Abstract
Background Effective and scalable community-based strategies are needed for identification and management of serious neonatal illness.
Methods As part of a community-based, cluster-randomized controlled trial of the impact of a package of maternal-neonatal health care, community health workers (CHWs) were trained to conduct household surveillance and to identify and refer sick newborns according to a clinical algorithm. Assessments of newborns by CHWs at home were linked to hospital-based assessments by physicians, and factors impacting referral, referral compliance and outcome were evaluated.
Results Seventy-three per cent (7310/10 006) of live-born neonates enrolled in the study were assessed by CHWs at least once; 54% were assessed within 2 days of birth, but only 15% were attended at delivery. Among assessments for which referral was recommended, compliance was verified in 54% (495/919). Referrals recommended to young neonates 0–6 days old were 30% less likely to be complied with compared to older neonates. Compliance was positively associated with having very severe disease and selected clinical signs, including respiratory rate ≥70/minute; weak, abnormal or absent cry; lethargic or less than normal movement; and feeding problem. Among 239 neonates who died, only 38% were assessed by a CHW before death.
Conclusions Despite rigorous programmatic effort, reaching neonates within the first 2 days after birth remained a challenge, and parental compliance with referral recommendation was limited, particularly among young neonates. To optimize potential impact, community postnatal surveillance must be coupled with skilled attendance at delivery, and/or a worker skilled in recognition of neonatal illness must be placed in close proximity to the community to allow for rapid case management to avert early deaths.
Keywords: Community health worker, neonatal illness, referral, surveillance, care seeking
KEY MESSAGES.
Well-trained and supervised community health workers are capable of conducting high-quality routine household surveillance of newborns. However, specific problems exist.
Critical challenges include the ability to reach newborns at delivery and in the immediate postnatal period, when most neonatal mortality occurs, and reluctance of families to comply with referral of preterm newborns.
Community postnatal surveillance must be coupled with skilled attendance at delivery, and/or a worker skilled in recognition of neonatal illness must be situated close to the community to allow for rapid case management.
Introduction
Two-thirds of births and most neonatal deaths occur at home, outside the formal health care system, in low and middle-income countries, where approximately 99% of an estimated 4 million global neonatal deaths occur (Lawn et al. 2005). To optimally advance neonatal health and survival along the continuum of care, interventions must be introduced at the domiciliary and community level while linking with the health care system for treatment of life-threatening illness (Darmstadt et al. 2000; Bhutta et al. 2005; Darmstadt et al. 2005; Haws et al. 2007; Darmstadt et al. 2008b). Community-based efficacy trials of maternal and neonatal care packages have shown reductions in neonatal or perinatal mortality, and some studies have utilized community-based workers to implement some or all elements of programmes (Bang et al. 1999; Manandhar et al. 2004; Bang et al. 2005c; Jokhio et al. 2005; Baqui et al. 2008; Kumar et al. 2008).
Determining effective delivery modes for interventions at scale in low-resource settings remains an unresolved question (Lawn et al. 2005; Lawn et al. 2006; Haws et al. 2007). In order to determine how to best involve existing health care systems in providing essential services, the quality, accessibility and community perception and acceptance of the systems must be further addressed. Various intervention models for the management of childhood illness by community health workers (CHWs) have been implemented, and basic management and facilitated referral by CHWs is a preferable model where access to health facilities is good and when concerns about cost, supplies, and antimicrobial resistance exits (Winch et al. 2005b). In this model, CHWs identify children with illness, make referral for facility-level care, and facilitate referral. Winch et al. (2005b) described key components of facilitated referral, including (1) promoting referral compliance, (2) monitoring referral and compliance, and (3) addressing barriers to compliance such as geographic and financial constraints. However, low referral compliance rates of 24–58% have generally been reported by studies—largely including older infants and children who were referred from first-level facilities to referral-level hospitals (Ganatra and Hirve 1994; Bhandari et al. 1996; Bakry et al. 1999; Kalter et al. 2003)—and few studies quantitatively assessed factors associated with compliance (Kalter et al. 2003). Furthermore, little is known about levels of and factors associated with compliance with referral by CHWs for neonatal illness.
The Project for Advancing the Health of Newborns and Mothers 2 (Projahnmo-2) was carried out in Mirzapur, Bangladesh, a rural sub-district of Dhaka division where a good-quality, relatively accessible and acceptable tertiary care hospital, Kumudini Hospital, exists (Bari et al. 2006). The project developed a service delivery strategy to promote neonatal health through home-based health education and routine household surveillance for neonatal illness by CHWs, but relied on referral to the hospital for management of serious illness. The primary purpose of this study was to examine outcomes of the surveillance programme, including (1) factors associated with coverage of postnatal assessment by CHWs, and (2) factors associated with compliance with referral by the CHWs.
Data and methods
Study population and design
Projahnmo-2 was a cluster-randomized, controlled intervention trial of a preventive and curative maternal-neonatal health care package, conducted between January 2004 and December 2006. The study area was rural, and the neonatal mortality rate (NMR) was estimated at 24 per 1000 live births in 2002. The area was served by a 750-bed, non-profit, private, referral-level hospital, Kumudini Hospital. The study population of about 292 000 was divided into 12 unions, which were randomly allocated to either control or intervention arm.
In the intervention arm, each union had six CHW areas, each of which consisted of about three villages (4000 population) and was served by one female CHW, with educational attainment of secondary school certification or higher. In total 48 CHWs participated during the 3-year intervention period. Average age was 27 years, 79% were married, and average length of schooling was 11 years. Qualification of CHWs was similar to that of Family Welfare Assistants, community-based primary health-care workers in the Bangladesh government health system, who also served a population of 4000 (Baqui et al. 2008). CHWs received an initial 35 days of training, including 6 days of field practice on pregnancy surveillance, essential newborn care, routine neonatal assessment, and management of illness based on a clinical algorithm adapted from the Bangladesh Young Infant Integrated Management for Childhood Illness (IMCI) algorithm. Detailed information on recruitment, training and retention of CHWs and their use of the clinical algorithm has been presented previously (Darmstadt et al. 2009a).
Community-level surveillance of serious illness by CHWs
In the intervention arm, the surveillance system was designed primarily to enable linking between community-level neonatal assessments by CHWs and outpatient and/or inpatient records at Kumudini Hospital. At the community-level, a unique Projahnmo number was assigned to each pregnant woman and her live-born neonate through pregnancy surveillance and postnatal visits as described below. CHWs identified pregnancies in their population through routine bimonthly household surveillance, and administered informed verbal consent to participate in the study. Pregnant women were subsequently visited at home twice during the antenatal period, typically around 18 and 34 weeks of the pregnancy, to promote birth and newborn care preparedness (BNCP), described in detail elsewhere (Darmstadt et al. 2009b). CHWs provided a BNCP card with a Projahnmo number and encouraged families to take the card for any hospital visit for expected newborns in the future. CHWs also gave a Labour Notification Card to each woman with instructions for a family member to present the card to the CHW when the pregnant woman started into labour.
