Abstract
Sputum induction, bronchoalveolar lavage, or gastric aspiration are often needed to produce adequate diagnostic respiratory samples from people with HIV in whom tuberculosis is suspected. Since these procedures are rarely appropriate in less-developed countries, we compared the performances of a simple string test and the gold-standard sputum induction. 160 HIV-positive adults under investigation for tuberculosis, and 52 asymptomatic HIV-positive control patients underwent the string test followed by sputum induction. The string test detected tuberculosis in 14 patients in whom this disease was suspected; sputum induction detected only eight of them (McNemar's test, p=0·03). These preliminary data suggest that the string test is safe and effective for retrieval of useful clinical specimens for diagnosis of pulmonary tuberculosis, and is at least as sensitive as sputum induction.
Diagnosis of tuberculosis in people with HIV, especially those with a low CD4 count, is complicated by an altered symptom spectrum. Thus, a productive cough, the source of diagnostic sputum samples in HIV-negative people, might not be prominent, necessitating the use of procedures such as sputum induction or gastric aspiration.
We postulated that the string test, previously used successfully for retrieval of enteropathogens such as Giardia lamblia1 and Salmonella typhi2 from the upper gastrointestinal tract, might be an effective alternative method to obtain swallowed sputum in such patients. The primary objective of this study was to compare the string test with sputum induction (the gold-standard comparator) for diagnosis of tuberculosis in HIV-positive patients who had proven smear negative for Mycobacterium tuberculosis from a previous sample. The study was approved by the ethics review boards of Asociación Benéfica Proyectos en Informática, Salud, Medicina, y Agricultura, Universidad Peruana Cayetano Heredia, Hospital Nacional Dos de Mayo, and the Johns Hopkins Bloomberg School of Public Health. Sample-size calculations were not done before the study.
Eligibility criteria for patients were: HIV positive, under investigation for pulmonary tuberculosis, unable to produce an adequate sputum sample, and previous inadequate sample Ziehl-Nielsen smear negative. Between April 1, 2002, and Oct 17, 2003, 228 consecutive HIV-positive eligible adult patients were enrolled at the Infectious and Tropical Diseases Service at Hospital Nacional Dos de Mayo, Lima, Peru. Though all previous sputum samples were of poor quality, 90 were sent for mycobacterial culture on Ogawa medium in the hospital microbiology department, all of which proved negative. During the same time, 52 HIV-positive control volunteers with no suspicion of tuberculosis and no previous sputum examination were also enrolled to allow estimation of the string test's specificity. All participants were provided with a study information sheet and gave written informed consent.
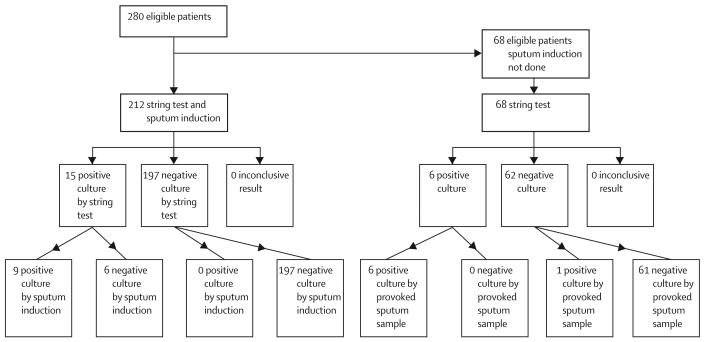
The study protocol required that each individual undergo the string test followed by sputum induction. In 68 of 280 cases the string test provoked a sufficiently strong expectorating cough upon withdrawal of the string that the investigator deemed the resulting sputum sample as adequate for culture, and cancelled the sputum induction procedure with the agreement of the patient (figure). Thus, paired data for string-test and nebuliser-induced sputum were available for 212 people—160 with suspected tuberculosis (median CD4 count 37 cells per mL) and 52 symptom-free HIV-positive controls (median CD4 count 102 cells per mL). Table 1 shows the symptoms of the study population.
Figure.
Standards for reporting of diagnostic accuracy (STARD) flowchart
Table 1.
