Abstract
Recent progress in molecular magnetic resonance imaging (MRI) provides the opportunity to image cells and cellular receptors using microparticles of iron oxide (MPIOs). However, imaging targets on vessel walls remains challenging owing to the quantity of contrast agents delivered to areas of interest under shear stress conditions. We evaluated ex vivo binding characteristics of a functional MRI contrast agent to ligand-induced binding sites (LIBSs) on activated glycoprotein IIb/IIIa receptors of human platelets, which were lining rupture-prone atherosclerotic plaques and could therefore facilitate detection of platelet-mediated pathology in atherothrombotic disease. MPIOs were conjugated to anti-LIBS single-chain antibodies (LIBS-MPIO) or control antibodies (control MPIO). Ex vivo binding to human platelet-rich clots in a dose-dependent manner was confirmed on a 3 T clinical MRI scanner and by histology (p < .05 for LIBS-MPIO vs control MPIO). By using a flow chamber setup, significant binding of LIBS-MPIO to a platelet matrix was observed under venous and arterial flow conditions, but not for control MPIO (p < .001). A newly generated MRI contrast agent detects activated human platelets at clinically relevant magnetic field strengths and binds to platelets under venous and arterial flow conditions, conveying high payloads of contrast to specific molecular targets. This may provide the opportunity to identify vulnerable, rupture-prone atherosclerotic plaques via noninvasive MRI.
Targeting contrast agents to specific molecules or cell types to increase the sensitivity of magnetic resonance imaging (MRI) provides a powerful tool for detection of epitopes important for disease progression and activity. Existing approaches use particles of iron oxide as well as gadolinium (Gd) preparations.1-3 Conjugation of Gd-containing paramagnetic particles to antibodies or peptidomimetics has been used to selectively image cellular receptors expressed in various disease conditions. For instance, this approach allowed imaging of angiogenesis in early-stage atherosclerosis with αvβ3-integrin-targeted Gd nanoparticles3,4 and of fibrin for intravascular thrombus detection.2,5 Shapiro and colleagues used microparticles of iron oxide (MPIOs) for cellular imaging and tracking. These MPIOs convey a payload of iron that is many orders of magnitude greater than iron nanoparticles and cause local magnetic field inhomogeneity extending for a distance ≈50 times the physical diameter of the microparticle.6 We recently extended the application of MPIO to molecular imaging in vivo using a contrast agent directed against vascular cell adhesion molecule 1 (VCAM-1) to identify acute brain inflammation.7
The potent contrast properties achieved by MPIOs suggest their use for imaging relatively low-abundant epitopes, such as found in the initiation of acute critical clinical conditions, which would extend the clinical applications of functional MRI. However, larger particle size is likely to be accompanied by increased buoyancy and momentum in flowing blood, posing a challenge to local accumulation under conditions of shear stress.
A clinically promising and interesting approach would be to selectively target activated platelets or platelet thrombi such as found on the surface of ruptured atherosclerotic plaques, which finally result in myocardial infarction or stroke. In contrast to fibrin thrombi, which form complex three-dimensional reticular structures with a high abundance of epitope, platelet thrombi may be partially occlusive and localized at the surface of a fissured or ruptured plaque, presenting a challenge to contrast delivery. The glycoprotein (GP) IIb/IIIa receptor mediates the final common pathway of platelet aggregation in this condition and is the key to thrombus formation.8 We recently developed a single-chain antibody that recognizes the ligand-induced binding sites (LIBS) of GP IIb/IIIa receptors that become exposed only on activation through receptor-ligand binding.9 Furthermore, we constructed a contrast agent consisting of LIBS single-chain antibodies conjugated to MPIO targeting activated mouse platelets, which has already been applied in an ex vivo mouse model of endovascular platelet aggregation.10
In this study, we extended the application of this contrast agent to image human platelets in vitro. We report the properties of the LIBS-MPIO contrast agent under venous and arterial flow conditions and demonstrate that MPIOs can be detected at clinically relevant magnetic field strengths, all of which are important for the future use of MPIO-based contrast agents in human applications.