CHWs, notified by the card, attended the delivery whenever possible, especially if the woman was primiparous or had birth spacing of <18 months, or visited the mother and newborn infant on the day of, or as close as possible to the time of delivery. Additional visits of each live-born neonate were scheduled on postnatal days 2, 5 and 8. During each of the postnatal visits, CHWs completed a standardized newborn assessment form, identified the presence of serious illness requiring referral to Kumudini Hospital, and made referral to the hospital as specified in the clinical algorithm. The complete algorithm is presented elsewhere (Darmstadt et al. 2009a).
Serious illnesses requiring urgent referral included: perinatal asphyxia, very severe disease (VSD), possible very severe disease (PVSD), significant jaundice on the first day of life (i.e. any jaundice in the first 24 hours), possible gonococcal eye infection [i.e. eye(s) discharging pus], diarrhoea (i.e. unusually loose, watery, frequent stools) with blood, and diarrhoea with severe dehydration (i.e. not able to drink or drinks poorly, lethargic or unconscious, or skin pinch goes back slowly over >2 seconds). A neonate was categorized to have VSD if she/he had one or more of eight signs and symptoms observed by a CHW, including: convulsion, respiratory rate ≥70/minute, severe chest in-drawing, axillary temperature >101.0°F (fever), axillary temperature <95.5°F (hypothermia), unconsciousness, many or severe skin pustules/blisters, or a single large area of pus/redness with swelling of skin, and umbilical redness extending to the abdominal skin. A neonate was classified to have PVSD if she/he had one or more of nine signs and symptoms. In 2005, after initial analysis of case fatality rates for each sign and symptom, a revision was made to include three of the original PVSD signs in the VSD category: weak, abnormal or absent cry; lethargic or less than normal movement; and not able to feed or suck at all (feeding problem). The original algorithm with eight signs for VSD and nine signs for PVSD was used from February 2004 to December 2005, while the new algorithm (i.e. eleven signs for VSD and six signs for PVSD) was introduced in October 2005 and used until the end of the study. Referral guidelines, however, were independent of these changes in illness classification algorithm, since both VSD and PVSD were managed by prompt referral.
For referred neonates, CHWs provided families a referral card with a Projahnmo number and facilitated transportation, if necessary, and all care at the hospital was free. If the family refused to be referred, the CHW continued to encourage referral but managed the neonate in the home according to the algorithm. The mean distance between each village and the hospital was 6.8 km (standard deviation [SD] 2.5, n = 122). Travel time to the hospital under usual circumstances was, on average, 70 minutes and did not exceed 2 hours. CHWs, based on the algorithm, also identified neonates with five minor illnesses requiring management at home and a follow-up visit (Darmstadt et al. 2009a). If the condition had not improved at the follow-up visit, CHWs recommended referral-level evaluation.
At the end of the first 28 days of life, CHWs re-visited all live-born neonates and recorded survival status. For neonates born elsewhere, such as at the maternal parental home, and who had not returned home yet by the end of the neonatal period, CHWs collected survival information through other household members (e.g. father) who were well updated about the neonate. For each death, age at death in days was collected.
Community-hospital linkage of surveillance
Hospital-level surveillance was conducted between February 2004 and December 2006 (Darmstadt et al. 2009c). Designated study staff recorded the address for each neonate who presented to Kumudini hospital, and ascertained whether the neonate came from the intervention arm. For all identified intervention-arm neonates, the Projahnmo number was verified through the BNCP or referral card that families carried with them, and information on outpatient visits and/or admissions was collected, regardless of whether or not the neonate had been assessed by a CHW before the hospital visits. There were 1817 outpatient visits and 835 admissions by intervention-arm neonates and the Projahnmo number was included in 1772 of the total outpatient records (98%) (from 1558 neonates) and 821 of the total admission records (98%) (from 785 neonates). We assumed an admission which occurred on the same date as or on the next day following an outpatient visit was a consequence of the same episode of illness, and considered each of these outpatient-inpatient pairs as one hospital visit. Using the 1772 outpatient and the 821 admission records, we identified a total of 2000 hospital visits records, including 593 outpatient-inpatient visits, 1179 outpatient-only (non-admitted), and 228 inpatient-only (admitted directly through the Emergency Department after clinic hours).
Hospital records were then linked with CHW assessment records using the Projahnmo number as an index variable. Consistency in sex recorded across CHW, outpatient and inpatient records was verified. We linked 1981 hospital records and 25 637 CHW assessment records for neonates born between February 2004 and November 2006, in order to ensure that the entire neonatal period of each neonate would have been included during the hospital-level surveillance period. We excluded 463 hospital records which occurred before any CHW assessment had been made, including 236 hospital records of 207 neonates who were never assessed by CHWs. Of the remaining 1518 hospital records, each record was linked to the most recent CHW assessment if there was more than one assessment for the index hospital visit. The mean interval between a CHW assessment and a hospital visit among 1518 CHW-hospital record pairs was 4.0 days (SD 5.4, median 1, range [0, 26]). We assumed only a hospital visit on the same date or on the next date following a CHW assessment (i.e. interval 0 or 1 day) was associated with the same episode of illness, and included only 805 CHW-hospital record pairs in the analysis. CHW-hospital record linkage, hereafter, refers to a record pair for which the interval between a CHW assessment and a hospital visit was only 0 or 1 day.
We defined compliance and self-referral among all CHW assessments, using CHW-hospital record linkage. Compliance referred to a hospital visit on the day of or the day following a CHW assessment for which a referral had been made (i.e. CHW-hospital record linkage identified following a referral recommendation). Self-referral referred to a hospital visit on the day of or the day following a CHW assessment for which a referral to the hospital had not been made (i.e. CHW-hospital record linkage identified after no referral recommendation). We assumed these were cases in which parents recognized danger signs of illnesses which developed after the CHW assessment, parents disagreed with a correct CHW assessment and referral recommendation, or parents disagreed with an incorrect CHW assessment and referral recommendation, although we cannot verify them using our data.
Statistical analysis
Factors associated with CHW assessment
In order to understand potential selection bias among the surveillance participants, we examined differential distribution of assessment status by selected maternal and neonatal background characteristics, using chi-square test. A P-value of 0.05 was considered statistically significant. CHW assessment status was examined using two neonatal-level outcomes: whether a neonate was assessed at least once during the neonatal period among all live births, and, among those who were ever assessed, whether the initial assessment was conducted within the first 2 days, which was shown in a study in northeastern Bangladesh to be critical for reducing neonatal mortality (Baqui et al. 2009). Background demographic and socio-economic characteristics included: child's sex, prematurity (gestational age <37 weeks), maternal age in years, primagravida, place of delivery (own home, maternal grandparents home, and health facility), maternal education (completed primary school or higher education), and living in a house with wall material of tin or cement, indicative of advanced economic status. We also examined the percentage of eligible neonates assessed with 95% confidence intervals (CI) across six unions and 36 CHW areas.
Factors associated with referral compliance
Among assessments for which CHWs recommended referral-level care, we examined associations between compliance—a hospital visit by the end of the next day following an assessment—and selected background and clinical characteristics. Demographic and socio-economic background characteristics included: child's sex, child's age at the time of referral (0–6 days vs. 7–27 days); mother's age at study enrolment (in years); parity (primagravida vs. non-primagravida); maternal education (less than primary school completion vs. more than primary school completion), and housing wall material (tin or cement vs. others). In addition, dummy variables for CHW area were included to control for varying physical accessibility to the hospital as well as any unobserved characteristics of CHWs.