Symptoms of participants
| Dry cough alone | 28 (18%) |
| Productive cough alone | 19 (12%) |
| Productive cough and weight loss | 16 (10%) |
| Dry cough and weight loss | 32 (20%) |
| Weight loss alone | 55 (34%) |
| Other symptoms* | 10 (6%) |
| Total with symptoms | 160 (100%) |
| Total without symptoms (control) | 52 |
| Total | 212 |
Percentages are of symptomatic participants.
Including fever, abdominal pain, chest pain, night sweats, haemoptysis, diarrhoea, chills, and poor appetite.
All 212 patients underwent the string test procedure followed (within 90 min) by sputum induction with nebulisation of 20% saline. No treatment was given between the two tests. The string test involved swallowing of the capsule (HDC Corporation, CA, USA), retention of the trailing string taped to the cheek for 4 h, then retrieval of the string through the mouth by steady gentle traction for 5 s. Retrieved string samples were put into sterile 15-mL Falcon tubes containing 1 mL of 0·9% saline, and samples of induced sputum were placed into sterile 50-mL pots. Strings were washed with normal saline, vortexed for 30 s, then centrifuged for 5 min after which the string was removed with sterile tweezers. The remaining liquid was then decontaminated in the same way as samples of induced sputum, by the 4% N-acetyl- L-cysteine sodium-hydroxide method. The sediment was resuspended to 2 mL with 0·2% bovine serum albumin. Every sample was separated for auramine staining and for culture in Löwenstein-Jensen and Middlebrook 7H9 broth (MODS).3 Experienced field research nurses did all procedures, and cultures were handled in our research laboratory by experienced laboratory technicians who were unaware of assignment, with double verification of all positive cultures.
15 of the 212 participants were diagnosed with tuberculosis during this study; 14 in whom this disease was suspected, and one asymptomatic control patient (table 2). In those with suspected tuberculosis (n=160), the string test yielded cultures that were positive for M tuberculosis in all 14 patients, whereas only eight were detected by induced sputum (McNemar's test, p=0·03). Two patients (1 and 4) had multidrug resistant isolates, one of which was detected only by the string test.
Table 2.
Diagnosis of tuberculosis in patients with HIV
| Patient | Symptoms | Previous culture | CD4 count | Induced sputum |
String test |
||||
|---|---|---|---|---|---|---|---|---|---|
| Auramine | Culture (LJ) | Culture (MODS) | Auramine | Culture (LJ) | Culture (MODS) | ||||
| 1 | Dry cough and weight loss | − | 30 | − | − | − | − | − | + |
| 2 | Dry cough and weight loss | − | 30 | − | ND | − | − | + | + |
| 3 | Dry cough and weight loss | − | 66 | − | − | + | − | + | + |
| 4 | Dry cough and weight loss, chest pain, abdominal pain, and poor appetite | − | 30 | + | + | + | + | + | + |
| 5 | Weight loss | − | 37 | − | + | + | − | + | + |
| 6 | Weight loss | − | 117 | − | + | + | − | + | + |
| 7 | Weight loss, night sweats, diarrhoea, chest pain, and abdominal pain | − | 30 | − | − | − | − | − | + |
| 8 | Productive cough, weight loss, fever, and chest pain | ND | 30 | − | + | + | − | + | C |
| 9 | Dry cough, weight loss, abdominal pain, and fever | − | 51 | + | + | + | + | + | + |
| 10 | Fever, chills, and poor appetite | − | 66 | − | − | − | − | + | + |
| 11 | Weight loss and chest pain | − | 44 | − | + | + | − | − | + |
| 12 | Dry cough, weight loss, chest pain, and poor appetite | − | 117 | − | − | − | − | + | + |
| 13 | Productive cough, weight loss, poor appetite, chills, and chest pain | ND | 30 | − | + | + | − | + | + |
| 14 | Productive cough, poor appetite, and abdominal pain | − | 30 | − | − | − | − | − | + |
| 15* | Asymptomatic | − | 226 | − | + | + | − | + | + |
LJ=Löwenstein-Jensen media. C=contaminated. ND=not done.
Patient from control group.