Materials and Methods
Single-Chain Antibody Generation and Conjugation to 1 μm MPIOs
The monoclonal antibody anti-LIBS 145 binds to GP IIb/IIIa only in its active conformation and demonstrates strong binding to adenosine diphosphate (ADP)-activated platelets in the presence of fibrinogen. Generation of anti-LIBS 145 has been described in detail elsewhere.11 For the irrelevant control antibody, a mutation of the heavy-chain CDR3 region of a platelet single-chain antibody was performed to achieve a nonbinding antibody for control purposes. The generation and purification of this antibody were performed in the same way as with the anti-LIBS-antibody.
Autofluorescent cobalt-functionalized MPIOs (diameter 1 μm; hydrodynamic diameter 1.2 μm) were conjugated to the histidine tag of either the anti-LIBS single-chain antibody or the control antibody following the manufacturer's protocol (Dynal Biotech, Oslo, Norway). In brief, 1 mg of beads was incubated with the LIBS antibody for 10 minutes at room temperature to bind approximately 10 μg of histidine-tagged antibody. The tube containing the suspension was then placed on a magnet until the beads had migrated to the side of the tube and the supernatant was discarded. This washing was repeated four times using a binding and washing buffer containing 50 mM NaP (pH 8), 300 mM NaCl, and 0.01% Tween 20. This resulted in the contrast agent of single-chain antibodies conjugated to MPIOs (LIBS-MPIO and control MPIO).
We worked in keeping with the manufacturer's protocol, which indicates that 1 mg of MPIOs binds approximately 10 μg of protein for a 30 kDa protein; the weight of one MPIO is 1 × 10−9 mg (containing 4.7 × 10−12 mmol Fe). Theoretically, 2 × 105 single-chain antibodies should bind to one MPIO. However, the protein binding capacity mediated by the cobalt molecules per MPIO is five times lower (as indicated by the manufacturer) to work with an excess of protein in the initial binding step; therefore, we expected 4 × 104 single-chain antibodies to bind per MPIO.
Using this calculation, 1 μg of LIBS-antibody should effectively bind to 20 μg of MPIOs. Therefore, the quantity of contrast agent applied is expressed by, for example, 1 μg antibody/20 μg of MPIOs or by 1 μg /20 μg LIBS-MPIOs.
Induction of Human Platelet-Rich Clots
Blood from a healthy volunteer taking no medication was anticoagulated with citric acid and centrifuged at 1,000 rpm for 10 minutes. Of the resulting platelet-rich plasma, 1 mL was incubated with 100 μL of ADP (möLab GmbH, Langenfeld, Germany), 88 μL of actin (Dade Behring, Marburg, Germany), and 25 μL of 1 M CaCl2 to induce coagulation. Samples were incubated for 12 minutes at 37°C in a water bath; the developed clots thereafter were incubated with three different doses of either LIBS-MPIO or control MPIO (1, 2, and 3 μg of total protein bound to 20g, 40, or 60 μg of MPIOs) and finally stored for another 30 minutes at 37°C ambient temperature under continuous rotation. Only a small percentage of LIBS-MPIOs was bound to the clots, as could be seen by the remaining MPIO-induced coloring of the supernatant. Clots were extensively washed with phosphate buffered saline (PBS) and transferred into 2% low melting point agarose (Cambrex, Rockland, ME) spiked with 2 mM of gadoteridol (ProHance, Bracco, Princeton, NJ) using wells of a standard enzyme-linked immunosorbent assay (ELISA) plate.
MRI of Human Platelet-Rich Clots
MRI of agarose-embedded clots was performed on a 3 T clinical scanner (Siemens Trio, Erlangen, Germany). The ELISA plate was placed within a standard wrist coil, with the longitudinal axes of the cone-shaped clots oriented horizontally and perpendicular to B0. A susceptibility-sensitive three-dimensional fast low angle shot (FLASH) sequence with an echo time/repetition time (TE/TR) of 9.1 ms/700 ms was run with a matrix of 72 × 512 × 512, leading to a resolution of 130 × 130 × 150 μm and a total acquisition time of 7 hours 57 minutes. In addition, a turbo spin echo (TSE) sequence with equal geometry and TE/TR of 20 ms/4,000 ms, leading to an acquisition time of 2 hours 50 minutes, was performed. TSE imaging was used as a morphologic reference and to facilitate discrimination of signal voids induced by noncontrast agent–based static inhomogeneities.
