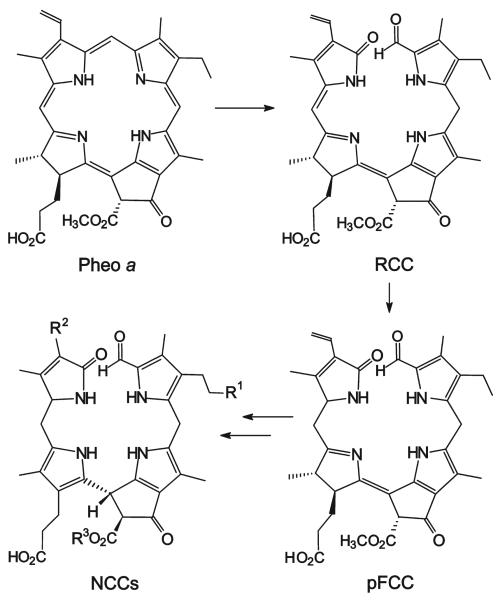
In autumn the degreening of leaves and the emergence of the fall colors are highly visible signs of leaf senescence, a form of programmed cell death in plants.[1] In the early 1990s the disappearance of chlorophyll in higher plants was considered an enigma.[2] When the products of chlorophyll breakdown were first identified in senescent leaves,[3] they turned out to be colorless tetrapyrroles, typified as “nonfluorescent” chlorophyll catabolites (NCCs).[4] In senescent leaves about 15 constitutionally different NCCs have been found, which have a common tetrapyrrolic skeleton but which differ in the structure or the site of attachment of their peripheral substituents.[4-8] Indeed, leaf senescence seems to involve a largely common and regulated pathway of chlorophyll breakdown,[9] in which NCCs appear to be the “final” products (Scheme 1).[4,9-11]
Scheme 1.
Outline of chlorophyll breakdown in senescent leaves.[11] The chlorophylls are degraded via pheophorbide a (Pheo a), “red” chlorophyll catabolite (RCC), and primary “fluorescent” chlorophyll catabolite (pFCC) to the “nonfluorescent” chlorophyll catabolites (NCCs, in which, typically, the residues R1, R2, and R3 vary).[11,13-15]
Degreening and the simultaneous appearance of appealing colors are also signs of fruit ripening.[1] However, the mechanism of chlorophyll breakdown and the structure of the catabolites in ripening fruit still remain to be clarified. Here we show that chlorophyll breakdown in ripening apples and pears also leads to NCCs. These were found to be identical to those from senescent leaves. In addition, the NCCs observed in both the peels and flesh of fruit were found to be remarkable antioxidants.
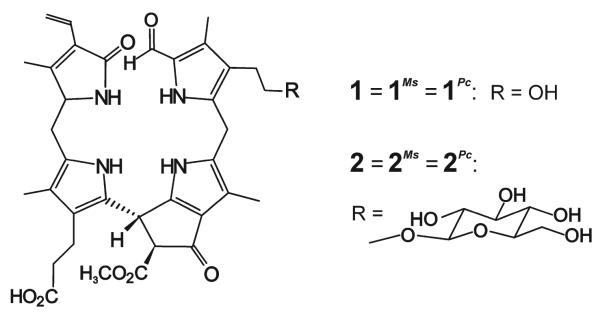
