Abstract
Background
Nosocomial transmission of tuberculosis remains an important public health problem. We created an in vivo air sampling model to study airborne transmission of tuberculosis from patients coinfected with human immunodeficiency virus (HIV) and to evaluate environmental control measures.
Methods
An animal facility was built above a mechanically ventilated HIV-tuberculosis ward in Lima, Peru. A mean of 92 guinea pigs were continuously exposed to all ward exhaust air for 16 months. Animals had tuberculin skin tests performed at monthly intervals, and those with positive reactions were removed for autopsy and culture for tuberculosis.
Results
Over 505 consecutive days, there were 118 ward admissions by 97 patients with pulmonary tuberculosis, with a median duration of hospitalization of 11 days. All patients were infected with HIV and constituted a heterogeneous group with both new and existing diagnoses of tuberculosis. There was a wide variation in monthly rates of guinea pigs developing positive tuberculin test results (0%–53%). Of 292 animals exposed to ward air, 159 developed positive tuberculin skin test results, of which 129 had laboratory confirmation of tuberculosis. The HIV-positive patients with pulmonary tuberculosis produced a mean of 8.2 infectious quanta per hour, compared with 1.25 for HIV-negative patients with tuberculosis in similar studies from the 1950s. The mean monthly patient infectiousness varied greatly, from production of 0–44 infectious quanta per hour, as did the theoretical risk for a health care worker to acquire tuberculosis by breathing ward air.
Conclusions
HIV-positive patients with tuberculosis varied greatly in their infectiousness, and some were highly infectious. Use of environmental control strategies for nosocomial tuberculosis is therefore a priority, especially in areas with a high prevalence of both tuberculosis and HIV infection.
Institutional transmission of tuberculosis is an important public health problem. Numerous outbreaks of tuberculosis have been reported in hospitals [1, 2], homeless shelters [3, 4], and correctional facilities [5, 6]. Nosocomial tuberculosis is particularly important in resource-limited settings where control measures are hardest to implement and tuberculosis burden is highest. Nosocomial tuberculosis is exacerbated by HIV infection, which increases susceptibility to tuberculosis infection [7] and progression to disease [8] and causes clinic attendance and hospitalization. HIV infection has an ill-defined effect on tuberculosis infectiousness, with conflicting results from household contact studies for HIV-positive and HIV-negative patients with tuberculosis [9]. Although delayed diagnosis because of atypical presentation may result in increased transmission of tuberculosis, reduced lung cavitation and decreased sputum bacillary load may reduce infectiousness in persons with HIV infection [10, 11].
Measuring transmission of tuberculosis is difficult. Tuberculosis rates among hospital staff are confounded by exposures outside of the workplace. Conventional mechanical air sampling for viable Mycobacterium tuberculosis has been unsuccessful [12], and PCR-based techniques [13, 14] also detect nonviable organisms. Much of our understanding of airborne transmission of tuberculosis is based on classic 1950s studies in which guinea pigs were exposed to exhaust air from a tuberculosis ward [15, 16]. By matching patient and guinea pig tubercular infection, using drug susceptibility and temporal exposure patterns, the investigators demonstrated airborne transmission of tuberculosis by droplet nuclei, as well as great variability in patient infectiousness and reduced infectiousness after the initiation of treatment.
Since the numerous hospital outbreaks associated with the tuberculosis resurgence during the 1980s and 1990s, the US Centers for Disease Control and Prevention developed guidelines for tuberculosis control in health care settings, which were recently updated [17]. Although use of these guidelines in North America has been paralleled by a decrease in reported nosocomial transmission of tuberculosis, the efficacy of individual interventions is unclear, because they are often implemented simultaneously and because of the difficulties in measuring transmission. It is important to determine which interventions are effective for reducing hospital-acquired infections, particularly in resource-limited settings. There has been renewed interest recently in upper-room UV light as an environmental control measure for tuberculosis [18, 19], but there have been no efficacy studies in a clinical setting. The aim of this study was to recreate the original air sampling model (from the 1950s) in the modern era of HIV infection and multidrug-resistant tuberculosis, to investigate the infectiousness of such patients, and to allow subsequent evaluation of environmental control measures for tuberculosis.
METHODS
Patient recruitment
The study was performed at Hospital Nacional Dos de Mayo (Lima, Peru). Persons with HIV infection with existing or suspected tuberculosis were treated in two 4-bed, negative-pressure rooms. All ward air was exhausted via ducts to the tuberculosis infectiousness facility on the roof. This ward operated normally as part of the tuberculosis-HIV service, and the study had no influence on patient admission, treatment, or duration of hospital stay.
Tuberculosis infectiousness facility
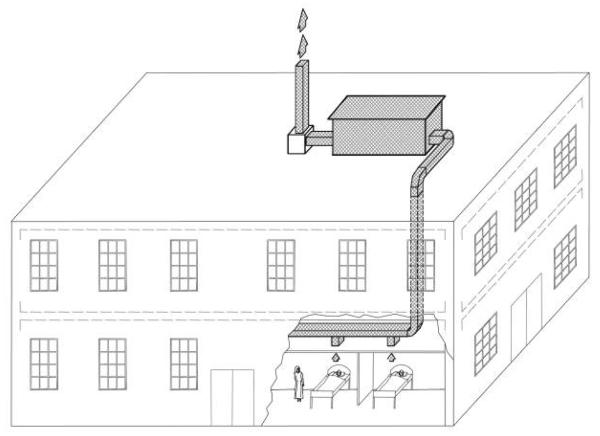
An airtight animal facility was constructed on the hospital roof (figure 1). Ward air passed through the animal facility before extractor fans exhausted it outside. Animals were housed in groups of 6–8. Animal cages had wire mesh drop-through floors to minimize the risk of horizontal tuberculosis spread via the fecal-oral route [20, 21]. Airflow from the ward and into the animal house was measured using an airflow capture hood (Alnor) on injection and extraction vents. Airflow patterns were assessed visually using smoke emitters (Regin HVAC Products).