MRI Data Analysis
Images were reconstructed perpendicular to the longitudinal axes of the clots, parallel to B0, to gain maximal and comparable susceptibility effects. For MPIO-induced signal extinction quantification, a representative clot incubated with LIBS-MPIO showing good MRI contrast properties and corresponding to regular LIBS-MPIO binding in histology was chosen as the standard for signal void quantification in all MRI measurements. These were performed with exactly the same MRI protocol. Contrast and brightness for this clot were adjusted using PhotoShop CS II (Adobe Systems, Munich, Germany), until a black and white mask of the clot was obtained. The only significant signal void appeared as a dark pixel, whereas the background signal appeared white. This allowed counting of the pixels in files, obtaining a resolution of 72 × 72 pixels/inch.
After transferring these standard contrast/brightness values to the other MRI pictures obtained, three reconstructed axial images per clot (n = 5 clots for each group) were evaluated. After zooming the masks, black pixels representing the MPIO-induced susceptibility artifacts in T2*-weighted MRI were quantified for each image, and the average value was quantified for each clot.
Histology of Clots: Quantification of MPIO Binding and Immunohistochemistry
After performing the MRI, specimens were dehydrated through graded ethanol solutions and Neo-clear (VMR, Birmingham, UK), paraffin embedded, and serially sectioned (10 μm thick). For optimal quantification, MPIOs were counted and averaged on four deparaffinized and rehydrated but unstained sections per clot. For platelet visualization with immunohistochemistry and proof of MPIO binding to areas of platelets only, deparaffinized, rehydrated sections were saturated in 1% H2O2 for 20 minutes, added to simmering citrate buffer, and boiled for 4 minutes in a pressure cooker for antigen retrieval. Specimens were washed in PBS Tween, incubated with protein block solution (DakoCytomation, Hamburg, Germany) for 4 hours, and incubated overnight at 4°C with either a monoclonal mouse antihuman CD62 antibody (1:100; R&D Systems, Abingdon, UK) or an irrelevant immunoglobulin G (IgG) serving as control. A biotinylated goat antimouse IgG (Vector, Burlingame, CA) served as a secondary antibody. Slides were washed with PBS, and peroxidase reaction was performed using Vectastain RTU Elite ABC reagent and Vector NovaRed (both from Vector). Finally, sections were dehydrated and mounted with Permount (Biomeda, Foster City, CA), and MPIO surface binding to platelets on the surface of the clot was evaluated on a light microscope.
Properties of the Contrast under Flow Conditions
Blood from healthy volunteers taking no medication was anticoagulated with citric acid and centrifuged at 1,000 rpm for 10 minutes. The resulting platelet-rich plasma was washed on sepharose columns and diluted with Tyrode's buffer (1:5), and 2 mL was added onto a fibrinogen-covered 35 mm culture dish (Corning Costar), which had been preincubated with 25 μg/mL fibrinogen overnight at 4°C and blocked with 1% bovine serum albumin (BSA) for 1 hour at 37°C. After 45 minutes' incubation at 37°C, the culture dishes were washed extensively with PBS.
The Glycotech (GlycoTech, Gaithersburg, MD) flow chamber was assembled with the 35 mm dish at the bottom of the resulting parallel plate flow chamber. The chamber and the tubing leading to and from the chamber were filled with LIBS-MPIO or control MPIO contrast agent (protein concentration of 0.1 μg/mL) diluted in PBS and 0.1% BSA to prevent nonspecific binding. Shear stress was applied with a Harvard apparatus syringe pump PHD 2000 system (Harvard Apparatus, Holliston, MA). The syringe pump was programmed to withdraw at 0.8 mL/min for 4 minutes and at 4.8 mL/min for 1 minute subsequently. The flow chamber was assembled with the Glycotech gasket C for all experiments. The shear rates calculated on the basis of gasket C (thickness 0.00254 cm, width 0.5 cm) and the subsequent applied withdrawal rates were 0.25 and 15 dyn/cm2, respectively. MPIO binding was observed online on a microscope, whereas the number of bound MPIOs was counted every minute and averaged from four fields of view per time point.
Statistical Methods
Data are expressed as mean ± standard error of the mean. Parametric data were compared using t-tests. Statistical significance was assigned to p < .05.
Results
Dose-Dependent MPIO Binding to Activated GP IIb/IIIa Receptors Detected with MRI at 3 T
T2*-weighted MRI of agarose-embedded clots using a 3 T clinical scanner revealed black areas of signal void on the surface of the clot, which are typical for the iron oxide–induced change in susceptibility. As shown in Figure 1A, signal void areas around the clot increased with a higher dose of LIBS-MPIO, whereas incubation of the clot with control MPIO did not show clear areas of signal void.