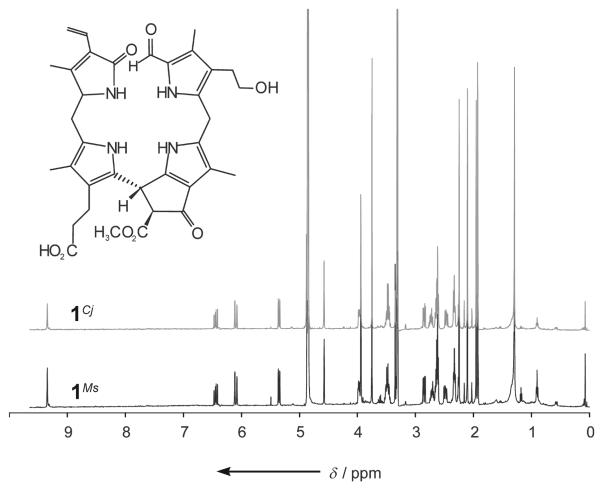
Extracts of freshly cut peels of a yellow pear (p1 in Figure 1) were analyzed by high-performance liquid chromatography (HPLC) with detection by UV/Vis spectroscopy (Figure 2) and found to contain two fractions having UV spectra characteristic of NCCs.[11, 12] In the mass spectrum of 1Pc, the less polar NCC from the pear (Pyrus communis, Pc), the [M+H]+ ion was observed at m/z 645.292 (m/zcalcd 645.292) and indicated the molecular formula to be C35H40N4O8. Further analysis (see the Supporting Information) helped identify 1Pc as the compound 1 shown in Scheme 2. NCC 1Pc proved to be identical to 1Cj, an NCC discovered in leaves of the tree Cercidiphyllum japonicum and named Cj-NCC-1.[5,7]
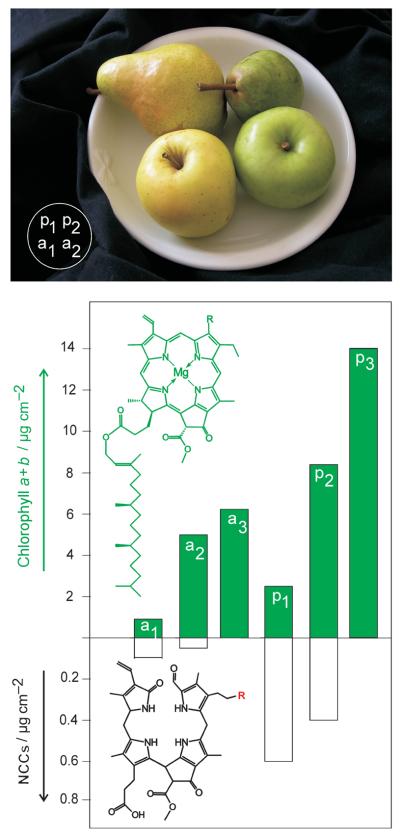
Figure 1.
Ripe fruit from the market contain “nonfluorescent” chlorophyll catabolites (NCCs). Top: Photo of the green and yellow pears and apples used here immediately before analysis (a1/a2: “Golden Delicious” apples; p1/p2: “Williamine” pears). Bottom: Relative amounts of chlorophylls (top section) and of NCCs (lower section, different scale) in the extract of the peels of ripe apples and pears (a1/a2 and p1/p2) and green unripe apples and pears(a3/p3, not photographed) picked three weeks before the usual harvest time.
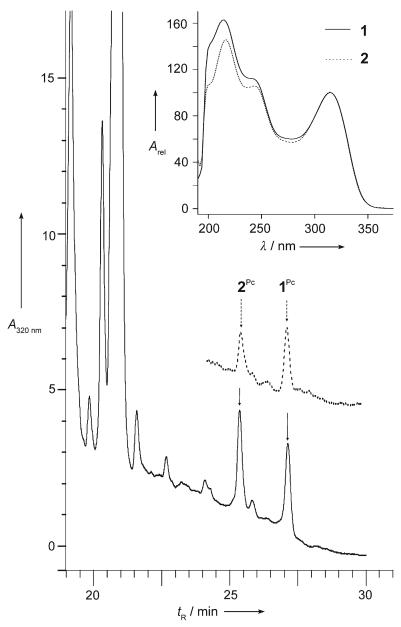
Figure 2.
Analysis of extracts of a ripe pear. Main panel: HPLC analysis of the extract from the peel (—) and the flesh (-----) of a ripe pear; the fractions corresponding to 1Pc and 2Pc are labeled. Inset: UV spectra of the HPLC fractions of 1Pc (= 1) and 2Pc (= 2) from the pear; identical spectra were obtained for the HPLC fractions of 1Ms and 2Ms (from apples), as well as of 1Cj and 2Nr (see the Supporting Information.).[5-7]
Scheme 2.
Structures of NCCs 1 and 2.