Figure 1.
Schematic representation of the animal facility on the roof of a tuberculosis ward. The HIV-tuberculosis ward is located on the ground floor, and exhaust air flowed through ductwork in the false ceiling up to the animal facility on the roof. Air passed over the guinea pigs and then passed through 2 extractor fans before being exhausted into the atmosphere through the chimney.
Animals
Outbred male and female Peruvian guinea pigs, weighing 600–1000 g, were maintained in quarantine for ≥1 month. All guinea pigs were skin tested at monthly intervals by intradermal injection with 100 U of purified protein derivative (PPD; Evans Vaccines). The diameter of induration was measured at 48 h. At least 2 negative monthly test results were required before transfer from quarantine to the hospital roof to ensure freedom from tuberculosis.
Experimental protocol
One hundred forty-four animals were added to the exposure facility initially, with an additional 148 added after 6 months. Total duration of ward air exposure was 505 days. Monthly tuberculin skin tests were continued, and those with positive reactions were removed for humane sacrifice and autopsy. A mean of 40 negative control guinea pigs were maintained in a separate rooftop facility ventilated with fresh air. An additional 13 animals were injected intramuscularly with 0.5 mL of Mycobacterium bovis sensitizing agent [22] (Center for Veterinary Biologics) to act as positive control animals for different PPD batches. Care of control animals was identical to that of animals exposed to ward air.
Diagnosis of tuberculosis in animals
Evidence of tubercular infection was sought in the lungs; bronchohilar, paratracheal, and mesenteric lymph nodes; spleen; and liver. Lesions were divided into halves, with one part stored in 10% formaldehyde and the other homogenized in 2 mL of sterile 0.9% saline for culture for M. tuberculosis. If no lesion suspicious for tuberculosis was detected, one-half of one diaphragmatic lung lobe and all bronchohilar lymph nodes were removed for homogenization and culture. Tissue homogenates were decontaminated for 15 min using NaOH-NALC [23], and the resulting mixture was centrifuged for 15 min at 17°C. The pellet was resuspended in 2 mL of 0.9% saline plus 0.2% bovine serum albumin (Sigma), and 125 μL of the specimen was cultured for M. tuberculosis using the microscopic-observation drug-susceptibility assay [24].
Patient and ward air infectiousness
Mean patient infectiousness per month was calculated using the Wells-Riley model of airborne infection [25], assuming guinea pig pulmonary ventilation to be 0.23 m3/day [15] and adjusting for non–ward air infiltration into ducts [26]. Ward periods relating to each monthly skin test were calculated considering a lag of 21 days prior to the test, reflecting the incubation period for tuberculosis in guinea pigs [15, 27].
Ethical approval
The study was approved by the institutional review boards at Hospital Dos de Mayo, Asociación Benéfica PRISMA (Lima), and Imperial College London Hammersmith Hospital Campus. Animal ethics approval was obtained from the Veterinary Medicine faculty at Universidad Nacional Mayor San Marcos (Lima) who supervised all animal work.
Statistical analyses
SPSS software, version 10 (SPSS), was used. Parametric data were compared using Student's t test, and nonparametric data were compared using the Mann-Whitney U test or Wilcoxon signed-ranks test. A χ2 test was used for data with binomial outcomes.
RESULTS
Patients
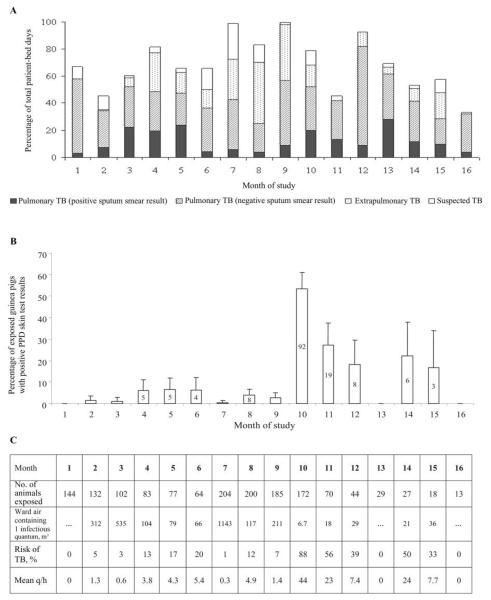
Over 505 days, there were 185 admissions to the negative-pressure rooms by 161 patients, resulting in a total of 2667 patient-days (mean bed occupancy, 66%). The median duration of hospitalization was 11 days (interquartile range, 6–21days). There were 118 admissions by 97 patients with pulmonary tuberculosis (1798 patient-days; 67%), 33 admissions by 30 patients with extrapulmonary tuberculosis (609 patient-days; 23%), and 34 admissions by 34 persons suspected of having tuberculosis but with no subsequent evidence of tuberculosis (260 patient-days; 10%). Forty-one admissions due to pulmonary tuberculosis (35%) were by patients with positive sputum smear results (figure 2A). All of the patients were HIV positive. Patients with pulmonary tuberculosis included those admitted for diagnosis and initiation of treatment, for treatment of adverse reactions, or for other complications of tuberculosis or HIV infection.
