2) homogenization with a glass mortar in saline to a volume of 4 mL (for acid-fast staining and culture). Decontamination was performed with an equal volume of 8 mg/mL n-acetyl-cysteine, 2% NaOH, and 2.9% sodium citrate for 15 min. The mixture was then diluted in 10 mL of PBS, centrifuged, and resuspended in 2 mL of 0.2% albumin.
Cultures of all specimens were performed in parallel with cultures of negative controls. For Löwenstein-Jensen culture, 200 μL of sample were inoculated onto each slope, incubated at 37°C, and examined 2 times per week (days 7–60). MODS cultures were performed using 4 wells of a 24-well plate, as described previously [6]. Specimens (500 μL) were inoculated into wells containing 1 mL each of a liquid culture solution (7H9-Middlebrook medium; 10% oleic acid, albumin, dextrose, and catalase; and polymixin [40 U/mL], amphotericin B [4 μg/mL], nalidixic acid [16 μg/mL], trimethoprim [4 μg/mL], and azlocilin [4 μg/mL] antimicrobial supplement). Plates were examined under an inverted-light microscope 2–5 times per week (days 4–60). To prevent cross-contamination and occupational exposure, closed plates were sealed inside plastic ziplock bags.
Stata, version 9.0 (Stata), was used for analysis. Contaminated specimens were considered to have negative culture results; thus, they would not falsely elevate sensitivity. Rates were compared by proportions using z tests, and times to diagnosis were compared using the log-rank test.
Results
One hundred eleven participants met the inclusion criteria and had thoracentesis performed and pleural biopsy specimens tested by Löwenstein-Jensen and MODS culture techniques. Twenty-two participants (20%) were able to provide a sputum specimen; specimens from 8 (36%) of these patients had positive MODS culture results. Seventy (63%) of 111 participants met the diagnostic standard of pleural tuberculosis, defined as pleural fluid and/or biopsy specimens that had positive results of acid-fast microscopic examination and/or culture for tuberculosis (table 1). Participants with pleural tuberculosis were significantly younger (median age, 26 years vs. 47 years; P < .01) and more likely to report constitutional symptoms, including fever (73% vs. 49%; P = .01), night sweats (60% vs. 34%; P = .01), and weight loss (84% vs. 71%; P = .09) than were those without a positive test result for tuberculosis. A greater percentage of patients with tuberculosis than of patients without proven tuberculosis had caseating necrosis found on culture of pleural biopsy specimens (46% vs. 13%; P < .001). Ten percent of those who did not receive a diagnosis of tuberculosis received a diagnosis of neoplasia.
Table 1. Demographic characteristics, patient symptoms, and results of culture of biopsy specimens for patients who received a diagnosis of pleural tuberculosis (TB), compared with patients who did not receive a diagnosis of pleural TB, after pleural biopsy and thoracentesis testing.
| Variable | Patients with confirmed pleural TB (n = 70) |
Patients who had a negative result of culture for TB (n = 41) |
P |
|---|---|---|---|
| Demographic characteristic | |||
| Male sexa | 75.7 | 69.3 | .4 |
| Age, median years ± SD (range)b | 26 ± 19.4 (17–80) | 47 ± 21.4 (17–89) | <.01 |
| Reported symptom | |||
| Fevera | 72.9 | 48.8 | .01 |
| Night sweatsa | 60.0 | 34.1 | .01 |
| Weight lossa | 84.2 | 70.7 | .09 |
| Mean no. of biopsies (range)c | 1.8 (1–4) | 1.4 (1–4) | .09 |
| Finding of culture of biopsy specimen | |||
| Presence of caseous necrosisa | 45.7 | 12.5 | <.01 |
| Neoplastic diagnosis | 0.0 | 9.8 | .01 |
NOTE. Data are percentage of patients, unless otherwise indicated.
Compared using Student’s t test.
Compared using Wilcoxon rank-sum test.
Compared using χ2 test.
