Abstract
The effects of HIV co-infection and multi-drug resistant tuberculosis (MDRTB) on tuberculosis prognosis are poorly defined. Therefore, we studied infectiousness and mortality of 287 tuberculosis patients treated with standard, directly observed, short-course therapy in the Peruvian community. During 6–17 months of treatment, 49 (18%) of patients died, of whom 48 (98%) had AIDS and 28 (57%) had MDRTB; 17/31 (55%) of MDRTB-patients with AIDS died within 2 months of diagnosis, before traditional susceptibility testing would have identified their MDRTB. Most non-MDRTB became smear- and culture-negative within 6 weeks of therapy, whereas most MDRTB remained sputum-culture-positive until death or treatment completion. HIV-negative patients with non-MDRTB had good outcomes. However, MDRTB was associated with prolonged infectiousness and HIV co-infection with early mortality, indicating a need for greater access to anti-retroviral therapy. Furthermore, early and rapid tuberculosis drug-susceptibility testing and infection control are required so that MDRTB can be appropriately treated early enough to reduce mortality and transmission.
INTRODUCTION
Tuberculosis kills 1.7 million people each year.1 Modern treatment regimens are generally associated with a good prognosis,2 but HIV co-infection3 and multi-drug resistant tuberculosis (MDRTB)4 have poorly defined effects upon treatment outcome5 and infectiousness.6 Peru has a model tuberculosis control program2 and an HIV prevalence < 0.5% but is one of the 30 highest tuberculosis-burden countries, with a tuberculosis incidence of 188/100,000 in the year 2003.2 MDRTB rates have recently exceeded 3% of all new cases and 9% of infectious sputum microscopy-positive patients, making Peru one of the eight highest MDRTB-burdened countries.4,7,8 As in most resource-poor settings, tuberculosis drug-susceptibility testing is restricted to high-risk groups, and facilities for respiratory isolation are sparse.7 In this setting, a prospective cohort study was performed to study the effects of HIV and MDRTB on tuberculosis infectiousness and mortality during tuberculosis therapy.
PATIENTS
All adults who were diagnosed consecutively by the Peruvian National Tuberculosis Control Program as having laboratory-proven pulmonary tuberculosis were invited to join the study over a 17-month period at two diagnostic centers. Informed written consent was given by 287 patients (Table 1): 223 (78%) patients were diagnosed at the Maria Auxiliadora general hospital outpatient clinic, where TB was diagnosed principally by sputum microscopy; and 64 (22%) patients were diagnosed concurrently at the infectious diseases unit at Dos de Mayo Hospital, the principal referral center for people with HIV infection in Lima, which serves an area including that of Maria Auxiliadora Hospital. National policy recommends a 12-month course of isoniazid preventive therapy for all HIV-seropositive people, and we considered this to have been taken if ≥ 3 months had been completed. Extrapulmonary tuberculosis was not studied. This project had national and international ethical approval, including A.B. Prisma, Peru, and Johns Hopkins Bloomberg School of Public Health, Baltimore, MD.
Table 1.
Clinical characteristics of the study population at diagnosis according to clinic
| Feature | All participants (n = 287) |
General clinic (n = 223) |
ID unit (n = 64) |
P value comparing hospitals |
|---|---|---|---|---|
| HIV seropositivity | 72 (25) | 8 (5) | 64 (100) | < 0.001 |
| MDRTB at time of diagnosis | 51 (18) | 21 (9) | 30 (47) | < 0.001 |
| Outpatient at time of diagnosis | 257 (90) | 223 (100) | 34 (53) | < 0.001 |
| Sputum smear positivity | 274 (96) | 223 (100) | 51 (80) | < 0.001 |
| Previous TB treatment | 60 (21) | 37 (17) | 23 (36) | 0.001 |
| Known TB exposure at home/work | 103 (36) | 83 (37) | 20 (31) | ns |
| Previous TB preventive therapy | 18 (6) | 3 (1) | 15 (23) | < 0.001 |
| Hospitalization in last 2 years | 45 (16) | 23 (10) | 22 (34) | < 0.001 |
| Male sex | 182 (63) | 130 (58) | 52 (81) | 0.001 |
| BCG scar(s) | 257 (90) | 199 (89) | 58 (91) | ns |
| Age in years: mean (SD) | 29 (11) | 28 (11) | 31 (8) | ns |
Note: The number (percentage) of patients with relevant clinical characteristics are shown for the whole study population and divided according to the hospital at which tuberculosis was diagnosed. Tuberculosis was treated within the same community health posts.
Abbreviations: HIV, human immunodeficiency virus; MDRTB, multi-drug resistant tuberculosis; TB, tuberculosis; BCG, Bacillus Calmette-Guerin; SD, standard deviation; ID, infectious diseases; ns, no significant difference.
METHODS
Investigations
For initial tuberculosis diagnosis, the Ministry of Health clinical laboratory performed Ziehl-Neelsen sputum microscopy and, for patients known to be HIV-positive, sputum culture on modified Ogawa medium with drug-susceptibility testing by the proportions method.
