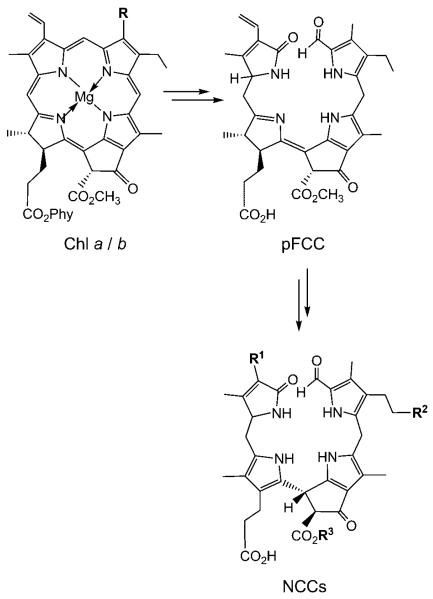
As revealed in the last two decades, chlorophyll breakdown in senescent leaves appears to occur by a largely common and well-controlled catabolic path, which rapidly furnishes non-fluorescent, colorless chlorophyll catabolites (NCCs) as “final” products.[1,2] Recently NCCs detected in ripe apples and pears were found to be the same as those in degreened leaves, suggesting chlorophyll catabolism in leaf senescence and fruit ripening to be similar (Scheme 1).[3,4]
Scheme 1.
Outline of chlorophyll breakdown in senescent plants. The chlorophylls a (R=CH3, Phy=phytyl) and b (R=CH=O, Phy=phytyl) are degraded by the primary “fluorescent” chlorophyll catabolite (pFCC) to “nonfluorescent” chlorophyll catabolites (NCCs, in which, typically, the residues R1, R2, and R3 vary).[4,5]
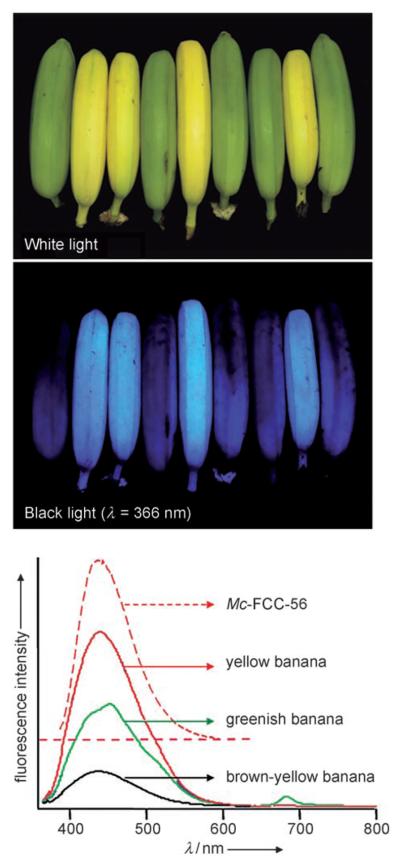
Chlorophylls disappear visually in the peels of ripening bananas (Musa cavendish), which develop a diagnostic bright yellow color, the “memory” color of bananas.[6] Surprisingly, we found ripe yellow bananas to appear blue under UV light and to display a previously unnoticed blue luminescence (Figure 1). Their blue luminescence was also found here to directly depend upon chlorophyll breakdown in the banana peels. It arises from abundant “fluorescent” catabolites of chlorophyll (FCCs),[1,7] which are intermediates of chlorophyll breakdown that are hardly detectable in higher plants.[1,8]
Figure 1.
Yellow bananas are blue luminescent. Top and middle: Yellow (ripe) and green (unripe) bananas under white light and under UV light at a wavelength of 366 nm. Bottom: Luminescence spectra (excitation at 350 nm) of intact bananas that are greenish (green line), bright yellow (red line), and brown-yellow (black line) and the spectrum of Mc-FCC-56 (in methanol, dashed red line).
