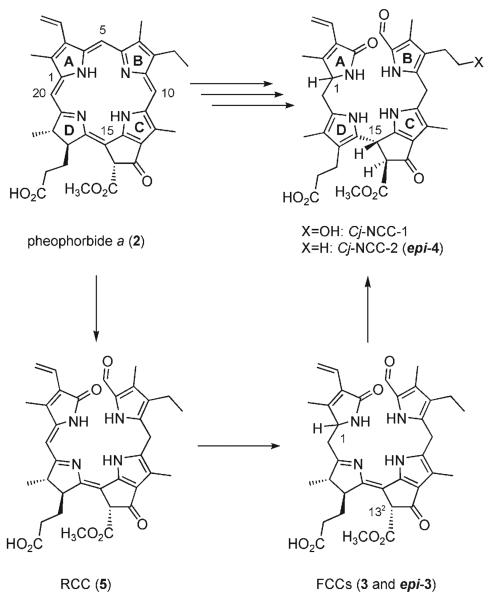
The breakdown of chlorophyll is the visible sign of leaf senescence, a form of programmed cell death in plants.[1,2] An estimated 109 tons of chlorophyll are degraded every year on this earth.[3] In this process, chlorophylls a and b (1a, 1b) are rapidly degraded via pheophorbide a (Pheo a, 2) to colorless nonfluorescent chlorophyll catabolites (NCCs), which were first identified in degreened leaves of barley (Scheme 1).[4]
Scheme 1.
Chlorophyll breakdown in senescent plants. The chlorophylls are degraded via pheophorbide a (Pheo a), the red chlorophyll catabolite (RCC), and fluorescent chlorophyll catabolites (FCCs) to nonfluorescent chlorophyll catabolites (NCCs).[5,6]
The NCCs are thought to be formed in the vacuoles in a fast and stereoselective isomerization from the fleetingly existent fluorescent chlorophyll catabolites (FCCs).[5, 6] Minute amounts of two FCCs were available from Pheo a (2) by in vitro enzymatic reactions, which allowed their identification as 3[7] and its (C1) epimer epi-3[8] (using enzyme preparations from oilseed rape and sweet pepper, respectively). However, the paucity of FCCs in plant extracts impeded studies on their chemistry and metabolism.
The disappearance of chlorophyll has also been associated with the change of color during fruit ripening.[6,9] Indeed, tetrapyrrolic NCCs were recently identified in apples and pears and shown to be remarkable antioxidants.[9] This previously unnoticed natural availability of NCCs in plant derived human nutrition calls attention to their possible physiological effects.[9] In this report, we describe a biomimetic synthesis of the FCCs 3 and epi-3 from the red chlorophyll catabolite (RCC, 5)[10] and their conversion to the NCCs 4 and epi-4 (Scheme 2). As RCC (5) is available from Pheo a (2),[10] this work completes the first partial synthesis of FCCs and NCCs (from 1a).
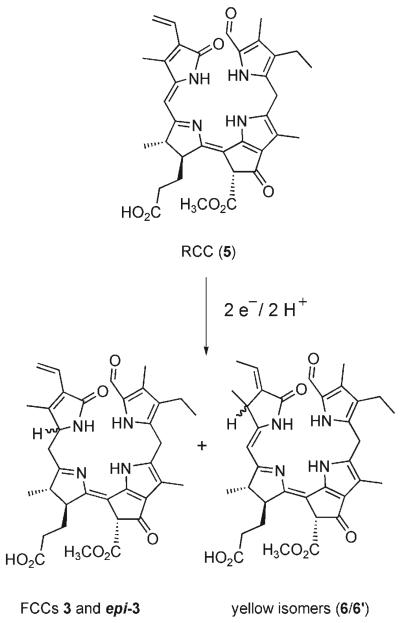
Scheme 2.
The natural FCCs 3 and epi-3 (and isomeric side products 6 and 6′) are obtained by biomimetic reduction of RCC (5).