For this study, two additional sputum samples were subjected to auramine microscopy9,10 and culture with both the 7H9 broth microscopic observation drug-susceptibility (MODS) assay11,12 and Lowenstein-Jensen slopes10 in the university research laboratory, and these results, that confirmed tuberculosis in all cases, were used for our analysis. MDRTB was defined as resistance to at least isoniazid (0.4 mg/mL) and rifampicin (1.0 mg/mL) in MODS and the microplate colorimetric assay.13 Chest radiographs were not studied.
At the general hospital, ELISA HIV antibody testing was done after counseling for 176 (79%) patients and was positive in 8 (5%) cases. The remaining 47 (21%) patients at the general hospital stated that they had no behavioral risk factors for HIV infection and did not have HIV tests. Because HIV is relatively uncommon in Peru, infecting 0.5% of the population and 1.7% of tuberculosis patients,7 these 47 patients were considered HIV-seronegative for all analyses. All 64 patients at the infectious diseases unit were HIV-seropositive by ELISA with confirmatory Western blot assay, but HIV clinical staging data, CD4 counts, and viral load measurements were not available for this study.
Treatment
All patients received the standard Peruvian treatment regimen with direct observation of tablet consumption at local health posts.2 Treatment was therefore unaffected by recruitment center. This study was not involved in patient care or treatment, but all laboratory results were rapidly communicated to patients and their clinicians, including the results of first-line drug susceptibilities. In line with Peruvian national policy, all patients received a complete trial of first-line tuberculosis therapy. Most patients were treated with 2 months of isoniazid (H), rifampicin (R), pyrazinamide (Z), and ethambutol (E) for 6 days/week, followed by 4 months of H and R for 2 days/week (2HRZE6/4HR2). Patients known to be co-infected with HIV and tuberculosis received 2HRZE6/7HR2. If tuberculosis had been treated previously, then streptomycin (S) was added: 1SHRZE6/2HRZE6/5HRE2. Only 12 patients received second-line drugs for a short term, due to cost and availability and these were administered in addition to the standard treatment regimen: ciprofloxacin alone in 5 cases, with ethionamide ± kanamycin and amoxicillin-clavulanate in 4 cases, and other regimens in 3 cases.
Follow-up
Recruitment took place from February 1999 to July 2000, and follow-up continued for each patient until completion of therapy (April 2001). After diagnosis and recruitment to the study, medical care and treatment were identical for all patients in the same community DOTS program, irrespective of the place of recruitment. Tuberculosis treatment adherence and survival at least to treatment completion were determined for 224 (78%) participants, and 217/224 (97%) completed tuberculosis treatment. Survival analysis was based upon the interval from tuberculosis treatment initiation until the first occurring date of: treatment completion, death; default from therapy; loss to follow-up; or end of study.
Infectiousness during follow-up was analyzed for both tuberculosis microscopy and culture as the time from initiation of therapy until the mid-point between the last positive result and the first of 2 consecutive negative sputum specimens, or the last positive result if there was no conversion.14 All participants provided diagnostic sputum samples, and we collected additional samples 2 weeks later from 108 (38%); after 1 month of treatment from 259 (90%); after 2 months treatment from 249 (86%); after 4 months treatment from 229 (78%); and later in treatment from 166 (54%).
Statistical analysis
Person-years of observation were calculated by recording the total number of surveillance days for each participant. For individuals lost to follow-up, person-time denominator data were censored at the time of the last visit. Continuous variables were compared using the Student’s t test or the Mann-Whitney U test. Categorical variables were compared using the χ2 test or Fisher’s exact test. Kaplan-Meier survival analysis was performed to evaluate time to conversion and time to death, and Cox proportional hazards regression analysis was performed to evaluate risk factors associated with conversion and mortality. The magnitude of associations of potential risk factors was expressed as hazard ratios (HR). Risk factors with P < 0.15 in univariate analyses were included in multiple regression analysis. All P values were two-sided. All statistical analyses were carried out using SPSS, version 13.0 (SPSS Corp., Chicago, IL).
RESULTS
Survival
The survival curves shown in Figure 1 demonstrate the effects of HIV and MDRTB on mortality: 49 (17%) of patients who were followed-up, died; 48 (67%) of 72 HIV-infected patients died compared with 1 (0.5%) of 215 HIV-negative patients (P < 0.0001), who died of hepatitis. MDRTB was diagnosed in 18% of all patients and in 71% of the patients who died.
Figure 1.
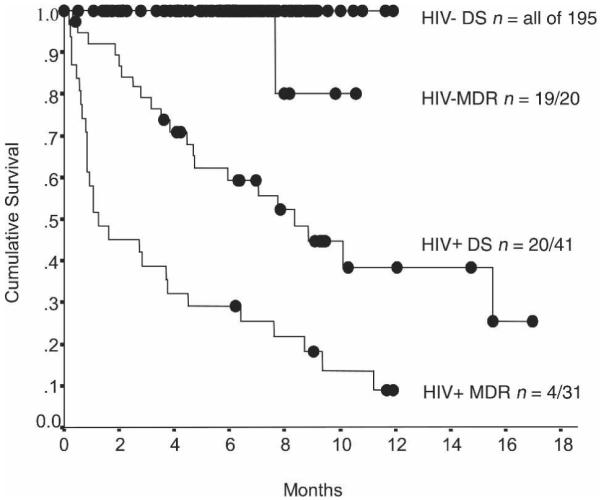
Kaplan-Meier analysis of mortality following commencement of tuberculosis therapy. The study population is divided according to HIV serology (HIV negative or positive) and tuberculosis drug resistance at the time of enrollment (MDR, strains resistant to isoniazid and rifampicin; DS, drug-susceptible, non-MDR strains); n, number of patients alive at the end of follow-up/the number of patients recruited to that diagnostic group; ●, patient whose follow-up ended at that time because of completion of or default from follow-up.