Figure 2.
Patient-bed occupancy, percentage of exposed guinea pigs with positive skin test reactions to purified protein derivative (PPD), and infectiousness of ward air, by study month. A, Bed occupancy for the 8-bed ward from which all exhaust air passed over the guinea pigs. B, Percentages (with 95% CIs) of exposed guinea pigs each month with positive PPD skin test results (≥7.5 mm induration at 48 h). C, Infectiousness of ward air. The mean infectiousness of patients with pulmonary tuberculosis (TB) in the ward per month (infectious quanta produced per hour [q/h]) was calculated using the Wells-Riley model of airborne infection [25]. Also shown is the volume of ward air in cubic meters in which 1 infectious quantum is calculated to have been suspended, assuming that 1 infectious quantum was responsible for each PPD-positive guinea pig. In addition, the mean daily risk of tubercular infection is shown for a health care worker breathing ward air without respiratory protection, estimated for each study month using the Wells-Riley model, assuming pulmonary ventilation to be 0.167 L/s [28] and the infectious TB dose for guinea pigs and health care workers to be equal.
Ward airflow
The mean total airflow leaving the ward (±SD) was 402±9.4 L/s, including 56.6±0.9 L/s from a small side room used in the mornings to administer intravenous medications to patients without tuberculosis. Mean total airflow entering the animal house (±SD) was 459.2±15.1 L/s—the difference reflecting outside air infiltration into ducts (mean rate of infiltration [±SD], 12%±3.5%). In smoke pattern testing, smoke entered the animal house, dispersing rapidly to both sides of the central partition without visible areas of stagnation.
Infectiousness of ward air
The proportion of guinea pigs with positive PPD testing results (induration, ≥7.5 mm) each month is shown in figure 2B. Attrition in total numbers reflects monthly removal of PPD-positive and -negative control animals for autopsy, as well as intercurrent deaths. There were no PPD-positive animals in months 1, 13, and 16, and there was just 1 in months 3 and 7. Ward air was highly infectious before the month 10 skin test, at which point 92 animals (53%) had positive PPD results. Estimated patient infectiousness, volume of ward air in which 1 infectious quantum was suspended, and daily risk of M. tuberculosis infection averaged over each study month using the Wells-Riley model are shown in figure 2C.
Detection of tuberculosis transmission via PPD skin testing
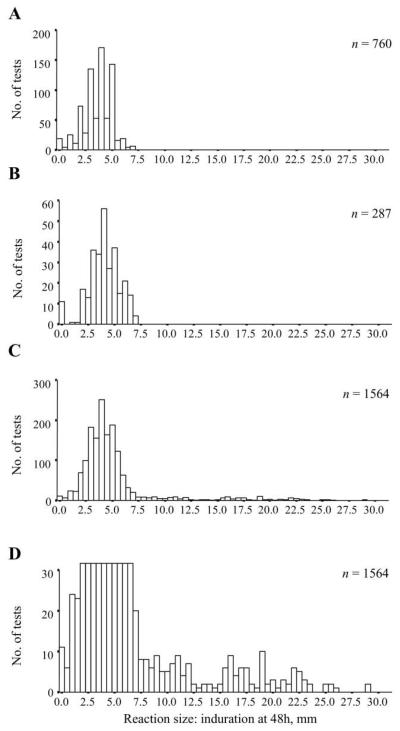
During the quarantine period, 760 PPD skin tests were performed for 308 animals. All reactions had a diameter <7.5 mm (mean±SD, 3.6±1.2 mm). Among unexposed negative control animals, 287 tests were performed for 50 animals. All reactions were <7.5 mm (mean±SD, 4.0±1.5 mm). Among ward air–exposed animals, 1564 tests were performed for 292 animals. The majority of reactions again were <7.5 mm, but larger reactions were now seen. Figure 3 shows the frequency distributions of PPD reactions among quarantined, unexposed negative control, and ward air–exposed animals. A putative cutoff of ≥7.5 mm for a positive test was selected.
Figure 3.
Tuberculin purified protein derivative (PPD) skin test responses in guinea pigs exposed and not exposed to ward air. The frequency distribution of induration diameter at 48 h for PPD skin tests is shown for quarantined animals (760 tests in 308 animals over 3 months) (A), unexposed negative control animals on the roof (287 tests in 50 animals over 13 months) (B), animals exposed to ward air (1564 tests in 292 animals over 16 months) (C), and animals exposed to ward air as in C, with the y axis truncated at 30 mm to emphasize the distribution of large responses ≥7.5 mm (D).
When males and females were compared, there was no significant difference in PPD responses (P = .2) or in rates of positive tests in exposed animals (both 10%; P = .7). All 13 animals challenged with Mycobacterium bovis sensitizing agent developed skin test responses ≥7.5 mm. The median reaction size for 93 tests conducted within 6 months of a sensitizing injection was 16 mm (interquartile range, 8.5–20 mm). Responses decreased over time but increased after rechallenge.
The infection data were not confounded by serial testing, because reaction size did not change over time. This was formally investigated in quarantined animals. In the first batch of animals, the mean reaction size (±SD) was 4.0±1.1 mm (in 148 tests) in the first month, 3.7±1.4 mm (in 147 tests) in the second month, and 3.6±1.3 mm (in 147 tests) in the third month. There was no significant difference between the first and second or second and third months. There was a statistically significant decrease in reaction size between the first and third months of 0.4 mm (P = .01), but this is not of biological importance. Similar data were obtained for the second batch of animals.