In order to control for clinical characteristics, a binary variable was constructed indicating whether the child had a serious illness (i.e. one or more of the seven illnesses requiring urgent referral described above). Further dichotomous variables were created indicating whether the child had each of the seven serious illnesses. For VSD, we used classifications assigned by the CHWs on the assessment form rather than a classification based on calculations using individual VSD signs noted by the CHW, since we aimed to assess referral compliance based on actual referrals recommended rather than on a theoretical basis calculated from the presence of the individual signs observed. Additional binary variables were constructed for each of the VSD signs, in order to examine differential compliance by individual sign/symptom, independent of the classification change.
Unit of analysis was an assessment, and a total of 919 assessments with a referral recommendation were analysed. Multiple referrals were recommended in 103 neonates, resulting in 277 referrals, accounting for 30% of observations. To address correlation within each neonate, we used the generalized estimating equation (GEE) model with binomial link (Zeger et al. 1988; Liang and Zeger 1993). We used exchangeable correlation structure for GEE estimation and Huber/White/sandwich estimator of variance to estimate standard errors. We estimated differential odds of compliance by background characteristics as well as serious illness status. Multivariable analyses included variables which had a P-value <0.2 in bivariable analysis. We further assessed additional differential in referral compliance by specific illness or individual sign of serious illnesses, by testing an interaction term of binary serious illness status and an individual illness or sign. A P-value of 0.05 was considered statistically significant.
Neonatal mortality by assessment and compliance status
To further understand differential characteristics by CHW assessment, referral and compliance, we estimated neonatal morality rates (NMR) (i.e. probability of dying during the first 28 days of life) according to six groups. Neonates who were never assessed by a CHW were categorized into: (1) those with no hospital record, and (2) those with ≥1 hospital record during the first 28 days of life. Neonates who were assessed at least once but never referred to the hospital were categorized into: (3) those with no self-referral, and (4) those with ≥1 self-referral (i.e. hospital record linked by the end of the next day following a CHW assessment where referral was not recommended). Finally, neonates who were assessed at least once and referred to the hospital in association with ≥1 of the assessment(s) were categorized into: (5) those with no compliance, and (6) those with ≥1 episode of compliance (i.e. hospital record linked by the end of the next day following a CHW assessment where referral was recommended). We calculated 95% CIs for NMR estimates. STATA 9.0 statistical software (Stata Corporation, College Station, TX, USA) was used for all analyses.
The study was approved by the Committee on Human Research at the Johns Hopkins Bloomberg School of Public Health, and the Ethical Review Committee and the Research Review Committee at ICDDR,B. The study was registered at clinicaltrials.gov, No. NCT00198627.
Results
Surveillance
Between February 2004 and November 2006, there were a total of 9937 deliveries resulting in 10 006 live births (Figure 1). CHWs were present for 15% of the 7570 home deliveries. The home delivery attendance rate was 16.0% (95% CI: 14.8–17.4%) among those classified as high-risk deliveries (i.e. primagravida or preceding birth interval <18 months) (n = 3179), higher than the estimate of 13.5% (12.5–14.5%) among the deliveries not identified as high-risk (n = 4391).
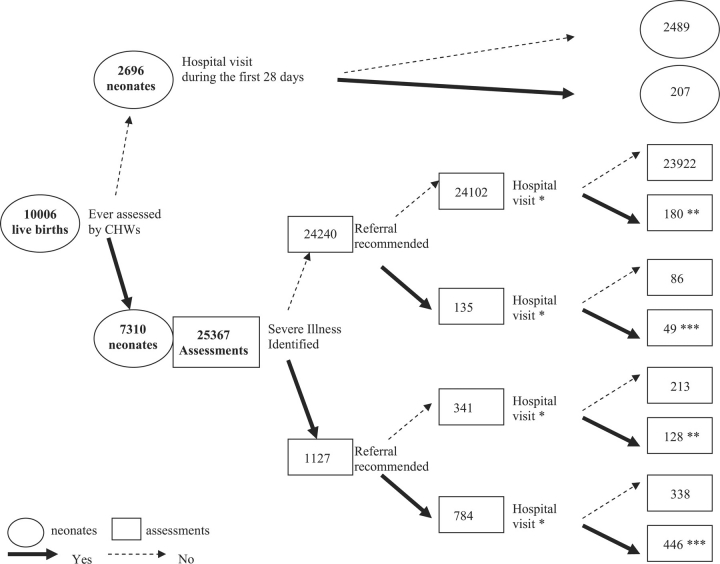
Figure 1.
Surveillance profile for live births born in the intervention arm, February 2004–November 2006. *Hospital visit by the end of next day following the assessment. **Self-referral. ***Compliance. Referral recommendation is missing in 5 assessments, of which 2 were linked with hospital visit records by the end of next day of the assessment.
Among the 10 006 neonates, 5453 received their first assessment on the same day or the day after birth and 7310 were assessed by CHWs at least once (assessment rate 73%), resulting in 25 367 assessments. The median number of CHW assessments per neonate was 4 (mean 3.5, SD 1.3, range [1, 12], n = 7310). Serious illness was identified in 1127 assessments (4%, 1127/25 367), and referral was recommended in 784 of these assessments (70%, 784/1127). Referral was also recommended in 135 assessments among those not categorized to have serious illness (0.6%, 135/24 240). In total, referral was recommended in 919 assessments (overall referral rate 4%, 919/25 367) for 745 neonates. Of those, 642 neonates received only one (n = 642 referrals) and 103 neonates received two or more referrals (n = 277 referrals).
Referral compliance rate was 54% (495/919) among the assessments for which referral was recommended. Self-referral was made in about 1% (308/24 443) of assessments without a referral recommendation. CHWs’ referral recommendation was missing in five assessments. Among the 2696 neonates who were never assessed by a CHW, 207 (8%) had one or more hospital records, 90% of which were within the first 2 days of life.
Differential characteristics by CHW assessment
Assessment status was significantly associated with place of birth. Among those born at their own home, 87% were assessed within the first 2 days and 95% were assessed at least once during the neonatal period (n = 3743) (Table 1). Overall, neonates whose mothers were younger, primipara, and with relatively higher socio-economic status—more common characteristics of women who delivered at their parental home compared with those who delivered at their own home in our study population (results not shown)—were less likely to be assessed by CHWs (Table 1).
Table 1.