The major predictor of mortality was HIV infection and there were also significant associations with: MDRTB; previous tuberculosis treatment; and lack of known exposure to a tuberculosis case at home or work (implying a greater probability of tuberculosis transmission outside of the home, including nosocomial transmission) (Table 2). HIV and MDRTB co-infection caused 55% mortality 2 months after diagnosis, and only 16% survived to complete 9 months of therapy. Considering each center separately, 45/64 (70%) of the patients diagnosed at the infectious diseases unit died during follow-up. In contrast, 4/223 (1.8%) of patients recruited at the general clinic died, 3 of whom were HIV-positive. However, in multiple regression analysis, the site of patient recruitment was not independently associated with mortality. This difference in mortality between hospitals was explained by HIV and MDR prevalence: prognosis was similarly poor for HIV-positive tuberculosis patients recruited from either hospital (Table 2).
Table 2.
Risk factor analysis for mortality
| Kaplan-Meier |
Cox regression |
||||
|---|---|---|---|---|---|
| Feature | Log-rank test | P value | Hazard ratio | 95% CI | P value |
| HIV seropositivity | 130 | < 0.001 | 99 | 13–730 | < 0.001 |
| MDRTB at time of diagnosis | 65 | < 0.001 | 2.5 | 1.4–4.5 | 0.002 |
| Previous TB treatment | 16 | 0.001 | 2.1 | 1.1–3.7 | 0.02 |
| Known exposure to TB at home/work | 4.5 | 0.03 | 0.5 | 0.3–1.0 | 0.05 |
| Diagnosed in the infectious diseases unit | 118 | < 0.001 | ns | ||
| Previous TB preventive therapy | 29 | < 0.001 | ns | ||
| Hospitalization in last 2 years | 26 | < 0.001 | ns | ||
| Male sex | 4.9 | 0.03 | ns | ||
| BCG scar(s) | 0.14 | Age | – | – | – |
| Age in years | – | – | ns | ||
Note: Relative risk of mortality associated with each patient’s initial clinical characteristics is shown for Kaplan-Meier survival analysis and in a Cox regression model, together with 95% confidence intervals.
Abbreviations: HIV, human immunodeficiency virus; MDRTB, multi-drug resistant tuberculosis; TB, tuberculosis; BCG, Bacillus Calmette-Guerin; SD, standard deviation; ID, infectious diseases; CI, confidence interval; ns, no significant difference; “–” signifies that analysis of that variable was not appropriate (see methods).
Infectiousness
Durations of sputum smear and culture positivity are shown in Figure 2. Most patients whose sputum initially grew MDRTB remained smear- and culture-positive throughout follow-up. In contrast, most non-MDRTB became smear- and culture-negative in the first 6 weeks of therapy. Cox regression analysis revealed MDRTB to be the main risk factor for prolonged positive sputum smear (HR = 3.6, 95% CI = 1.9–6.6, P < 0.001) and culture (HR = 3.8, 95% CI = 2.1–6.9, P < 0.0001). As patient age increased, so did the duration of positive sputum smear (P = 0.04) and culture (P = 0.05). Although HIV co-infection had borderline associations with prolonged positive sputum smear and culture in univariate analysis, neither approached statistical significance in multiple regression analysis that included the effect of MDRTB. Mortality was also associated with prolonged positive sputum smear (HR = 14, 95% CI = 6.7–27, P < 0.001) and culture (HR = 8, 95% CI = 3.9–15, P < 0.001).
Figure 2.
Kaplan-Meier analysis of infectiousness following commencement of TB therapy. Infectiousness is assessed as sputum smear positivity in a and as sputum culture positivity in b. The study population is divided according to HIV serology (HIV negative or positive) and tuberculosis drug resistance (“MDR” refers to strains resistant to isoniazid and rifampicin; “DS” refers to all other strains) at the time of enrollment; n, number of patients known to be sputum-smear- or culture-negative by the end of follow-up/the number of patients recruited to that diagnostic group; “●” signifies end of follow-up because of completion of or default from the study.
MDRTB
Fifty-one (18%) of 287 patients had MDRTB, and 31/51 (61%) of them were HIV-positive. Of all 72 HIV-positive patients, 31 (43%) had MDRTB. In addition to the drug-susceptibility data from our university laboratory, susceptibilities were also available from the Ministry of Health clinical laboratory for 32 (50%) of patients at the infectious diseases unit, and there was complete concordance in all except 3 cases. Twelve (30%) of the 40 MDRTB patients became culture-negative on treatment, despite 6 of them receiving only standard first-line tuberculosis therapy and 8 of them being HIV-negative.
