Abstract
The Wnt signaling pathway is frequently deregulated in cancer due to mutations in the genes encoding APC, β-catenin and axin. To identify small molecule inhibitors of Wnt signaling as potential therapeutics, a diverse chemical library was screened using a TCF-reporter cell line in which the activity of the pathway was induced at the level of the Disheveled protein. A series of deconvolution studies was used to focus on 3 compound series that selectively killed cancer cell lines with constitutive Wnt signaling. Activities of the compounds included the ability to induce degradation of β-catenin that had been stabilized by a GSK-3 inhibitor. This screen illustrates a practical approach to identify small molecule inhibitors of Wnt signaling that can seed the development of agents suitable to treat patients with Wnt-dependent tumors.
Introduction
The Wnt signaling pathway is activated by Wnt ligands at multiple stages of metazoan development and controls the differentiation and/or proliferation of stem cells in multiple tissues. The ‘canonical’ Wnt/β–catenin pathway is activated following Wnt ligand binding to a complex comprising Frizzled (Fz) and LRP5/6 receptors and ultimately activates β–catenin/TCF dependent transcription (Fig. 1a). Key steps in the pathway include the formation of a ligand-activated receptor complex, the inhibition of the intracellular β–catenin turnover and the formation of a nuclear β–catenin/TCF transcription complex. Wnt/β–catenin signalling activates (and represses) transcription of genes whose promoters contain binding sites for TCF-transcription factors. Target genes include key developmental and oncogenic targets (reviewed in (1)).
Fig. 1. Primary screen.
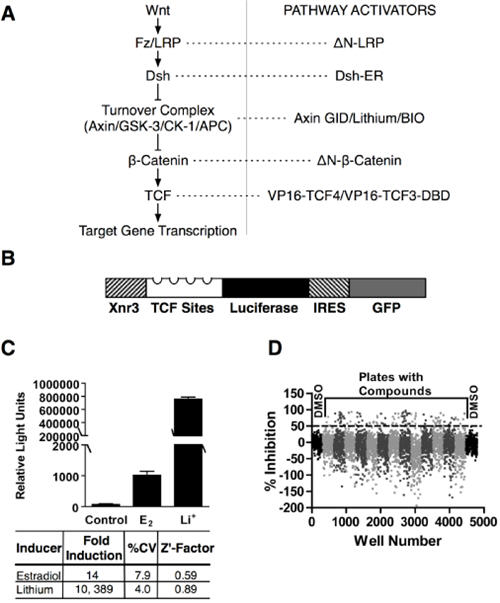
a. pathway activators (see text) that were used to induce Wnt/β-catenin signaling at distinct levels. b. the reporter construct used in the human epithelial HEK293-based cell line. The bicistronic TCF-luciferase-IRES-GFP reporter contained the Wnt response element from the Xnr3 promoter and four TCF binding sites. c. fold induction, coefficients of variation, and Z'-factors following 24 h estradiol and lithium induction. d. subset of thirteen 384-well plates (alternate shades of gray) and DMSO control plates (black) from the primary screen data. The dotted line indicates 50% inhibition of estradiol induction.
The Wnt signaling pathway is considered a key therapeutic target for cancer (1). Mutations to ‘core’ Wnt signaling components including β–catenin, APC and Axin inappropriately activate the β-catenin/TCF branch of the Wnt signaling pathway in cancers arising in a number of tissues including colon and liver. Many other cancers show evidence of inappropriate Wnt pathway activation including raised levels of β–catenin (2). Unfortunately, the known intracellular components of the ‘core’ Wnt pathway are not good targets for small molecule inhibitors, as many interactions comprise extended protein-protein interfaces. Furthermore, drugs that target ‘core components’ may induce side-effects since the function of key components (e.g. β–catenin) is required for tissue homeostasis in multiple organs including the immune system and intestine (3).
Small molecules that target ‘non-core’ Wnt signaling components have recently been identified (4). Of particular note, ICG001 blocks the interaction between β–catenin and the ‘non-core’ CBP protein leading to a reduction of adenoma formation in mouse models of colon cancer (5). The β–catenin-CBP interaction was shown to promote stem/progenitor marker expression whilst the related β–catenin-p300 interaction, which was not subject to blockade by ICG001, promoted expression of genes involved in proliferative responses such as c-myc (6). The key point from this example is that compounds that target ‘non-core’ components may be able to regulate distinct subsets of therapeutically relevant transcriptional targets. The recently discovered small molecules XAV939 and IWR2 inhibit Wnt signalling by blocking tankyrase activity that is required for degradation of the β–catenin scaffold protein Axin (3, 7).
One of the main challenges in targeting ‘non-core’ Wnt signalling molecules is knowing which protein to target. A large number of potential target genes that regulate Wnt/β–catenin signalling have been identified in whole genome RNAi screens, mass spectrometry and yeast 2-hybrid studies (8-10) and it remains to be seen which protein should be the focus of drug discovery efforts. To overcome this problem, we used a cell-based assay to screen a diverse library of small molecules for regulators of TCF-dependent transcription. We identified a number of chemical regulators that functioned at distinct levels within the Wnt signaling cascade.
Materials and Methods
Materials
The Cancer Research UK Centre for Cancer Therapeutics compound library was used for the primary screen. CCT031374 analogues were either obtained from this library or from commercial sources. Preparation of compounds to follow-up the initial hit matter is described in the Supplementary Material. ΔN-LRP, ΔN-β-catenin, VP16-TCF4, Tau-1N4R, c-myc-luciferase and survivin-luciferase vectors were kind gifts of K. Brennan, P. Polakis, A. García de Herreros, S. Lovestone, B. Vogelstein and R. Moon respectively. Human tumor cell lines were obtained from ATCC, grown for less than 20 passages and tested regularly for mycoplasma infection (Lonza Mycoalert).
Reporter Cell Line
A fragment of the Xnr3 enhancer (−180 to −60), four TCF consensus-binding sites and a c-Fos minimal promoter were inserted into the pUB-bsd blasticidin resistance plasmid (Invitrogen). The luciferase gene and the IRES-GFP-SV40 polyA sequences from pIRES-hrGFP-2a (Stratagene) were inserted downstream of the promoter (Fig.1b). The reporter vector was transfected into a stable HA-Dvl2-ER (estrogen receptor) expressing HEK293 cell line (11). Blasticidin-resistant cells were treated with 9mM lithium chloride (LiCl) for 16 hours to induce Wnt-dependent expression of GFP. Two rounds of fluorescent activated cell sorting (FACS) were used to enrich for high GFP expressing cell clones. The 7df3 clone was used as the reporter line in the primary screen.