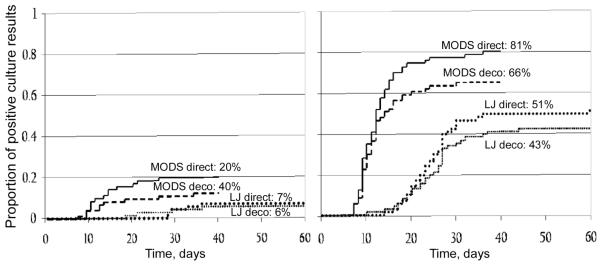
MODS culture was significantly more sensitive than Löwenstein-Jensen culture for direct culture of biopsy specimens (sensitivity, 81% vs. 51%; P < .001), decontaminated culture of biopsy specimens (sensitivity, 66% vs. 43%; P = .007), and direct culture of pleural fluid specimens (20% vs. 7.1%; P = .027). MODS culture tended to be more sensitive than Löwenstein-Jensen culture of decontaminated pleural fluid specimens (sensitivity, 14% vs. 5.7%; P = .09) (figure 1). Direct culture tended to be more sensitive than decontaminated culture, and this was statistically significant for MODS culture of biopsy specimens (81% vs. 67%; P = .04). The sensitivity of auramine microscopic examinations was significantly greater for pleural biopsy specimens than for pleural fluid specimens (P < .001). The sensitivity of auramine microscopic examination of pleural biopsy specimens was 20% (14 of 70 auramine microscopic examinations of biopsy specimens yielded positive results) and of pleural fluid specimens was 0.0% (0 of 70 auramine microscopic examinations of pleural fluid specimens yielded positive results).
Figure 1.
Time to positive culture results and sensitivity of microscopic-observation drug-susceptibility (MODS) and Löwenstein-Jensen (LJ) cultures of pleural fluid specimens (left) and pleural biopsy specimens (right) from 70 patients with proven pleural tuberculosis. Percentages shown represent the sensitivity of the method, in which any positive test result was the reference standard. Deco, decontaminated culture; direct, nondecontaminated culture.
Contamination rates were lower for MODS culture than for Löwenstein-Jensen culture for the most sensitive culture method, direct culture of pleural biopsy specimens (contamination rate, 2.9% vs. 11%; P = .05). Of all other cultures performed for the diagnostic reference group, only 1 was contaminated—decontaminated MODS culture of a pleural biopsy specimen. All negative-control cultures yielded negative culture results.
MODS culture provided a diagnosis more rapidly than did Löwenstein-Jensen culture for all testing methods (figure 1). The relative rapidity of MODS culture, compared with Löwenstein-Jensen culture, was most notable for the most sensitive culture method (i.e., direct culture of pleural biopsy specimens; median time to diagnosis, 11 days vs. 24 days; P < .001).
Discussion
There is a great need for a cost-effective, rapid, and sensitive method to diagnose pleural tuberculosis in patients who have negative findings of examination of a sputum smear specimen. Löwenstein-Jensen culture is probably the most commonly used culture method worldwide, and we report that, compared with this technique, MODS culture provided a diagnosis in one-half of the time. Moreover, MODS culture was associated with a lower rate of contamination and was more than twice as sensitive as Löwenstein-Jensen culture for pleural fluid specimens and almost twice as sensitive as Löwenstein-Jensen culture for pleural biopsy specimens. Direct culture was more sensitive than decontaminated culture. These results are consistent with previous reports [6, 7] and are particularly notable in an era in which extrapulmonary tuberculosis is a major and growing worldwide problem.
To date, there is no accepted and cost-effective methodology for diagnosing pleural tuberculosis that provides reliable detection and drug-susceptibility information. Acid-fast staining is of little value for pleural tuberculosis because of the high concentration of bacilli required for diagnosis [1]. Standard culture methods, such as Löwenstein-Jensen culture, are, likewise, hampered by a low sensitivity of ~60% for biopsy specimens [1] and 25%–37% for fluid specimens [4]. Recently developed, rapid diagnostic methods involving testing of pleural fluid specimens for adenosine deaminase, lysozyme, and IFN-γ [2–5] rely on specialized laboratory techniques that are rarely available in resource-poor settings and do not establish drug susceptibility. PCR may determine diagnosis and drug susceptibility, but it has variable sensitivity (20%–80%) and is prone to false-positive results attributable to laboratory cross-contamination [2, 3, 8]. Finally, use of automated mycobacterial broth culture systems allows relatively rapid results and drug-susceptibility testing [9], but expense limits the use of these systems in most regions of endemicity.