Risk factors for MDRTB
MDRTB was associated in multiple regression (all P ≤ 0.01) with previous tuberculosis treatment (HR = 2.8, 95% CI = 1.3–5.9), previous tuberculosis preventive therapy (HR = 7.9, 95% CI = 2.3–27), and current tuberculosis diagnosis made at the infectious diseases unit (HR = 4.9, 95% CI = 2.4–10). To evaluate the independent effect of recruitment center, data were also analyzed separately according to recruitment clinic. The general hospital had 21 (9%) of the MDRTB patients, but none of their recorded characteristics was significantly associated with drug resistance (all P > 0.1). At the infectious diseases unit, where all patients were HIV-positive, 30 (47%) of them had MDRTB. Multiple regression analysis revealed that MDRTB was associated with previous tuberculosis preventive therapy (HR = 16, 95% CI = 2.8–85, P = 0.002) and recent hospitalization (HR = 6.8, 95% CI = 1.9–24, P = 0.003).
DISCUSSION
This study demonstrates the profound impact of HIV infection and multi-drug resistance on survival and infectiousness in tuberculosis patients. Survival throughout treatment was almost universal in HIV-negative individuals, irrespective of drug susceptibilities. However, most HIV-positive patients co-infected with tuberculosis died during therapy, and most of those co-infected with MDRTB died in the first 2 months of treatment. Conventional drug-susceptibility testing used throughout the less-developed world would have identified MDRTB before death in only a minority of these cases, whereas inexpensive, recently reported techniques could have more rapidly provided this information and allowed appropriate therapy to be instituted.7,12,15 Most patients with MDRTB remained infectious throughout therapy, which is a concern because facilities for respiratory isolation of inpatients or outpatients are limited in less-developed countries.
Mortality
Two-thirds of the HIV-positive tuberculosis patients died during follow-up, which is a rate that is greater than in most reports, even after allowing for differences in follow-up3,16 and MDRTB.17,18 However, there are reports of similarly high mortality,19 and Peruvian mortality during treatment of first episodes of AIDS-associated tuberculosis approached this level, falling from 63% to 39% from 1996 to 1999.20,21 The cause of death in these patients could not be confirmed to be AIDS-associated tuberculosis in these published studies nor in the present research because autopsies were not performed; it is therefore possible that other AIDS-related diseases contributed to these high mortality rates. Delayed diagnosis,22,23 minimal access to anti-retroviral drugs,24 and co-existent infections17 may also have contributed. However, the median survival time of these patients at the infectious diseases unit was 61 months from the time of HIV diagnosis but < 6 months from the diagnosis of tuberculosis co-infection, indicating the major risk associated with a tuberculosis diagnosis in HIV-positive people in this setting.
In contrast, there was minimal mortality in HIV-negative patients, including those with MDRTB, reflecting the renowned Peruvian tuberculosis program. For > 10 years, this program has provided > 99% of the Peruvian population all five components of the World Health Organization DOTS policy: government commitment to sustained TB control; bacteriological diagnosis; standardized short-course chemotherapy regimens under close supervision; a proper drug supply system; and recording/reporting of cases and results.2,5,18
It is noteworthy that most of the HIV-positive and HIV-negative participants were outpatients at the time that tuberculosis was diagnosed, and only a minority had been hospitalized in the past 2 years (Table 1), implying that tuberculosis was not acquired during hospitalization for other terminal diseases.
Infectiousness
Although HIV status was the principle determinant of survival, MDRTB was the main determinant of infectiousness. Of those with MDRTB, approximately half of the HIV-negative and almost all of the HIV-positive patients remained sputum smear- and/or culture-positive throughout treatment. Follow-up sputum culture data are rarely available in less-developed countries, although most tuberculosis occurs there. It is significant that sputum cultures almost exactly mirrored sputum smears, implying that positive sputum smears did not generally result from mycobacteria that had been killed by treatment. When sensitive broth culture is used, sputum smear-positivity usually indicates culture-positivity.25 This implies that sputum smears are reliable indicators of the duration of infectiousness.
HIV co-infection did not significantly influence the duration of infectiousness during treatment.14 This contrasts with countries with very little MDRTB, where prolonged culture-positivity is infrequent.4 Peru has high tuberculosis and MDRTB prevalences.2,7,26-30 In this setting, conventional treatment of MDRTB for immunocompetent people was associated with good survival but frequently maintained infectiousness. Although we did not study secondary tuberculosis in contacts or radiographic data to assess disease severity, it is well established that sputum smear and culture positivity are risk factors for tuberculosis and MDRTB dissemination.6 As a result of this research, the infectious diseases unit is now providing same-day sputum tuberculosis microscopy for HIV-positive patients with respiratory symptoms, rapid initiation of therapy after diagnosis, respiratory isolation of smear-positive patients, and increased use of personal respirator masks.
MDRTB rates
The MDRTB rates found for HIV co-infected patients in the present study were similar to recent Lima-based research,30 but MDRTB rates were higher than nationally,20 even in the general clinic where we recruited unselected, predominantly HIV-negative residents. However, in much of the less-developed world, including Peru, drug-susceptibility testing is restricted to high-risk groups, including HIV co-infection and those who have failed several months of first-line tuberculosis therapy. Our results imply that many patients with MDRTB and unrecognized HIV co-infection die before they meet these criteria, so MDRTB may be under-diagnosed.