Primary Screen
63,000 structurally-diverse, low molecular weight compounds were added individually to reporter cells at 20μM two hours prior to addition of 10μM β-estradiol in 384-well plates. Luciferase assays were carried out 24h after β-estradiol addition using SteadyGlo reagent (Promega). Activities of primary hits were reconfirmed in further luciferase assays and counter-screened for inhibition of luciferase enzyme (EasyLite kinase reagent, Perkin Elmer) and non-specific cell growth inhibition (Celltiter Blue, Promega).
Secondary Assays
Hit compounds (20μM) were assayed in transiently-transfected HEK293 cells co-transfected with Topflash (Wnt reporter, Upstate), TK-renilla (control) and CMV-driven HA-Dvl2-ER expression plasmids. Twenty-four hours after transfection, cells were stimulated with 3μM β-estradiol or treated with the GSK-3 inhibitor 6-bromoindirubin-3′-oxime (BIO; Calbiochem). Luciferase activity was measured after 24h using DualGlo reagents (Promega). For deconvolution assays, HEK293 cells were co-transfected with Topflash and CMV-lacZ reporter (control) plasmids together with constitutively-active ‘core’ components of the pathway: ΔN-LRP, Axin-GID, ΔN-β-catenin and VP16-TCF4. Compounds were added 24h after transfection and reporter activity analyzed a further 24h later using BrightGlo and BetaGlo (Promega). Luciferase activity was normalized to β-galactosidase reporter activity. Experiments with c-myc and survivin-luciferase reporters were carried out in HEK293 cells using Axin-GID as the inducer. SW480 cells were co-transfected with Topflash and CMV-lacZ reporter plasmids 24h prior to compound addition. Reporter assays were conducted after a further 24h.
Growth Inhibition (GI50)
Cells were seeded to give a density of 25% confluence after 24h in either 96 or 384 well tissue culture plates. Compounds (0.05 -100μM) were added at 24h, Cell Titre Blue or WST-1 (Roche) reagent was added after 72h (HT29, SW480 and HCT116) or 96h (SNU475 and CCD841Co).
β-Catenin Stability Experiments
Mouse L-cells were cultured in DMEM/10% FBS and exposed to either 7.5μM BIO, 9mM LiCl, 50% Wnt-3a conditioned medium (12) or 50μM MG-132 (Merck Biosciences) and with compound for 6h prior to medium replacement as indicated. β-catenin was assayed as previously described (13). The following antibodies were used: β-catenin and GSK-3β (BD-Transduction laboratories), β-actin (Sigma). The BD-Transduction Laboratories β-catenin antibody was used for immunocytochemistry as previously described (14).
For pulse-chase analyses, L-cells were treated throughout with 7.5μM BIO. Cells were first starved in medium lacking methionine and cysteine for 1h then labeled for 1h with 5 MBq per 25 cm2 flask of 35S-labelled methionine / cysteine. Cells were chased with unlabelled medium for the indicated periods. Cells were lysed and immunoprecipitation was carried out using 2 μg/sample monoclonal β-catenin antibody and 50 μl of Protein G coated beads (GE Healthcare). After 3 washes in lysis buffer, bound proteins were eluted in SDS sample buffer, separated by electrophoresis and analyzed as previously described (15).
Phosphorylated Tau Experiment
HEK293 cells were transfected with GSK3β and/or Tau 1N4R expression vectors (16). After 48h, cells were exposed to 5μM BIO for 8h, after which they were treated with 20μM CCT031374 and 5μM BIO for the indicated time periods. Immunoblotting for GSK-3β, phospho and non-phospho Tau antibodies (BT2, AT270, Autogen Bioclear) was carried out as previously described (16). Band peak areas were calculated using ImageJ (Scion). The phospho-Tau peak areas were normalized to the total Tau levels.
GST-E-Cadherin Pulldown of β-Catenin
The β-catenin binding region of E-cadherin (amino acids 730-882) was fused in frame with the GST coding region of pGEX-5X-2 (GE Healthcare). GST-E-cadherin fusion protein was expressed and purified using Glutathione Sepharose 4 Fast Flow beads (GE Healthcare). Ten cm dishes of HEK293 and SW480 cells were, treated with CCT031374 and/or BIO and lysed to give a final volume of 1ml cell extract. 5μg GST-E-cadherin was combined with 500μl of cell extract and precipitated following addition of glutathione beads. Bound β-catenin was detected by immunoblotting.
ES Cell Quantitative PCR For LEF1
H9 human ES cells were cultured on feeders using standard culture methods (17). Using a modification of (17), neurogenic embryoid bodies were generated by culturing H9 colony fragments in suspension in neuralizing medium (ADF (Invitrogen 12634010) containing 10 μM SB431542 (TGF-β Inhibitor, Tocris) and 10uM Y-27632 (ROCK inhibitor, Calbiochem) for the first 48 hours, On day 8, medium was supplemented with BIO (5μM) and/or CCT031374 (20μM) for 24h. RNA was extracted with Rneasy (Qiagen). cDNA was synthesized with Superscript II Rnase H- RT (Invitrogen). Quantitative PCR reactions were performed in DyNAmoHS master mix (New England Biolabs) using the LEF1 primers: 5′accagattcttggcagaagg 3′ and 5′cagaccagcctggataaagc 3′.
Xenopus Studies
Xenopus laevis embryos were obtained, degelled and cultured using standard procedures (18) and staged according to Nieuwkoop and Faber (NF) (19). Test compound or DMSO (control) was added at the 4-16 cell stage. Embryos were scored at NF stages 35-38. For the animal cap assay, dissected Xenopus embryo animal caps were treated with 300 mM LiCl for 10 minutes followed by 2.5h exposure to CCT036477 at the indicated concentrations or with DMSO control (indicated with dash). RNA extraction and RT-PCR conditions were carried out as described (20).