MODS culture is a nonproprietary laboratory culture technique for detection of M. tuberculosis that was recently developed in Peru. It is more sensitive than standard techniques (particularly for evaluating pleural biopsy specimens), and it is capable of establishing a diagnosis approximately twice as often as standard Löwenstein-Jensen culture, presumably because of its use of sensitive culture broth and a large sample inoculum. MODS culture also provides a more timely result than does Löwenstein-Jensen culture, significantly diminishing time to both positive and negative results. MODS culture also allows for concurrent determination of drug susceptibilities. Although we did not perform testing for drug resistance concurrently with testing for tuberculosis in this study, all samples were retrospectively tested for drug susceptibilities by MODS culture; 7.1% of the samples were multidrug resistant. This is consistent with the 9% rate of multidrug-resistant tuberculosis [10] and 364 cases of tuberculosis per 100,000 population [11] in communities local to the community evaluated in this study; these rates are among the highest rates worldwide in regions with a low prevalence of HIV infection and are much higher than the Peruvian national rates. Rapid drug-susceptibility testing is of crucial importance for people living with HIV infection [10]. Finally, at US$2 per sample for material costs, MODS culture is similar in price to Löwenstein-Jensen culture and is considerably less costly than automated mycobacterial culture.
The most notable shortcomings of MODS culture are the requirements for an inverted-light microscope and for laboratory training to recognize the characteristic patterns of mycobacterial cord growth. Another concern is biosafety with regard to the manipulation of infectious liquid cultures, although these are completely sealed from the time that they are set-up until sterilization by autoclaving [6].
Limitations of this study include the variable number of biopsies performed for each patient and the difficulty in defining a diagnostic standard for tuberculosis. Diagnostic yield increases with increasing number of pleural biopsy specimens [12]; thus, culture sensitivities might have been limited by the number of biopsy specimens obtained per participant. Furthermore, samples had to be diluted sufficiently to compare tests. In clinical practice, use of a single technique would allow culture without dilution, thereby increasing sensitivity. Defining a gold standard for the diagnosis of tuberculosis is challenging. Although we did not include automated mycobacterial culture as a comparison test, other authors have reported that MODS culture has similar sensitivity [6]. More extensive testing may have defined a diagnosis for a larger proportion of the study population, especially if techniques such as inducing sputum were used to allow more cases of tuberculosis to be diagnosed by sputum culture without invasive testing. We defined the diagnostic standard for tuberculosis as confirmation of the presence of mycobacteria in the pleural specimen by microscopic examination or culture to ensure high specificity, because cases of caseating granulomas caused by nonmycobacterial pathogens occur relatively frequently in Peru. However, we also reanalyzed the data with less-strict diagnostic criteria, reallocating the 5 patients with caseating necrosis who had negative microscopic examination findings and culture results to the group of patients with pleural tuberculosis. This resulted in slightly diminished sensitivity for all culture techniques but no change in the significantly higher sensitivity of MODS culture, compared with Löwenstein-Jensen culture (sensitivity for direct culture of biopsy specimens, 74% vs. 44%; P = .003).
In summary, we report that, compared with Löwenstein-Jensen culture, direct MODS culture of pleural biopsy specimens has approximately twice the sensitivity and provides a diagnosis in one-half of the time. Clinicians who must diagnose extrapulmonary tuberculosis should consider using this technique to improve diagnostic capabilities, especially in resource-poor settings, where automated culture is unlikely to be sustainable. Such improvements in diagnostic methods would facilitate adequate treatment of patients with pleural tuberculosis, potentially diminishing the morbidity and mortality associated with the disease.
Acknowledgments
We thank Dr. Carlos Saavedra, Dr. Juan Sosa, Dr. Gina Aguillar, and other physicians at the Hospital Nacional Dos de Mayo for their invaluable assistance with patient recruitment and care.