In this study, patients with initially unrecognized MDRTB completed a trial of first-line therapy despite MDRTB being diagnosed during first-line therapy. These MDRTB results were provided routinely by the Ministry of Health for all new tuberculosis diagnoses in HIV-positive and some other patients and by our research for all patients. This initial trial of first-line therapy is explained by the observations that (1) first-line therapy includes at least two drugs to which most MDRTB is susceptible and consequently much MDRTB appears to be cured by first-line therapy5,31-33; (2) second-line MDRTB therapy is expensive and toxic34; (3) failure of first-line therapy reliably predicts MDRTB35; and (4) conventional drug-susceptibility testing identifies MDRTB too late to influence the first months of therapy.10 Hence, the patients with MDRTB in this study first had to survive failing 6–9 months of first-line therapy before receiving specific MDRTB therapy. This was associated with mortality and prolonged MDRTB infectiousness, in contrast to the excellent prognoses for MDRTB treated with specific therapy in Peru.34,36 The present results imply that rapid, early MDRTB testing is a priority and that, when MDRTB is diagnosed, appropriate therapy should be instituted urgently, without awaiting completion of a trial of second-line therapy. Second-line antibiotics for MDRTB and highly active anti-retroviral therapy have both become much more widely available in Peru,34 and rapid MDRTB tests,15 including the MODS assay that we used,11,12 have become approved components of the Peruvian National Tuberculosis Control policy.37
Recently, a community-based MDRTB treatment program achieved a cure rate of 73% among 75 patients.34 Although this represents a breakthrough in MDRTB care, antibiotic costs were US dollars (USD) 15,681 per patient, a price beyond the reach of most programs. Also, this selected patient group had survived a median of 4 years of intermittent therapy before this therapy, demonstrating chronic disease. Similarly, a standardized regimen of MDRTB treatment administered by the Peruvian tuberculosis program cured 48% of MDRTB patients.36 Together, these results indicate potential to prevent mortality where resources exist for rapid testing and specific MDRTB therapy.
MDRTB is associated with previous tuberculosis treatment,4,30 and we also found an association with previous isoniazid preventive therapy, unlike other studies.30,38-40 This could be explained by a covariate, such as duration of AIDS, increasing the likelihood of both preventive therapy and nosocomial MDRTB exposure. Alternatively, isoniazid preventive monotherapy could have increased the risk of subsequent isoniazid and rifampicin resistance. A recent study of the risk factors for MDRTB for HIV-positive and negative patients in Lima30 and our present study both demonstrated associations between MDRTB and past tuberculosis treatment, hospitalizations, and care in centers for people with HIV. This recent case-control study was not designed to test for HIV effects on MDRTB risk, and therefore it is important to note that in the present study HIV co-infection was not independently associated with MDRTB risk.
Reliability
Studies of this kind can be confounded by laboratory cross-contamination,41 although care was taken to prevent this, including using closed culture wells.11 Evidence that sputum results diagnosed disease rather than contamination was provided by (1) association with mortality; (2) agreement between drug-susceptibility testing in separately collected samples processed in different laboratories; and (3) concordance between independent microscopy and culture in our laboratory.
Implications
Most patients with MDRTB remained infectious throughout suboptimal therapy designed for drug-susceptible tuberculosis. Greater use of drug-susceptibility testing would allow appropriate therapy, when available, to improve prognosis34,42 and potentially reduce MDRTB transmission. Early MDRTB diagnosis may also prevent therapy with inappropriate regimes from causing MDRTB strains to develop additional resistance.43 Rapid culture and drug-susceptibility testing would be required for appropriate therapy to be instituted before most mortality occurred. Techniques suitable for less-developed countries, such as the MODS assay that we used, can provide drug susceptibilities in 5–14 days for USD 1–2 per sample,11,12 early and inexpensive enough to potentially reduce mortality. Another priority is improved infection control to reduce nosocomial transmission. Anti-retroviral drugs may considerably improve24 the grave prognoses that we identified for patients with tuberculosis and HIV co-infection, and availability has recently increased significantly in Peru.
CONCLUSION
This research demonstrated excellent prognoses for HIV-negative individuals and rapid resolution of infectiousness for patients with non-MDRTB. However, MDRTB patients had prolonged infectiousness and, with HIV co-infection, more than 50% mortality in the first 2 months of therapy. This study therefore demonstrates the importance of early and rapid drug-susceptibility testing, improved infection control measures, and early anti-retroviral therapy so that patients with MDRTB and HIV can receive appropriate therapy early enough to reduce their mortality and potential to infect others.
Acknowledgments
We are grateful to Maria Prado at Maria Auxiliadora Hospital and Nurys Cabanillas, Dr. Jorge Arevalo, and Dr. Marcos Ñavincopa at Dos de Mayo Hospital; to Dr. Lawrence Moulton for statistical assistance; to Dr. David AJ Moore for editorial assistance; and to Patricia Fuentes for laboratory assistance. We gratefully acknowledge the Peruvian Ministry of Health for approval of and collaboration in this project.