The mass spectrum of 2Pc, the more polar NCC from the pear, displayed the ion [M+H]+ at m/z 807.341 (m/zcalcd 807.345), consistent with the molecular formula C41H50N4O13. Further analysis by spectroscopy proved 2Pc to be identical to 2Nr (Scheme 2), an NCC from extracts of tobacco leaves (Nicotiana rustica) and named Nr-NCC-2.[6]
NCCs 1Pc and 2Pc were present in amounts of about 300 ng cm−2 each (or roughly 7 μgg−1) in peels of the yellow pear p1. Only about 7% of the original chlorophylls were accounted for by the two NCCs in the yellow peel relative to the amount of chlorophylls in the peel of green pears (p3) (about 14 μgcm−2, see the Supporting Information). Analysis of extracts from the peeled flesh of another ripe pear by HPLC and UV/Vis spectroscopy indicated it to contain the two NCCs 1Pc and 2Pc also (Figure 2, inset). The NCCs 1Pc and 2Pc were more abundant in the fruit flesh near the skin (≈1.2 μgg−1) than in a sample from an inner layer (<0.2 μgg−1). Indeed, in flesh from near the green peel, chlorophylls were also found (≈10 μgg−1. NCCs thus made up roughly 10 % of the chlorophyll available in this part of the green fruit.
In extracts from senescent leaves of the pear tree the two NCCs 1Pc and 2Pc were also found, with estimated amounts of about 29 μgcm−2 (1Pc) and 4.9 μgcm−2 (2Pc) (see the Supporting Information). Green leaves from the pear tree contained about 50 μgcm−2 of chlorophylls (a and b), indicating that the two NCCs account for close to 70% of the green pigments available in the green leaf.
Freshly cut and extracted yellow peels of a ripe apple (a1 in Figure 1) were also found to harbor two compounds bearing the characteristics of the NCCs identified in the pear. The less polar and more abundant NCC from the peel of apple (Malus sylvestris), 1Ms, was identical to 1Pc (and to 1Cj, see Figure 3 and the Supporting Information). Likewise, 2Ms, the more polar and less abundant NCC in the ripe apple a1, was identical to the NCC 2Pc. A more systematic study showed apples to contain the NCC 1Ms (and less of 2Ms) not only in the skin, but also in the flesh of the fruit, with a concentration profile similar to that in the pear. Likewise, degreened leaves from the apple tree contained significant amounts of 1Ms and smaller quantities of 2Ms.
Figure 3.
1H NMR spectra (500 MHz; in CD3OD, 26°C) of 1Ms (bottom) and 1Cj (top) are identical.
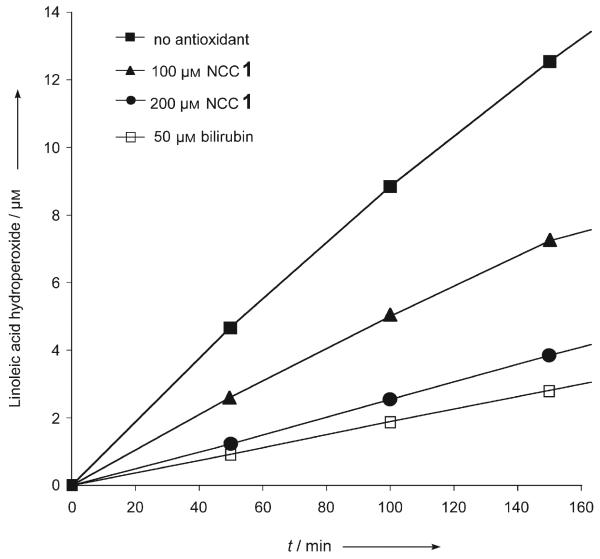
NCC 1, the less polar of the two chlorophyll catabolites from fruit, was tested in the standard autoxidation experiment used for the analysis of bilirubin[16] (Figure 4). The rate of formation of hydroperoxides of linoleic acid was monitored by HPLC analysis as a function of time and of the concentration of the added antioxidant.[16] In the presence of NCC 1, the rate of formation of hydroperoxides of linoleic acid was significantly reduced. The peroxy radical scavenging effect of 1 is only slightly inferior to that of bilirubin.[16] This result may be of particular interest since bilirubin is a tetrapyrrolic heme catabolite structurally related to NCCs.[11] Bilirubin is not only a remarkable antioxidant,[16] but it was also reported recently to be a cytoprotective agent relevant in the reduction of coronary heart diseases, retinal damage, and cancer mortality.[17]
Figure 4.