The FCCs 3 and epi-3 were both obtained from electrochemical reduction of RCC (5)[10] by application of a method developed for the related reduction of the RCC methyl ester (Me-5).[11] RCC (5) (8 mg, 12.8 μmol) was reduced at an Hg electrode at −1.3V versus a 0.1n calomel reference electrode in deoxygenated methanolic solution. After 2.07 F mol−1 had been consumed, the crude reaction mixture was neutralized and analyzed by reversed-phase HPLC (see Figure S1 in the Supporting Information). Four fluorescent fractions were observed and isolated, which had UV/Vis absorptions characteristic of FCCs. The two main products were identified by comparison with authentic samples as 3 (0.66 mg, 1.05 μmol; 8.2 %) and epi-3 (0.64 mg, 1.02 μmol; 8.0 %).[7,8] The two minor fractions 3′ (0.17 μmol; 1.3%) and epi-3′ (0.15 μmol; 1.2 %) were indicated to be 132-epimers of 3 and epi-3, respectively, on the basis if their slow conversion in solution to 3 and epi-3 (by epimerization of the β-keto ester moiety at C132, see e.g. Ref. [12]). Along with 3 and epi-3 and their two minor stereoisomers, the mixture of reduction products also contained several fractions with yellow nonfluorescent reduction products. The main representatives (6 and 6′) of these compounds were further analyzed and were indicated to be 2,32-reduction products of 5, that is, constitutional isomers of the FCCs 3 and epi-3, analogous to the reduction side products obtained earlier from the reduction of the methyl ester Me-5[11] (see Scheme 2 and Figure S1 in the Supporting Information).
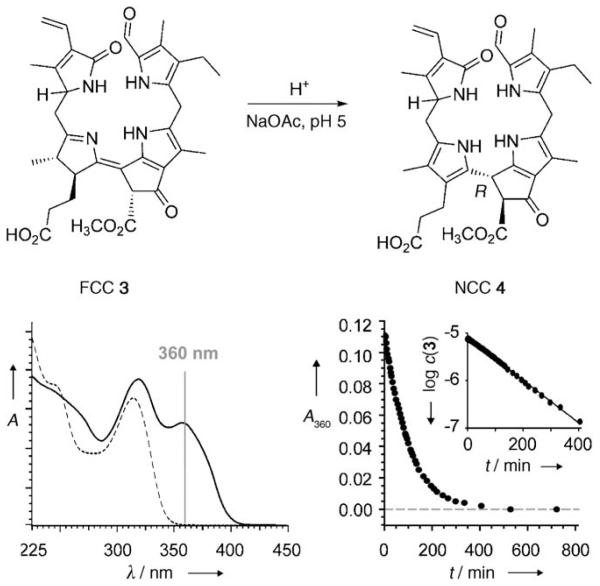
In an earlier non-enzymatic isomerization reaction with epi-3[8] in acidic medium, the NCC epi-4 was obtained as a single reaction product.[5] The stereoselectivity of this reaction was ascribed to an intramolecular protonation by the propionic acid function extending from C17 at ring D.[5] Exploiting this inherent reactivity of the FCCs, we directly acidified the reaction mixture of an electrochemical reduction of 5 (7.5 mg, 12 μmol) with acetate buffer (pH 4.9) and stored it at room temperature under inert gas. Disappearance of the FCCs (3, 3′, epi-3, and epi-3′, originally present in similar ratios as noted above) and appearance of NCCs were analyzed by analytical HPLC. After an overall reaction time of 18.7 h, only insignificant amounts of FCCs remained (HPLC with fluorescence detection), and the mixture contained two main fractions with UV/Vis spectra characteristic of NCCs (as well as the fractions of yellow side products, such as 6 and 6′, see the Supporting Information).
Purification by semipreparative HPLC led to 0.5 mg (7 % yield) each of two main NCCs, isolated as powders and identified as 4 and epi-4 as follows: Chromatographic and 1H NMR spectroscopic correlations allowed the less polar NCC to be identified with epi-4, the NCC obtained from isomerization of epi-3.[5] The slightly more polar NCC was also indicated by its UV/Vis, NMR, and mass spectra (see Experimental Section) to be a 31,32-didehydro-1,4,5,10,15,20,22,24-octahydro-132-methoxycarbonyl-4,5-secophytoporhpyrinate. Compared to the spectra of epi-4, the 1H NMR spectra of this NCC showed mainly significant chemical shift differences for signals that could be assigned to protons attached to rings A and D. These data thus support the identification of this NCC as 4, the (C1) epimer of epi-4. Whereas epi-4 was shown earlier to be the same as Cj-NCC-2 from leaves of Cercidiphyllum japonicum,[5] the isomeric NCC 4 remains to be identified in natural plants.