Financial support: This research was supported by Wellcome Trust fellowships in Clinical Tropical Medicine (to A.R.E. & C.A.E.); USAID TB Award (HRN-5986-A-00-6006-00); Fogarty-NIH AIDS Training Program (3T22-TW00016-05S3); NIH ITREID (grant 5D43-TW00910); and NIAID (01637).
Footnotes
Conflict of Interest: All authors declare that they have no conflict of interest in relation to this work. Dr. Eduardo Ticona was previously head of the Peruvian National Tuberculosis Control Program.
Disclaimer: The opinions and assertions made by the authors do not reflect the official position or opinion of the U.S. Department of the Army, or the Henry M. Jackson Foundation Inc., or any other organization listed.
REFERENCES
- 1.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Ravigli-one MC, Dye C. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organisation . Global tuberculosis control: surveillance, planning, financing. Geneva: 2006. WHO Report 2005. [Google Scholar]
- 3.Mukadi YD, Maher D, Harries A. Tuberculosis case fatality rates in high HIV prevalence populations in sub-Saharan Africa. AIDS. 2001;15:143–152. doi: 10.1097/00002030-200101260-00002. [DOI] [PubMed] [Google Scholar]
- 4.Espinal MA, Laszlo A, Simonsen L, Boulahbal F, Kim SJ, Reniero A, Hoffner S, Rieder HL, Binkin N, Dye C, Williams R, Raviglione MC. Global trends in resistance to antituberculosis drugs. World Health Organization-International Union against Tuberculosis and Lung Disease Working Group on Anti-Tuberculosis Drug Resistance Surveillance. N Engl J Med. 2001;344:1294–1303. doi: 10.1056/NEJM200104263441706. [DOI] [PubMed] [Google Scholar]
- 5.Espinal MA, Kim SJ, Suarez PG, Kam KM, Khomenko AG, Migliori GB, Baez J, Kochi A, Dye C, Raviglione MC. Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countries. JAMA. 2000;283:2537–2545. doi: 10.1001/jama.283.19.2537. [DOI] [PubMed] [Google Scholar]
- 6.Behr MA, Warren SA, Salamon H, Hopewell PC, Ponce de Leon A, Daley CL, Small PM. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet. 1999;353:444–449. doi: 10.1016/s0140-6736(98)03406-0. [DOI] [PubMed] [Google Scholar]
- 7.Anonymous . Tuberculosis en el Peru: informe anual, 2004. Direccion General de Salud de las Personas, Ministerio de Salud del Peru; Lima, Peru: 2005. [Google Scholar]
- 8.Vasquez-Campos L, Asencios-Solis L, Leo-Hurtado E, Quispe-Torres N, Salazar-Lindo E, Bayona J, Becerra MC. Drug resistance trends among previously treated tuberculosis patients in a national registry in Peru, 1994–2001. Int J Tuberc Lung Dis. 2004;8:465–472. [PubMed] [Google Scholar]
- 9.Singh NP, Parija SC. The value of fluorescence microscopy of auramine-stained sputum smears for the diagnosis of pulmonary tuberculosis. Southeast Asian J Trop Med Public Health. 1998;29:860–863. [PubMed] [Google Scholar]
- 10.Kubica K. Public health mycobacteriology: a guide for the level III laboratory. USDHHS: Centers for Disease Control; Atlanta: 1985. [Google Scholar]
- 11.Caviedes L, Lee TS, Gilman RH, Sheen P, Spellman E, Lee EH, Berg DE, Montenegro-James S, The Tuberculosis Working Group in Peru Rapid, efficient detection and drug susceptibility testing of Mycobacterium tuberculosis in sputum by microscopic observation of broth cultures. J Clin Microbiol. 2000;38:1203–1208. doi: 10.1128/jcm.38.3.1203-1208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore DA, Mendoza D, Gilman RH, Evans CA, Hollm Delgado MG, Guerra J, Caviedes L, Vargas D, Ticona E, Ortiz J, Soto G, Serpa J, Tuberculosis Working Group in Peru Microscopic observation drug susceptibility assay, a rapid, reliable diagnostic test for multidrug-resistant tuberculosis suitable for use in resource-poor settings. J Clin Microbiol. 2004;42:4432–4437. doi: 10.1128/JCM.42.10.4432-4437.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franzblau SG, Witzig RS, McLaughlin JC, Torres P, Madico G, Hernandez A, Degnan MT, Cook MB, Quenzer VK, Ferguson RM, Gilman RH. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J Clin Microbiol. 1998;36:362–366. doi: 10.1128/jcm.36.2.362-366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Telzak EE, Fazal BA, Pollard CL, Turett GS, Justman JE, Blum S. Factors influencing time to sputum conversion among patients with smear-positive pulmonary tuberculosis. Clin Infect Dis. 1997;25:666–670. doi: 10.1086/513772. [DOI] [PubMed] [Google Scholar]
