SUMMARY
SETTING
The tuberculin skin test (TST) is widely used as a diagnostic or screening test for Mycobacterium tuberculosis infection and disease. A peri-urban shanty-town in the desert hills of south Lima, Peru, highly endemic for tuberculosis, and where bacille Calmette-Guérin (BCG) vaccine had been given in multiple doses until 1995.
OBJECTIVE
To analyze the effect of multiple BCG vaccines on TST in a community-based setting.
DESIGN
Point-prevalence survey of TST reactions of 572 people aged 6–26 years from 255 households. TST reactions were compared to the observed number of BCG scars and other potential risk factors (age, living with a TST-positive person, and contact with active tuberculosis).
RESULT
People with two or more scars had significantly larger reactions, even after adjusting for potential risk factors. The adjusted population attributable fraction of being TST-positive and having two or more BCG scars was 26%.
CONCLUSION
There is no demonstrated benefit of repeat BCG vaccination. We therefore recommend that physicians take into consideration the number of BCG scars when interpreting the TST and that programs give no more than one BCG vaccination.
Keywords: Mycobacterium tuberculosis, tuberculin skin test, purified protein derivative, BCG vaccine, vaccination
Tuberculosis (TB) is of major importance in developing countries. Ninety-five per cent of tuberculosis cases and 98% of tuberculosis deaths are in developing countries, and 75% of these cases are in the economically productive age group.1 Poverty, poorer health care, and migration make TB control difficult in developing countries. In industrialized countries, a large percentage of tuberculosis cases are among recent immigrants. In the United States, for example, nearly 50% of TB cases are among immigrants.2
Although Peru has recently been removed from the World Health Organization's (WHO) list of 23 highburden countries, TB is still a major problem in this country.3 The reported incidence in 2000 was 134 per 100 000 population;4 however, the WHO estimates the annual incidence at 228/100 000,3 and epidemiological studies in a Lima shantytown have demonstrated even higher incidences (364/100 000).5
Recent studies have shown that bacille Calmette-Guérin (BCG) is effective in the prevention of tuberculous meningitis and disseminated TB, but its protective effect against pulmonary TB is controversial.6,7 The results of a recent randomized trial of multiple BCG vaccination show no benefit of two or more vaccinations.8 Since 1995 the WHO has recommended a single dose of the BCG vaccine at or soon after birth in all countries with a high incidence of TB infection.9 However, multiple BCG vaccines are still given in 39 intermediate- and high-incidence countries as a part of their standard TB control programs.10
Peru's national BCG vaccination program has undergone numerous modifications. The program was initiated in 1962 and originally included one intradermal dose for newborns (0.1 mg live bacillus in 0.1 ml, Pasteur Institute, Paris, France). The program was modified in the 1970s to include three vaccinations given at 5-year intervals up to age 15, as it was thought that the immunity provided by BCG vaccination wanes within 5 years. The third vaccination, given at age 15, was dropped from the program in 1979, and the second, given at age 10, was finally dropped in 1996. The program, however, was administered erratically.
The purpose of our study was to analyze the effect of multiple BCG vaccinations on tuberculin skin test (TST) reaction size in an endemic, community-based setting. Several studies have examined the effects of multiple BCG vaccines on TST reaction size.11-18 None of these studies included household surveys, and thus could not assess the effect of household contact with a person with TST-positive reaction (Table 1). Two studies in Canada and the Philippines, in which household contact with an active tuberculosis case was considered as an independent risk factor for adjustment, failed to demonstrate an independent effect of multiple vaccinations.12,15 The absence of significant effect in these studies, however, may be due to their limited sample size.
Table 1.
Studies of multiple BCG vaccination on tuberculin skin test
| Author | Year | Country | Population | Age, years | Participants, n |
|---|---|---|---|---|---|
| Sepulveda et al.11 | 1989 | Chile | Medical students | 19 (mean) | 208 |
| Young et al.12 | 1992 | Canada | Canadian Indian children | 1–15 | 701 |
| Menzies et al.13 | 1992 | Canada | Schoolchildren, adults | grade 6 & 10, 18–25 years | 4 629 |
| Ildirim et al.14 | 1995 | Turkey | Schoolchildren | 6–12 | 3 548 |
| Lao et al.15 | 1999 | Philippines | Schoolchildren | 5 (mean) | 284 |
| Chee et al.16 | 2001 | Singapore | Schoolchildren | 12,16 | 266 005 |
| Kuyucu et al.17 | 2001 | Turkey | Schoolchildren | 7–14 | 2 810 |
| Bierrenbach et al.18 | 2003 | Brazil | Schoolchildren | 14 | 1 148 |
BCG = bacille Calmette-Guérin.