The data were also not affected by the introduction of a second batch of animals to monitor patient infectiousness. The first batch had been exposed to ward air for 6 months when the second batch began exposure. Subsequently, 38 (14%) of 265 skin test results were positive in the first group, which was almost identical to 95 (14%) of 671 test results being positive in the second group (P = .9). Thus, there was no accumulation in the exposure chamber over time of animals resistant to tuberculosis because of the removal of PPD-positive susceptible animals.
Analysis of guinea pig infection
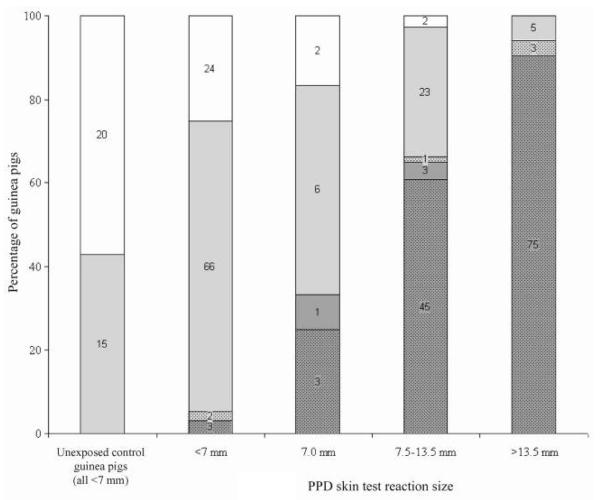
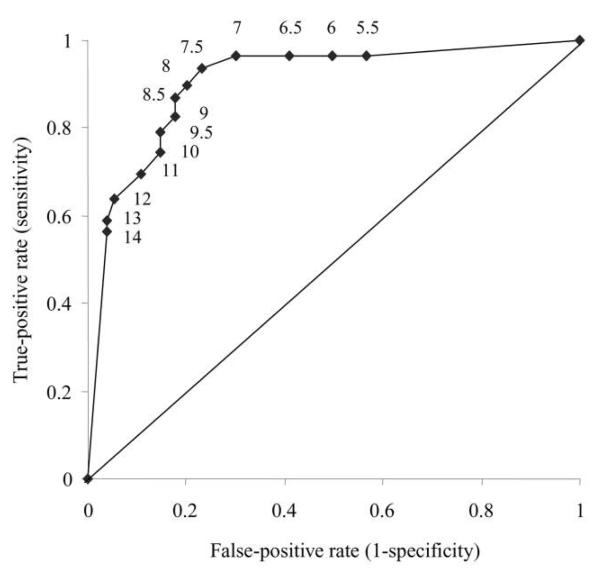
Autopsy and culture results are shown in table 1. A macroscopic lung primary focus and/or enlarged, hard bronchohilar or paratracheal lymph nodes with evidence of caseation were considered to be direct evidence of tuberculosis acquired by the airborne route. One hundred thirty-five of 285 animals had a positive culture result for M. tuberculosis in at least 1 tissue, which comprised bronchohilar or paratracheal lymph nodes (52 [93%] of 56 specimens positive for M. tuberculosis); lung (44 [81%] of 54 specimens positive for M. tuberculosis); spleen (58 [61%] of 95 specimens positive for M. tuberculosis); or combined lung/lymph node (70 [99%] of 71 specimens positive for M. tuberculosis). Autopsy and culture results for unexposed and exposed animals revealed a good relation to PPD results (figure 4). A receiver operating characteristic [29] curve characterizing the relative sensitivity and specificity of different cutoffs for skin test positivity is shown in figure 5. The point closest to the top-left corner is usually selected as the cutoff (in this case, 8.5 mm). A cutoff of ≥7.5 mm was adopted, because responses of ≥7.5 mm were seen only in exposed animals, and this cutoff gave increased sensitivity with only a small decrease in specificity, compared with the cutoff of 8.5 mm.
Table 1.
Autopsy and tuberculosis culture results for guinea pigs that were killed after administration of a monthly tuberculin purified protein derivative (PPD) skin test or that died between skin tests (intercurrent deaths).
| Positive autopsy results |
Negative autopsy results |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PPD status | Pulmonary primary focus present |
Primary focus absent |
Total no. of guinea pigs |
Culture- positive |
Culture- negative |
No culturea |
Total no. of guinea pigs |
Total no. of guinea pigs |
|||
| Culture- positive |
Culture- negative |
No culturea |
Culture- positive |
Culture- negative |
|||||||
| Positive | 116 | 3 | 2 | 4 | 0 | 125 | 4 | 30 | 0 | 34 | 159 |
| Negative | 6 | 1 | 0 | 0 | 0 | 7 | 2 | 98 | 1 | 101 | 108 |
| Intercurrent deaths | 1 | 0 | 3 | 0 | 0 | 4 | 2 | 18 | 1 | 21 | 25 |
| Total | 123 | 4 | 5 | 4 | 0 | 136 | 8 | 146 | 2 | 156 | 292 |
NOTE. Data are no. of guinea pigs. A positive autopsy result was defined as the presence of a macroscopic hard, white pulmonary primary focus and/or enlarged, hard, caseating bronchohilar or paratracheal lymph nodes.
No cultures performed for operational reasons.
Figure 4.