Financial support. Wellcome Trust fellowships in Clinical Tropical Medicine (to D.A.J.M., A.R.E., and C.A.E.), United States Agency for International Development Tuberculosis Award (HRN-5986-A-00–6006-00), Fogarty–National Institutes of Health (NIH) AIDS Training Program (3T22-TW00016–05S3), NIH International Training and Research in Emerging Infectious Diseases (5D43-TW00910), National Institute of Allergy and Infectious Diseases (01637), Innovation For Health And Development, and Innovation for Health and Development.
Footnotes
Presented in part: The Imperial College London Wellcome Centre for Clinical Tropical Medicine Annual Scientific Meeting, Lima, Peru, February 2007, and the International Union Against Tuberculosis and Lung Disease Annual Conference, Cape Town, South Africa, November 2007.
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Berger HW, Mejia E. Tuberculous pleurisy. Chest. 1973;63:88–92. doi: 10.1378/chest.63.1.88. [DOI] [PubMed] [Google Scholar]
- 2.de Wit D, Maartens G, Steyn L. A comparative study of the polymerase chain reaction and conventional procedures for the diagnosis of tuberculous pleural effusion. Tuber Lung Dis. 1992;73:262–7. doi: 10.1016/0962-8479(92)90130-C. [DOI] [PubMed] [Google Scholar]
- 3.Querol JM, Minguez J, Garcia-Sanchez E, Farga MA, Gimeno C, Garcia-de-Lomas J. Rapid diagnosis of pleural tuberculosis by polymerase chain reaction. Am J Respir Crit Care Med. 1995;152:1977–81. doi: 10.1164/ajrccm.152.6.8520765. [DOI] [PubMed] [Google Scholar]
- 4.Valdes L, Alvarez D, San Jose E, et al. Tuberculous pleurisy: a study of 254 patients. Arch Intern Med. 1998;158:2017–21. doi: 10.1001/archinte.158.18.2017. [DOI] [PubMed] [Google Scholar]
- 5.Villegas M, Labrada L, Saravia N. Evaluation of polymerase chain reaction, adenosine deaminase, and interferon-gamma in pleural fluid for the differential diagnosis of pleural tubercuolosis. Chest. 2000;118:1355–64. doi: 10.1378/chest.118.5.1355. [DOI] [PubMed] [Google Scholar]
- 6.Moore DA, Evans CA, Gilman RH, et al. Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N Engl J Med. 2006;355:1539–50. doi: 10.1056/NEJMoa055524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gopi A, Madhavan SM, Sharma SK, Sahn SA. Diagnosis and treatment of tuberculous pleural effusion in 2006. Chest. 2007;131:880–9. doi: 10.1378/chest.06-2063. [DOI] [PubMed] [Google Scholar]
- 8.Nagesh BS, Sehgal S, Jindal SK, Arora SK. Evaluation of polymerase chain reaction for detection of Mycobacterium tuberculosis in pleural fluid. Chest. 2001;119:1737–41. doi: 10.1378/chest.119.6.1737. [DOI] [PubMed] [Google Scholar]
- 9.Maartens G, Bateman ED. Tuberculous pleural effusions: increased culture yield with bedside inoculation of pleural fluid and poor diagnostic value of adenosine deaminase. Thorax. 1991;46:96–9. doi: 10.1136/thx.46.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawai V, Soto G, Gilman RH, et al. Tuberculosis mortality, drug resistance, and infectiousness in patients with and without HIV infection in Peru. Am J Trop Med Hyg. 2006;75:1027–33. [PMC free article] [PubMed] [Google Scholar]
- 11.Sanghavi DM, Gilman RH, Lescano-Guevara AG, Checkley W, Cabrera LZ, Cardenas V. Hyperendemic pulmonary tuberculosis in a Peruvian shantytown. Am J Epidemiol. 1998;148:384–9. doi: 10.1093/oxfordjournals.aje.a009657. [DOI] [PubMed] [Google Scholar]
- 12.Kirsch CM, Kroe DM, Azzi RL, Jensen WA, Kagawa FT, Wehner JH. The optimal number of pleural biopsy specimens for a diagnosis of tuberculous pleurisy. Chest. 1997;112:702–6. doi: 10.1378/chest.112.3.702. [DOI] [PubMed] [Google Scholar]