- 15.Solis LA, Shin SS, Han LL, Llanos F, Stowell M, Sloutsky A. Validation of a rapid method for detection of M. tuberculosis resistance to isoniazid and rifampin in Lima, Peru. Int J Tuberc Lung Dis. 2005;9:760–764. [PMC free article] [PubMed] [Google Scholar]
- 16.El-Sadr WM, Perlman DC, Denning E, Matts JP, Cohn DL. A review of efficacy studies of 6-month short-course therapy for tuberculosis among patients infected with human immunodeficiency virus: differences in study outcomes. Clin Infect Dis. 2001;32:623–632. doi: 10.1086/318706. [DOI] [PubMed] [Google Scholar]
- 17.Fischl MA, Uttamchandani RB, Daikos GL, Poblete RB, Moreno JN, Reyes RR, Boota AM, Thompson LM, Cleary TJ, Lai S. An outbreak of tuberculosis caused by multiple-drug-resistant tubercle bacilli among patients with HIV infection. Ann Intern Med. 1992;117:177–183. doi: 10.7326/0003-4819-117-3-177. [DOI] [PubMed] [Google Scholar]
- 18.Frieden TR, Sterling T, Pablos-Mendez A, Kilburn JO, Cauthen GM, Dooley SW. The emergence of drug-resistant tuberculosis in New York City. N Engl J Med. 1993;328:521–526. doi: 10.1056/NEJM199302253280801. [DOI] [PubMed] [Google Scholar]
- 19.Fischl MA, Daikos GL, Uttamchandani RB, Poblete RB, Moreno JN, Reyes RR, Boota AM, Thompson LM, Cleary TJ, Oldham SA, et al. Clinical presentation and outcome of patients with HIV infection and tuberculosis caused by multiple-drug-resistant bacilli. Ann Intern Med. 1992;117:184–190. doi: 10.7326/0003-4819-117-3-184. [DOI] [PubMed] [Google Scholar]
- 20.Tuberculosis en el Peru: informe 2000. Direccion General de Salud de las Personas, Ministerio de Salud del Peru; Lima, Peru: 2001. Publication 9972-776-12-3 ed. [Google Scholar]
- 21.Tuberculosis en el Peru: informe 1999. Direccion General de Salud de las Personas, Ministerio de Salud del Peru; Lima, Peru: 2000. [Google Scholar]
- 22.Kramer F, Modilevsky T, Waliany AR, Leedom JM, Barnes PF. Delayed diagnosis of tuberculosis in patients with human immunodeficiency virus infection. Am J Med. 1990;89:451–456. doi: 10.1016/0002-9343(90)90375-n. [DOI] [PubMed] [Google Scholar]
- 23.Pablos-Mendez A, Sterling TR, Frieden TR. The relationship between delayed or incomplete treatment and all-cause mortality in patients with tuberculosis. JAMA. 1996;276:1223–1228. doi: 10.1001/jama.1996.03540150025026. [DOI] [PubMed] [Google Scholar]
- 24.Kirk O, Gatell JM, Mocroft A, Pedersen C, Proenca R, Brettle RP, Barton SE, Sudre P, Phillips AN, EuroSIDA Study Group JD Infections with Mycobacterium tuberculosis and Mycobacterium avium among HIV-infected patients after the introduction of highly active antiretroviral therapy. Am J Respir Crit Care Med. 2000;162:865–872. doi: 10.1164/ajrccm.162.3.9908018. [DOI] [PubMed] [Google Scholar]
- 25.Long R, Bochar K, Chomyc S, Talbot J, Barrie J, Kunimoto D, Tilley P. Relative versus absolute noncontagiousness of respiratory tuberculosis on treatment. Infect Control Hosp Epidemiol. 2003;24:831–838. doi: 10.1086/502145. [DOI] [PubMed] [Google Scholar]
- 26.Sanghavi DM, Gilman RH, Lescano-Guevara AG, Checkley W, Cabrera LZ, Cardenas V. Hyperendemic pulmonary tuberculosis in a Peruvian shantytown. Am J Epidemiol. 1998;148:384–389. doi: 10.1093/oxfordjournals.aje.a009657. [DOI] [PubMed] [Google Scholar]
- 27.Madico G, Gilman RH, Checkley W, Cabrera L, Kohlstadt I, Kacena K, Diaz JF, Black R. Community infection ratio as an indicator for tuberculosis control. Lancet. 1995;345:416–419. doi: 10.1016/s0140-6736(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 28.Getchell WS, Davis CE, Gilman J, Urueta G, Ruiz-Huidobro E, Gilman RH. Basic epidemiology of tuberculosis in Peru: a prevalence study of tuberculin sensitivity in a Pueblo joven. Am J Trop Med Hyg. 1992;47:721–729. doi: 10.4269/ajtmh.1992.47.721. [DOI] [PubMed] [Google Scholar]
- 29.Suarez PG, Watt CJ, Alarcon E, Portocarrero J, Zavala D, Canales R, Luelmo F, Espinal MA, Dye C. The dynamics of tuberculosis in response to 10 years of intensive control effort in Peru. J Infect Dis. 2001;184:473–478. doi: 10.1086/322777. [DOI] [PubMed] [Google Scholar]