Study population characteristics, and neonatal assessment status by background characteristics, Mirzapur, Bangladesh (n = 10 006)
| All |
Assessed at least once |
Never assessed |
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
Age at initial assessment |
|||||||||
|
Day 0–1 |
Day 2 or later |
||||||||
| (n = 5453) |
(n = 1857) |
(n = 2696) |
|||||||
| n | n | (%) | n | (%) | P-valuea | n | (%) | P-valueb | |
| Delivery place | |||||||||
| Own home | 3743 | 3241 | (86.6) | 314 | (8.4) | 0 | 188 | (5.0) | 0.004 |
| Maternal grandparents' home | 3827 | 2085 | (54.5) | 353 | (9.2) | 1389 | (36.3) | ||
| Health facility | 2004 | 216 | (10.8) | 1084 | (54.1) | 704 | (35.1) | ||
| Missing | 432 | 11 | (2.5) | 6 | (1.4) | 415 | (96.1) | ||
| Sex | |||||||||
| Male | 4676 | 2737 | (58.5) | 833 | (17.8) | 0.018 | 1106 | (23.7) | 0.037 |
| Female | 5016 | 2816 | (56.1) | 922 | (18.4) | 1278 | (25.5) | ||
| Missing | 314 | 0 | 0.0 | 2 | (0.6) | 312 | (99.4) | ||
| Gestational age (weeks) | |||||||||
| <37 | 1676 | 932 | (55.6) | 268 | (16.0) | 0.923 | 476 | (28.4) | 0.544 |
| ≥37 | 8005 | 4363 | (54.5) | 1427 | (17.8) | 2215 | (27.7) | ||
| Missing | 325 | 258 | (79.4) | 62 | (19.1) | 5 | (1.5) | ||
| Agec | |||||||||
| <20 | 2105 | 958 | (45.5) | 387 | (18.4) | 0 | 760 | (36.1) | 0 |
| 20–29 | 6596 | 3732 | (56.6) | 1140 | (17.3) | 1724 | (26.1) | ||
| ≥30 | 1297 | 856 | (66.0) | 229 | (17.7) | 212 | (16.3) | ||
| Missing | 8 | ||||||||
| Parity | |||||||||
| Multiparae | 5541 | 3477 | (62.8) | 858 | (15.5) | 0 | 1206 | (21.8) | 0 |
| Primiparae | 4244 | 1929 | (45.5) | 850 | (20.0) | 1465 | (34.5) | ||
| Missing | 221 | 147 | (66.5) | 49 | (22.2) | 25 | (11.3) | ||
| Maternal education | |||||||||
| None | 1801 | 1219 | (67.7) | 283 | (15.7) | 0 | 299 | (16.6) | 0 |
| Some primary | 1066 | 688 | (64.5) | 167 | (15.7) | 211 | (19.8) | ||
| Completed primary school | 7139 | 3646 | (51.1) | 1307 | (18.3) | 2186 | (30.6) | ||
| Housing wall material | |||||||||
| Raw materials | 1853 | 1174 | (63.4) | 292 | (15.8) | 0 | 387 | (20.9) | 0 |
| Tin or cement | 8134 | 4372 | (53.7) | 1462 | (18.0) | 2300 | (28.3) | ||
| Missing | 19 | ||||||||
aP-value for chi-square test between background characteristics and age at initial assessment (day 0–1 vs. day 2 or later), among those ever–assessed.
bP-value for chi-square test between background characteristics and assessment status (ever vs. never assessed).
cMother's age at the time of study enrolment during pregnancy.
Among 3743 neonates born at their own home, however, assessment coverage did not vary by maternal background characteristics or neonatal sex (results not shown). Nevertheless, assessment status varied significantly by gestational age among those born at their own home: about 83% (80–86%; n = 852) of preterm neonates were ever-assessed, significantly lower than 88% (86–89%; n = 2837) among term neonates. Assessment coverage also varied across unions, ranging from 68% in Banail to 79% in Ajgana (Table 2).
Table 2.
Variation in community health worker assessment rates and compliance for referral recommendation across six unions
| Union | No. of neonates |
Assessment rate |
No. of referrals |
Compliance rate |
||||
|---|---|---|---|---|---|---|---|---|
| Assessed at least once |
Age at initial assessment: day 0–1 |
|||||||
| % | (95% CI) | % | (95% CI) | % | (95% CI) | |||
| Ajgana | 1723 | 79.0 | (77.0–80.9) | 61.9 | (59.6–64.2) | 152 | 34.9 | (27.3–43.0) |
| Bahuria | 1693 | 73.9 | (71.7–76.0) | 56.8 | (54.4–59.1) | 166 | 48.2 | (40.4–56.1) |
| Banail | 1680 | 68.5 | (66.2–70.7) | 51.5 | (49.1–54.0) | 149 | 47.0 | (38.8–55.3) |
| Bhatgram | 1772 | 74.8 | (72.7–76.8) | 56.4 | (54.1–58.8) | 147 | 62.6 | (54.2–70.4) |
| Jamurki | 1757 | 71.9 | (69.7–74.0) | 50.9 | (48.5–53.2) | 208 | 68.3 | (61.5–74.5) |
| Warshi | 1381 | 69.4 | (66.9–71.9) | 55.4 | (52.7–58.0) | 97 | 59.8 | (49.3–69.6) |
| TOTAL | 10 006 | 919 | ||||||
Factors associated with compliance
Further background information on the neonates by assessment and referral compliance status is shown in Table 3. Among 919 assessments in which CHWs recommended referral, 784 (85%) had serious neonatal illness, 19 (2%) had minor illness requiring only a follow-up CHW visit, and 116 (13%) had neither serious nor minor illness, yet a referral recommendation was made. Union-level variation in compliance was substantially higher than that in assessment coverage, ranging from 35% in Ajgana and 68% in Jamurki (Table 2), and there was significant within-union variation across CHW areas in Ajgana and Jamurki (chi-square test results not shown).
Table 3.