Figure 1.
A, T2*-weighted 3 T magnetic resonance imaging of agarose-embedded clots; iron oxide microparticle (MPIO)-induced susceptibility effects appear as low signal (black) areas on the surface of clots exposed to ligand-induced binding site (LIBS)-MPIO. Contrast effects appear to be dose dependent, whereas incubation of the clot with control MPIO does not result in clear signal extinction. B, Quantification of black areas confirmed a significantly higher signal void and therefore MPIO binding, depending on the antibody concentration for LIBS-MPIO.
Quantification of dark areas (Figure 1B) showed a significantly higher signal void depending on antibody concentrations (incubation with 1 μg/20 mg LIBS-MPIO vs 2μg/40 μg LIBS-MPIO: p < .01; 2 μg/40 μg LIBS-MPIO vs 3μg/60 μg LIBS-MPIO: p < .01). For control MPIO, there was significantly lower binding for all three concentrations of protein compared with LIBS-MPIO (p < .01 for 1 μg/ 20 μg, 2 μg/40 μg, and 3 μg/60 μg of protein for control MPIO vs LIBS-MPIO).
Evidence of LIBS-MPIO Binding to Platelet Areas Only
As demonstrated in Figure 2A, platelet aggregates can be found on the clot surface and in the center of the clot. Between those platelet islands, a reticular network consisting of fibrin and plasma proteins can be observed. The specificity of the monoclonal mouse antihuman CD62P antibody is demonstrated in the comparison between sections incubated with anti-CD62P (see Figure 2A) and sections incubated with irrelevant IgG (Figure 2B); both sections were obtained from a clot incubated with LIBS-MPIO. MPIO binding happens only on the site of platelet aggregation without nonspecific binding to the surface fibrin network, as shown by the arrows (see Figure 2, A and B). In a high-power view of a superficial platelet aggregate (Figure 2C), dense LIBS-MPIO aggregation can be observed (black arrows). This section has not been immunostained to ensure detection of MPIO aggregation, but the blue-appearing structure (red arrow) shows the typical appearance of platelet aggregates in this model.
Figure 2.
A, Platelet aggregates detected by CD62 immunohistochemistry on the clot surface and in the center of the clot. The specificity of the monoclonal mouse antihuman CD62 antibody to platelet areas is demonstrated in comparison with B, which has been stained with a nonspecific IgG antibody; both sections (A and B) were obtained from a clot incubated with ligand-induced binding site iron oxide microparticles (LIBS-MPIO). MPIO binding happens only on the site of platelet aggregation, without nonspecific binding to the surface fibrin network, as shown by the arrows in A and B. In a nonstained high-power view of a superficial platelet aggregate in C, dense LIBS-MPIO aggregation (black arrows) can be observed only at areas of platelet aggregation located directly on the surface of the clot (red arrows).
Figure 3 demonstrates the results of MPIO quantification in sections from human platelet-rich clots. Increasing amounts of LIBS-MPIO antibody construct incubated with clots result in a significantly stronger MPIO binding (p < .01 for 1 μg/20 μg LIBS-MPIO vs 2 μg/40 μg LIBS-MPIO, p < .05 for 2 μg/40 μg vs 3 μg/60 μg LIBS-MPIO), whereas MPIO binding in the control MPIO–incubated clots is always significantly lower compared with LIBS-MPIO (p < .01 for all doses of control MPIO vs LIBS-MPIO).
Figure 3.
Results of iron oxide microparticle (MPIO) quantification in sections from human platelet-rich clots. Increasing amounts of ligand-induced binding site (LIBS) antibody incubated with clots result in a significantly stronger MPIO binding, whereas MPIO binding in the control MPIO–incubated clots is always significantly lower compared with LIBS-MPIO.