STUDY POPULATION AND METHODS
Las Pampas de San Juan de Miraflores is a shantytown comprised of fifty communities and a total population of 40 000. Located in the desert hills of south Lima, these communities consist mainly of migrants of low socio-economic status from the Andean highlands. Living conditions tend to be crowded, sanitation systems inadequate, and access to potable water limited. Shantytowns account for 30% of the 8 million inhabitants of Lima. Epidemiological data on TB in this region have been reported previously.19,20 The same BCG strain was used throughout the period. There has never been any community- or school-based revaccination program based on TST in Peru.
A sample size of 397 households in Las Pampas was randomly selected from 1649 households using a census performed by the Asociación Benéfica Proyectos en Informática, Salud, Medicina y Agricultura (AB PRISMA) in 2000. A household was defined as a group of people living together and sharing common living spaces (kitchen, bedroom and living room). Within each household, all individuals except those with a history of TB, or those who had received a TST in the past 6 months, were invited to participate in the study. There is no screening or active case finding of tuberculosis using TST in these communities. Written informed consent was obtained from participants, or, in the case of those aged under 18, by a parent or guardian.
Subjects were surveyed with structured questionnaires regarding exposure to tuberculosis and overall health. The Mantoux skin test was given intradermally with a 1 ml syringe and 25-gauge needle, using 0.1 mm of five tuberculin units (Tubersol, Connaught Laboratories, Inc, Ontario, Canada) on the volar surface of one forearm. All skin tests were performed and read by a trained research nurse after 48–72 h and supervised by one of the authors (MS). The diameter of indurations along and transverse to the longitudinal axis of the arm were measured by the ballpoint pen method21 and recorded in mm. BCG scars were counted on both arms. Participants with mean induration sizes ≥ 10 mm in both directions were considered to be TST-positive following recommendations from both the American Thoracic Society for persons born in countries with high prevalence of tuberculosis and groups with poor access to health care22 and the Peruvian Ministry of Health.23 Patients with a positive TST and symptoms associated with tuberculosis underwent serial sputum examinations and a chest X-ray. Children aged <15 years in recent contact with someone with active tuberculosis were referred to the national tuberculosis control program to rule out active TB or to start on preventive therapy for latent TB infection. The study was approved by the ethical review boards of AB PRISMA and the Johns Hopkins University Bloomberg School of Public Health.
Exclusion
Household members aged ≥ 27 years were excluded from the analysis because of possible confusion between smallpox and BCG vaccination scars. Smallpox vaccination terminated in Peru in 1974. Children aged <6 years were also excluded because of the low probability of having multiple BCG vaccines. However, they and subjects aged > 26 years were included for TST data for the variable ‘presence of another person with TST-positive reaction in the same household’.
Statistical analysis
The χ2 test, Fisher's exact tests, and Kruskal-Wallis H test (a non-parametric test),24 were used to compare the distribution of the variables in the BCG categorical groups. The Cuzick non-parametric test25 was used to evaluate trends across ordered groups. Body mass index (BMI) was also compared in each BCG group as it is related to anergy and vulnerability of developing active tuberculosis.15,26
Because TST reactions were not normally distributed, we used multivariate ordinal logistic regression with the partial proportional odds model to calculate the adjusted odds ratio (OR) of having an increased TST reaction size among different categories of BCG scars, with the size of the TST reaction coded as 1 (0 mm), 2 (1–4 mm), 3 (5–9 mm), 4 (10–14 mm), and 5 (≥ 15 mm). The adjusted variables included age, history of living with a person with active TB, and presence of another TST-positive person in the same household. Logistic regression analysis was applied to calculate the adjusted OR of being TST-positive using cut-off points of 10 mm and 15 mm and adjusting for potential confounders.