Autopsy and culture results for negative control guinea pigs not exposed to ward air and guinea pigs exposed to ward air, according to tuberculin purified protein derivative (PPD) skin test reaction size. Autopsies were graded as normal (no shading); abnormal, with nonspecific changes (light gray shading); or abnormal, with changes pathognomonic for pulmonary tuberculosis (dark gray shading). The numbers of guinea pigs that were culture-positive for Mycobacterium tuberculosis are denoted by dots in the graph for each category of autopsy result. Two PPD-positive animals and 1 PPD-negative animal did not have cultures performed and were excluded from this figure.
Figure 5.
Receiver operating characteristic curve for 100 U of tuberculin purified protein derivative (PPD) skin test in guinea pigs, on the basis of sensitivity and specificity calculated from combined autopsy and culture data for 267 animals exposed to ward air. The area under the curve is 0.91, suggesting a test of excellent accuracy. As indicated by the adjacent numbers, the points on the graph correspond to PPD cutoff points for positive tests of ≥14, 13, 12, 11, 10, 9.5, 9, 8.5, 8, 7.5, 7, 6.5, 6, and 5.5 mm.
DISCUSSION
This study used a robust method of studying tuberculosis transmission to demonstrate that the infectiousness of air from a tuberculosis-HIV ward was highly variable. During the majority of the study, ward air was relatively safe, causing few infections in the guinea pigs, but at other times, the air was highly infectious. Patient infectiousness appeared highly variable among this heterogeneous group of patients with tuberculosis and HIV infection, as seen in patients with tuberculosis but without HIV infection in similar studies conducted during the 1950s.
Marked variability in the infectiousness of air from this tuberculosis-HIV ward was demonstrated. During the first 9 months, ward air was of relatively low infectiousness, with <7% of exposed guinea pigs developing positive skin test results each month. Furthermore, no PPD-positive animals were observed in months 13 and 16. In contrast, ward air was highly infectious prior to month 10, when 92 animals (53% of the colony) were PPD-positive. This variation is not accounted for by changes in bed occupancy, in patient bed-days for patients with positive sputum smear results, or by grade of smear result positivity (data not shown). Environmental factors, such as relative humidity (highest in winter, study months 13–14), are also unlikely to explain this variation. This variability of infectiousness concurs with the studies by Riley et al. [15, 16, 30], in which tuberculosis transmission occurred from a minority of patients, and also with more recent work studying aerosols generated by coughing patients with tuberculosis [31].
Estimated using the airborne infection model, the mean infectiousness of patients with pulmonary tuberculosis each month varied from production of 0–44 infectious quanta per hour (1 quantum being the “infectious dose” for tuberculosis [25]). The mean infectiousness during the whole study was 8.2 infectious quanta per hour, >6 times that calculated for the heterogeneous mix of patients without HIV infection studied by Riley et al. [25] during the 1950s and 1960s. This mean infectiousness, however, masks variability in infectiousness between patients [30]. In the studies during the 1950s and 1960s, a patient with laryngeal tuberculosis accounted for many infections in guinea pigs (estimated production, 60 infectious quanta per h [25]), and treatment was deferred in some patients to study the effect on infectiousness. There was no evidence of laryngeal tuberculosis in our study, and in contrast to the studies written or cowritten by Riley [15, 16, 30], all patients in our study were immunosuppressed due to HIV infection. DNA fingerprinting of patient and guinea pig M. tuberculosis strains may allow investigation of determinants of patient infectiousness, such as cough frequency. Although the Wells-Riley model has limitations, such as assuming steady-state conditions [32], it is useful for comparisons with published infectiousness values using the same model [25, 32], as well as for quantifying monthly variations in our study.
The potential for air in HIV-tuberculosis wards to be highly infectious underscores the need for effective implementation of administrative tuberculosis control measures, such as prompt identification, isolation, and treatment of patients with tuberculosis. Furthermore, the need for environmental control measures to reduce tuberculosis transmission is highlighted. Natural ventilation, which can provide high rates of air exchange for little or no cost and is applicable across a wide variety of health care settings, including waiting rooms and emergency departments, is an important environmental control strategy in low-resource settings [33, 34] in which burdens of tuberculosis and HIV infection are highest. However, with inherent limits in the protection afforded by dilutional ventilation [28], there is a need for new environmental control strategies and for further development of existing measures, such as upper-room UV light. Community transmission of tuberculosis is also important, and it is not possible to say whether the patients in our study were likely to be more or less infectious than community patients, owing to factors such as advancement of disease, cough hygiene practices, and treatment. The identification of highly infectious patients and of the duration of infectiousness would facilitate optimal use of scarce isolation facilities in lowresource settings and might perhaps allow targeted treatment of latent infection with M. tuberculosis.
To accurately determine infectiousness, we have optimized this in vivo air sampling model. A PPD test cutoff of ≥7.5 mm of induration was defined, which was well supported by the absence of responses >7 mm in 1047 tests of quarantined and negative control animals and by the receiver operating characteristic curve. The area under this curve was 0.91, reflecting a test of excellent accuracy [29]. By selecting 7.5 mm instead of 8.5 mm as the cutoff, greater sensitivity is gained with only a small loss in specificity. This helps minimize the chance of animals with tuberculosis remaining in the exposure chamber and risking horizontal tuberculosis transmission between animals. Of the 4 of 13 animals with responses of 7 mm that were found to have tuberculosis, 3 went on to develop skin test responses ≥7.5 mm while awaiting autopsy.