Of the 160 symptomatic participants under investigation for tuberculosis, about a third reported a dry cough, and roughly half reported significant weight loss. Of the 14 subsequently found to have tuberculosis, six (43%) reported dry cough and 12 (86%) weight loss; only one (11) had a history of prior tuberculosis. The string test was very well tolerated by all patients. However, 191 (90%) reported brief, transient nausea at the moment of string withdrawal, and 42 (20%) retched
Using the string test and MODS, we detected microbiologically confirmed disease in 14 (9%) of 160 patients with suspected tuberculosis. Such people would have remained undiagnosed or been diagnosed only on clinical grounds in most developing world settings where sputum induction and culture is rarely an option. The availability of an isolate and of drug susceptibility data was particularly important for the two patients with multidrug resistant infection (and their close contacts) who, if treated empirically, would otherwise have received inappropriate first-line treatment. Even with access to sputum induction, six people would have remained undiagnosed. These six (in addition to the other eight) reported substantial clinical improvement after the start of treatment, which suggests that the cultures were true-positives not the result of cross-contamination or other errors.
We also detected M tuberculosis from the string test and induced sputum of one of the 52 HIV-positive control patients who had no symptoms of pulmonary tuberculosis. Though this finding could be a false-positive, the fact that both MODS and Löwenstein-Jensen cultures of both samples were positive suggests otherwise. This result is more probably an early case of tuberculosis detected in an HIV-positive patient who was yet to develop pronounced symptoms. However, our data suggest that the string test does not have a role in routine investigation of asymptomatic HIV-positive patients.
Our preliminary findings suggest that the string test is a safe method for retrieval of useful clinical specimens for diagnosis of pulmonary tuberculosis, and is at least as sensitive as the acknowledged gold-standard sputum induction.4 The procedure is well tolerated, has minimum risk for patients undergoing investigation, and has no appreciable risk for other hospital patients and staff (by contrast with sputum induction). The string test now warrants prompt assessment in children with suspected tuberculosis for whom retrieval of adequate diagnostic respiratory samples is also generally problematic, and further large-scale comparative efficacy studies to more precisely define sensitivity and specificity.
Acknowledgments
This study was jointly funded by the Office of Health, Infectious Diseases, and Nutrition, Global Health Bureau, US Agency for International Development, under the terms of Award No # HRN-5986- A-00-6006-00, GHS-A-00-03-00019-00, Global Research Activity Cooperative Agreement, NIH/NIAID (T35A107646), The Wellcome Trust and RG-ER Fund. The funding sources had no role in the study design, data collection, data interpretation, data analysis, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. The opinions expressed herein are those of the author(s) and do not necessarily reflect the views of the US Agency for International Development.
Footnotes
Conflict of interest statement
We declare that we have no conflict of interest.
References
- 1.Beal CB, Viens P, Grant RG, Hughes JM. A new technique for sampling duodenal contents: demostration of upper small-bowel pathogens. Am J Trop Med Hyg. 1970;19:349–52. doi: 10.4269/ajtmh.1970.19.349. [DOI] [PubMed] [Google Scholar]
- 2.Gilman RH, Islam S, Rabbani B, Ghosh H. Identification of gallbladder typhoid carriers by a string device. Lancet. 1979;1:795–96. doi: 10.1016/s0140-6736(79)91316-3. [DOI] [PubMed] [Google Scholar]
- 3.Caviedes L, Lee T-S, Gilman RH, et al. Rapid, efficient detection and drug susceptibility testing of Mycobacterium tuberculosis in sputum by microscopic observation of broth cultures. J Clin Microbiol. 2000;38:1203–08. doi: 10.1128/jcm.38.3.1203-1208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conde MB, Soares SL, Mello FC, et al. Comparasion of sputum induction with fiberoptic bronchoscopy in the diagnosis of tuberculosis: experience at an Acquired Immune Deficency Syndrome Reference Center in Rio de Janeiro, Brazil. Am J Respir Crit Care Med. 2000;162:2238–40. doi: 10.1164/ajrccm.162.6.2003125. [DOI] [PubMed] [Google Scholar]