“Fruit NCC” 1 and bilirubin as antioxidants. Inhibition of linoleic acid autoxidation by 1, and by bilirubin[16] (see the Experimental Section and the Supporting Information for details).
Our identification in fruit of two nonfluorescent chlorophyll catabolites (NCCs) provides first structural insights into the fate of chlorophyll during fruit ripening. Apples and pears were found to contain the same two NCCs (1 and 2). Their amounts correlated roughly with apparent fruit ripening (Figure 1). NCCs were more abundant in the fruit peel but also occurred in the flesh of the fruit. One ripe pear contained about 300 μg of NCCs, accounting for about 7–10% of the green pigments in an unripe pear.
NCCs in the two ripe fruits are identical to those in senescent leaves of the fruit trees. The more polar NCC 2 is derived (in a formal sense) from the less polar one (1) by glucosylation. Related relationships also exist between NCCs from degreened leaves.[8,11, 12] Our observations indicate a common biochemical path of chlorophyll catabolism in fruit ripening and leaf senescence and support the view that degreening in senescent leaves and in ripening fruit shows similarities in chlorophyll breakdown.[1,19,20]
Degreening has been proposed to be an important detoxification process in senescent plants, in which the (potentially) phototoxic green plant pigments are destroyed and colorless catabolites are formed.[9,10] Indeed, the absence of activities of chlorophyll catabolizing enzymes (linked with the “accelerated cell death” genes acd-1 and acd-2) in Arabidopsis thaliana correlated with the observation of necrotic lesions.[21] The intermediary catabolites Pheo a and RCC are photoactive compounds, whose binding and transformation in the course of “complete” chlorophyll breakdown was suggested to help protect the senescent plant.[22] Consistent with this view, the endogenous “detoxification” process in senescent leaves leads to the colorless NCCs, as the final products[10,11] and which account for the major part of the chlorophylls present in green leaves.[7,18]
In the fruit, the recovered NCCs accounted for only a minor portion of the degraded chlorophylls. While their fate in fruit is not known, NCCs were shown here to feature effective antioxidant properties. This may suggest a further physiological role of the NCCs in senescent plants and fruit in helping to inhibit the decline of vital functions.[23]
Fruits is among the most basic components of human nutrition. The availability of NCCs in fruit calls attention to their possible physiological relevance for humans and higher animals. Many plant-derived compounds (vitamins, antioxidants) are seen as beneficial constituents of human nutrition.[24-26] However, most of the questions about the mechanisms by which food components are likely to reduce the risk of (chronic) disease, remain unanswered.[27] The disease-preventing effects of apples and pears are mostly associated with flavonoids and their antioxidative activity,[27-31] and the fruit peels appear to be specifically rich sources.[27,32, 33] The discovery of the NCCs as components of ripe fruit is thus of particular interest. Remarkably, chlorophyll is now seen as being phototoxic (and it is assumed not to be absorbed by the intestinal tract), and an ATP-binding cassette (ABC) drug transporter specifically removes photoactive Pheo a in mammals.[34] In contrast, NCCs, the overlooked colorless tetrapyrroles and natural antioxidants in fruit, may possess beneficial physiological properties. The occurrence of NCCs in ripe fruit might thus give a new turn[32] to the meaning of the popular saying: “An apple a day keeps the doctor away.”[35]
Experimental Section
Isolation and spectroscopic characterization of NCCs 1Pc and 2Pc from pear peels (Pyrus communis): Ripe “Williamine” pears (from the farmers' market in Innsbruck) were peeled, and the peels (450 g, fresh weight) were blended and extracted (for details see the Supporting Information). The collected raw extracts were separated by preparative HPLC, and two fractions, collected at 15.5 min and 19 min, were “desalted” and isolated. Pure samples of the NCCs 1Pc (312 μg) and 2Pc (190 μg) were obtained and found to be identical to 1Cj[5, 7] and 2Nr,[6] respectively, by HPLC and spectroscopic analysis (see the Supporting Information).