Zebrafish Studies
Zebrafish embryos were collected after natural spawning. Embryos were treated from the 16 cell stage until mid gastrulation (8h post fertilization) with 20μM of the compound CCT036477 in 1% DMSO/fish water or with DMSO equivalent. Control and experimental embryos were imaged when at the post-gastrulation somitic stage (48h post fertilization).
Results
Optimization and outcome of high-throughput screen
To identify novel small-molecule regulators of the Wnt signaling pathway, a HEK293 based reporter cell line (7df3) was generated to allow the inducible induction of TCF-dependent transcription. The rationale for this approach was twofold. Firstly, inducible induction of the Wnt pathway was predicted to minimize positive and negative feedback from transcriptional targets, thereby simplifying the process of target deconvolution. Secondly, the cell line did not require Wnt signaling for proliferation, thereby allowing the rapid distinction of non-specific growth inhibitors and compounds acting specifically on the Wnt pathway.
A bicistronic reporter coding for firefly luciferase and GFP under the control of a promoter containing 4 canonical TCF binding sites and the Wnt-responsive region from the Xenopus Xnr3 promoter (21) (Fig. 1b) was introduced into HEK293 cells that already contained an integrated Disheveled - estrogen receptor fusion (Dvl2-ER (11)). Previous studies showed that estradiol induction of Dvl2 activity raised β-catenin levels within 30min in these cells (11). Clones that showed tight regulation of TCF-dependent transcription in response to estradiol and the GSK-3 inhibitor LiCl (Fig. 1c), were identified by FACS sorting.
Assays in a 384-well format showed reproducible 14-fold and 10, 400-fold induction with estradiol and LiCl respectively with low coefficients of variation (%CV) and high Z′-factors (estradiol, 0.59; LiCl 0.89; Fig.1c). Importantly, the cell line also showed robust behavior with multiple cellular passages over more than two years. For the primary screen, 63,040 chemically diverse compounds were analyzed by sequential addition of compound (20μM in 0.2% DMSO) and estradiol 24h prior to luciferase assay. Data from a subset of 384 well plates from the primary assay (Fig.1d) illustrate the range of responses to compound addition. The 1, 902 compounds that showed >50% inhibition were reanalyzed (n=2) and counter-screened to exclude direct inhibitors of luciferase enzyme activity and to remove acutely cytotoxic compounds. At this stage, 306 molecules were selected for subsequent analysis (Fig. 2a).
Fig 2. Hit triage and deconvolution.
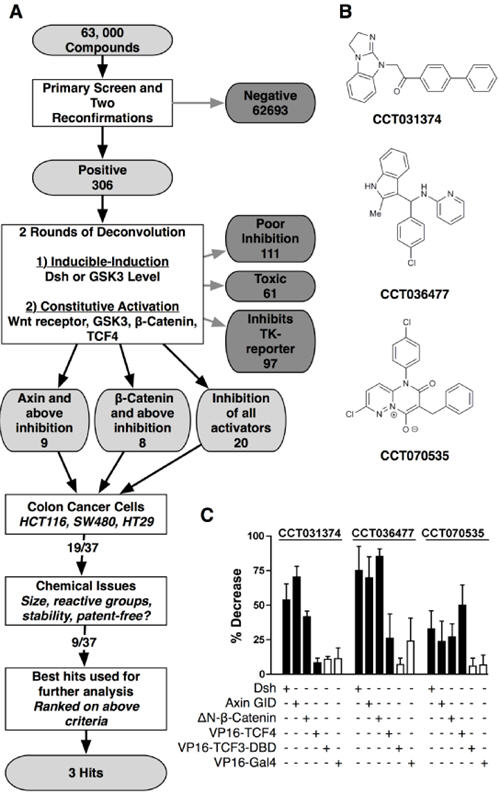
a. Compound attrition flow diagram leading to selected hits. b. Structures of the three hit compounds CCT031374, CCT036477 and CCT070535. c. Summary of the effects of 30μM CCT031374, CCT036477 and CCT070535 on TCF-dependent transcription induced at different levels of the Wnt pathway. Control Gal4-luciferase reporter activity was induced by VP16-Gal4.
Hit Triage and Mechanistic Deconvolution Assays
To identify molecules that specifically blocked TCF-dependent transcription, compounds with a greater than 5:1 ratio of activity against a TCF-luciferase versus a TK -renilla luciferase reporter were selected following co-transfection with a Dvl-2-ER expression plasmid in HEK293 cells. Thirty seven compounds showed a greater than a 5:1 specificity ratio together with low toxicity and high TCF- activity as originally detected in the 7df3 reporter line (Fig.2a). All 37 hits showed activity against TCF-dependent transcription that was induced by treatment of HEK293 cells with the GSK-3 inhibitor BIO (22).
To identify the point in the canonical Wnt signaling pathway at which the compounds acted, the pathway was activated at distinct levels by expression of activating cDNAs (see Fig. 1a). At the receptor level, TCF-dependent transcription was induced by expression of a dominantly-active N-terminal deletion of the LRP6 co-receptor, ΔN-LRP6 (23). Activation at the Disheveled level involved expression of Dvl-2. At the GSK-3 level, transcription was activated by expression of the GSK-3 binding domain of Axin (Axin-GID) which acts to titrate GSK-3 from the endogenous Axin protein (24). A non-degradable, β-catenin with an N-terminal deletion (ΔN-89-β-catenin) (25) activated transcription downstream of the β-catenin turnover complex and a TCF4-VP16 fusion protein was used to probe compound activity at the level of the nuclear transcription factor. All 37 compounds blocked activation by Axin-GID: 9 of these failed to block ΔN-β-catenin or TCF4-VP16 (i.e. they act at the level of Axin), a further 8 blocked ΔN-β-catenin but failed to block TCF4-VP16 (i.e. they act at the level of β-catenin) while 20 compounds blocked all three activators (Fig.2a). Results from a subset of the deconvolution assays is shown for 3 compounds (Fig.2b, c).