The significance of PPD-positive animals with negative autopsy and culture results is of interest. Of 159 exposed guinea pigs with PPD responses ≥7.5 mm, 30 (19%) had negative culture and autopsy results (figure 4). A study by Mills et al. [35], in which Riley was a researcher, similarly identified such a group of animals. One possible explanation is reduced strain virulence, and infection of guinea pigs by the intramuscular or airborne route with clinical M. tuberculosis strains has shown considerable differences in virulence [36-42]. For reduced M. tuberculosis strain virulence, mycobacterial numbers in spleen or primary lung lesions may decrease to <100 colony-forming units by 6 weeks [40], and a lung focus may potentially resolve over time. Because of the monthly PPD system and the ~21-day interval from infection to PPD conversion [27], an animal may have been infected for 3–7 weeks by the time the skin test was administered. If infection occurred with a reduced-virulence strain, by the time of autopsy, there may have been little macroscopic evidence of tuberculosis, and mycobacterial levels may have been less than detection limits. Variation in the airborne fitness or dose of inhaled bacilli may also contribute to the discordance between culture and skin test results. Experimental airborne infection of guinea pigs with clinical M. tuberculosis strains has shown variability in lesion-inducing efficiency, defined as the number of colony-forming units required to be inhaled to produce a macroscopic primary focus [40], which ranged from 1, for fully virulent, to 4, for poorly virulent strains. It is possible that a guinea pig infected with fewer droplet nuclei than the lesion-inducing efficiency number for that strain may develop a positive skin test result without autopsy or culture evidence of tuberculosis. Therefore, if only guinea pigs with macroscopic evidence of tuberculosis are included, assessments of patient infectiousness may be underestimations.
There are 2 additional potential confounders of the data. First, 5 animals had false-negative PPD results, of which 3 had tuberculosis at autopsy, including a grossly underweight animal that may have developed anergy due to extensive disease. The remaining 2 animals with apparently false-negative PPD results may have represented laboratory cross-contamination or, alternatively, early tubercular infection prior to PPD conversion. Second, horizontal tuberculosis transmission between animals inside the facility may confound these studies of patient infectiousness. Tuberculosis transmission may occur between guinea pigs by the respiratory and fecal-oral routes [20, 21]. However, drop-through cages were installed to prevent coprophagy. Importantly, the spatial distribution of tubercular infection in the animal facility was random, consistent with airborne infection from patients in the ward and not with horizontal spread.
In summary, this study has demonstrated marked variability in infectiousness of air from an HIV-tuberculosis ward with a heterogeneous mix of patients. Average patient infectiousness each month varied greatly, as did the estimated risk of nosocomial tuberculosis transmission to a health care worker on the ward. This highlights the importance of the continuing need for administrative and environmental control measures for reducing institutional tuberculosis transmission. This air sampling model is currently in use, evaluating upper-room ultraviolet light and negative air ionization to prevent airborne transmission of tuberculosis.
Acknowledgments
We thank the staff and patients of the Servicio de Enfermedades Infecciosas y Tropicales at Hospital Nacional Dos de Mayo (Lima, Peru) for their invaluable and continued support in this and ongoing studies. We thank Miguel Gil Saavedra for veterinary support and Patricia Fuentes, Pilar Navarro, Jorge Coronel, and other staff at the Laboratorio de Investigación y Desarrollo at Universidad Peruana Cayetano Heredia for processing of specimens. We thank SAEG Peru SA, for engineering support, and Antonio Quispe, for creating figure 1.
Financial support. Sir Halley Stewart Trust, the Sir Samuel Scott of Yews Trust, the Wellcome Trust (A.R.E., D.A.J.M., C.A.E., J.S.F., and R.H.G. are funded by the Wellcome Trust [United Kingdom], and A.R.E., D.A.J.M., and C.A.E. have Wellcome Trust Clinical Tropical Medicine Research Fellowships), USAID (HRN-5986-A-00-6006-00, GHS-A-00-03-00019-00 to R.H.G.), and Global Research Activity Cooperative Agreement from National Institutes of Health (T35AI-07646 to R.H.G.).