To estimate the attributable risk of a positive TST from having two or more BCG scars in the study population we used the method described by Zhang and Yu.27 BCG scar count was considered as both a categorical variable (no scar, one scar, two scars and three or more scars) and a binominal variable (having two or more scars or less than two scars).
All statistical tests were interpreted in a two-tailed fashion to estimate P values and 95% confidence intervals (CI). Binary logistic regression analysis was performed using Stata statistical software package (version 7.0, Stata Corporation, College Station, TX, USA). The multivariate ordinal logistic regression with the partial proportional odds model was performed using SAS-NT software (version 8.1, SAS Institute, Cary, NC, USA).
RESULTS
Three hundred and ninety-seven households were visited over 6 months. Of these, 307 (77%) agreed to participate in the study, and 700 individuals aged 6 to 26 years from 280 households were identified. Among the 700 individuals, both TST results and information on the observed number of BCG scars on both arms were available from 588 (84%) individuals. Sixteen of these 588 individuals had a history of tuberculosis disease and were excluded from the study. None reported receiving a TST in the previous 6 months. The final study population was comprised of 572 individuals from 255 households. These 572 individuals were divided into four groups based on the number of BCG scars observed. Sixty-eight (12%) had no BCG scars, 310 (54%) had one scar, 164 (29%) had two scars and 30 (5%) had three or more scars. As shown in Table 2, those with one scar accounted for the largest percentage among groups and had the youngest mean age (P < 0.001, χ2 test). The group without BCG scars had the lowest proportion of people who had completed secondary school (P = 0.003, χ2 test). The male to female ratio, mean BMI (≥ 15 years), mean household income, presence of known TB contact and presence of another TST-positive person in the household were all similar across groups (Table 2).
Table 2.
Characteristics of participants with tuberculin skin test by numbers of BCG scars in Las Pampas, Lima
| Number of BCG scars |
|||||
|---|---|---|---|---|---|
| Feature | No scar | One scar | Two scars | ≥3 scars | P value |
| Participants, n | 68 | 310 | 164 | 30 | |
| Age group, years | |||||
| 6–10 | 25 | 165 | 25 | ||
| 11–15 | 7 | 69 | 81 | 18 | |
| 16–20 | 12 | 35 | 35 | 7 | |
| 21–26 | 24 | 41 | 23 | 5 | |
| Mean age, years | 15.5 | 12.1 | 14.8 | 15.4 | <0.001* |
| Female/male ratio | 1.3 | 1.0 | 1.0 | 0.9 | 0.625† |
| Mean body mass index (≥15 years) | 23.5 | 23.1 | 22.9 | 23.3 | 0.299* |
| Mean household income, $ | 187 | 177 | 181 | 168 | 0.266* |
| Secondary school completed (≥18 years), % | 41.4 | 70.5 | 78.6 | 90 | 0.003† |
| Having a history of living with a patient with active tuberculosis within last 5 years, % | 14.7 | 9.4 | 11.7 | 16.7 | 0.419† |
| Presence of another TST-positive person in the household, % | 49.3 | 60.9 | 63.8 | 66.7 | 0.188† |
| Tuberculin skin test reaction size, n (%) | |||||
| 0 mm | 34 (50.0) | 124 (40.0) | 42 (25.6) | 5 (16.7) | <0.001† |
| 1–4 mm | 23 (33.8) | 128 (41.3) | 57 (34.8) | 9 (30.0) | 0.324† |
| 5–9 mm | 4 (5.9) | 22 (7.1) | 20 (12.2) | 4 (13.3) | 0.172 † |
| 10–14 mm | 1 (1.5) | 13 (4.2) | 16 (9.7) | 7 (23.3) | <0.001† |
| ≥15 mm | 6 (8.8) | 23 (7.4) | 29 (17.7) | 5 (16.7) | 0.005† |
Kruskal-Wallis H test.
χ2 test.
BCG = bacille Calmette-Guérin; TST = tuberculin skin test.