Hit Selection
Selective anti-proliferative activity for human tumor cell lines with Wnt pathway activating oncogenic β-catenin, APC or AXIN deletions (HT29 (42), SW480 (39), HCT116 (40) and SNU475 (41)) compared to a non-transformed epithelial cell line was used as a criterion in combination with chemical tractability to focus on a subset of 9 and subsequently 3 compounds for further analysis (Table 1, Fig. 2b, Fig. S1). The set of 3 compounds (CCT070535, CCT036477, and CCT031374; see Fig.2b) were selected based on a combination of the following criteria: metabolic stability (compound stability in mouse liver microsomes; Fig. S2), low growth inhibitory activity in non-tumor control cells, promoter-specificity (TCF versus TK) and the availability of commercially available analogues. A key feature in this selection was the clarity of the deconvolution response to different Wnt pathway activators since unambiguous activity suggested that the mechanism of compound action could be tracked in subsequent assays (Fig.2c). All 3 compounds blocked HCT116 human colon cancer cell proliferation by inducing apoptosis as shown by caspase 3 activity assays (Fig. S3) but CCT031374 induced almost two times more caspase activity than the other compounds.
Table 1.
Inhibition of 7dF3 reporter activity (IC50) and growth inhibition (GI50) of hit compounds
| Compound | Reporter IC50 (μM) |
HT29 (APC mutant) GI50 (μM) |
HCT116 (oncogenic β- catenin, GI50 (μM) |
SW480 (APC mutant) GI50 (μM) |
SNU475 (Axin mutant) GI50 (μM) |
CCD841Co (Control) GI50 (μM) |
|---|---|---|---|---|---|---|
| CCT007812 | 6.2 | 11.0 | 11.1 | 46.7 | 32.0 | >>44 |
| CCT014939 | 20.5 | >100 | >100 | >100 | 28.5 | >>44 |
| CCT020435 | 14.1 | 10.0 | 12.5 | 9.1 | ND | >>44 |
| CCT028492 | 2.7 | 8.0 | 14.1 | 11.7 | ND | ND |
| CCT031374 | 6.1 | 11.5 | 13.9 | 13.2 | 9.6 | >>44 |
| CCT036098 | 21.5 | >100 | 31.9 | 27.9 | 17.3 | >>44 |
| CCT036477 | 4.6 | 17.9 | 17.7 | 33.3 | ND | >>100 |
| CCT038407 | 5.3 | 3.0 | 2.3 | 2.5 | 21.1 | >>44 |
| CCT070535 | 5.1 | 17.6 | 11.1 | 11.8 | 13.4 | >>44 |
NOTE: HT29 (42), SW480 (39), and HCT116 (40) are colon carcinoma cell lines. SNU475 is a hepatocellular carcinoma cell line (29). CCD841Co is a colonic epithelial cell line whose use as a control has been described previously (5). Abbreviation: ND, not done. >>No growth inhibition at the highest concentration tested (indicated).
Alteration of β-catenin Stability
To assess whether the compounds altered β-catenin levels or localization, mouse L-cells were treated with each compound together with the GSK-3 inhibitor, BIO to block β-catenin degradation. Following 8h incubation, control BIO-treated cells showed a strong increase in total β-catenin levels that was associated with raised nuclear and cytosolic pools as determined by immunocytochemistry (Fig. 3a,b). Of the top nine compounds only CCT031374 prevented BIO-induced accumulation of β-catenin (Fig. 3a). The blockade of β-catenin accumulation by CCT031374 was accompanied by a reduction in both nuclear and cytosolic β-catenin pools (Fig. 3b, Fig. S4, Fig. S5). In U2OS GFP-β-catenin human osteosarcoma cells (Bioimage, Thermo-Fisher), addition of CCT031374 induced formation of GFP-β-catenin aggregates (Fig. S3b) possibly due to sequestration by subcellular organelles, a phenotype that was occasionally observed with endogenous β-catenin in mouse L-cells (Fig. S4c). By contrast, CCT036477 did not alter β-catenin levels but blocked transcription at the β-catenin level, although not by blocking β-catenin's interaction with the histone acetyltransferases CBP or p300 (Fig. S6). Compound CCT070535, which blocked TCF-dependent transcription at the TCF level, did not alter BIO-induced levels of β-catenin, but did increase the levels of nuclear β-catenin (Fig. S4B).
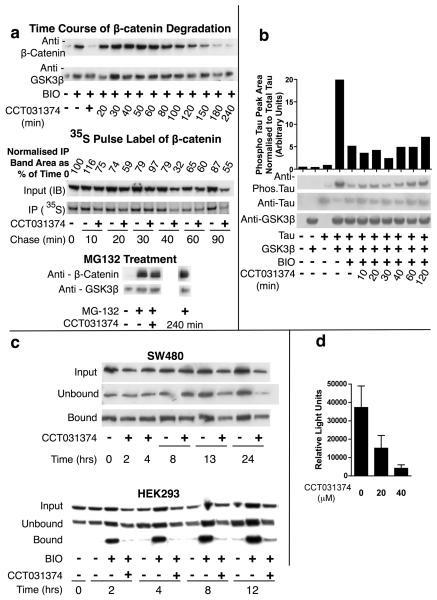
Fig 3. Prevention of β-catenin stabilisation by CCT031374.
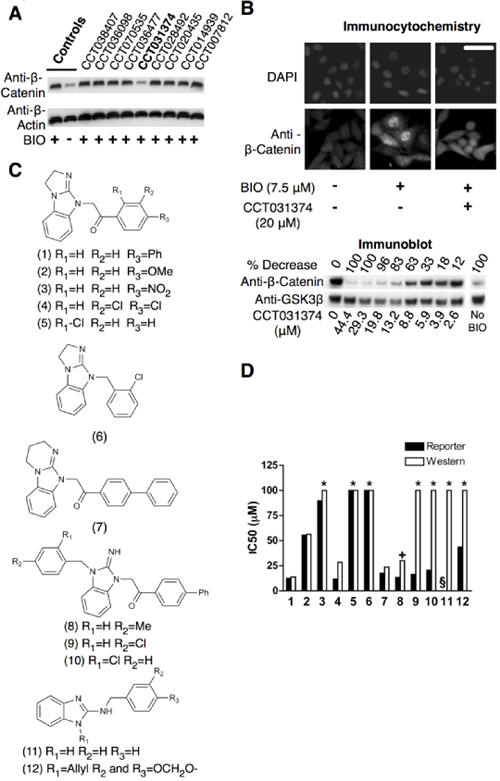
a. β-catenin abundance in Western blots of lysates from mouse L-cells treated for 6h with 7.5μM BIO and 20μM of the indicated hit compounds. b. Decreased abundance of β-catenin in immunostained mouse L-cells and in Western blots of lysates from mouse L-cells treated with indicated concentrations of CCT031374 and 7.5μM BIO for 8h. Decreases of relative β-catenin/β-actin ratios in the Western blot were normalised to BIO (100%) and control (0%). Bar: 30μm c. Structures of analogues of CCT031374. CCT031374 is compound (1). EN300-05350 [compound (11)] is a structurally unrelated control of similar lipophilicity, pKa and H-bonding. d. IC50 values from reporter cell assays and Western blots of lysates from mouse L-cell using CCT031374 and its analogues. Key: Asterisks - no effect at the maximum concentration used; +. Compound (8) was toxic to mouse L-cells at this concentration (30μM); §. assay not done.