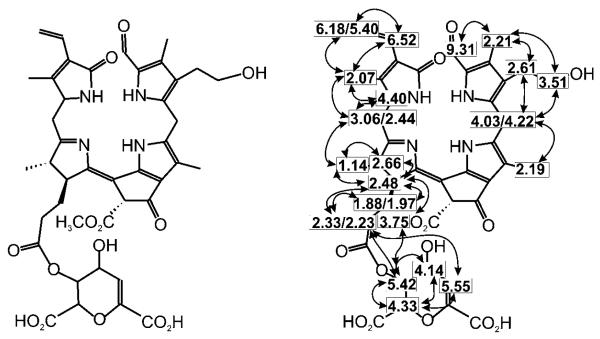
Unpeeled yellow bananas showed blue fluorescence with a maximum near 450 nm, as did extracts from fresh peelings of yellow bananas. The luminescence of such extracts was also similar to that of a solution of the main banana FCC in methanol (Mc-FCC-56 in Figure 1). Indeed, when fresh extracts from banana peelings were analyzed by high-performance liquid chromatography (HPLC), about a dozen luminescent components with absorbance spectra typical of FCCs (maxima near 317 and 358 nm) were revealed.[1] The mass spectrum of Mc-FCC-56, the most abundant FCC, showed its most intense ion at m/z 831.27. This value, consistent with a molecular formula of C42H46O14N4, was confirmed by a high-resolution mass spectrum of Mc-FCC-56, which exhibited a signal of the pseudo-molecular ion of the sodium salt at m/z 853.289 (m/zcalc [C42H46O14N4Na]+ 853.291). The structure of Mc-FCC-56 was determined by multidimensional NMR spectroscopy. The 1H NMR spectrum showed a doublet and three singlets at high field (of methyl groups), a singlet at δ=3.75 ppm (methyl ester), as well as signals due to vinyl and formyl groups at low field (see Figure S1 in the Supporting Information). Homo- and heteronuclear correlations (from 1H,1H COSY, 1H,1H ROESY, 1H,13C HSQC, and 1H,13C HMBC spectra[9]) allowed the assignment of all non-exchangeable H atoms and of 41 (of the 42) C atoms. Using correlations from 1H,1H NOE spectra the constitution of Mc-FCC-56 was then deduced as a 31,32-didehydro-82-hydroxy-132-(methoxycarbonyl)-173-[5′-daucyl]-1,4,5,10,17,18,20,22-octahydro-4,5-dioxo-4,5-seco-(22 H)-phytoporphyrin (Figure 2).[10] This analysis indicated the propionate side chain of Mc-FCC-56 to be esterified with a cyclic unit with the constitution of daucic acid,[11] and the terminal carbon (C82) of the side chain at position 8 to carry a hydroxy group.
Figure 2.
Elucidation of the structure of Mc-FCC-56 by NMR analysis. Constitutional formula of Mc-FCC-56 and assigned H atoms with homonuclear correlations (dashed lines: COSY, bold lines: NOESY). The spectra were measured at 600 MHz in CD3OD at 25°C. For the corresponding figure with 1H,13C correlations see Figure S2 in the Supporting Information.
The presence of a propionate ester in Mc-FCC-56 is a striking structural revelation. Such a modification of the propionate function is unprecedented in the known FCCs, but it occurs in several major FCC fractions from the banana peels, which were also isolated and analyzed (see Figure 3, Experimental Section, and the Supporting Information). The presence of the ester function helps to rationalize the observed, unprecedented persistence of Mc-FCC-56 and its analogues in the banana peels: All natural chlorophyll catabolites from plants have, so far, featured a free acid function,[1,5] which was shown to induce the remarkably rapid and stereospecific isomerization of FCCs to the nonfluorescent NCCs,[8,12] as the “last” step of chlorophyll breakdown in higher plants. The rapid further conversion of typical FCCs explains their fleeting existence in leaves and their (so far) elusive nature in ripening fruit.[4,8,12] In contrast, Mc-FCC-56 only underwent slow isomerization under comparable conditions. In acidic solution, Mc-FCC-56 gave two main NCCs, Mc-NCC-55 and Mc-NCC-58, which were indicated by their CD spectra to be stereoisomeric at the C15 meso position (see Figure S3 in the Supporting Information). Slow, not stereo-selective isomerizations were similarly observed for synthetic methyl esters of FCCs also.[12] Clearly, the ester group inhibits the conversion of Mc-FCC-56 (and of related FCC esters) to an NCC and helps to disrupt the usual mechanism of chlorophyll catabolism.