LIBS-MPIO Binds to Platelets under Venous and Arterial Flow Conditions
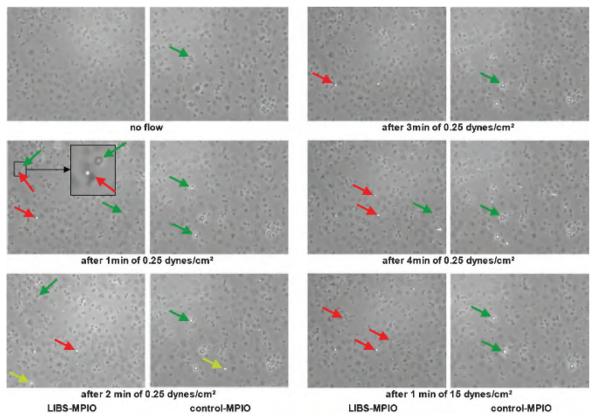
Two different continuous flow conditions were simulated in the flow chamber: a slow venous flow (0.25 dyn/cm2) and a flow similar to that observed in arteries (15 dyn/cm2). MPIO binding was observed every minute; the microscopic view of MPIO binding is demonstrated in Figure 4 for six different time points (no flow; 0.25 dyn/cm2 after 1 minute, 2 minutes, 3 minutes, and 4 minutes; and after 1 minute of 15 dyn/cm2). The red arrows indicate the typical appearance of MPIOs as white dots without a halo, which must be differentiated from nonactivated platelets with a larger diameter and a large dark halo (green arrow and inset); the yellow arrow indicates a flowing MPIO.
Figure 4.
Flow chamber experiments under venous flow conditions (0.25 dyn/cm2) and strong arterial flow conditions (15 dyn/cm2). Microscopic view of iron oxide microparticle (MPIO) binding for six different time points (no flow; 0.25 dyn/cm2 after 1 minute, 2 minutes, 3 minutes, and 4 minutes; and 1 minute of 15 dyn/cm2). The inset shows a zoomed area of interest with a neighboring MPIO (red arrow) and a platelet (green arrow). The MPIO appears as a white and round structure, which is also attached on the surface of a flattened and therefore activated platelet (dark structure underneath). Conversely, the platelet marked with the green arrow is not flattened but has a round morphology, which is slightly bigger than the MPIO and has a dark halo. This corresponds to a platelet, which is not flattened but only partially attached to the well plate and is therefore not yet completely activated; the number of such nonattached structures increases with higher flow and shear stress. The yellow arrow indicates a flowing MPIO. Ligand-induced binding site MPIO binding increases over time and remains constant after switching from venous to arterial flow conditions, whereas only modest background binding of control MPIOs to activated platelets was observed.
A constant increase occurred in LIBS-MPIO binding 2 minutes after flow initiation (Figure 5), as well as a further significant increase after 3 and 4 minutes (p < .001). Only modest background binding was observed for control MPIO. After switching from venous to arterial flow, binding remained almost constant, without a significant wash-off effect, after 1 minute of arterial flow.
Figure 5.
Quantification of iron oxide microparticles (MPIOs) under venous and arterial flow conditions. At venous flow, a constant increase in ligand-induced binding site (LIBS)-MPIO binding 2 minutes after flow initiation can be observed with only modest background binding for control MPIO. After switching from venous to arterial flow, MPIO binding remained almost constant, without a significant wash-off effect, after 1 minute of arterial flow.
Discussion
Imaging of cellular receptors is a promising method for functional MRI, providing insights into pathologic processes at a molecular level. Targeted contrast agents consisting of receptor-targeted antibodies and particles with high payload of MRI-detectable contrast could provide an opportunity for detection of disease-specific epitopes found on diseased vessel walls. We report the properties of an MPIO-based contrast agent targeted to LIBSs of GP IIb/IIIa receptors on activated human platelets. Ruptured atherosclerotic plaques in coronary or cerebral vessels but also vulnerable, rupture-prone plaques are lined with activated platelets12,13 and thus appear to be detectable via noninvasive MRI.
The generated contrast agent is based on the highly versatile single-chain antibody format.14,15 An important advantage of this new technology is its low immunogenicity owing to the lack of Fc regions, which makes its application in humans very attractive. Furthermore, disguised but specific epitopes such as LIBS are far easier to target by such small-sized proteins. Single-chain antibodies can be produced in large quantities and based on bacterial production at low prices and can be tailored in size for various purposes.14,15 The ability of the anti-LIBS single-chain antibody to target effector molecules to activated platelets in a highly selective manner was recently demonstrated.16 The ability of LIBS-MPIO to selectively bind to human platelets is demonstrated here by immunohistochemistry and histology. Binding of LIBS-MPIO significantly increases with larger doses of contrast agent, which simultaneously can be detected by a typical signal void in T2*-weighted MRI. Notably, we were able to image LIBS as a functional epitope using clinically relevant field strengths of 3 T.