Exploratory Studies with CCT031374 Series Compounds
The IC50 for CCT031374 activity in blocking BIO-induced β-catenin stabilization in L-cells was similar to that determined in the TCF-reporter cell line and transient assays (Fig.3b, S3a), suggesting that the compound may interfere with TCF-dependent transcription by blocking the function of β-catenin. The activity of several CCT031374 analogues showed both TCF-reporter and BIO/ β-catenin L-cell inhibition (Fig.3d). The biphenyl-keto-methyl analogues (7, 8, 9, 10 in Fig. 3c) retained potency in the reporter cell assay comparable to that of CCT031374 (compound 1 in Fig. 3d) but compounds 9 and 10 were significantly less potent in the L-cell β-catenin stabilization assay (Fig. 3c). Compounds 9 and 10 have low permeability in PAMPA permeability assays (Fig. S7), which may confound the interpretation of compound activity across different cell types. The remaining compounds (2-6 in Fig. 3c) are less lipophilic close analogues of CCT031374 with equivalent or lower potency (higher IC50 values) in the reporter and mouse L-cell assays. Because of the lipophilic basic nature of the compound series, compounds 11 and 12 (Fig. 3c) with a minimal core scaffold of similar pKa and lipophilicity were tested as controls. Compounds 11 and 12 did not destabilize β-catenin in mouse L-cells, a finding consistent with the hypothesis that CCT031374 activity on β-catenin levels is not solely due to a non-specific effect driven by the highly lipophilic and basic compounds.
To further characterize the mechanism of action of CCT031374, a series of time-course analyses were performed (Fig.4a; S8A,B). Although there appeared to be a transient increase of β-catenin (Fig. 4a), this was due to change of medium as this was also seen in the control analysis (Fig. S8a). When the β-catenin band peak areas were normalized to that of loading control (Fig. S8b), addition of CCT031374 was seen to reduce levels of BIO-stabilized β-catenin within 60 minutes. Pulse chase analyses showed that CCT031374 increased β-catenin degradation, within as little as 10 minutes of compound addition (Fig.4a). By contrast, β-catenin stabilized with the proteasome inhibitor MG132 was not degraded upon addition of CCT031374, suggesting that proteasomal degradation may act downstream of CCT031374 in lowering β-catenin levels (Fig.4a). Surprisingly, β-catenin stabilized by treatment of cells with soluble Wnt-3A and LiCl (9mM) was resistant to degradation induced by CCT031374 (Fig S9). Direct comparison was not straightforward since Wnt-3A and LiCl induction resulted in significantly lower levels of β-catenin than with BIO induction (Fig.4a).
Fig 4. Mechanism of action of CCT031374.
a. CCT031374 and β-catenin degradation in mouse L-cells. Western blots and autoradiographs of lysates of mouse L-cells that were exposed to BIO (7.5μM) for 6h prior to addition of CCT031374 (20μM) in the presence of BIO for the times shown. CCT031374 (20μM) increased turnover of 35S-pulse labelled β-catenin. BIO (7.5μM) was present throughout labelling and chase periods. Western blots of lysates from mouse L-cells after 240min of treatment with 20μM CCT031374 after 6 hours of 50μM MG132 proteasomal inhibitor or 6 hours of coincubation of CCT031374 with 50μM MG132 (+). b. CCT031374 does not prevent BIO's inhibition of Tau phosphorylation by GSK3. Western blots of lysates from HEK293 cells that had been transfected with expression plasmids for Tau and GSK3β as indicated and had been treated with BIO (7.5μM) for 4h or with both BIO and CCT031374 (20μM) for the indicated time periods. Relative phosphorylated: total Tau levels were quantified. c. CCT031374 decreased ‘free’ β-catenin levels in HEK293 cells but not in SW480 cells. ‘Free’ β-catenin was isolated from cell lysates by pull down with a GST-E-cadherin fusion protein. d. CCT031374 treatment of SW480 cells decreased TCF-dependent transcription of Topflash luciferase reporter. SW480 cells were transfected with Topflash reporter, then exposed to compound for 24h. Reporter activity was normalised to co-transfected TK-renilla luciferase activity.
To determine whether CCT031374 reversed the action of BIO against other GSK-3 targets, an assay for GSK-3 dependent Tau phosphorylation was used (Fig.4b). As expected, BIO was able to reduce GSK-3 dependent Tau phosphorylation on Thr-181, a well-characterized GSK-3 phosphorylation site (42). The addition of CCT031374 largely failed to reduce the BIO block of Tau phosphorylation, although there was a minor reversal of BIO action after 2h treatment. This low level ‘response’ to CCT031374 occurred much later than the effect of CCT031374 on β-catenin levels suggesting that the action against β-catenin was a strong, immediate response to compound action (Fig.4b). Consistent with the Tau phosphorylation data, CCT031374 did not inhibit recombinant GSK3 in a kinase assay (Fig S10). In addition, CCT031374 had no activity in biochemical assays for PP1, PP2A and Tyrosine Phosphatase enzyme activity (Fig. S11).