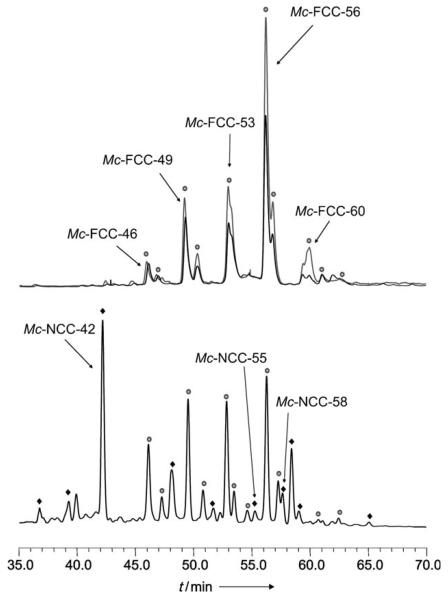
Figure 3.
HPLC analysis of freshly prepared extracts of yellow banana peels. Fractions with characteristic luminescence or absorbance are tentatively identified (and marked) as specific FCC or NCC fractions. Top: Chromatogram with detection of luminescence at 450 nm; black trace: extract of ethylene-ripened bananas; gray trace: extract of naturally ripened bananas. Bottom: Chromatogram with detection of the absorbance at 320 nm (see the Supporting Information for experimental details).
Extracts of peels of commercial ethylene-treated and naturally ripened, yellowing bananas (Musa cavendish, see the Experimental Section and the Supporting Information) all contained a similar assortment and quantity of the major FCCs (Figure 3 and the Supporting Information). Thus, the unique pattern of FCCs and FCC esters (such as Mc-FCC-56), as observed in the bananas from the market, is not diagnostic of ethylene ripening. Freshly degreened (yellow) leaves of bananas also contained several fractions with absorbance and luminescence characteristics of FCCs. However, exploratory HPLC analyses indicated the main leaf FCCs to differ from those in the banana peels (see Figure S5 in the Supporting Information).Ongoing work will help to clarify the structures of these still largely unknown fluorescent catabolites of chlorophyll.
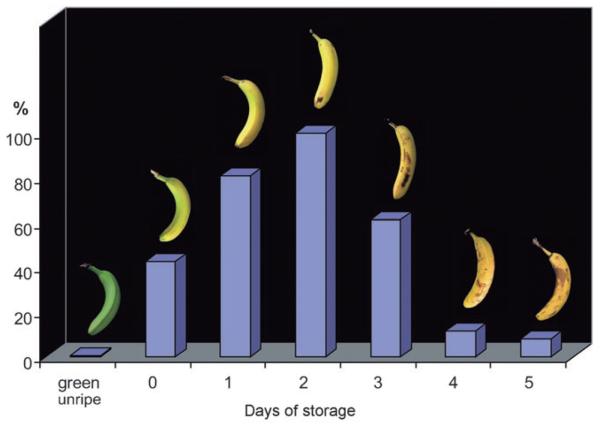
When intact, ripening bananas were analyzed for their absorbance and luminescence characteristics, the distribution and intensity of the blue luminescence was similar to that of the banana extracts (Figure 1). Indeed, unripe, green bananas were barely visible under UV light, showed little luminescence, and contained only small amounts of FCCs. Apparently the fluorescing catabolites accumulated in banana peels in the earlier phase of ripening, in parallel with breakdown of chlorophylls and the development of a bright yellow color. During further ripening, when the bananas became dull yellow, the amounts of FCCs diminished and fluorescence decreased. The intensity of the blue luminescence of whole bananas, as well as of extracts of their peels, correlated with chlorophyll breakdown, and showed maximal intensity at an intermediate degree of ripening (Figures 1 and 4).