For a more thorough mechanistic investigation, the isomerization of the FCCs 3 and epi-3 to the NCCs 4 and epi-4 was analyzed at room temperature as a function of pH. The disappearance of the FCCs was monitored by the UV absorbance at 360 nm and it followed pseudo first-order kinetics (Figure 1). An HPLC analysis with observation at 320 nm (where FCCs and NCCs absorb) was carried out in parallel. Samples of 3 and epi-3 were thus dissolved in buffered aqueous solutions and stored at 26°C. The isomerization of the two FCCs was monitored in the pH range 3.5–7.0 (for 3) and 4.0–6.0 (for epi-3). At pH 4.0, 3 and epi-3 disappeared with apparent first-order rate constants of 0.02 min−1 and 0.039 min−1, respectively. At pH 6.0, each of the two FCCs disappeared about 12 times more slowly. The two FCCs eventually converted cleanly at pH ≈ 5 into the NCCs 4 and epi-4, as observed by HPLC (see Table S1 and Figure S2 in the Supporting Information).
Figure 1.
Top: Acid-induced stereoselective isomerization of FCC 3 to the NCC 4 parallels the biomimetic isomerization of FCC epi-3 to NCC epi-4.[5] Bottom: UV/Vis spectral (left, —: trace at t = 0 min and -----: trace at t = 427 min) and kinetic analysis (right) of the isomerization of 3 to 4 observed at room temperature and pH 5.
The derived relationship of the rate versus pH could be explained by the effective participation of a proton donor with a pKa near 5.0. Whereas, at pH < 4.0 protonation from the solution may be a significant factor, in weakly acidic medium, the critical step in the isomerization of the FCCs to the corresponding NCCs is suggested to occur by a stereoselective intramolecular protonation at C15 by the propionic acid function at C17.[5] A structural model indicates the protonation to take place on the β face, leading to 15R configuration at C15. The characteristics of the CD spectra of all known natural NCCs are consistent with a common configuration at C15, which has been derived from the model to be R.[5,13]
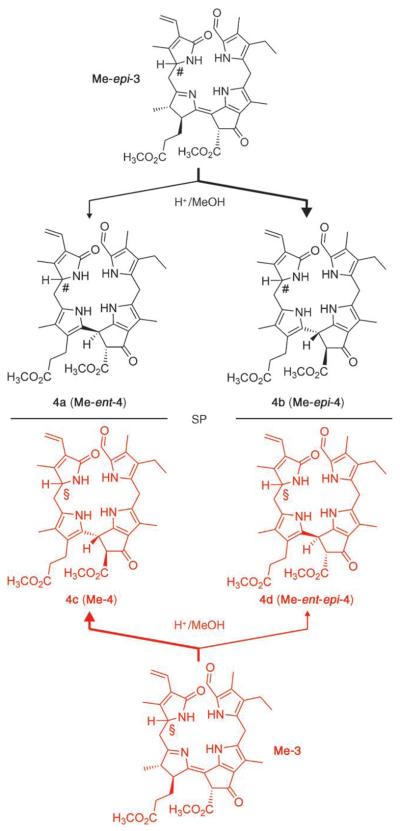
To further characterize the putative role of the propionic acid function in the isomerization of FCCs to NCCs, we also studied the behavior of the FCC methyl esters (Me-3 and Me-epi-3), which were available from partial synthesis.[11] Strongly acidic conditions were required to observe an isomerization of Me-3 and Me-epi-3 at a practical rate. When dissolved in 0.3m trifluoroacetic acid (TFA) in methanol, Me-epi-3 disappeared with half-life (t1/2) of ca. 6 h at room temperature. Me-epi-3 thus isomerized about 20 times more slowly under these conditions than epi-3 at pH 4.0. Storage of an Ar-saturated solution of 1.08 mg (1.68 μmol) of Me-epi-3 in 0.3m TFA in methanol at ambient temperature for 18 h led to about 67% conversion and two fractions with UV characteristics of NCCs, as analyzed by HPLC. Upon purification by HPLC, 0.28 mg (0.4 μmol; 26%) of fraction 4b was obtained, which was identified (as Me-epi-4) by comparison with the authentic methyl ester of Cj-NCC- 2.[5] From the other fraction (0.08 mg, 0.12 μmol; 7%), the slightly more polar 4a was isolated (Scheme 3).
Scheme 3.
Acid-induced isomerization of the FCC methyl esters Me-3 and Me-epi-3 shows reduced stereoselectivity and provides two diastereomeric pairs of NC enantiomers (Me-4/Me-ent-4 and Me-epi-4/Me-ent-epi-4). The identical configuration at C1 in Me-3 and the NCCs 4c and 4d is indicated by §, the opposite common configuration of Me-epi-3 and 4a and 4b by the symbol #. Molecular models support a tentative assignment of S when the configuration at C1 is § and R when the configuration at C1 is #. SP = symmetry plane, bold arrows lead to the major products.