An important advantage of MPIOs compared with a nanoparticle-based approach is that it is not necessary to deliver very large numbers of particles to establish strong contrast effects. Shapiro and colleagues described MRI detection of MPIO in single cells for cellular imaging.17 Depending on the size of the MPIO, T2* effects were readily detected from single MPIO at 50 mm resolution, and significant signal effects could be detected at resolutions as low as 200 μm.6 The detection of MPIOs in single cells in an animal-based in vivo model was recently described by the same group.17 Furthermore, we were able to show that targeted MPIOs can detect VCAM-1 expression in acute brain inflammation in vivo in mice with high sensitivity and excellent contrast properties.7 These studies confirm the concept of in vivo targeting of cells and cellular receptors with MPIO-based contrast agents.
To the best of our knowledge, we have described for the first time the application of a targeted contrast agent against human epitopes using MPIOs and MRI at clinically relevant field strengths. Although resolution achieved in routine clinical examinations is not as high as described in our in vitro approach with long acquisition times, the detection of platelet thrombi with exposure of a large number of LIBS epitopes using an MPIO-based contrast agent could be a promising strategy.
One possible limitation for the application of large iron oxide–based particles is the balance between ligand affinity and shear stress exerted on the micrometer-sized particle. Owing to shear stress, especially under arterial flow conditions, binding of individual particles is unlikely to be as efficient as binding of nanometer-sized contrast agents. As shown here in flow chamber experiments, LIBS-MPIO binds and remains bound under venous and arterial flow conditions, although the density of MPIO binding is relatively low (see Figure 5). However, owing to the contrast effect achieved even by a single MPIO, effective labeling with MPIOs is a reasonable proposition. This should overcome the conclusion drawn in a study of targeting platelet thrombi with ultrasmall superparamagnetic iron oxide (SPIO)s coupled to an arginine, glycine, aspartic acid (RGD) peptide, in which the achievable resolution and sensitivity were seen as a potential limitation to the usefulness of active vascular targeting in MRI.18 Given that we image a very specific target of platelet activation but aim for the sensitive detection of low numbers of activated platelets, low numbers of contrast particles have to provide sufficient MRI contrast. This might be difficult to achieve with ultrasmall SPIOs. However, micron-sized particles provide a much larger signal void, far beyond their own spatial dimensions, such that sufficient MRI contrast is generated by low numbers of bound contrast particles. Future in vivo setups based on iron oxide particles will also need a careful experimental setup with imaging sequences performed before and after contrast agent application to differentiate between signal effect and artifacts. This is an important issue with respect to the negative contrast caused by iron oxides. We purposely did not seek positive contrast using Gd chelates as we were interested in the higher relaxivities of iron oxides that could deliver more sensitive imaging of low numbers of activated platelets.
Future applications of the LIBS-MPIO contrast agent could involve noninvasive imaging of platelet adhesion to the vascular wall and thrombus formation under in vivo conditions in animals and humans. The feasibility of MPIOs for in vivo imaging was recently demonstrated by VCAM-1-targeted MPIOs in acute brain inflammation7 and atherosclerotic plaque detection in mice19 after injection of a total dose of 4 × 108 MPIOs. Using scan times of 1 hour, MPIOs could readily be detected throughout inflamed brain vessels by MRI with excellent contrast properties. Therefore, specific imaging of receptors with shorter scanning times than applied in this study seems possible using MPIO; the size of the MPIOs and their magnetic properties are an important feature as they cause a signal void that far exceeds their diameter.20 Initial biodistribution data show that after injection, MPIOs were found in lungs, kidneys, liver, and spleen 30 minutes and 24 hours after intravenous injection in mice with sequestration in the spleen after 24 hours, suggesting rapid clearance.19 Rapid clearance of circulating MPIOs is an important advantage in imaging of vascular receptors since background signal is low owing to the fast clearance of nonbound, circulating MPIOs.