As CCT031374 blocked the growth of a number of cancer cell lines including SW480 colon cancer cells (Table 1), it was anticipated that the compound would reduce free β-catenin levels within these cells. Surprisingly, a pull down assay with GST-E-cadherin to detect free β-catenin levels found that these were not altered by CCT031374 (Fig 4c). By contrast, CCT031374 blocked free β-catenin levels in BIO-stimulated HEK293 cells (Fig 4c). Despite the lack of effect on β-catenin levels, CCT031374 reduced TCF-dependent transcription in SW480 cells (Fig. 4d). Similarly, CCT031374 did not alter levels of free β-catenin resulting from overexpression of ΔN-β-catenin or Axin-GID in HEK293 cells (Fig S12) but did decrease TCF-dependent transcription (Fig. 2c). Taken together, these data show that reductions in β-catenin levels are not required for CCT031374-mediated inhibition of TCF-dependent transcription
Compound Activity Against Known Wnt Targets
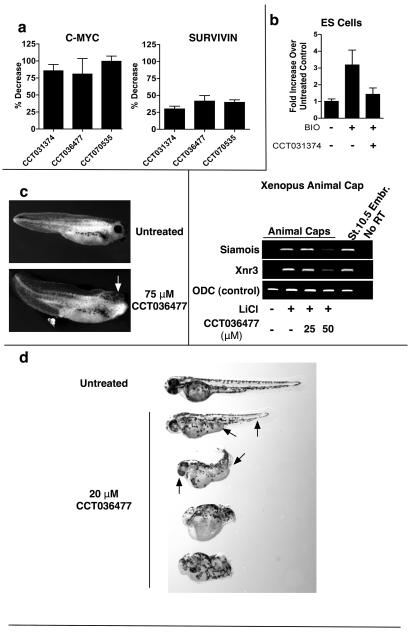
The activities of CCT031374, CCT036477 and CCT070535 were tested against expression of known Wnt target genes using myc and survivin-luciferase reporter readouts (Fig. 5a). Each compound showed activity against both promoters, but responses were greatest against the c-myc promoter. CCT031374 also showed activity against endogenous LEF-1 mRNA levels in human neurogenic embryoid bodies (hNEBs) in which Wnt signaling had been induced by the GSK-3 inhibitor, BIO (Fig. 5b). CCT036477 was toxic to the hNEBs while CCT070535 showed a weak, non-significant response. Expression of Axin2, another Wnt target gene (39), was not significantly induced by BIO in the hNEBs (data not shown).
Fig 5. Effect of compounds on Wnt target gene expression and development.
a. HEK293 cells were transfected with CMYC-luc or SURVIVIN-luc promoter-reporter constructs together with Axin-GID inducer and exposed to 40μM compound for 24h. Reporter activity was normalised to expression from a co-transfected CMV-lacZ reporter. b. CCT031374, at 20 μmol/L, repressed BIO-induced LEF1 expression in human neurogenic embryoid bodies exposed to 3 μmol/L BIO and CCT031374 for 24 h. The relative abundance of LEF1 was normalized to ACTB. CCT036477 decreased Siamois and Xnr3 expression in Xenopus animal cap assays. Animal caps were treated with 0.3 mol/L LiCl for 10 min, then incubated in medium with or without CCT036477 for 3 h. RNA from NF stage 10.5 embryos provided a second positive control.
c. CCT036477 inhibited the development of Xenopus anterior head structures such as the eye (indicated with an arrow) and the cement gland. Embryos were exposed to DMSO or 75μM CCT036477 from the 4-16 cell stage to NF stage 38. d. CCT036477 induced head and tail patterning defects (indicated with arrows) in Zebrafish embryos. Zebrafish embryos were exposed to 1% DMSO or 20μM CCT036477 from fertilisation until mid-gastrulation. A range of mild to severe head patterning and tail patterning phenotypes were seen.
In Vivo Activity of Hit Compounds
To obtain an integrated view of compound activity in multiple developmental stages, compounds were added to the medium of Xenopus and Zebrafish embryos during development. In these assays, CCT036477 showed the strongest phenotypic effects on both Xenopus and Zebrafish development. When added to Xenopus laevis embryos at the 4-16 cell stage, CCT036477 ventralized embryos and interfered with primary axis formation (Fig. 5c) as has previously been shown for inhibitors of Wnt signaling (1). Consistent with this observation, CCT036477 reduced expression of two well-characterized Wnt target genes (Siamois and Xnr3) in animal cap assays (Fig. 5b). CCT036477 addition to Zebrafish embryos at the 16-cell stage also induced axis defects leading to phenotypes (Fig. 5d) that have been associated with alterations to Wnt signaling (40).
Discussion
In the current study, we showed that a highly sensitive Wnt reporter cell line can be used to identify small molecule inhibitors of Wnt signaling. The cell based screen described here was similar to previous studies by Emami and colleagues (2004)(5), Huang and colleagues (2009)(7) and Chen and colleagues (2009)(3) in that it relied on the identification of compounds that blocked the activity of an integrated TCF-luciferase reporter. However, the present study used a reporter cell line that had an inactive basal TCF-reporter that could be induced through the activation of a Dishevelled-estrogen receptor fusion protein following addition of estrogen. The advantage of this strategy is that Wnt signaling can be transiently-induced and that the cell response to inhibition of the pathway is less likely to be dependent on complex positive and negative feedback pathways commonly found in cancer cell lines (41-39). Furthermore, cell lines whose growth is dependent on the Wnt pathway have been shown to undergo apoptosis (as seen for CCT031374, CCT036477 and CCT070535; Fig. S3) following Wnt pathway inhibition and this can be difficult to distinguish from non-specific cell killing during high throughput screening.
Multiple compounds were identified in the primary screen that showed specificity for the TCF-reporter when compared to control promoters. A hit triage/deconvolution cascade was designed to identify the most promising candidates amongst these compounds for further study. These assays included growth inhibitory activity against tumor cell lines with activated Wnt signaling. However, a key criterion was the ‘clarity’ of the deconvolution response such that reproducible, mechanistically consistent readouts were identified that allowed the logical pursuit of the compound's mechanism of action to distinct points in the Wnt pathway. From a total of 63, 040 compounds, 9 compounds that operated at either the β-catenin or TCF levels of the pathway were selected for further investigation. Downstream assays focused on 2 compounds that operated at the β-catenin level and one that operated at the TCF level.