Background and clinical characteristics and unadjusted differential compliance rate among assessments with referral recommendation (n = 919)
|
Assessments |
Compliance rate |
||||
| n | (%) | n | (%)a | (95% CI) | |
| Total | 919 | 495 | 53.9 | (50.6–57.1) | |
| Background characteristics | |||||
| Sex | |||||
| Female | 431 | (46.9) | 210 | 48.7 | (43.9–53.6) |
| Male | 488 | (53.1) | 285 | 58.4 | (53.9–62.8) |
| Age at assessment (day) | |||||
| 0–6 | 554 | (60.3) | 272 | 49.1 | (44.9–53.3) |
| 7–27 | 365 | (39.7) | 223 | 61.1 | (55.9–66.1) |
| Gestational age (week) | |||||
| <37 | 251 | (27.3) | 116 | 46.2 | (39.9–52.6) |
| ≥37 | 627 | (68.2) | 354 | 56.5 | (52.5–60.4) |
| Missing | 41 | (4.5) | 25 | 61.0 | (44.5–75.8) |
| Mother's education | |||||
| < primary school completion | 326 | (35.5) | 162 | 49.7 | (44.1–55.3) |
| ≥ primary school completion | 593 | (64.5) | 333 | 56.2 | (52.1–60.2) |
| Housing wall material | |||||
| Katch, wood, other | 220 | (23.9) | 97 | 44.1 | (37.4–50.9) |
| Tin or cement | 698 | (76.0) | 397 | 56.9 | (53.1–60.6) |
| Missing | 1 | (0.1) | 1 | – | |
| Union | |||||
| Ajgana | 152 | (16.5) | 53 | 34.9 | (27.3–43.0) |
| Bahuria | 166 | (18.1) | 80 | 48.2 | (40.4–56.1) |
| Banail | 149 | (16.2) | 70 | 47.0 | (38.8–55.3) |
| Bhatgram | 147 | (16.0) | 92 | 62.6 | (54.2–70.4) |
| Jamurki | 208 | (22.6) | 142 | 68.3 | (61.5–74.5) |
| Warshi | 97 | (10.6) | 58 | 59.8 | (49.3–69.6) |
| Clinical characteristics | |||||
| One or more of the 7 illnesses requiring urgent referral | |||||
| No | 135 | (14.7) | 49 | 36.3 | (28.2–45.0) |
| Yes | 784 | (85.3) | 446 | 56.9 | (53.3–60.4) |
| 7 illnesses requiring urgent referral | |||||
| Asphyxia | 33 | (3.6) | 19 | 57.6 | (39.2–74.5) |
| Very Severe Disease (VSD) | 382 | (41.6) | 246 | 64.4 | (59.4–69.2) |
| Possible VSD | 457 | (49.7) | 240 | 52.5 | (47.8–57.2) |
| Significant jaundice on the first day of life | 15 | (1.6) | 7 | – | |
| Gonococcal eye infection | 40 | (4.4) | 24 | 60.0 | (43.3–75.1) |
| Diarrhoea with blood in stool | 0 | 0.0 | – | – | |
| Diarrhoea with severe dehydration | 2 | (0.2) | 2 | – | |
| 11 individual signs of VSD | |||||
| Convulsion | 13 | (1.4) | 10 | – | – |
| Respiratory rate ≥70/min | 119 | (12.9) | 89 | 74.8 | (66.0–82.3) |
| Severe chest in drawing present | 17 | (1.8) | 11 | – | |
| Temperature >101°F | 26 | (2.8) | 19 | 73.1 | (52.2–88.4) |
| Temperature <95.5°F | 87 | (9.5) | 45 | 51.7 | (40.8–62.6) |
| Unconscious | 2 | (0.2) | 2 | – | |
| Many or severe skin pustules | 65 | (7.1) | 39 | 60.0 | (47.1–72.0) |
| Umbilical redness extending to the skin | 13 | (1.4) | 9 | – | |
| Weak, abnormal or absent cry | 54 | (5.9) | 41 | 75.9 | (62.4–86.5) |
| Lethargic or less than normal movement | 100 | (10.9) | 64 | 64.0 | (53.8–73.4) |
| Feeding problem | 139 | (15.1) | 91 | 65.5 | (56.9–73.3) |
aCompliance rate was not calculated if the denominator was less than 25.
Table 4 presents odds ratios of compliance by background and clinical characteristics, based on multivariable GEE logistic regression models, controlling for CHW areas. Referrals recommended to young neonates 0–6 days old were 30% less likely to be complied with compared with referrals among older neonates. Male sex was positively associated with compliance, with marginal significance (P-value <0.1). Odds of compliance were more than twice as high among referrals for whom serious illness was identified than among referrals without serious illness. Further, compliance was positively associated with interaction terms of serious illness and VSD (Table 4, Model 2) or selected individual signs, including respiratory rate ≥70/minute; weak, abnormal or absent cry; lethargic or less than normal movement; and feeding problem (Table 4, Models 3–6), suggesting that odds of compliance increased additionally among referrals with these conditions, even among those with serious illness.
Table 4.
Odds ratio of successful compliance by selected background and clinical characteristics: multivariable logistic regression (n = 877) a,b
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
Model 5 |
Model 6 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | |
| Neonatal characteristics | ||||||||||||
| Sex | ||||||||||||
| Female | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Male | 1.34 | (0.98–1.83) | 1.37 | (0.99–1.87) | 1.27 | (0.93–1.75) | 1.36 | (0.99–1.87) | 1.37 | (0.99–1.88) | 1.32 | (0.96–1.81) |
| Age at assessment (day) | ||||||||||||
| 0–6 | 0.70 | (0.51–0.96) | 0.70 | (0.51–0.96) | 0.72 | (0.52–0.99) | 0.67 | (0.49–0.92) | 0.67 | (0.49–0.92) | 0.67 | (0.48–0.92) |
| 7–27 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Gestational age (week) | ||||||||||||
| <37 | 0.76 | (0.53–1.10) | 0.75 | (0.52–1.10) | 0.80 | (0.55–1.16) | 0.72 | (0.49–1.05) | 0.71 | (0.49–1.03) | 0.75 | (0.52–1.09) |
| ≥37 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Socio-economic characteristics | ||||||||||||
| Mother's education | ||||||||||||
| <primary school completion | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| ≥primary school completion | 1.17 | (0.84–1.63) | 1.20 | (0.86–1.69) | 1.15 | (0.82–1.61) | 1.16 | (0.83–1.62) | 1.17 | (0.84–1.64) | 1.16 | (0.83–1.62) |
| Housing wall material | ||||||||||||
| Katch, wood, other | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Tin or cement | 1.14 | (0.76–1.71) | 1.14 | (0.76–1.71) | 1.15 | (0.77–1.73) | 1.20 | (0.81–1.80) | 1.19 | (0.79–1.79) | 1.17 | (0.78–1.75) |
| Clinical characteristics | ||||||||||||
| Serious illness | ||||||||||||
| No | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Yes | 2.37 | (1.49–3.77) | 1.78 | (1.09–2.90) | 2.15 | (1.35–3.42) | 2.27 | (1.42–3.64) | 2.18 | (1.36–3.50) | 2.24 | (1.41–3.58) |
| Interaction term of serious illness and: | ||||||||||||
| Very Severe Disease | 1.90 | (1.34–2.69) | ||||||||||
| Respiratory rate ≥70/min | 2.24 | (1.30–3.89) | ||||||||||
| Weak, abnormal or absent cry | 3.73 | (1.76–7.91) | ||||||||||
| Lethargic or less than normal movement | 2.42 | (1.44–4.09) | ||||||||||
| Feeding problem | 1.71 | (1.07–2.73) | ||||||||||
aGeneralized Equation Estimation model with an exchangeable correlation structure was used in order to address intracluster correlation at neonate-level. In total, 42 observations were excluded from the analysis due to missing covariates, resulting in 877 assessments from 710 neonates.
bCHW-area dummy variables were included in each model, but results are not shown.
cUrgent referral conditions included following 7 illnesses: perinatal asphyxia, very severe disease, possible very severe disease, significant jaundice on the first day of life, possible gonococcal eye infection, diarrhoea with blood, and diarrhoea with severe dehydration.
dInteraction term of having urgent referral condition and Very Severe Disease (VSD) as well as each of the 11 VSD signs were examined. Only significant interaction terms are presented.
Differential neonatal mortality rate
Overall NMR in the entire surveillance population was 23.9 per 1000 live births. NMR was significantly higher among 2696 neonates never assessed (55.3 per 1000) than among 7310 neonates assessed by CHWs at least once (12.3 per 1000) (Table 5). Furthermore, in the never-assessed group, nearly 40% of neonates who were admitted to the hospital at least once (n = 108) died (results not shown) and NMR among those who did not visit the hospital during the neonatal period was 43.4 per 1000, also significantly higher than the overall NMR in the ever-assessed group (Table 5).
Table 5.