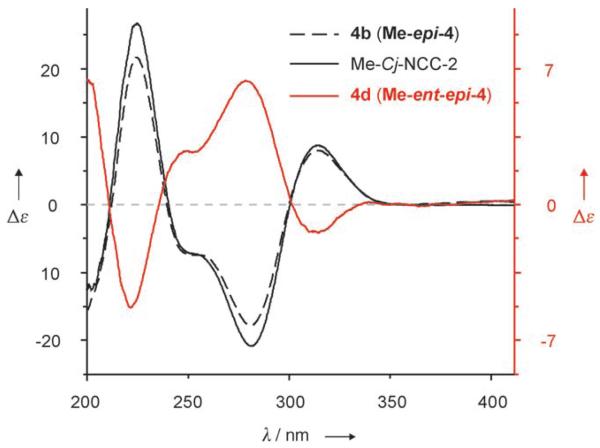
Similar treatment of an Ar-saturated solution of Me-3 (0.77 mg, 1.2 μmol) in 0.3m TFA in methanol at room temperature led to isomerization, too (64% conversion), giving two fractions with UV characteristics of NCCs. The CD spectrum of the major isomerization product 4c (160 μg, 0.25 μmol; 21% yield) was similar to the spectra of authentic Me-epi-4 and of the natural NCCs.[13] However, the CD spectrum of the minor fraction, 4d (90 μg, 0.14 μmol; 12%), was nearly the mirror image (and was similar to that of the minor isomer 4a from isomerization of Me-epi-3, see Scheme 3 and Figure 2). In fact, as 4b (Me-epi-4) and 4d had identical (500 MHz 1H NMR) spectra, and their CD spectra were nearly mirror images of each other, they were identified as enantiomers. On the same basis, 4a was identified as the enantiomer of 4c(Me-4). In contrast to the stereospecific, high-yielding isomerization of the FCCs 3 and epi-3, acid-induced isomerization of the methyl esters Me-3 and Me-epi-3 was much slower and less stereoselective: the NCC methyl esters Me-4 and Me-epi-4 and their (C15) epimers 4d (Me-ent-epi-4) and 4a (Me-ent-4) were obtained in a range of ratios from 2:1 to 4:1. Indeed, blocking the intramolecular protonation resulted in a lack of stereoselectivity, which opens up a remarkable (formal) first path to the enantiomers of the natural NCCs by partial synthesis.
Figure 2.
CD spectra of the synthetic NCC methyl esters 4b and 4d, and of the methyl ester of the natural NCC Cj-NCC-2, which is identical to Me-epi-4. All spectra recorded in methanol.
In this study we report a two-step biomimetic partial chemical synthesis of NCCs from RCC (5). Its first step opens the door to both natural (C1-epimeric) FCCs (3 and epi-3)by single electron and proton transfer reactions (in an electrochemical reduction).[6,11] During natural chlorophyll breakdown, this reaction is mediated by two distinct classes of ferredoxin-dependent RCC reductases, which introduce the new stereocenter at C1 with opposite configuration.[7,8] These reductases have been suggested to catalyze the formation of FCCs by a series of proton and electron transfer steps,[11,14] a mechanism also similar to that of the (homologous) bilin reductases.[16] The second step represents an efficient, stereoselective transformation of the FCCs to the NCCs 4 and epi-4, representatives of the two known epimeric lines of natural NCCs. The stereoselectivity and rate of this “natural” access by FCC isomerization are compatible with the hypothetic analogous processes in the vacuoles.[5,6,17]
Based on the efficiency of this chemical reaction, we have proposed this last catabolic step to occur spontaneously in the acidic environment of the vacuole rather than to require an enzyme.[5] On the other hand, the observed stability of FCCs in pH-neutral medium may provide a time window for the hypothetical modification of FCCs by cytosolic enzymes.[18,19] Such reactions were inferred from the structures of NCCs and were suggested to be relevant for transport of FCCs into the vacuoles.
