Abstract
Apoptosis triggered by p53 upon DNA damage secures removal of cells with compromised genomes, and is thought to prevent tumorigenesis. In contrast, we provide evidence that p53-induced apoptosis can actively drive tumor formation. Mice defective in p53-induced apoptosis due to loss of its proapoptotic target gene, puma, resist γ-irradiation (IR)-induced lymphomagenesis. In wild-type animals, repeated irradiation injury-induced expansion of hematopoietic stem/progenitor cells (HSCs) leads to lymphoma formation. Puma−/− HSCs, protected from IR-induced cell death, show reduced compensatory proliferation and replication stress-associated DNA damage, and fail to form thymic lymphomas, demonstrating that the maintenance of stem/progenitor cell homeostasis is critical to prevent IR-induced tumorigenesis.
Keywords: Apoptosis, p53, BH3-only proteins, stem cells, γ-irradiation, cancer
Tumorigenesis is a multistep process that can be initiated or accelerated by genetic lesions caused by DNA damage. These oncogenic events facilitate neoplastic transformation by the selection of disease-initiating “cancerous stem cells” against anti-oncogenic mechanisms, such as apoptosis, cellular senescence, and/or maintenance of genomic stability (Rossi et al. 2008). All of these defense mechanisms depend to a significant extent on the tumor suppressor p53, the gene most frequently inactivated in human cancer (Vousden and Lane 2007). p53's ability to promote apoptosis in response to DNA damage is considered critical for tumor suppression, but induction of cellular senescence or the maintenance of genomic integrity is considered to contribute (Vousden and Lane 2007). Apoptosis induced in response to DNA damage is mediated mainly by the p53 target gene puma, but other members of the “BH3-only” subgroup of Bcl2 proteins, including bim and noxa, can contribute (Erlacher et al. 2005; Michalak et al. 2008). Puma and Bim show nonredundant functions in lymphocyte apoptosis; e.g., in cell death induced by cytokine deprivation, glucocorticoids, and DNA damage (Erlacher et al. 2006). Notably, thymocytes and mature T and B cells from bim−/−puma−/− double-deficient mice resist γ-irradiation (IR)-induced apoptosis more potently than lymphocytes from puma−/−noxa−/− mice, although bim, in contrast to noxa, is not a direct p53 target gene (Erlacher et al. 2006; Michalak et al. 2008). Loss of BH3-only proteins, most frequently Bim, has been documented in human cancer, and is associated with impaired drug responsiveness and/or poor prognosis (Frenzel et al. 2009). In line with a critical role for BH3-only protein-mediated apoptosis in tumor suppression, loss of Puma or Bim accelerates oncogene-driven tumorigenesis in mice (Egle et al. 2004; Garrison et al. 2008; Michalak et al. 2009). Here, we investigated the contribution of these two BH3-only proteins to tumor suppression in response to DNA damage in gene-ablated mice, and provide evidence that p53-induced apoptosis, executed via activation of Puma, can promote IR-induced lymphomagenesis.
Results and Discussion
Loss of puma prevents IR-induced thymic lymphoma formation
Exposure of mice to repeated low-dose IR drives lymphoma formation through a mechanism suppressed by p53 (Kaplan and Brown 1952; Kemp et al. 1994). Whole-body IR of mice induced the DNA damage response regulators p53, p21, Puma (but not Bim), and γ-phosphorylated H2AX, as well as hallmarks of apoptosis such as activated caspase-3 and cleaved PARP in thymocytes. Interestingly, H2AX was still found phosphorylated in puma−/− or p53−/− cells 24 h after irradiation, indicating that damaged cells are still present in these but successfully cleared in wild-type mice (Supplemental Fig. 1A). As expected (Macleod et al. 1995; Michalak et al. 2008), p53-deficient thymocytes failed to induce p21 or Puma, or to fully process caspase-3. Puma−/− cells also poorly induced p21, despite normal stabilization of p53—a phenomenon not observed when recapitulated ex vivo (Supplemental Fig. 1A,B), suggesting that this effect was not cell-autonomous. Nonetheless, puma−/− thymocytes halted their cell cycle as efficiently as wild-type cells, evidenced by the fast drop of phospho-histone 3 (pH3)-positive mitotic cells. In addition, the percentage of thymocytes that incorporated BrdU into DNA following IR was reduced in wild-type, bim−/−, and puma−/− mice to a similar extent, suggesting that these mouse mutants show a largely normal DNA damage response. Consistent with previous observations, p53−/− cells failed to arrest in G1 (Brugarolas et al. 1995), but showed a largely normal G2 arrest (Supplemental Fig. 2A,B).