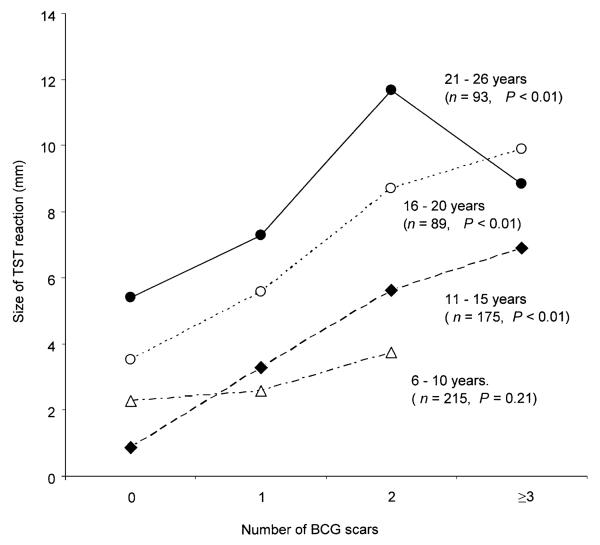
Figure 1 shows the mean TST reaction size by age group and number of BCG scars. There was a stepwise increase in TST reaction size with increasing number of BCG scars which, when stratified by age groups, remained significant for each group (P <0.01, Cuzick non-parametric trend test), except for those aged 6–10 years (P = 0.21). The effect of two or more BCG scars on the TST reaction size was still significant after adjusting for age, history of living with a patient with active TB, and the presence of another TST-positive person in the household, using an ordinal logistic model (Table 3). Level of education was not found to be associated with a positive TST by logistic regression.
Figure 1.
Mean tuberculin skin test (TST) reaction size by age group and number of BCG scars in Las Pampas, Lima. P values were calculated using the Cuzick non-parametric trend test. BCG = bacille Calmette-Guérin.
Table 3.
Adjusted odds ratio (AOR) for having a larger reaction size and a positive tuberculin skin test (TST)
| Feature | Ordinal logistic regression* AOR‡ (95%CI) |
Binary logistic regression† AOR‡ (95%CI) |
|---|---|---|
| BCG vaccination | ||
| No BCG | Ref | Ref |
| One BCG scar | 1.9 (1.1–2.4)§ | 2.2 (0.8–5.7) |
| Two BCG scars | 3.5 (1.9–4.3)¶ | 4.8 (1.9–12.5)# |
| ≥3 BCG scars | 4.9 (2.1–6.2)¶ | 7.8 (2.4–25.4)# |
| Age | ||
| Six years of age | Ref | Ref |
| Effect of each additional year of age | 1.1 (1.06–1.13)¶ | 1.2 (1.1–1.3)¶ |
| Education (≥18 years)** | ||
| <10 years (incomplete secondary school or less) | Ref | Ref |
| ≥10 years (secondary school or more) | 0.8 (0.4–1.6) | 0.5 (0.2–1.2) |
| History of living with a patient with active tuberculosis | ||
| No | Ref | Ref |
| Yes | 2.0 (1.2–3.3)# | 3.8 (2.0–7.6)¶ |
| Presence of another TST-positive person in the household | ||
| No | Ref | Ref |
| Yes | 1.4 (1.0–2.0)§ | 2.7 (1.6–4.8)# |
TST reaction size was categorized as 0, 1–4, 5–9 10–14 and 15 mm.
TST reactivity was defined as positive (≥10 mm) and negative (<10).
AORs are adjusted by all other variables except ‘education’ for all participants.
P < 0.05.
P < 0.001.
P < 0.01.
Variable ‘education’ was adjusted for all other variables for participants aged more than 18 years.
Ref = reference group.