Limitations
In this study, we used commercially available MPIOs for targeting histidine-tagged single-chain antibodies. The polystyrene coating of these specific particles is not suitable for use in humans owing to the potential toxicity of the coating. However, work continues on the development of micron-sized biodegradable contrast particles for clinical use.21-24 Studies in mice suggest that MPIOs are safe. In the previously described study of VCAM targeting, no abnormal behavior was observed in these animals after the intravenous application of VCAM-MPIO, and we have not seen evidence of tissue infarction, inflammation, or hemorrhage.7
The scanning times in our approach last longer than clinical applications would allow. However, we aimed for optimal spatial resolution in this proof of principle approach on a clinical scanner, which also allowed us to correlate quantification of signal extinction by MRI with histologic findings. Initial experience with MRI protocols of VCAM imaging in mice allows scanning times of around 1 hour even with higher resolutions than described in this approach, providing excellent contrast properties.7 These data are promising and encourage further studies of in vivo MRI with MPIOs but will definitely need further optimizing before being transferred into clinical settings.
Another important issue is the time point of possible future thrombus in vivo imaging. In a previous approach, we observed vascular clearance of MPIOs targeting VCAM in brain inflammation hours after injection of the contrast agent7; however, we were able to obtain excellent contrast and VCAM targeting before this time point. As many cells are involved in the pathology of thrombus formation under in vivo conditions, an early time point of imaging after injection of the contrast agent might be reasonable before nonspecific vascular clearance of MPIOs begins.
Conclusions
LIBS-MPIO provides the opportunity to specifically detect activated human platelets at clinically relevant MRI field strengths in vitro. LIBS-MPIO binds to platelets under venous and arterial flow conditions, providing high payloads of contrast agent to highly function-specific molecular epitopes. Given that activated platelets are markers of vulnerable, rupture-prone atherosclerotic plaques, the newly described contrast agent holds promise for allowing identification of those plaques in MRI and initializing preventive measures. These are important results for future use of MPIO-based contrast agents in human applications and encourage further studies on the use of MPIO-based functional contrast agents in general.
References
- 1.Ruehm SG, Corot C, Vogt P, et al. Magnetic resonance imaging of atherosclerotic plaque with ultrasmall superparamagnetic particles of iron oxide in hyperlipidemic rabbits. Circulation. 2001;103:415–22. doi: 10.1161/01.cir.103.3.415. [DOI] [PubMed] [Google Scholar]
- 2.Flacke S, Fischer S, Scott MJ, et al. Novel MRI contrast agent for molecular imaging of fibrin: implications for detecting vulnerable plaques. Circulation. 2001;104:1280–5. doi: 10.1161/hc3601.094303. [DOI] [PubMed] [Google Scholar]
- 3.Winter PM, Morawski AM, Caruthers SD, et al. Molecular imaging of angiogenesis in early-stage atherosclerosis with alpha(v)beta3-integrin-targeted nanoparticles. Circulation. 2003;108:2270–4. doi: 10.1161/01.CIR.0000093185.16083.95. [DOI] [PubMed] [Google Scholar]
- 4.Kooi ME, Cappendijk VC, Cleutjens KB, et al. Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation. 2003;107:2453–8. doi: 10.1161/01.CIR.0000068315.98705.CC. [DOI] [PubMed] [Google Scholar]
- 5.Spuentrup E, Buecker A, Katoh M, et al. Molecular magnetic resonance imaging of coronary thrombosis and pulmonary emboli with a novel fibrin-targeted contrast agent. Circulation. 2005;111:1377–82. doi: 10.1161/01.CIR.0000158478.29668.9B. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro EM, Skrtic S, Sharer K, et al. MRI detection of single particles for cellular imaging. Proc Natl Acad Sci U S A. 2004;101:10901–6. doi: 10.1073/pnas.0403918101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McAteer MA, Sibson N, von zur Muhlen C, et al. In vivo magnetic resonance imaging of acute brain inflammation using microparticles of iron oxide. Nat Med. 2007;13:1253–8. doi: 10.1038/nm1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peter K, Ahrens I, Schwarz M, et al. Distinct roles of ligand affinity and cytoskeletal anchorage in alphaIIbbeta3 (GP IIb/IIIa)-mediated cell aggregation and adhesion. Platelets. 2004;15:427–38. doi: 10.1080/09587100410001723179. [DOI] [PubMed] [Google Scholar]