Neonatal mortality rate (NMR) (per 1000 live births) by assessment, referral recommendation and hospital record linkage status (n = 10 006)
| Live births | Neonatal deaths | NMR | (95% CI) | |
|---|---|---|---|---|
| Never assessed by CHWs | 2696 | 149 | 55.3 | (46.9–64.6) |
| No hospital record | 2489 | 108 | 43.4 | (35.7–52.2) |
| Hospital record | 207 | 41 | 198.1 | (146.0–259.0) |
| Assessed by CHWs at least once | 7310 | 90 | 12.3 | (9.9–15.1) |
| Never referred to the hospital | 6560 | 26 | 4.0 | (2.6–5.8) |
| No self-referrala | 6278 | 22 | 3.5 | (2.2–5.3) |
| Self-referrala at least once | 282 | 4 | 14.2 | (3.9–35.9) |
| Referral was recommended at least once | 745 | 60 | 80.5 | (62.0–102.5) |
| No complianceb | 269 | 23 | 85.5 | (55.0–125.5) |
| Complianceb at least once | 476 | 37 | 77.7 | (55.3–105.6) |
| Referral information is missing | 5 | 4 | – | – |
| Total | 10 006 | 239 | 23.9 | (20.9–26.9) |
aSelf-referral: hospital record linked by the end of the next day following a CHW assessment where referral was not recommended.
bCompliance: hospital record linked by the end of the next day following a CHW assessment where referral was recommended. – NMR was not estimated due to small sample size.
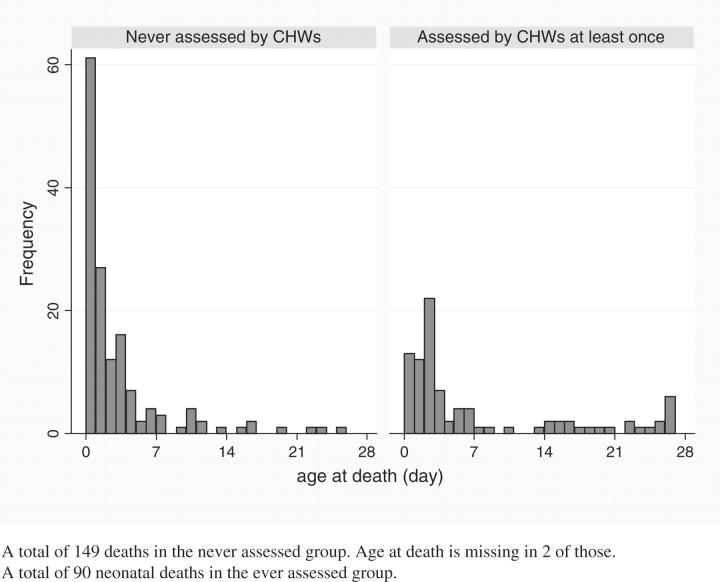
Figure 2 shows the markedly different distributions of age-at-death in days by CHW assessment status. In the never-assessed group, 41% (33–50%) and 60% (51–68%) of deaths occurred by the end of the first and second day, respectively, significantly higher than the cumulative proportions in their assessed counterparts, 14% (8–23%) and 28% (19–38%), respectively. Among the 239 neonates who died, only 90 (38%) were assessed by CHWs at least once prior to death. Furthermore, CHWs were able to assess only 18% and 33% of neonates who died on day 0 and during the first week of life, respectively.
Figure 2.
Distribution of age at death (day) by community health worker assessment status (n = 239).
Table 5 further shows NMR by referral and hospital visit status, among those who had a CHW assessment at least once. NMR among neonates who were never referred to the hospital was 4.0 per 1000, significantly lower than the NMR of 80.5 among those for whom referral was recommended at least once.
Discussion
Surveillance for serious illness, conducted in the intervention arm of the Projahnmo-2 trial, had a number of unique strengths. As reported previously, CHWs identified and classified serious illness with high sensitivity and specificity (Darmstadt et al. 2009a). For the referred neonates, the hospital was accessible in about an hour on average, cost was free (borne by the project) and, if necessary, transportation was arranged for free as well, facilitating referrals and the decision to seek care as much as possible. Formative research suggested positive community perception of the quality of care at Kumudini hospital, a tertiary care centre which was relatively well staffed and equipped (Bari et al. 2006). Thus, we attempted to address each of the three major delays in care seeking: problem recognition and decision to seek care, reaching a facility, and receipt of quality care (Barnes-Josiah et al. 1998). Finally, a unique link system between the community and the hospital enabled us to verify care seeking and clinical outcomes following CHWs’ assessments and referral recommendations.
Despite rigorous efforts to reach all live-born neonates, only 54% of target neonates received the initial CHW assessment on day 0 or 1 of life and only 73% of all the neonates were assessed ever during the neonatal period. Moreover, only about 15% of newborns were attended at delivery. A similar home-based newborn care programme implemented in Gadchiroli, India—where a CHW covered a population of approximately 1000—reached a coverage of 84% for home-delivery attendance and 93% for newborn assessment (Bang et al. 1999; Bang et al. 2005a; Bang et al. 2005b). Our data suggest that two main barriers for achieving high coverage of immediate newborn care through CHW visits may be difficulties in attending deliveries, and failure to reach those who died very early. With a CHW to population ratio of 1 : 4000 (similar to the primary health care worker-to-population ratio in the Bangladesh governmental health system), integrating delivery and immediate neonatal care, for example, through a skilled birth attendant, may be the only promising approach to avert early neonatal deaths during or very early after delivery. In addition, in communities where delivering at the maternal home is a common custom, particularly for primagravid women whose neonates have a substantially increased mortality risk associated with both first-order birth as well as young maternal age (Mahy 2003), the temporary change of residence around delivery time needs to be considered in programme design in order to ensure that neonates are reached on or immediately after delivery. However, in a programme with wide coverage, this issue would be mitigated. Programmes need to assure service availability for new mothers and neonates through a responsive and complete labour notification system, regardless of whether the delivery occurred in the home of the parents or grandparents.
Overall compliance rate was estimated to be only 54% among those with referral recommendations, indicating that substantial barriers remained for care seeking even though the programme was designed to eliminate major barriers such as danger sign recognition, cost and access to the hospital. First, significantly lower odds of compliance among neonates 0–6 days of age suggests that unique barriers exist for young neonates, such as the cultural norm to seclude the mother and baby after delivery as a means of protecting them (Winch et al. 2005), maternal postpartum condition, and, possibly difficulty in assessment of certain signs (e.g. respiratory rate) among young neonates (Darmstadt et al. 2009a), leading to reduced confidence on the part of the family in the CHW's recommendation. It is crucial to address these barriers among young neonates, in whom 80% of all neonatal deaths occurred in our population. The finding also suggests that CHW surveillance systems should consider the feasibility of including home-based treatment in intervention packages, especially for young neonates given the low compliance rate, even in a setting where a good-quality, accessible and acceptable health facility exists in the community. Options include initiation of therapy at home coupled with facilitated referral and continuation of therapy at the hospital, or if referral is refused, treatment of serious illness at home (Bang et al. 1999; Bang et al. 2005c; Baqui et al. 2008).