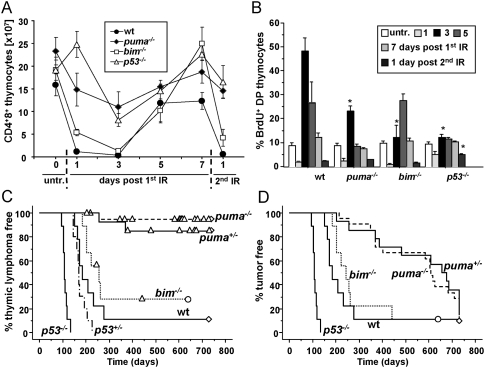
The numbers of CD4+8+ (DP) thymocytes in wild-type mice dropped to ∼10% of untreated controls within 24 h after a single dose of IR, and mobilization of progenitors replenished the organ before the next irradiation cycle. Depletion of DP thymocytes after IR was mildly delayed in bim−/− mice, consistent with our previous finding (Erlacher et al. 2005), but cell numbers also dropped to ∼10% on day 3 post-IR. Strikingly, thymic cellularity never fell below 50% in γ-irradiated mice lacking Puma or p53 (Fig. 1A). Thymocyte numbers rebounded in mice of all genotypes prior to subsequent irradiation. Together, these observations indicate that puma−/− thymocytes resist IR-induced apoptosis nearly as potently as p53−/− ones.
Figure 1.
Loss of Puma delays IR-induced lymphomagenesis. Wild-type, puma−/−, and p53−/− mice 4–6 wk of age were exposed to 1.75 Gy of whole-body IR, and were sacrificed at the indicated time points. (A) The number of CD4+8+ thymocytes was quantified by cell counting and cell surface staining, followed by flow cytometric analysis. Each data point represents the mean ± SEM of three to six animals per genotype and time point. Statistically significant differences in cell number were observed between wild-type versus puma−/− mice on days 1 and 3 after the first IR and day 1 after the second IR (P < 0.025); between wild-type versus bim−/− mice on day 1 (P = 0.005); and between wild-type and p53−/− mice on days 1, 3, and 7 after the first IR and day 1 after the second IR (P < 0.014). Untreated puma−/− mice also showed increased cellularity compared with the other genotypes (P < 0.001). (B) Mice of the indicated genotypes were exposed to 1.75 Gy of IR at the indicated time points and were injected i.p. with BrdU 4 h before sacrifice. The percentage of cycling CD4+8+ thymocytes was defined by combined staining of cell surface markers, followed by intracellular staining of BrdU, followed by flow cytometric analysis. Data points represent the mean ± SEM of three to six animals per genotype and time point. Statistically significant differences in BrdU uptake were observed between wild-type versus puma−/− mice on day 3 (P = 0.003), wild-type versus bim−/− on day 3 (P < 0.015), and wild-type versus p53−/− at day 3 after the first IR and day 1 after the second IR (P < 0.02). Untreated controls were not different across genotypes. Cohorts of wild-type (n = 16), puma+/− (n = 12), puma−/− (n = 20), bim−/− (n = 9), and p53−/− (n = 9) mice were subjected to fractionated IR (four times at1.75 Gy) at weekly intervals, starting at 4 wk of age, and were monitored for the development of tumors up to 730 d. (C) Kaplan-Meier analysis of thymic lymphoma-free survival of mice of the indicated genotypes. (D) Tumor-free survival of cohorts of mice shown in C. Mean survival in days ± SE: 201 ± 9 for wild type versus 570 ± 52 for puma+/− versus 558 ± 38 for puma−/− (Fig. 2A,B); P < 0.0001. Circles represent mice still alive, triangles represent mice that developed tumors distinct from thymic lymphomas, and diamonds represent mice that were tumor-free at the end of the observation period; i.e., 730 d.