- 9.Schwarz M, Rottgen P, Takada Y, Peter K. Single-chain antibodies for the conformation-specific blockade of activated platelet integrin alphaIIbbeta3 designed by subtractive selection from naive human phage libraries. FASEB J. 2004;18:1704–6. doi: 10.1096/fj.04-1513fje. [DOI] [PubMed] [Google Scholar]
- 10.von zur Muhlen C, Peter K, Ali Z, Choudhury RP. Magnetic resonance imaging of platelets on wire-injured mouse femoral arteries using activation-specific anti-GP IIb/IIIa single chain antibodies conjugated to microparticles of iron oxide [abstract] J Am Coll Cardiol. 2007;49(Suppl):108. [Google Scholar]
- 11.Schwarz M, Katagiri Y, Kotani M, Peter K. Reversibility versus persistence of GPIIb/IIIa blocker-induced conformational change of GPIIb/IIIa (alphaIIbbeta3, CD41/CD61) J Pharmacol Exp Ther. 2004;308:1002–11. doi: 10.1124/jpet.103.058883. [DOI] [PubMed] [Google Scholar]
- 12.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115:3378–84. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massberg S, Brand K, Gruner S, Gawaz M. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J Exp Med. 2002;196:887–96. doi: 10.1084/jem.20012044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwarz M, Meade G, Stoll P, et al. Conformation-specific blockade of the integrin GPIIb/IIIa: a novel antiplatelet strategy that selectively targets activated platelets. Circ Res. 2006;99:25–33. doi: 10.1161/01.RES.0000232317.84122.0c. [DOI] [PubMed] [Google Scholar]
- 15.Eisenhardt SU, Schwarz M, Schallner N, et al. Generation of activation-specific human anti-alphaMbeta2 single-chain antibodies as potential diagnostic tools and therapeutic agents. Blood. 2007;109:3521–8. doi: 10.1182/blood-2006-03-007179. [DOI] [PubMed] [Google Scholar]
- 16.Stoll P, Bassler N, Hagemeyer CE, Peter K. Targeting ligand-induced binding sites on GPIIb/IIIa via single-chain antibody allows effective anticoagulation without bleeding time prolongation. Arterioscler Thromb Vasc Biol. 2007;27:1206–12. doi: 10.1161/ATVBAHA.106.138875. l. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro EM, Sharer K, Skrtic S, Koretsky AP. In vivo detection of single cells by MRI. Magn Reson Med. 2006;55:242–9. doi: 10.1002/mrm.20718. [DOI] [PubMed] [Google Scholar]
- 18.Johansson LO, Bjornerud A, Ahlstrom HK, et al. A targeted contrast agent for magnetic resonance imaging of thrombus: implications of spatial resolution. J Magn Reson Imaging. 2001;13:615–8. doi: 10.1002/jmri.1086. [DOI] [PubMed] [Google Scholar]
- 19.McAteer MA, Schneider JE, Ali ZA, et al. Magnetic resonance imaging of endothelial adhesion molecules in mouse atherosclerosis using dual-targeted microparticles of iron oxide. Arterioscler Thromb Vasc Biol. 2008;28:77–83. doi: 10.1161/ATVBAHA.107.145466. 2007 [Epub Oct 25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shapiro EM, Skrtic S, Koretsky AP. Sizing it up: cellular MRI using micron-sized iron oxide particles. Magn Reson Med. 2005;53:329–38. doi: 10.1002/mrm.20342. [DOI] [PubMed] [Google Scholar]
- 21.Hemmingsson A, Carlsten J, Ericsson A, et al. Relaxation enhancement of the dog liver and spleen by biodegradable superparamagnetic particles in proton magnetic resonance imaging. Acta Radiol. 1987;28:703–5. [PubMed] [Google Scholar]
- 22.Hamoudeh M, Fessi H. Preparation, characterization and surface study of poly-epsilon caprolactone magnetic microparticles. J Colloid Interface Sci. 2006;300:584–90. doi: 10.1016/j.jcis.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 23.Chen HH, Le Visage C, Qiu B, et al. MR imaging of biodegradable polymeric microparticles: a potential method of monitoring local drug delivery. Magn Reson Med. 2005;53:614–20. doi: 10.1002/mrm.20395. [DOI] [PubMed] [Google Scholar]
- 24.Zhu D, White RD, Hardy PA, et al. Biocompatible nanotemplate-engineered nanoparticles containing gadolinium: stability and relaxivity of a potential MRI contrast agent. J Nanosci Nanotechnol. 2006;6:996–1003. doi: 10.1166/jnn.2006.169. [DOI] [PubMed] [Google Scholar]