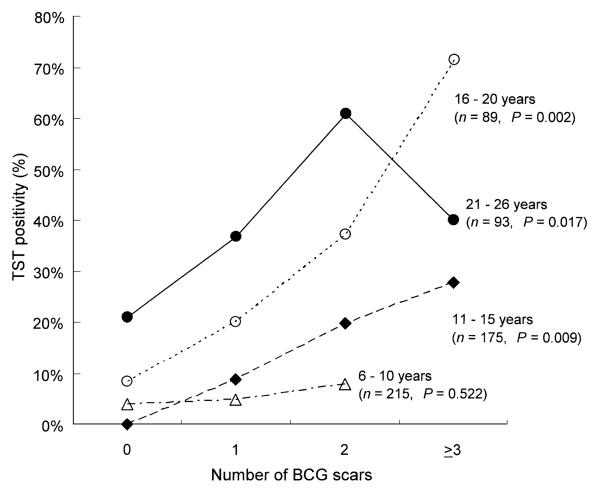
Figure 2 shows the percentage of TST-positive reactors (reaction size ≥10 mm vs. negative <10 mm) by number of BCG scars. The percentage of TST positives significantly increased from the group with no scar to those with three or more scars in each group (P <0.017), except for those aged 6–10 years (P = 0.522). As shown in Table 3, after adjusting for age group, history of living with a patient with active TB, and the presence of another TST-positive person in the household, TST positivity was still strongly associated with having two or three or more scars (OR 5.4, 95%CI 2.0–14.2 and OR 8.3, 95%CI 2.5–27.8, respectively). It was not significantly associated with having one scar. The effect of multiple BCGs on TST positivity remained after changing the cut-off point of a positive TST from 10 to 15 mm. After adjusting for the above variables, the population attributable fraction of TST positivity from having two or more BCG scars was 26%.
Figure 2.
Tuberculin skin test (TST) positivity by age group and number of BCG scars in Las Pampas, Lima. TST reactivity was defined as positive (≥ 10 mm) and negative (< 10 mm). P values were tested for linear trend of the log odds. BCG = bacille Calmette-Guérin.
DISCUSSION
TST has been a useful diagnostic test to identify persons infected with TB. However, with the rise in reported numbers of TB cases and the continued use of multiple BCG vaccines in many countries, the need for better guidelines for interpretation of the TST has become more apparent.
In this study, we found that with an increasing number of BCG scars, the prevalence of positive TST also increased among children of school age and young adults. Increasing TST positivity is associated with increased age at BCG vaccination and decreasing time since last BCG vaccination.28 Age is therefore an important confounder. In our study, even after adjusting for age, the effect of multiple BCG vaccinations remained, even in the oldest age group (age 21–26 years) who had received their last vaccination 10–15 years before. The findings of this study are consistent with other studies of more selected populations.13-15
The TST-positive prevalence was higher in older age groups regardless of the number of BCG scars, probably due to the increased frequency of latent TB infection with increased age.
Many factors affect a person's response to the TST, such as strain and dose of BCG vaccination, age at vaccination, number of years since last vaccination, and frequency of TST.28-30 Therefore, all studies examining the relationship of BCG and TST are constrained by the above variables and are most valid when used locally.31 Nevertheless, the strong association between the number of BCG scars and TST positivity appears to be true world-wide.
In Peru, in this population, scarring after BCG is a highly reliable indicator. Our group has demonstrated that 99% of children given a BCG at or soon after birth will have a scar.32 In countries with more severe malnutrition and low birth weights, others have noted a decrease in scarring after BCG vaccination.33
Based on our data, we recommend that physicians in developing countries and those who care for immigrants from these countries must take into account the number of BCG scars, and not simply a history of BCG vaccination when interpreting TST readings. BCG given at birth does not change the proportion of TST-positive results when the cut-off point is 10 mm, despite a significant increase in size of TST reaction.34 Thus a single BCG given at birth is less likely to distort the interpretation of the TST. There appears to be no benefit in giving additional doses of BCG for tuberculosis prevention.6,7 We therefore recommend that physicians take into consideration the number of BCG scars when interpreting the TST and that programs give no more than one BCG vaccination.
Acknowledgements
We would like to thank Drs Lawrence Moulton, Caryn Bern, and Margaret Kosec for their advice on this paper and comments on the manuscript, Ms Eleana Sanchez and Ms Lilia Cabrera for data collection, Mr Marco Varela for data management and Ms JB Phu and Ms D Sara for technical assistance. We also thank the communities of Las Pampas de San Juan de Miraflores for their cooperation.
This study was supported by the USAID–Tuberculosis Award HRN-5986-A-00-6006-00, the NIH ITREID grant 5D43-TW00910, the Fogarty-NIH AIDS training program 3T22-TW00016-05S3, the NIAID tutorial training grant 5T35-AI07646-02, and the anonymous RG-ER fund. M Saito is funded by St Luke's Life Science Institute. M Z Levy is a Howard Hughes Pre-Doctoral Fellow. C A Evans is funded by a Wellcome Trust Fellowship.