Figure 4.
Relative total amount of fluorescent chlorophyll catabolites (FCCs) in extracts of peels of green unripe, of fresh ethylene-treated bananas (at day 0), and of ethylene-treated bananas that ripened further during one to five days of storage at ambient temperature.
In ripening bananas the observed temporal accumulation of FCCs can be rationalized as a striking consequence of a unique biosynthetic esterification, as found in the polar FCCs Mc-FCC-46, Mc-FCC-49, Mc-FCC-53, Mc-FCC-56, which accounted for more than 80% of the FCCs found in the peels (see Figure 3 and Figure S4 in the Supporting Information). This esterification inhibits the natural further isomerization of FCCs to NCCs.[8,12] Indeed, we found only small amounts of Mc-NCC-55 and Mc-NCC-58, the two epimeric Mc-NCCs formed by acid-catalyzed isomerization of Mc-FCC-56. The other, more abundant NCC fractions from the banana peels were determined by spectroscopic means to all carry a free propionic acid function, a feature of the previously analyzed NCCs.[1] In particular, Mc-NCC-42, a major NCC from the banana peels, was identified by HPLC, 1H NMR spectroscopy, and mass spectrometry to be So-NCC-2, an NCC recently found in degreened leaves of spinach.[13]
Surprisingly, the blue luminescence of bananas apparently has been entirely overlooked. Most humans would even consider the idea of a blue banana to be unappetizing.[6] The major contributors to our visual perception of ripe bananas are carotenoids.[14] However, as shown here, FCCs are a source of blue luminescence, and as in vivo optical brighteners[15] they contribute to the bright yellow appearance of freshly ripe bananas. FCCs are remarkably abundant in banana peels, and these luminescent products of the natural breakdown of chlorophyll are temporal indicators of ripeness (see Figure 4): When observed under 366 nm light, ripe bananas are blue (see Figure 1). Apparently, luminescence has not been analyzed previously during fruit ripening and in correlation with chlorophyll breakdown. In earlier work with banana peels only an uncharacterized fraction was described as an NCC on account of its UV-absorbance maximum near 320 nm.[16] Weak blue and green fluorescence in higher plants has been associated with cell wall components.[17] Plant chlorophylls are known to be very weakly luminescent in intact protein assemblies, and the (in vivo) appearance of their red fluorescence is generally diagnosed as a symptom of metabolic stress in leaves.[18]
FCCs were first described about ten years ago as short-lived intermediates of chlorophyll breakdown in higher plants[7,19] and were occasionally also detected in extracts of artificially degreened leaves.[20] In yellow peels and leaves of bananas (Musa cavendish), FCCs were identified here as abundant sources of easily seen in vivo luminescence in higher plants. Chlorophyll breakdown in bananas differs from that in other higher plants analyzed so far.[1,5, 19] Exploratory investigations on fresh yellow banana leaves further suggest the major catabolites from the leaves to differ from those in the (banana) fruit (see Figure S5 in the Supporting Information). These findings, the striking structural features of the major FCCs from banana peels, and the observed accumulation in the peels can be explained by two possible considerations:
Fluorescent intermediates of chlorophyll breakdown[7,19] are a newly discovered source for color in plants. The color of fruit is particularly important for the specific interaction with frugivorous animals. Indeed, many animals have a larger window of vision in the UV region (see Ref.[21, 22]), and the blue luminescence of bananas may give them the distinct signal that the banana fruit is ripe.
The accumulation of FCCs in the banana peels may also be related to possible internal roles in the fruit. Indeed, chlorophyll breakdown may not merely be an important detoxification process spitting out NCCs as its “final” products, as believed until recently:[1] The discovery of NCCs in fruit, their accumulation in senescent leaves, and their properties as antioxidants suggested consideration of a further possible role of these abundant tetrapyrrolic products of chlorophyll breakdown.[3] They may help sustain the viability of ripening fruit and senescing leaf cells. Phytobilins, the structurally related tetrapyrroles from natural heme breakdown, have important biological roles in plants and other photosynthetic organisms.[23,24] Bilirubin has also been identified very recently as a cytoprotective component in mammals.[25]
We are addressing some of these intriguing questions in our ongoing work with FCCs and bananas.