Second, our analyses suggest that family's perception regarding specific illnesses and signs may play an important role in care seeking. However, family's perception regarding these signs may not necessarily be related with mortality risk. A study examined the population attributable risk fraction (PAF) of neonatal mortality in the Mirzapur surveillance population and reported a PAF of 0.29 for feeding problem, 0.18 for lethargy, 0.10 for weak, abnormal or absent cry, and 0.05 for respiratory rate ≥70/minute (Darmstadt et al. 2008a), four individual signs associated with increased odds of compliance in our analyses. However, while severe hypothermia (<95.5°F) and moderate-severe hypothermia (<97.5°F) had a PAF of 0.27 and 0.46, respectively (Darmstadt et al. 2008a), there was no differential odds of compliance related with these signs. Thus, programmatic emphasis on recognition of danger signs and promotion of care seeking must include more specific information on the importance of these signs.
Further, NMR differentials by assessment, referral and compliance further confirm limitations and strengths of the surveillance. First, differential NMR by assessment status suggests the surveillance failed to reach the most vulnerable neonates, who died before the first assessment could be made. This has important implications for home-based strategies to reach newborns, since our pregnancy and delivery surveillance systems and home visitation programme were intensively implemented, including supervision, in a setting in which families had high acceptance of the CHW home visits and high regard for the hospital, and yet most deaths occurred outside of our reach. A major limiting factor seemed to be that a CHW covered approximately three villages, and thus timely notification of labour and attendance at the delivery were limited, especially at night, and thus the ability of the CHW to intervene at or immediately after delivery.
Second, among the ever-assessed neonates, the NMR difference by referral implies that CHWs’ administration of the clinical algorithm properly screened seriously ill neonates. Both CHWs’ classification of VSD and the clinical algorithm were validated previously (Darmstadt 2008a; Darmstadt et al. 2009a), and our CHWs’ management of illness following the clinical algorithm—70% for correctly recommending referral (n = 1127) and 99.4% for correctly not recommending referral (n = 24 240)—is better than overall performance of most IMCI workers (Huicho et al. 2008).
Lastly, no significant NMR difference by compliance status among those referred needs careful interpretation since we were not able to examine severity of condition by compliance status and care seeking outside of Kumudini hospital. If compliance to Kumudini hospital as well as care seeking outside the hospital was irrespective of severity of condition, a lower NMR in the compliance group would be expected due to the treatment. However, although certain signs associated with mortality risk such as moderate hypothermia were negatively associated with compliance with referral, the mean number of individual signs and symptoms of severe illness was 1.36 (1.26–1.44) among referrals complied with, significantly higher than 1.01 (0.93–1.10) among non-compliant counterparts. Thus, it is likely that referrals were complied with among relatively sicker neonates and that the NMR without referral and treatment among them would have been higher than what we observed, however, this cannot be proven.
Our paper highlights the need for additional understanding of several aspects of community surveillance and case management for illness. First, qualitative studies on local understanding of hypothermia will be essential to promote recognition and promotion of care seeking for this critical sign (Darmstadt et al. 2006). Second, about 30% of assessments which were categorized to have serious illness were not referred and factors associated with CHWs’ adherence to the clinical algorithm in guiding referral recommendations needs to be further studied. In addition, 27% of those who were classified not to have serious illness but referred were born before 37 gestational weeks (results not shown). This suggests that additional understanding is needed regarding whether a separate algorithm is necessary for preterm neonates for clinical as well as programmatic reasons. Finally, implications of temporary residence change around delivery need to be further examined. Even though our data suggest that mothers who gave birth at their parents’ home tend to belong to a higher socio-economic group, discontinuity of care and unfamiliarity with a new health care system may be barriers for care seeking during the critical early postnatal period.
In conclusion, well-trained and supervised CHWs are capable of conducting high-quality routine household surveillance of newborns (Darmstadt et al. 2009a), and previous evaluations of this strategy have shown significant potential to reduce neonatal mortality through identification and referral of sick newborns for care at hospital (Bhutta et al. 2005; Darmstadt et al. 2005; Haws et al. 2007; Baqui et al. 2008; Bhutta et al. 2008). However, critical barriers include the ability to reach newborns at delivery and in the immediate postnatal period when the vast majority of neonatal mortality occurs, and community reluctance to comply with referral of preterm newborns. For optimal impact, community surveillance must be coupled with skilled attendance at delivery, and/or accommodate the presence of a worker skilled in recognition of neonatal illness within the village or in close proximity to the community to allow for rapid assessment, and potentially also the early initiation of treatment at home, in the immediate postnatal period. In addition, in many health systems contexts, the same cadre of workers that provides community-based case management of childhood illness will need to be utilized to also provide care for neonates, since a newborn-specific health care provider is unlikely to be feasible. Thus, further evidence is needed to inform strategies for integrated case management of neonatal and childhood illness.
Acknowledgements
This study was supported by the Wellcome Trust—Burroughs Wellcome Fund Infectious Disease Initiative 2000, and the Office of Health, Infectious Diseases and Nutrition, Global Health Bureau, United States Agency for International Development (USAID) through the Global Research Activity Cooperative agreement with the Johns Hopkins Bloomberg School of Public Health (award HRN-A-00-96-90006-00). Support for data analysis and manuscript preparation was provided by the Saving Newborn Lives program through a grant by the Bill & Melinda Gates Foundation to Save the Children-US.