References
- 1.World Health Organization . Guidelines for the management of drug-resistant tuberculosis. WHO; Geneva, Switzerland: 1997. [Google Scholar]
- 2.Centers for Disease Control and Prevention Tuberculosis morbidity among U.S.-born and foreign-born populations—United States, 2000. MMWR. 51:101–104. 101. [PubMed] [Google Scholar]
- 3.World Health Organization . Global tuberculosis control. WHO report 2001. WHO; Geneva, Switzerland: 2001. [Google Scholar]
- 4.Ministerio de Salud . Tuberculosis en el Perú: Informe 2000. Dirección General de Salud de las Personas; Lima, Perú: 2001. [Google Scholar]
- 5.Sanghavi DM, Gilman RH, Lescano-Guevara AG, Checkley W, Cabrera LZ, Cardenas V. Hyperendemic pulmonary tuberculosis in a Peruvian shantytown. Am J Epidemiol. 1998;148:384–389. doi: 10.1093/oxfordjournals.aje.a009657. [DOI] [PubMed] [Google Scholar]
- 6.Colditz GA, Brewer TF, Berkey CS, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]
- 7.Leung CC, Tam CM, Chan SL, Chan-Yeung M, Chan CK, Chang KC. Efficacy of the BCG revaccination programme in a cohort given BCG vaccination at birth in Hong Kong. Int J Tuberc Lung Dis. 2001;5:717–723. [PubMed] [Google Scholar]
- 8.Karonga Prevention Trial Group Randomized controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Lancet. 1996;348:17–24. [PubMed] [Google Scholar]
- 9.World Health Organization Global Tuberculosis Programme and Global Programme on Vaccines. Statement on BCG revaccination for the prevention of tuberculosis. Wkly Epidemiol Rec. 1995;70:229–231. [PubMed] [Google Scholar]
- 10.Fine P, Carneiro I, Milstein J, Clements C. Issues relating to the use of BCG in immunization programmes: a discussion document. WHO; Geneva, Switzerland: 1999. [Google Scholar]
- 11.Sepulveda RL, Ferrer X, Latrach C, Sorensen RU. The influence of Calmette-Guérin bacillus immunization on the booster effect of tuberculin testing in healthy young adults. Am Rev Respir Dis. 1990;142:24–28. doi: 10.1164/ajrccm/142.1.24. [DOI] [PubMed] [Google Scholar]
- 12.Young TK, Mirdad S. Determinants of tuberculin sensitivity in a child population covered by mass BCG vaccination. Tubercle Lung Dis. 1992;73:94–100. doi: 10.1016/0962-8479(92)90062-O. [DOI] [PubMed] [Google Scholar]
- 13.Menzies R, Vissandjee B. Effect of bacille Calmette-Guérin vaccination on tuberculin reactivity. Am Rev Respir Dis. 1992;145:621–625. doi: 10.1164/ajrccm/145.3.621. [DOI] [PubMed] [Google Scholar]
- 14.Ildirim I, Hacimustafaoglu M, Ediz B. Correlation of tuberculin induration with the number of Bacillus Calmette-Guérin vaccines. Pediatr Infect Dis J. 1995;14:1060–1063. doi: 10.1097/00006454-199512000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Lao LY, De Guia T. Tuberculin skin testing: determinants and reaction. Respirology. 1999;4:311–317. doi: 10.1046/j.1440-1843.1999.00198.x. [DOI] [PubMed] [Google Scholar]
- 16.Chee CB, Soh CH, Boudville IC, Chor SS, Wang YT. Interpretation of the tuberculin skin test in Mycobacterium bovis BCG-vaccinated Singaporean schoolchildren. Am J Respir Crit Care Med. 2001;164:958–961. doi: 10.1164/ajrccm.164.6.2101093. [DOI] [PubMed] [Google Scholar]
- 17.Kuyucu N, Kuyucu S, Bakirtas A, Karacan C. BCG revaccination and tuberculin reactivity. Indian J Pediatr. 2001;68:21–25. doi: 10.1007/BF02728851. [DOI] [PubMed] [Google Scholar]
- 18.Bierrenbach AL, Cunha SS, Barreto ML, et al. Tuberculin reactivity in a population of schoolchildren with high BCG vaccination coverage. Rev Panam Salud Publica. 2003;13:285–293. doi: 10.1590/s1020-49892003000400003. [DOI] [PubMed] [Google Scholar]