IR-triggered cell death was followed by a proliferative burst in the thymi of wild-type mice, peaking at day 3 after treatment, indicating massive mobilization of progenitors from the bone marrow and compensatory proliferation within the thymus (Fig. 1B; Supplemental Fig. 2B). Bim deficiency caused a delayed proliferative burst, reaching its maximum at day 5 post-IR, in correlation with the delayed thymocyte depletion (Fig. 1A). In contrast, mice lacking Puma or p53 showed a strongly reduced proliferative response (Fig. 1B).
Following cohorts of mice treated with four weekly doses of IR, we observed that loss of p53 accelerated thymic lymphoma formation, confirming a previous study (Kemp et al. 1994). Loss of Bim had no effect on tumor latency (P = 0.17). Remarkably, loss of one or both alleles of puma abrogated thymic lymphoma formation induced by IR (Fig. 1C). Long-term surveillance and histopathological assessment revealed that, very late in follow-up, a portion of puma+/− and puma−/− mice developed different malignancies, including high-grade and low-grade lymphomas, carcinomas, and sarcomas, but others were still without signs of pathology when analyzed after 730 d (Fig. 1D; Supplemental Fig. 3). Notably, in untreated animals, loss of Puma did not reduce spontaneous tumorigenesis or extend lifespan, suggesting that irradiation injury is necessary to unmask this unexpected tumor-suppressive process in puma−/− mice (Supplemental Table1; Supplemental Fig. 3).
Lack of T-cell apoptosis upon DNA damage fails to prevent lymphomagenesis
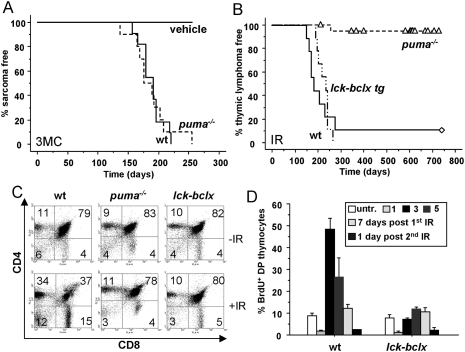
According to the “initiator–promoter” model of tumor formation, these observations suggest that enhanced proliferation of stem/progenitor cells triggered by apoptosis of differentiated cells and subsequent creation of space acts as a strong promoter of tumor formation by expanding progenitors that carry oncogenic lesions induced by DNA damage. If leukocyte depletion in the thymus was critical for lymphomagenesis, overexpression of Bcl-xL in the T-cell lineage should prevent tumorigenesis after IR. Similarly, Puma deficiency should fail to delay tumor formation triggered by DNA damage in radio-resistant tissues lacking a compensatory proliferative response. The latter was addressed using intramuscular (i.m.) injection of the hydrocarbon 3-Methylcholanthrene (3-MC), a carcinogen that causes DNA damage by forming bulky adducts, promoting formation of fibrosarcomas, suppressed by p53 (Garcia-Cao et al. 2002). Fibrosarcomas formed within 7 mo in wild-type and puma−/− mice alike (Fig. 2A), suggesting that the protection from tumorigenesis observed with loss of Puma needs tissue depletion and the generation of space. In contrast, overexpression of Bcl-xL in thymocytes and T cells (Chao et al. 1995) did not significantly delay the formation of thymic lymphomas in lck-bcl-x transgenic mice exposed to IR (Fig. 2B). This was surprising, given that overexpression of Bcl-xL prevented cell death as well as compensatory proliferation in the thymus after IR as efficiently as loss of Puma (Fig. 2C,D; Supplemental Fig. 4A). Together, this suggested that generation of space by T-cell apoptosis cannot be the sole critical factor driving tumorigenesis, and that the “tumor-initiating cell”—kept in check by the absence of cell death in puma−/− mice—must be either a very early thymic progenitor in which the lck