CCT031374 acted at the β-catenin level based on the observation that it blocked TCF-dependent transcription induced by a stabilized form of β-catenin (N-terminal deletion of 89 amino acids (40)), but not by a constitutively active TCF-VP16 fusion protein. Consistent with this mechanism, CCT031174 increased the degradation of endogenous wild-type β-catenin in mouse L-cells that had been stabilized by the GSK-3 inhibitor BIO. CCT031374 did not increase β-catenin N-terminal phosphorylation in the presence of BIO-treated cells (data not shown), suggesting that a pathway independent of phospho-β-catenin binding to β-TRCP induced increased degradation. A number of alternative β-catenin degradation routes have been described. These include pathways involving APC and the Siah1 ubiquitin ligase (41, 42), the presenilin/PKA complex (39) and the protease calpain (40). CCT031374 failed to induce β-catenin degradation products characteristic of calpain-induced proteolysis (40) (data not shown) suggesting that distinct novel pathways are involved in its mechanism of action.
Surprisingly, the block of TCF-dependent transcription in SW480 colon cancer cells by CCT031374 was not accompanied by a corresponding reduction in β-catenin protein levels (Fig.4F). Moreover, levels of transfected stabilized β-catenin (ΔN-89-β-catenin) were not reduced in HEK293 cells treated with CCT031374 (Fig.S12), despite the fact that the compound blocked TCF-dependent transcription in these cells (Fig.2b). Taken together these data argue that the degradation of β-catenin may not be necessary for the blocking effect of CCT031374 on TCF-dependent transcription in all cellular contexts, suggesting that CCT031374 operates through more than one mechanism. A similar correlation between β-catenin stability and TCF-dependent transcription was observed in studies of the prolyl oligopeptidase Pin-1. Pin-1 activity correlated with both β-catenin stability and TCF-dependent transcription in HeLa cells by modulating its interaction with APC (41). By contrast, in PTEN-deficient LNCaP cells, Pin-1 altered TCF-dependent transcription without altering β-catenin levels by blocking β-catenin interactions with the androgen receptor (42). CCT031374 may similarly function through molecular pathways that can be coupled to protein degradation in selected contexts.
Of the three compound series studied, only CCT036477 showed clear activity in vivo where it blocked development of Zebrafish and Xenopus embryos and expression of Wnt target genes. CCT031374 and CCT070535 did not show clear responses in these systems and it is currently unclear whether the compounds reached the required concentration to block signaling in these in vivo models or whether their lack of in vivo activity reflects a specificity for cells with oncogenically activated Wnt signaling. Overall, these studies have identified novel small molecules that target distinct levels of Wnt signal transduction pathway. Further studies with these compounds (see Fig. S13) could lead to the identification of molecular targets of therapeutic benefit in multiple tumor types with activated Wnt signaling and further investigations will be published in due course.
Supplementary Material
Acknowledgements
Teresa Brooks, Paul Rogers helped with reporter cell line optimization, Perihan Nalbant for help with the GFP-β-catenin assay and Bethan Lloyd-Lewis assisted with the intensity quantitation of immunocytochemistry images. Work at Cardiff University and The Cancer Research UK Centre for Cancer Therapeutics was supported by CR-UK grant numbers C368/A5410, C368/A7505 and C309/A2187, C309/A8274, C309/A8365 respectively. NHS funding supported work at the NIHR Biomedical Research Centre, The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust. Paul Workman is a CR-UK Life Fellow.
References
- 1.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–80. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Cowin P, Rowlands TM, Hatsell SJ. Cadherins and catenins in breast cancer. Curr Opin Cell Biol. 2005;17:499–508. doi: 10.1016/j.ceb.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Chen B, Dodge ME, Tang W, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nature chemical biology. 2009;5:100–7. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ewan KB, Dale TC. The potential for targeting oncogenic WNT/beta-catenin signaling in therapy. Current drug targets. 2008;9:532–47. doi: 10.2174/138945008784911787. [DOI] [PubMed] [Google Scholar]
- 5.Emami KH, Nguyen C, Ma H, et al. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected] Proc Natl Acad Sci U S A. 2004;101:12682–7. doi: 10.1073/pnas.0404875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyabayashi T, Teo JL, Yamamoto M, McMillan M, Nguyen C, Kahn M. Wnt/beta-catenin/CBP signaling maintains long-term murine embryonic stem cell pluripotency. Proc Natl Acad Sci U S A. 2007;104:5668–73. doi: 10.1073/pnas.0701331104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang SM, Mishina YM, Liu S, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–20. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 8.DasGupta R, Kaykas A, Moon RT, Perrimon N. Functional genomic analysis of the Wnt-wingless signaling pathway. Science. 2005;308:826–33. doi: 10.1126/science.1109374. [DOI] [PubMed] [Google Scholar]
- 9.Tian Q. Proteomic exploration of the Wnt/beta-catenin pathway. Current opinion in molecular therapeutics. 2006;8:191–7. [PubMed] [Google Scholar]
- 10.Mosimann C, Hausmann G, Basler K. Beta-catenin hits chromatin: regulation of Wnt target gene activation. Nat Rev Mol Cell Biol. 2009;10:276–86. doi: 10.1038/nrm2654. [DOI] [PubMed] [Google Scholar]
- 11.Smalley MJ, Signoret N, Robertson D, et al. Dishevelled (Dvl-2) activates canonical Wnt signalling in the absence of cytoplasmic puncta. J Cell Sci. 2005;118:5279–89. doi: 10.1242/jcs.02647. [DOI] [PubMed] [Google Scholar]