Determination of the antioxidant activity of NCC 1:[16] Solutions of linoleic acid, azoisobutyronitrile (AIBN), 1 (isolated as 1Cj,[5, 7]) and bilirubin were mixed and diluted to obtain the desired final concentrations (0.15 m linoleic acid, 2 mm AIBN, and 0–200 μm of 1 or bilirubin). The air-saturated mixtures were kept at 37°C, and the formation of linoleic acid hydroperoxides was monitored by UV/Vis spectroscopy and HPLC analysis (Figure 4, see the Supporting Information).
Supplementary Material
Footnotes
We thank Stefan Hoertensteiner (University of Zürich) and Josef Dallavia (Laimburg) for helpful discussions, Sigrid Gschoesser for recording NMR spectra, and Simone Moser and Sonja Berger for experimental help. This work was supported by the Austrian Science Foundation (FWF, project no. P-16097 and P-19596).
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.Dangl JL, Dietrich RA, Thomas H. In: Biochemistry & Molecular Biology of Plants. Buchanan BB, Gruissem W, Jones RL, editors. Rockville, MD: 2001. pp. 1044–1100. Am. Soc. Plant Physiol. [Google Scholar]
- 2.Brown SB, Houghton JD, Hendry GAF. In: Chlorophylls. Scheer H, editor. CRC Press; Boca Raton: 1991. pp. 465–489. [Google Scholar]
- 3.Kräutler B, Jaun B, Bortlik K, Schellenberg M, Matile P. Angew. Chem. 1991;103:1354–1357. [Google Scholar]; ; ; Angew. Chem. Int. Ed. Engl. 1991;30:1315–1318. [Google Scholar]
- 4.Kräutler B, Matile P. Acc. Chem. Res. 1999;32:35–43. [Google Scholar]
- 5.Oberhuber M, Berghold J, Breuker K, Hörtensteiner S, Kräutler B. Proc. Natl. Acad. Sci. USA. 2003;100:6910–6915. doi: 10.1073/pnas.1232207100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berghold J, Eichmüller C, Hörtensteiner S, Kräutler B. Chem. Biodiversity. 2004;1:657–668. doi: 10.1002/cbdv.200490057. [DOI] [PubMed] [Google Scholar]
- 7.Curty C, Engel N. Phytochemistry. 1996;42:1531–1536. [Google Scholar]
- 8.Müller T, Moser S, Ongania KH, Pru inska A, Hörtensteiner S, Kräutler B. ChemBioChem. 2006;7:40–42. doi: 10.1002/cbic.200500268. [DOI] [PubMed] [Google Scholar]
- 9.Matile P, Hörtensteiner S, Thomas H. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999;50:67–95. doi: 10.1146/annurev.arplant.50.1.67. [DOI] [PubMed] [Google Scholar]
- 10.Hörtensteiner S. Annu. Rev. Plant Biol. 2006;57:55–77. doi: 10.1146/annurev.arplant.57.032905.105212. [DOI] [PubMed] [Google Scholar]
- 11.Kräutler B, Hörtensteiner S. In: Chlorophylls and Bacteriochlorophylls: Biochemistry, Biophysics, Functions and Applications. Scheer H, Grimm B, Porra R, Rüdiger W, editors. Vol. 25. Springer; Dordrecht, The Netherlands: 2006. pp. 237–260. [Google Scholar]
- 12.Berghold J, Müller T, Ulrich M, Hörtensteiner S, Kräutler B. Monatsh. Chem. 2006;137:751–763. [Google Scholar]
- 13.Mühlecker W, Ongania KH, Kräutler B, Matile P, Hörtensteiner S. Angew. Chem. 1997;109:401–404. [Google Scholar]; Angew. Chem. Int. Ed. Engl. 1997;36:401–404. [Google Scholar]
- 14.Hörtensteiner S, Wüthrich KL, Matile P, Ongania KH, Kräutler B. J. Biol. Chem. 1998;273:15 335–15 339. doi: 10.1074/jbc.273.25.15335. [DOI] [PubMed] [Google Scholar]