Propionate esterification, as in the FCC methyl esters (Me-3 and Me-epi-3), made FCC derivatives more resistant against acid-induced isomerization. Me-3 and Me-epi-3 converted only under more forcing conditions to methyl esters of NCCs: 4 and epi-4 were obtained, and also their enantiomers, ent-4 and ent-epi-4 (Scheme 3). As such methyl esters can be hydrolyzed easily with catalysis by porcine liver esterase (see the Supporting Information and Ref. [10]), this finding has opened a pathway to the (so far unknown) enantiomers of natural NCCs. Our experiments indicate that FCC esterification leads to more persistent FCCs and that their acid-induced isomerization to NCCs proceeds with low stereoselectivity. Indeed, accumulation of FCCs in some ripening fruit was recently detected in our labs and appears to be correlated with a modification of their propionic acid group in FCCs, giving an unexpected first hint for the possible occurrence of FCC esters.
Our investigations provide a synthetic path to colorless tetrapyrrolic chlorophyll catabolites, such as both of the natural FCCs as well as NCCs and some of their non-natural analogues. The chemical synthesis of FCCs and NCCs may enable their use, for example, as substrates/reference materials in studies of their conversion by plant extracts and in other (e.g. pharmacological) investigations. NCCs were recently shown to exist in fruit and to be effective natural antioxidants.[9] These findings have drawn our attention to possible physiological effects of the NCCs, which were considered earlier to be mere detoxification products.[20] Clearly, chemical synthesis is a key approach, setting a foundation for a better understanding of the metabolism and an eventual physiological role of chlorophyll catabolites.
Experimental Section
Spectroscopic data for NCC 4: UV/Vis (MeOH/potassium phosphate (50 mm, pH 7.0) = 6.5/3.5 v/v): λmax (rel. ε) = 314 (0.64), 232 (0.70), 216 (1.00) nm; FAB-MS (glycerin matrix): m/z (%): 667.3 (14) [M+K]+, 652.3 (58), 651.3(97) [M+Na]+, 631.3 (31), 630.3 (49), 629.3 (100) [M+H]+, 597.3 (32) [M−MeOH+H]+, 505.3 (62) [M−ring A + H]+; 1H NMR (500 MHz, 2 mm in [D4]MeOH, 26°C, TMS): δ = 0.99 (m, 3H; H3C(82)), 1.89 (s, 3H; H3C(21)), 1.94 (s, 3H; H3C(181)), 2.08 (s, 3H; H3C(121)), 2.25 (s, 3H; H3C(71)), 2.34 (m, 2 H; H2C(172)), 2.42 (dd,3J(H,H) = 7.8 Hz,15.6 Hz, 2H; H2C(81)), 2.51 (dd, 3J(H,H) = 8.8 Hz,14.6 Hz, 1 H; HAC(20)), 2.66 (m, 1 H; HBC(171), 2.73 (m, 1 H; HBC(171)), 2.79 (dd, 3J(H,H) = 5.9 Hz,14.6 Hz, 1 H; HBC(20)), 3.74 (s, 3H; H3C(135)), 3.91 (s, 2 H; H2C(10)), 4.07 (dd, 3J(H,H) = 5.9 Hz,7.8 Hz, 1 H; HC(1)), 4.91 (s, 1 H; HC(15)), 5.38 (dd, 3J(H,H) = 2.0 Hz,11.7 Hz, 1 H; HAC(32)), 6.10 (dd, 3J(H,H) = 2.0 Hz,17.6 Hz, 1 H; HBC(32)), 6.44 (dd, 3J(H,H) = 11.7 Hz, 17.6 Hz, 1 H; HC(31)), 9.36 ppm (s, 1 H, HC(5)); 13C NMR (125 MHz, 2 mm in [D4]MeOH, 26°C, TMS) from HSQC data: δ = 8.6 (71), 9.0 (121), 9.1 (181), 12.3(21), 15.1 (82), 17.4 (81), 21.9 (171), 23.5 (10), 30.3 (20), 37.1 (15), 39.6 (172), 52.6 (135), 61.5 (1), 67.5 (132), 111.9 (12), 115.3 (18), 119.4 (32), 120.7 (17), 124.2 (16), 124.2 (19), 125.6 (13), 125.9 (8), 127.5 (31), 128.4 (3), 129.0 (6), 133.8 (11), 134.4 (7), 137.5 (9), 156.9 (2), 161.4 (14), 171.5 (133), 174.5 (4), 181.2 ppm (173).
The Supporting Information contains all experimental data, including synthetic procedures, spectroscopic data, and identification of the synthesized compounds, as well as additional figures concerning the synthesis of FCCs (Figure S1), HPLC analysis (Figure S2), and a kinetic description of the tautomerization of FCCs to NCCs (Table S1) and the tautomerization of FCC methyl esters (Figure S3).