References
- Bakry N, Laabid A, De Brouwere V, Dujardin B. [Why patients do not comply with reference decision made by general practitioners?] Revue d'Épidémiologie et de Santé Publique. 1999;47(Suppl. 2):2S65–2S74. [PubMed] [Google Scholar]
- Bang AT, Bang RA, Baitule SB, Reddy MH, Deshmukh MD. Effect of home-based neonatal care and management of sepsis on neonatal mortality: field trial in rural India. The Lancet. 1999;354:1955–61. doi: 10.1016/S0140-6736(99)03046-9. [DOI] [PubMed] [Google Scholar]
- Bang AT, Bang RA, Baitule SB, Reddy HM, Deshmukh MD. Management of birth asphyxia in home deliveries in rural Gadchiroli: the effect of two types of birth attendants and of resuscitating with mouth-to-mouth, tube-mask or bag-mask. Journal of Perinatology. 2005a;25(Suppl. 1):S82–S91. doi: 10.1038/sj.jp.7211275. [DOI] [PubMed] [Google Scholar]
- Bang AT, Bang RA, Stoll BJ, Baitule SB, Reddy HM, Deshmukh MD. Is home-based diagnosis and treatment of neonatal sepsis feasible and effective? Seven years of intervention in the Gadchiroli field trial (1996 to 2003) Journal of Perinatology. 2005b;25(Suppl. 1):S62–S71. doi: 10.1038/sj.jp.7211273. [DOI] [PubMed] [Google Scholar]
- Bang AT, Reddy HM, Deshmukh MD, Baitule SB, Bang RA. Neonatal and infant mortality in the ten years (1993 to 2003) of the Gadchiroli field trial: effect of home-based neonatal care. Journal of Perinatology. 2005c;25(Suppl. 1):S92–107. doi: 10.1038/sj.jp.7211277. [DOI] [PubMed] [Google Scholar]
- Baqui AH, El-Arifeen S, Darmstadt GL, et al. Effect of community-based newborn-care intervention package implemented through two service-delivery strategies in Sylhet district, Bangladesh: a cluster-randomised controlled trial. The Lancet. 2008;371:1936–44. doi: 10.1016/S0140-6736(08)60835-1. [DOI] [PubMed] [Google Scholar]
- Baqui AH, Ahmed S, El Arifeen S, et al. Effect of timing of first postnatal care home visit on neonatal mortality in Bangladesh: an observational cohort study. British Medical Journal. 2009;339:b2826. doi: 10.1136/bmj.b2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari S, Mannan I, Rahman MA, et al. Trends in use of referral hospital services for care of sick newborns in a community-based intervention in Tangail District, Bangladesh. Journal of Health, Population and Nutrition. 2006;24:519–29. [PMC free article] [PubMed] [Google Scholar]
- Barnes-Josiah D, Myntti C, Augustin A. The “three delays” as a framework for examining maternal mortality in Haiti. Social Science & Medicine. 1998;46:981–93. doi: 10.1016/s0277-9536(97)10018-1. [DOI] [PubMed] [Google Scholar]
- Bhandari N, Bahl R, Bhatnagar V, Bhan MK. Treating sick young infants in urban slum setting. The Lancet. 1996;347:1774–5. [PubMed] [Google Scholar]
- Bhutta ZA, Darmstadt GL, Hasan BS, Haws RA. Community-based interventions for improving perinatal and neonatal health outcomes in developing countries: a review of the evidence. Pediatrics. 2005;115(2 Suppl.):519–617. doi: 10.1542/peds.2004-1441. [DOI] [PubMed] [Google Scholar]
- Bhutta ZA, Memon ZA, Soofi S, et al. Implementing community-based perinatal care: results from a pilot study in rural Pakistan. Bulletin of the World Health Organization. 2008;86:452–9. doi: 10.2471/BLT.07.045849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmstadt GL, Black RE, Santosham M. Research priorities and postpartum care strategies for the prevention and optimal management of neonatal infections in less developed countries. Pediatric Infectious Disease Journal. 2000;19:739–50. doi: 10.1097/00006454-200008000-00014. [DOI] [PubMed] [Google Scholar]
- Darmstadt GL, Bhutta ZA, Cousens S, et al. Evidence-based, cost-effective interventions: how many newborn babies can we save? The Lancet. 2005;365:977–88. doi: 10.1016/S0140-6736(05)71088-6. [DOI] [PubMed] [Google Scholar]
- Darmstadt GL, Kumar V, Yadav R, et al. Introduction of community-based skin-to-skin care in rural Uttar Pradesh, India. Journal of Perinatology. 2006;26:597–604. doi: 10.1038/sj.jp.7211569. [DOI] [PubMed] [Google Scholar]
- Darmstadt GL, Baqui AH, Choi Y, et al. 2008a. Validation of clinical algorithm to identify neonates with severe illness during routine household visits in rural Bangladesh. unpublished manuscript. [DOI] [PubMed] [Google Scholar]
- Darmstadt GL, Walker N, Lawn JE, et al. Saving newborn lives in Asia and Africa: cost and impact of phased scale-up of interventions within the continuum of care. Health Policy and Planning. 2008b;23:101–17. doi: 10.1093/heapol/czn001. [DOI] [PubMed] [Google Scholar]
- Darmstadt GL, Baqui AH, Choi Y, et al. Validation of community health workers' assessment of neonatal illness in rural Bangladesh. Bulletin of the World Health Organization. 2009a;87:12–19. doi: 10.2471/BLT.07.050666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmstadt GL, Baqui AH, Choi Y, et al. 2009b. Changes in knowledge, newborn care practice, and neonatal mortality: a cluster-randomised controlled trial of a package of community-based newborn care interventions in Mirzapur, Bangladesh. unpublished manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmstadt GL, Saha S, Choi Y, et al. Population-based incidence and etiology of culture-proven, community-acquired neonatal sepsis in Mirzapur, Bangladesh: implications for treatment strategies. Journal of Infectious Diseases. 2009c doi: 10.1086/605473. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganatra B, Hirve S. Male bias in health care utilization for under-fives in a rural community in western India. Bulletin of the World Health Organization. 1994;72:101–4. [PMC free article] [PubMed] [Google Scholar]
- Haws RA, Thomas AL, Bhutta ZA, Darmstadt GL. Impact of packaged interventions on neonatal health: a review of the evidence. Health Policy and Planning. 2007;22:193–215. doi: 10.1093/heapol/czm009. [DOI] [PubMed] [Google Scholar]
- Huicho L, Scherpbier RW, Nkowane AM, Victora CG. How much does quality of child care vary between health workers with differing durations of training? An observational multicountry study. The Lancet. 2008;372:910–16. doi: 10.1016/S0140-6736(08)61401-4. [DOI] [PubMed] [Google Scholar]
- Jokhio AH, Winter HR, Cheng KK. An intervention involving traditional birth attendants and perinatal and maternal mortality in Pakistan. New England Journal of Medicine. 2005;352:2091–9. doi: 10.1056/NEJMsa042830. [DOI] [PubMed] [Google Scholar]
- Kalter HD, Salgado R, Moulton LH, et al. Factors constraining adherence to referral advice for severely ill children managed by the Integrated Management of Childhood Illness approach in Imbabura Province, Ecuador. Acta Paediatrica. 2003;92:103–10. doi: 10.1111/j.1651-2227.2003.tb00478.x. [DOI] [PubMed] [Google Scholar]
- Kumar V, Mohanty S, Kumar A, et al. Effect of community-based behaviour change management on neonatal mortality in Shivgarh, Uttar Pradesh, India: a cluster-randomized, controlled trial. The Lancet. 2008;372:1151–62. doi: 10.1016/S0140-6736(08)61483-X. [DOI] [PubMed] [Google Scholar]
- Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: when? Where? Why? The Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- Lawn JE, Cousens SN, Darmstadt GL, et al. 1 year after The Lancet Neonatal Survival Series—was the call for action heard? The Lancet. 2006;367:1541–7. doi: 10.1016/S0140-6736(06)68587-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Regression analysis for correlated data. Annual Review of Public Health. 1993;14:43–68. doi: 10.1146/annurev.pu.14.050193.000355. [DOI] [PubMed] [Google Scholar]
- Mahy M. Childhood Mortality in the Developing World: A Review of Evidence from the Demographic and Health Surveys. Calverton, MD: ORC Macro; 2003. [Google Scholar]
- Manandhar DS, Osrin D, Shrestha BP, et al. Effect of a participatory intervention with women's groups on birth outcomes in Nepal: cluster-randomised controlled trial. The Lancet. 2004;364:970–9. doi: 10.1016/S0140-6736(04)17021-9. [DOI] [PubMed] [Google Scholar]
- Winch PJ, Alam MA, Akther A, et al. Local understandings of vulnerability and protection during the neonatal period in Sylhet District, Bangladesh: a qualitative study. The Lancet. 2005a;366:478–85. doi: 10.1016/S0140-6736(05)66836-5. [DOI] [PubMed] [Google Scholar]
- Winch PJ, Gilroy KE, Wolfheim C, et al. Intervention models for the management of children with signs of pneumonia or malaria by community health workers. Health Policy and Planning. 2005b;20:199–212. doi: 10.1093/heapol/czi027. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–60. [PubMed] [Google Scholar]