- 15.Rüdiger W. In: The Porphyrin Handbook. Kadish KM, Smith KM, Guilard R, editors. Vol. 13. Elsevier Science; Amsterdam: 2003. pp. 71–108. [Google Scholar]
- 16.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 17.Baranano DE, Rao M, Ferris CD, Snyder SH. Proc. Natl. Acad. Sci. USA. 2002;99:1609–1698. doi: 10.1073/pnas.252626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mühlecker W, Kräutler B. Plant Physiol. Biochem. 1996;34:61–75. [Google Scholar]
- 19.Moser D, Matile P. J. Plant Physiol. 1997;150:759–761. [Google Scholar]
- 20.Giovannoni J. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001;52:725–749. doi: 10.1146/annurev.arplant.52.1.725. [DOI] [PubMed] [Google Scholar]
- 21.Mach JM, Castillo AR, Hoogstraten R, Greenberg JT. Proc. Natl. Acad. Sci. USA. 2001;98:771–776. doi: 10.1073/pnas.021465298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao N, Greenberg JT. Plant Cell. 2006;18:397–411. doi: 10.1105/tpc.105.036251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matile P. In: Regulation of Photosynthesis. Aro E-M, Andersson B, editors. Kluwer Academic Publishers; Dordrecht: 2001. pp. 277–296. [Google Scholar]
- 24.Sabate J, Rajaram S. Am. J. Clin. Nutr. 2003;78:501S. [Google Scholar]
- 25.Willett WC. Science. 1994;264:532–537. doi: 10.1126/science.8160011. [DOI] [PubMed] [Google Scholar]
- 26.Riboli E, Lambert R. IARC Press; Lyon: 2002. (Scientific Publication No. 156 ed.). [PubMed] [Google Scholar]
- 27.Boyer J, Liu RH. Nutr. J. 2004;3:5–19. doi: 10.1186/1475-2891-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis PA, Polagruto JA, Valacchi G, Phung A, Soucek K, Keen CL, Gershwin ME. Exp. Biol. Med. 2006;231:594–598. doi: 10.1177/153537020623100514. [DOI] [PubMed] [Google Scholar]
- 29.Tsao R, Yang R, Xie S, Sockovie E, Khanizadeh S. J. Agric. Food Chem. 2005;53:4989–4995. doi: 10.1021/jf048289h. [DOI] [PubMed] [Google Scholar]
- 30.Liu RH, Liu J, Chen B. J. Agric. Food Chem. 2005;53:2341–2343. doi: 10.1021/jf058010c. [DOI] [PubMed] [Google Scholar]
- 31.Lotito SB, Frei B. Free Radical Biol. Med. 2004;36:201–211. doi: 10.1016/j.freeradbiomed.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Eberhardt MV, Lee CY, Liu RH. Nature. 2000;405:903–904. doi: 10.1038/35016151. [DOI] [PubMed] [Google Scholar]
- 33.Leontowicz M, Gorinstein S, Leontowicz H, Krzeminski R, Lojek A, Katrich E, Ciz M, Martin-Belloso O, Soliva-Fortuny R, Haruenkit R, Trakhtenberg S. J. Agric. Food Chem. 2003;51:5780–5785. doi: 10.1021/jf030137j. [DOI] [PubMed] [Google Scholar]
- 34.Jonker JW, Buitelaar M, Wagenaar E, van der Valk MA, Scheffer GL, Scheper RJ, Plosch T, Kuipers F, Elferink RPJO, Rosing H, Beijnen JH, Schinkel AH. Proc. Natl. Acad. Sci. USA. 2002;99:15649–15654. doi: 10.1073/pnas.202607599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Titelman GY, editor. Random House Dictionary of Popular Proverbs and Sayings. 1996 A Welsh saying originating from the 19th century. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.