- 19.Madico G, Gilman RH, Checkley W, et al. Community infection ratio as an indicator for tuberculosis control. Lancet. 1995;345:416–419. doi: 10.1016/s0140-6736(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 20.Getchell WS, Davis CE, Gilman J, Urueta G, Ruiz-Huidubro E, Gilman RH. Basic epidemiology of tuberculosis in Peru: a prevalence study of tuberculin sensitivity in a Pueblo joven. Am J Trop Med Hyg. 1992;47:721–729. doi: 10.4269/ajtmh.1992.47.721. [DOI] [PubMed] [Google Scholar]
- 21.Jordan TJ, Sunderam G, Thomas L, Reichman LB. Tuberculin reaction size measurement by the pen method compared to traditional palpation. Chest. 1987;92:234–236. doi: 10.1378/chest.92.2.234. [DOI] [PubMed] [Google Scholar]
- 22.The American Thoracic Society and the Centers for Disease Control and Prevention Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med. 2000;161:1376–1395. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 23.Dirección del Programa Nacional de Control de Enfermedades Transmisibles Control de la Tuberculosis . Actualización en la técnica de aplicación, lectura e interpretación de la prueba de tuberculina. Directiva No 003-2000-PCT. Ministry of Health; Lima, Peru: 2000. [Google Scholar]
- 24.Kruskal WH, Wallis WA. Use of ranks in one-criterion variance analysis. J Am Statist Assoc. 1952;47:583–621. [Google Scholar]
- 25.Stata Corp . Stata Reference Manuel. 1 A–G. Stata Press; College Station, TX: 1999. Release 6. [Google Scholar]
- 26.Hoge CW, Fisher L, Donnell HD, Jr, et al. Risk factors for transmission of Mycobacterium tuberculosis in a primary school outbreak: lack of racial difference in susceptibility to infection. Am J Epidemiol. 1994 Mar 1;139:520–530. doi: 10.1093/oxfordjournals.aje.a117035. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 28.Menzies D. What does tuberculin reactivity after bacillus Calmette-Guérin vaccination tell us? Clin Infect Dis. 2000;31(Suppl 3):S71–S74. doi: 10.1086/314075. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control Use of BCG vaccines in the control of tuberculosis: A joint statement by the ACIP and the Advisory Committee for Elimination of Tuberculosis. MMWR. 1988;37:663–664. 669–675. [PubMed] [Google Scholar]
- 30.Sepulveda R, Burr C, Ferrer X, Sorensen RU. Booster effect of tuberculin testing in healthy 6-year-old school children vaccinated with Bacillus Calmette-Guérin at birth in Santiago, Chile. Pediatr Infect Dis J. 1998;7:578–581. [PubMed] [Google Scholar]
- 31.Wang L, Turner MO, Elwood RK, Schulzer M, FitzGerald JM. A meta-analysis of the effect of Bacille Calmette Guérin vaccination on tuberculin skin test measurements. Thorax. 2002;57:804–809. doi: 10.1136/thorax.57.9.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santiago EM, Lawson E, Gillenwater K, et al. A prospective study of bacillus Calmette-Guérin scar formation and tuberculin skin test reactivity in infants in Lima, Peru. Pediatrics. 2003;112:298–302. doi: 10.1542/peds.112.4.e298. [DOI] [PubMed] [Google Scholar]
- 33.Rani SH, Vijayalakshni V, Sunil K, Lakshmi KA, Suman LG, Murthy KJ. Cell mediated immunity in children with scar-failure following BCG vaccination. Indian Pediatr. 1998;35:569–571. [PubMed] [Google Scholar]
- 34.Karalliedde S, Katugaha LP, Uragoda CG. Tuberculin response of Sri Lankan children after BCG vaccination at birth. Tubercle. 1987;68:33–38. doi: 10.1016/0041-3879(87)90005-5. [DOI] [PubMed] [Google Scholar]