Footnotes
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Edlin BR, Tokars JI, Grieco MH, et al. An outbreak of multidrug-resistant tuberculosis among hospitalized patients with the acquired immunodeficiency syndrome. N Engl J Med. 1992;326:1514–21. doi: 10.1056/NEJM199206043262302. [DOI] [PubMed] [Google Scholar]
- 2.Ikeda RM, Birkhead GS, DiFerdinando GT, Jr, et al. Nosocomial tuberculosis: an outbreak of a strain resistant to seven drugs. Infect Control Hosp Epidemiol. 1995;16:152–9. doi: 10.1086/647077. [DOI] [PubMed] [Google Scholar]
- 3.Curtis AB, Ridzon R, Novick LF, et al. Analysis of Mycobacterium tuberculosis transmission patterns in a homeless shelter outbreak. Int J Tuberc Lung Dis. 2000;4:308–13. [PubMed] [Google Scholar]
- 4.Dwyer B, Jackson K, Raios K, Sievers A, Wilshire E, Ross B. DNA restriction fragment analysis to define an extended cluster of tuberculosis in homeless men and their associates. J Infect Dis. 1993;167:490–4. doi: 10.1093/infdis/167.2.490. [DOI] [PubMed] [Google Scholar]
- 5.Mohle-Boetani JC, Miguelino V, Dewsnup DH, et al. Tuberculosis outbreak in a housing unit for human immunodeficiency virus-infected patients in a correctional facility: transmission risk factors and effective outbreak control. Clin Infect Dis. 2002;34:668–76. doi: 10.1086/338815. [DOI] [PubMed] [Google Scholar]
- 6.Valway SE, Greifinger RB, Papania M, et al. Multidrug-resistant tuberculosis in the New York State prison system, 1990-1991. J Infect Dis. 1994;170:151–6. doi: 10.1093/infdis/170.1.151. [DOI] [PubMed] [Google Scholar]
- 7.Theuer CP, Hopewell PC, Elias D, Schecter GF, Rutherford GW, Chaisson RE. Human immunodeficiency virus infection in tuberculosis patients. J Infect Dis. 1990;162:8–12. doi: 10.1093/infdis/162.1.8. [DOI] [PubMed] [Google Scholar]
- 8.Markowitz N, Hansen NI, Hopewell PC, et al. Incidence of tuberculosis in the United States among HIV-infected persons. The Pulmonary Complications of HIV Infection Study Group. Ann Intern Med. 1997;126:123–32. doi: 10.7326/0003-4819-126-2-199701150-00005. [DOI] [PubMed] [Google Scholar]
- 9.Cruciani M, Malena M, Bosco O, Gatti G, Serpelloni G. The impact of human immunodeficiency virus type 1 on infectiousness of tuberculosis: a meta-analysis. Clin Infect Dis. 2001;33:1922–30. doi: 10.1086/324352. [DOI] [PubMed] [Google Scholar]
- 10.Nunn P, Mungai M, Nyamwaya J, et al. The effect of human immunodeficiency virus type-1 on the infectiousness of tuberculosis. Tuber Lung Dis. 1994;75:25–32. doi: 10.1016/0962-8479(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 11.Elliott AM, Hayes RJ, Halwiindi B, et al. The impact of HIV on infectiousness of pulmonary tuberculosis: a community study in Zambia. AIDS. 1993;7:981–7. doi: 10.1097/00002030-199307000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Nardell EA. Air sampling for tuberculosis—homage to the lowly guinea pig. Chest. 1999;116:1143–5. doi: 10.1378/chest.116.4.1143. [DOI] [PubMed] [Google Scholar]
- 13.Mastorides SM, Oehler RL, Greene JN, Sinnott JT, Kranik M, Sandin RL. The detection of airborne Mycobacterium tuberculosis using micropore membrane air sampling and polymerase chain reaction. Chest. 1999;115:19–25. doi: 10.1378/chest.115.1.19. [DOI] [PubMed] [Google Scholar]
- 14.Schafer MP, Fernback JE, Jensen PA. Sampling and analytical method development for qualitative assessment of airborne mycobacterial species of the Mycobacterium tuberculosis complex. Am Ind Hyg Assoc J. 1998;59:540–6. doi: 10.1080/15428119891010712. [DOI] [PubMed] [Google Scholar]
- 15.Riley RL, Mills CC, O'Grady F, Sultan LU, Wittestadt F, Shivipuri DN. Infectiousness of air from a tuberculosis ward—ultraviolet irradiation of infected air: comparative infectiousness of different patients. Am Rev Respir Dis. 1962;85:511–25. doi: 10.1164/arrd.1962.85.4.511. [DOI] [PubMed] [Google Scholar]
- 16.Riley RL, Mills CC, Nyka W, et al. Aerial dissemination of pulmonary tuberculosis: a two-year study of contagion in a tuberculosis ward: 1959. Am J Epidemiol. 1995;142:3–14. doi: 10.1093/oxfordjournals.aje.a117542. [DOI] [PubMed] [Google Scholar]
- 17.Jensen PA, Lambert LA, Iademarco MF, Ridzon R. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54:1–141. [PubMed] [Google Scholar]
- 18.Xu P, Peccia J, Fabian P, et al. Efficacy of ultraviolet germicidal irradiation of upper-room air in inactivating bacterial spores and Mycobacteria in full-scale studies. Atmos Environ. 2003;37:405–19. [Google Scholar]
- 19.Xu P, Kujundzic E, Peccia J, et al. Impact of environmental factors on efficacy of upper-room air ultraviolet germicidal irradiation for inactivating airborne mycobacteria. Environ Sci Technol. 2005;39:9656–64. doi: 10.1021/es0504892. [DOI] [PubMed] [Google Scholar]
- 20.Lurie M. The effect of eliminating exposure to enteric infection on the incidence and course of tuberculosis acquired by normal guinea pigs confined with tuberculous cage mates. J Exp Med. 1930;51:753–68. doi: 10.1084/jem.51.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lurie M. The route of infection in naturally acquired tuberculosis of the guinea pig. J Exp Med. 1930;51:769–76. doi: 10.1084/jem.51.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Supplemental assay 636 for the evaluation of batches of PPD using guinea pigs. National Veterinary Services Laboratory (previously Centre for Veterinary Biologics Laboratory); Ames, Iowa: 1998. [Google Scholar]