- 12.Willert K, Brown JD, Danenberg E, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–52. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 13.Kishida S, Yamamoto H, Kikuchi A. Wnt-3a and Dvl induce neurite retraction by activating Rho-associated kinase. Mol Cell Biol. 2004;24:4487–501. doi: 10.1128/MCB.24.10.4487-4501.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smalley MJ, Sara E, Paterson H, et al. Interaction of axin and Dvl-2 proteins regulates Dvl-2-stimulated TCF-dependent transcription. Embo J. 1999;18:2823–35. doi: 10.1093/emboj/18.10.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salghetti SE, Kim SY, Tansey WP. Destruction of Myc by ubiquitin-mediated proteolysis: cancer-associated and transforming mutations stabilize Myc. Embo J. 1999;18:717–26. doi: 10.1093/emboj/18.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lovestone S, Reynolds CH, Latimer D, et al. Alzheimer's disease-like phosphorylation of the microtubule-associated protein tau by glycogen synthase kinase-3 in transfected mammalian cells. Curr Biol. 1994;4:1077–86. doi: 10.1016/s0960-9822(00)00246-3. [DOI] [PubMed] [Google Scholar]
- 17.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 18.Sive HL, Grainger RM, Harland RM. Early development of Xenopus laevis : a laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 2000. [Google Scholar]
- 19.Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin) : a systematical and chronological survey of the development from the fertilized egg till the end of metamorphosis. Garland Publishing; New York: 1994. [Google Scholar]
- 20.Samuel LJ, Latinkic BV. Early activation of FGF and nodal pathways mediates cardiac specification independently of Wnt/beta-catenin signaling. PLoS ONE. 2009;4:e7650. doi: 10.1371/journal.pone.0007650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKendry R, Hsu SC, Harland RM, Grosschedl R. LEF-1/TCF proteins mediate wnt-inducible transcription from the Xenopus nodal-related 3 promoter. Dev Biol. 1997;192:420–31. doi: 10.1006/dbio.1997.8797. [DOI] [PubMed] [Google Scholar]
- 22.Polychronopoulos P, Magiatis P, Skaltsounis AL, et al. Structural basis for the synthesis of indirubins as potent and selective inhibitors of glycogen synthase kinase-3 and cyclin-dependent kinases. J Med Chem. 2004;47:935–46. doi: 10.1021/jm031016d. [DOI] [PubMed] [Google Scholar]
- 23.Brennan K, Gonzalez-Sancho JM, Castelo-Soccio LA, Howe LR, Brown AM. Truncated mutants of the putative Wnt receptor LRP6/Arrow can stabilize beta-catenin independently of Frizzled proteins. Oncogene. 2004;23:4873–84. doi: 10.1038/sj.onc.1207642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraser E, Young N, Dajani R, et al. Identification of the Axin and Frat binding region of glycogen synthase kinase-3. J Biol Chem. 2002;277:2176–85. doi: 10.1074/jbc.M109462200. [DOI] [PubMed] [Google Scholar]
- 25.Munemitsu S, Albert I, Rubinfeld B, Polakis P. Deletion of an amino-terminal sequence beta-catenin in vivo and promotes hyperphosporylation of the adenomatous polyposis coli tumor suppressor protein. Mol Cell Biol. 1996;16:4088–94. doi: 10.1128/mcb.16.8.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith KJ, Johnson KA, Bryan TM, et al. The APC gene product in normal and tumor cells. Proc Natl Acad Sci U S A. 1993;90:2846–50. doi: 10.1073/pnas.90.7.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishisho I, Nakamura Y, Miyoshi Y, et al. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253:665–9. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- 28.Morin PJ, Sparks AB, Korinek V, et al. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science. 1997;275:1787–90. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 29.Satoh S, Daigo Y, Furukawa Y, et al. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nature Genet. 2000;24:245–50. doi: 10.1038/73448. [DOI] [PubMed] [Google Scholar]
- 30.Goedert M, Jakes R, Crowther RA, et al. Epitope mapping of monoclonal antibodies to the paired helical filaments of Alzheimer's disease: identification of phosphorylation sites in tau protein. Biochem J. 1994;301(Pt 3):871–7. doi: 10.1042/bj3010871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–83. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lekven AC, Thorpe CJ, Waxman JS, Moon RT. Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev Cell. 2001;1:103–14. doi: 10.1016/s1534-5807(01)00007-7. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Sancho JM, Aguilera O, Garcia JM, et al. The Wnt antagonist DICKKOPF-1 gene is a downstream target of beta-catenin/TCF and is downregulated in human colon cancer. Oncogene. 2005;24:1098–103. doi: 10.1038/sj.onc.1208303. [DOI] [PubMed] [Google Scholar]
- 34.Li TW, Ting JH, Yokoyama NN, Bernstein A, van de Wetering M, Waterman ML. Wnt activation and alternative promoter repression of LEF1 in colon cancer. Mol Cell Biol. 2006;26:5284–99. doi: 10.1128/MCB.00105-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caldwell GM, Jones CE, Soon Y, Warrack R, Morton DG, Matthews GM. Reorganisation of Wnt-response pathways in colorectal tumorigenesis. Br J Cancer. 2008;98:1437–42. doi: 10.1038/sj.bjc.6604327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci U S A. 1995;92:3046–50. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J, Stevens J, Rote CA, et al. Siah-1 mediates a novel beta-catenin degradation pathway linking p53 to the adenomatous polyposis coli protein. Mol Cell. 2001;7:927–36. doi: 10.1016/s1097-2765(01)00241-6. [DOI] [PubMed] [Google Scholar]
- 38.Matsuzawa SI, Reed JC. Siah-1, SIP, and Ebi collaborate in a novel pathway for beta-catenin degradation linked to p53 responses. Mol Cell. 2001;7:915–26. doi: 10.1016/s1097-2765(01)00242-8. [DOI] [PubMed] [Google Scholar]
- 39.Hino S, Tanji C, Nakayama KI, Kikuchi A. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase stabilizes beta-catenin through inhibition of its ubiquitination. Mol Cell Biol. 2005;25:9063–72. doi: 10.1128/MCB.25.20.9063-9072.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li G, Iyengar R. Calpain as an effector of the Gq signaling pathway for inhibition of Wnt/beta -catenin-regulated cell proliferation. Proc Natl Acad Sci U S A. 2002;99:13254–9. doi: 10.1073/pnas.202355799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryo A, Nakamura M, Wulf G, Liou YC, Lu KP. Pin1 regulates turnover and subcellular localization of beta-catenin by inhibiting its interaction with APC. Nat Cell Biol. 2001;3:793–801. doi: 10.1038/ncb0901-793. [DOI] [PubMed] [Google Scholar]
- 42.Chen SY, Wulf G, Zhou XZ, Rubin MA, Lu KP, Balk SP. Activation of beta-catenin signaling in prostate cancer by peptidyl-prolyl isomerase Pin1-mediated abrogation of the androgen receptor-beta-catenin interaction. Mol Cell Biol. 2006;26:929–39. doi: 10.1128/MCB.26.3.929-939.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.