Experimental Section
Isolation and spectroscopic characterization of Mc-FCC-56: Peels of yellow ethylene-ripened bananas (Musa cavendish, from supermarkets in Innsbruck) were deep-frozen in liquid nitrogen and extracted with cold methanol. Purification by chromatography with an RP-18 column followed by an anion-exchange column, separation by semipreparative HPLC, and desalting with SepPak cartridges (see the Supporting Information) gave an analytically pure sample of Mc-FCC-56, which was isolated as a pale yellow solid. UV/Vis (methanol/water 9:1, c=5.8 × 10−5m, λmax/nm (log ε)): 237 (4.40), 317 (4.29), 358 (4.10); Fluorescence (methanol, c=4.2 × 10−6 m, excitation at 350 nm) λmax at 447 nm (see Figure 1 and Figure S6 in the Supporting Information). MS (ESI pos): m/z 870.26 (4), 869.23 (7, [M+K]+), 856.33 (6), 855.32 (12), 854.31 (28), 853.24 (43, [M+Na]+), 834.34 (6), 833.36 (22), 832,32 (67), 831.27 (100, [M+H]+), 678.20 (2, [M-ring B+H]+), 667.15 (4, [M−C7H7O6+Na]+), 645.25 (9, [M−C7H7O6+H]+); HRMS (ESI, methanol): m/z 853.289 (m/zcalc[C42H46O14N4Na]+=853.291).
Supplementary Material
Footnotes
Dedicated to Professor Hans Gruber on the occasion of his 80th birthday
We thank Georg Kontaxis and Robert Konrat (Max Perutz Laboratories, Vienna) for heteronuclear NMR measurements. Our work was supported by the National Science Foundation of Austria (FWF P-19596) and the National Science Foundation of the USA (NSF-CHE-04-15516, NSF-CHE-07-17518).
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.200803189.
References
- 1.Kräutler B, Hörtensteiner S. In: Chlorophylls and Bacteriochlorophylls. Grimm B, Porra R, Rüdiger W, Scheer H, editors. Springer; Dordrecht: 2006. pp. 237–260. [Google Scholar]
- 2.Kräutler B, Jaun B, Bortlik K, Schellenberg M, Matile P. Angew. Chem. 1991;103:1354–1357. [Google Scholar]; Angew. Chem. Int. Ed. Engl. 1991;30:1315–1318. [Google Scholar]
- 3.Müller T, Ulrich M, Ongania KH, Kräutler B. Angew. Chem. 2007;119:8854–8857. doi: 10.1002/anie.200703587. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2007;46:8699–8702. [Google Scholar]
- 4.Kräutler B. Photochem. Photobiol. Sci. 2008 doi: 10.1039/b802356p. DOI: 10.1039/b802356p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kräutler B. In: Progress in the Chemistry of Organic Natural Products. Kinghorn AD, Falk H, Kobayashi J, editors. Vol. 89. Springer; Vienna: 2008. pp. 1–43. [Google Scholar]
- 6.Hansen T, Olkkonen M, Walter S, Gegenfurtner KR. Nat. Neurosci. 2006;9:1367–1368. doi: 10.1038/nn1794. [DOI] [PubMed] [Google Scholar]
- 7.Mühlecker W, Ongania KH, Kräutler B, Matile P, Hörtensteiner S. Angew. Chem. 1997;109:401–404. [Google Scholar]; Angew. Chem. Int. Ed. Engl. 1997;36:401–404. [Google Scholar]
- 8.Oberhuber M, Berghold J, Breuker K, Hörtensteiner S, Kräutler B. Proc. Natl. Acad. Sci. USA. 2003;100:6910–6915. doi: 10.1073/pnas.1232207100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kessler H, Gehrke M, Griesinger C. Angew. Chem. 1988;100:507–554. [Google Scholar]; Angew. Chem. Int. Ed. Engl. 1988;27:490–536. [Google Scholar]
- 10.Atom numbering is based on nomenclature of chlorophylls, see Scheer H. In: Chlorophylls and Bacteriochlorophylls. Grimm B, Porra R, Rüdiger W, Scheer H, editors. Springer; Dordrecht: 2006. pp. 1–26. Provisional short names of FCCs or NCCs using two-letter code of plant species (Mc=Musa cavendish) and retention times (min) under standard analytical HPLC (e.g. Mc-FCC-56).