- 30.Campos PE, Suarez PG, Sanchez J, Zavala D, Arevalo J, Ticona E, Nolan CM, Hooton TM, Holmes KK. Multidrug-resistant Mycobacterium tuberculosis in HIV-infected persons, Peru. Emerg Infect Dis. 2003;9:1571–1578. doi: 10.3201/eid0912.020731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singla R, Al-Sharif N, Al-Sayegh MO, Osman MM, Shaikh MA. Influence of anti-tuberculosis drug resistance on the treatment outcome of pulmonary tuberculosis patients receiving DOTS in Riyadh, Saudi Arabia. Int J Tuberc Lung Dis. 2002;6:585–591. [PubMed] [Google Scholar]
- 32.Bonnet M, Sizaire V, Kebede Y, Janin A, Doshetov D, Mirzoian B, Arzumanian A, Muminov T, Iona E, Rigouts L, Rusch-Gerdes S, Varaine F. Does one size fit all? Drug resistance and standard treatments: results of six tuberculosis programmes in former Soviet countries. Int J Tuberc Lung Dis. 2005;9:1147–1154. [PubMed] [Google Scholar]
- 33.DeRiemer K, Garcia-Garcia L, Bobadilla-del-Valle M, Palacios-Martinez M, Martinez-Gamboa A, Small PM, Sifuentes-Osornio J, Ponce-de-Leon A. Does DOTS work in populations with drug-resistant tuberculosis? Lancet. 2005;365:1239–1245. doi: 10.1016/S0140-6736(05)74812-1. [DOI] [PubMed] [Google Scholar]
- 34.Mitnick C, Bayona J, Palacios E, Shin S, Furin J, Alcantara F, Sanchez E, Sarria M, Becerra M, Fawzi MC, Kapiga S, Neuberg D, Maguire JH, Kim JY, Farmer P. Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru. N Engl J Med. 2003;348:119–128. doi: 10.1056/NEJMoa022928. [DOI] [PubMed] [Google Scholar]
- 35.Becerra MC, Freeman J, Boyona J, Shin SS, Kim JY, Furin JJ, Werner B, Sloutsky A, Timperi R, Wilson ME, Pagano M, Farmer PE. Using treatment failure under effective directly observed short-course chemotherapy programs to identify patients with multidrug resistant tuberculosis. Int J Tuberc Lung Dis. 2000;4:108–114. [PubMed] [Google Scholar]
- 36.Suarez PG, Floyd K, Portocarrero J, Alarcon E, Rapiti E, Ramos G, Bonilla C, Sabogal I, Aranda I, Dye C, Raviglione M, Espinal MA. Feasibility and cost-effectiveness of standardised second-line drug treatment for chronic tuberculosis patients: a national cohort study in Peru. Lancet. 2002;359:1980–1989. doi: 10.1016/S0140-6736(02)08830-X. [DOI] [PubMed] [Google Scholar]
- 37.Anonymous . Norma Tecnica de Salud para el Control de la Tuberculosis. Direccion General de Salud de las Personas, Ministerio de Salud del Peru; Lima, Peru: 2006. [Google Scholar]
- 38.Hawken MP, Meme HK, Elliott LC, Chakaya JM, Morris JS, Githui WA, Juma ES, Odhiambo JA, Thiong’o LN, Kimari JN, Ngugi EN, Bwayo JJ, Gilks CF, Plummer FA, Porter JD, Nunn PP, McAdam KP. Isoniazid preventive therapy for tuberculosis in HIV-1-infected adults: results of a randomized controlled trial. AIDS. 1997;11:875–882. doi: 10.1097/00002030-199707000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Gordin F, Chaisson RE, Matts JP, Miller C, de Lourdes Garcia M, Hafner R, Valdespino JL, Coberly J, Schechter M, Klukowicz AJ, Barry MA, O’Brien RJ. Rifampin and pyrazinamide vs isoniazid for prevention of tuberculosis in HIV-infected persons: an international randomized trial. Terry Beirn Community Programs for Clinical Research on AIDS, the Adult AIDS Clinical Trials Group, the Pan American Health Organization, and the Centers for Disease Control and Prevention Study Group. JAMA. 2000;283:1445–1450. doi: 10.1001/jama.283.11.1445. [DOI] [PubMed] [Google Scholar]
- 40.Hanson ML, Comstock GW, Haley CE. Community isoniazid prophylaxis program in an underdeveloped area of Alaska. Public Health Rep. 1967;82:1045–1056. [PMC free article] [PubMed] [Google Scholar]
- 41.Burman WJ, Reves RR. Review of false-positive cultures for Mycobacterium tuberculosis and recommendations for avoiding unnecessary treatment. Clin Infect Dis. 2000;31:1390–1395. doi: 10.1086/317504. [DOI] [PubMed] [Google Scholar]
- 42.Farmer P, Kim JY. Community-based approaches to the control of multidrug resistant tuberculosis: introducing DOTS-plus. BMJ. 1998;317:671–674. doi: 10.1136/bmj.317.7159.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Furin JJ, Becerra MC, Shin SS, Kim JY, Bayona J, Farmer PE. Effect of administering short-course, standardized regimens in individuals infected with drug-resistant Mycobacterium tuberculosis strains. Eur J Clin Microbiol Infect Dis. 2000;19:132–136. doi: 10.1007/s100960050445. [DOI] [PubMed] [Google Scholar]