- 23.World Health Organization . Laboratory services in TB control. Parts I–III. World Health Organization; Geneva: 1998. [Google Scholar]
- 24.Caviedes L, Lee TS, Gilman RH, et al. Rapid, efficient detection and drug susceptibility testing of Mycobacterium tuberculosis in sputum by microscopic observation of broth cultures. The Tuberculosis Working Group in Peru. J Clin Microbiol. 2000;38:1203–8. doi: 10.1128/jcm.38.3.1203-1208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riley RL, Nardell EA. Clearing the air: the theory and application of ultraviolet air disinfection. Am Rev Respir Dis. 1989;139:1286–94. doi: 10.1164/ajrccm/139.5.1286. [DOI] [PubMed] [Google Scholar]
- 26.Escombe AR. The detection and prevention of airborne tuberculosis transmission. Imperial College London; London: 2006. [PhD thesis] [Google Scholar]
- 27.Fok JS, Ho RS, Arora PK, Harding GE, Smith DW. Host-parasite relationships in experimental airborne tuberculosis. V. Lack of hematogenous dissemination of Mycobacterium tuberculosis to the lungs in animals vaccinated with bacille Calmette-Guérin. J Infect Dis. 1976;133:137–44. doi: 10.1093/infdis/133.2.137. [DOI] [PubMed] [Google Scholar]
- 28.Nardell EA, Keegan J, Cheney SA, Etkind SC. Airborne infection: theoretical limits of protection achievable by building ventilation. Am Rev Respir Dis. 1991;144:302–6. doi: 10.1164/ajrccm/144.2.302. [DOI] [PubMed] [Google Scholar]
- 29.Luna-Herrera J, Martinez-Cabrera G, Parra-Maldonado R, et al. Use of receiver operating characteristic curves to assess the performance of a microdilution assay for determination of drug susceptibility of clinical isolates of Mycobacterium tuberculosis. Eur J Clin Microbiol Infect Dis. 2003;22:21–7. doi: 10.1007/s10096-002-0855-5. [DOI] [PubMed] [Google Scholar]
- 30.Sultan L, Nyka W, Mills C, O'Grady F, Wells W, Riley RL. Tuberculosis disseminators: a study of the variability of aerial infectivity of tuberculous patients. Am Rev Respir Dis. 1960;82:358–69. doi: 10.1164/arrd.1960.82.3.358. [DOI] [PubMed] [Google Scholar]
- 31.Fennelly KP, Martyny JW, Fulton KE, Orme IM, Cave DM, Heifets LB. Cough-generated aerosols of Mycobacterium tuberculosis: a new method to study infectiousness. Am J Respir Crit Care Med. 2004;169:604–9. doi: 10.1164/rccm.200308-1101OC. [DOI] [PubMed] [Google Scholar]
- 32.Beggs CB, Noakes CJ, Sleigh PA, Fletcher La, Siddiqi K. The transmission of tuberculosis in confined spaces: an analytical review of alternative epidemiological models. Int J Tuberc Lung Dis. 2003;7:1015–26. [PubMed] [Google Scholar]
- 33.World Health Organization . World Health Organization guidelines for the prevention of tuberculosis in healthcare facilities in resource-limited settings. World Health Organization; Geneva: 1999. [Google Scholar]
- 34.Escombe AR, Oeser CC, Gilman RH, et al. Natural ventilation for the prevention of airborne contagion. PLoS Med. 2007;4:e68. doi: 10.1371/journal.pmed.0040068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mills CC, O'Grady F, Riley RL. Tuberculin conversion in the “naturally infected” guinea pig. Bull Johns Hopkins Hosp. 1960;106:36–45. [PubMed] [Google Scholar]
- 36.Prabhakar R, Venkataraman P, Vallishayee RS, et al. Virulence for guinea pigs of tubercle bacilli isolated from the sputum of participants in the BCG trial, Chingleput District, South India. Tubercle. 1987;68:3–17. doi: 10.1016/0041-3879(87)90003-1. [DOI] [PubMed] [Google Scholar]
- 37.Mitchison DA, Wallace JG, Bhatia AL, Selkon JB, Subbaiah TV, Lancaster MC. A comparison of the virulence in guinea-pigs of South Indian and British tubercle bacilli. Tubercle. 1960;41:1–22. doi: 10.1016/s0041-3879(60)80019-0. [DOI] [PubMed] [Google Scholar]
- 38.Mitchison DA, Bhatia AL, Radhakrishna S, Selkon JB, Subbaiah TV, Wallace JG. The virulence in the guinea-pig of tubercle bacilli isolated before treatment from South Indian patients with pulmonary tuberculosis. I. Homogeneity of the investigation and a critique of the virulence test. Bull World Health Organ. 1961;25:285–312. [PMC free article] [PubMed] [Google Scholar]
- 39.Bhatia AL, Csillag A, Mitchison DA, Selkon JB, Somasundaram PR, Subbaiah TV. The virulence in the guinea-pig of tubercle bacilli isolated before treatment from South Indian patients with pulmonary tuberculosis. Comparison with virulence of tubercle bacilli from British patients. Bull World Health Organ. 1961;25:313–22. [PMC free article] [PubMed] [Google Scholar]
- 40.Balasubramanian V, Wiegeshaus EH, Smith DW. Growth characteristics of recent sputum isolates of Mycobacterium tuberculosis in guinea pigs infected by the respiratory route. Infect Immun. 1992;60:4762–7. doi: 10.1128/iai.60.11.4762-4767.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohn ML, Davis CL. Infectivity and pathogenicity of drug-resistant strains of tubercle bacilli studied by aerogenic infection of guinea pigs. Am Rev Respir Dis. 1970;102:97–100. doi: 10.1164/arrd.1970.102.1.97. [DOI] [PubMed] [Google Scholar]
- 42.Gangadharam PR, Cohn ML, Davis CL, Middlebrook G. Infectivity and pathogenicity of Indian and British strains of tubercle bacilli studied by aerogenic infection of guinea pigs. Am Rev Respir Dis. 1963;87:200–5. doi: 10.1164/arrd.1963.87.2.200. [DOI] [PubMed] [Google Scholar]