Supplementary Material
Footnotes
We thank K. Breuker, K.-H. Ongania, and S. Gschösser for spectroscopic measurements and acknowledge financial support from the Austrian Science Funds (FWF P-16097 and P-19596).
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.Matile P, Hörtensteiner S, Thomas H, Kräutler B. Plant Physiol. 1996;112:1403. doi: 10.1104/pp.112.4.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matile P. In: Regulation of Photosynthesis. Aro E-M, Andersson B, editors. Kluwer Academic Publishers, Dordrecht; The Netherlands: 2001. p. 277. [Google Scholar]
- 3.Hendry GAF, Houghton JD, Brown SB. New Phytol. 1987;107:255. doi: 10.1111/j.1469-8137.1987.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 4.Kräutler B, Jaun B, Bortlik K, Schellenberg M, Matile P. Angew. Chem. 1991;103:1354. [Google Scholar]; ; ; Angew. Chem. Int. Ed. Engl. 1991;30:1315. [Google Scholar]
- 5.Oberhuber M, Berghold J, Breuker K, Hörtensteiner S, Kräutler B. Proc. Natl. Acad. Sci. USA. 2003;100:6910. doi: 10.1073/pnas.1232207100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kräutler B, Hörtensteiner S. In: Chlorophylls and Bacteriochlorophylls: Biochemistry, Biophysics, Functions and Applications. Grimm B, Porra R, Rüdiger W, Scheer H, editors. Vol. 25. Springer, Dordrecht, The Netherlands: 2006. p. 237. [Google Scholar]
- 7.Mühlecker W, Ongania KH, Kräutler B, Matile P, Hörtensteiner S. Angew. Chem. 1997;109:401. [Google Scholar]; ; ; Angew. Chem. Int. Ed. Engl. 1997;36:401. [Google Scholar]
- 8.Mühlecker W, Kräutler B, Moser D, Matile P, Hörtensteiner S. Helv. Chim. Acta. 2000;83:278. [Google Scholar]
- 9.Müller T, Ulrich M, Ongania KH, Kräutler B. Angew. Chem. 2007;119:8854. doi: 10.1002/anie.200703587. [DOI] [PMC free article] [PubMed] [Google Scholar]; ; ; Angew. Chem. Int. Ed. 2007;46:8699. [Google Scholar]
- 10.Kräutler B, Mühlecker W, Anderl M, Gerlach B. Helv. Chim. Acta. 1997;80:1355. [Google Scholar]
- 11.Oberhuber M, Kräutler B. ChemBioChem. 2002;3:104. doi: 10.1002/1439-7633(20020104)3:1<104::AID-CBIC104>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 12.Berghold J, Müller T, Ulrich M, Hörtensteiner S, Kräutler B. Monatsh. Chem. 2006;137:751. [Google Scholar]
- 13.Kräutler B. In: The Porphyrin Handbook. Kadish KM, Smith KM, Guilard R, editors. Vol. 13. Elsevier Science; Oxford: 2003. p. 183. [Google Scholar]
- 14.Wüthrich KL, Bovet L, Hunziker PE, Donnison IS, Hörtensteiner S. Plant J. 2000;21:189. doi: 10.1046/j.1365-313x.2000.00667.x. [DOI] [PubMed] [Google Scholar]
- 15.Rodoni S, Mühlecker W, Anderl M, Kräutler B, Moser D, Thomas H, Matile P, Hörtensteiner S. Plant Physiol. 1997;115:669. doi: 10.1104/pp.115.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frankenberg N, Lagarias JC. In: The Porphyrin Handbook. Kadish KM, Smith KM, Guilard R, editors. Vol. 13. Elsevier Sicence; Oxford, UK: 2003. p. 211. [Google Scholar]
- 17.Hörtensteiner S, Kräutler B. Photosynth. Res. 2000;64:137. doi: 10.1023/A:1006456310193. [DOI] [PubMed] [Google Scholar]
- 18.Pruzinska A, Tanner G, Aubry S, Anders I, Moser S, Müller T, Ongania K-H, Kräutler B, Youn J-Y, Liljegren SJ, Hörtensteiner S. Plant Pathol. 2005;139:52. doi: 10.1104/pp.105.065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mühlecker W, Kräutler B. Plant Physiol. Biochem. 1996;34:61. [Google Scholar]
- 20.Hörtensteiner S. Annu. Rev. Plant Biol. 2006;57:55. doi: 10.1146/annurev.arplant.57.032905.105212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.