- 11.Lichtenthaler FW, Nakamura K, Klotz J. Angew. Chem. 2003;115:6019–6023. doi: 10.1002/anie.200352718. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2003;42:5838–5843. doi: 10.1002/anie.200352718. [DOI] [PubMed] [Google Scholar]
- 12.Oberhuber M, Berghold J, Kräutler B. Angew. Chem. 2008;120:3100–3104. doi: 10.1002/anie.200705330. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem. Int. Ed. 2008;47:3057–3061. doi: 10.1002/anie.200705330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.a) Oberhuber M, Berghold J, Mühlecker W, Hörtensteiner S, Kräutler B. Helv. Chim. Acta. 2001;84:2615–2627. [Google Scholar]; b) Berghold J, Breuker K, Oberhuber M, Hörtensteiner S, Kräutler B. Photosynth. Res. 2002;74:109–119. doi: 10.1023/A:1020991023248. [DOI] [PubMed] [Google Scholar]
- 14.Seymour GB. In: Biochemistry of Fruit Ripening. Seymour GB, Taylor JE, Tucker GA, editors. Chapman and Hall; London: 1993. pp. 83–106. [Google Scholar]
- 15.Zollinger H. Color Chemistry. Synthesis Properties and Applications of Organic Dyes and Pigments. 3rd ed. Helvetica Chimica Acta/Wiley-VCH, Zürich/Weinheim; 2003. pp. 366–377. [Google Scholar]
- 16.Drury R, Hörtensteiner S, Donnison I, Bird CR, Seymour GB. Physiol. Plant. 1999;107:32–38. [Google Scholar]
- 17.Lichtenthaler HK, Schweiger J. J. Plant Physiol. 1998;152:272–282. [Google Scholar]
- 18.Lichtenthaler HK, Miehe JA. Trends Plant Sci. 1997;2:316–320. [Google Scholar]
- 19.Kräutler B, Matile P. Acc. Chem. Res. 1999;32:35–43. [Google Scholar]
- 20.Pružinska A, Tanner G, Aubry S, Anders I, Moser S, Müller T, Ongania K-H, Kräutler B, Youn J-Y, Liljegren SJ, Hörtensteiner S. Plant Physiol. 2005;139:52–63. doi: 10.1104/pp.105.065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zollinger H. Farbe. Eine multidisziplinäre Betrachtung. Wiley-VCH; Zürich: 2005. [Google Scholar]
- 22.Arnold KE, Owens IPF, Marshall NJ. Science. 2002;295:92–92. doi: 10.1126/science.295.5552.92. [DOI] [PubMed] [Google Scholar]
- 23.Frankenberg N, Lagarias JC. In: The Porphyrin Handbook. Kadish KM, Smith KM, Guilard R, editors. Vol. 13. Elsevier; Oxford: 2003. pp. 211–235. [Google Scholar]
- 24.Frankenberg-Dinkel N, Terry MJ. In: Tetrapyrroles: Birth, Life and Death. Warren MJ, Smith AG, editors. Landes Bioscience; Austin: 2008. pp. 208–219. [Google Scholar]
- 25.Baranano DE, Rao M, Ferris CD, Snyder SH. Proc. Natl. Acad. Sci. USA. 2002;99:16093–16098. doi: 10.1073/pnas.252626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.