promoter is still silent, or an even less differentiated hematopoietic progenitor. This idea is consistent with the observation that vav-bcl-2 transgenic mice overexpressing Bcl-2 in all hematopoietic cells, including hematopoietic stem/progenitor cells (HSCs), fail to develop thymic lymphomas upon IR (Michalak et al. 2010), and the observation that IR-induced tumorigenesis can be suppressed by the transfer of healthy bone marrow into irradiated recipients or bone marrow shielding (Kominami and Niwa 2006). Therefore, we reasoned that IR damage in stem/progenitor cells selects for a pool of cells carrying oncogenic lesions that provide a proliferative advantage, acquiring additional alterations during differentiation and expansion. This may facilitate transformation most frequently in the thymus, presumably due to the high risk for chromosomal translocations arising during the recombination of T-cell receptors (Liao and Van Dyke 1999). Alternatively, most stem/progenitor cells actually die after IR, and the few survivors are forced to replenish the periphery to avoid fatal hypoplasia, thereby accumulating further DNA damage due to replication stress. Such primed precancerous cells are usually cleared by a functional DNA damage response. This model is consistent with the high frequency of p53 inactivation observed in IR-induced thymic lymphomas from wild-type and p53+/− mice (Brathwaite et al. 1992; Kemp et al. 1994), and a recent study describing that irradiation strongly selects for p53-deficient hematopoietic progenitors (Marusyk et al. 2010).
Figure 2.
Lack of protection from tumorigenesis by loss of Puma in the absence of tissue depletion or by overexpression of Bcl-x in the T-lymphoid linage. (A) Cohorts of adult wild-type (n = 10) and puma−/− (n = 11) mice received a single injection of 3-MC or vehicle (seven mice per genotype) i.m. in the gluteus. Local tumor growth was monitored over 255 d, and animals were sacrificed when tumors reached ≥1 cm in size. (B) Cohorts of lck-bcl-x tg (n = 9) mice were monitored for tumorigenesis triggered by fractionated IR, and were monitored for the development of thymic lymphomas over time. Triangles represent mice that developed tumors distinct from thymic lymphomas, and diamonds represent mice that were tumor-free at 730 d. (C) Wild-type, puma−/−, and lck-bcl-x tg mice were exposed to a single dose of IR (1.75 Gy). Animals were sacrificed after 24 h, and thymic single-cell suspensions were counted and stained for cell surface markers, followed by flow cytometric analysis. Representative dot blots from three independent experiments and animals per genotype defining the changes in the percentages of CD4+8+ thymocytes upon IR are shown. (D) Quantification of BrdU incorporation and S-phase activity in lck-bcl-x tg transgenic mice, performed as described in Figure 1B. Statistically significant differences in BrdU uptake were observed between wild-type versus bcl-x tg mice on day 3 (P = 0.007).
Lack of stem cell apoptosis and compensatory proliferation in irradiated puma−/− mice
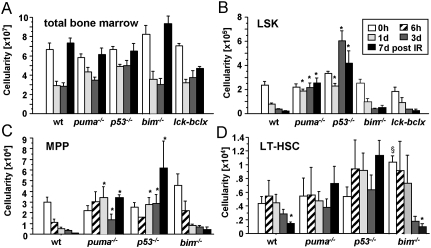
We monitored bone marrow dynamics upon IR and analyzed the HSC-containing lin−Sca-1+c-kit+ (LSK) population, as well as the (lin−Sca1+ckit+CD48−CD150−) multipotent progentiors (MPPs) and (lin−Sca1+ckit+CD48−CD150+) fraction, highly enriched for long-term HSCs (LT-HSCs). The percentages and numbers of LSK cells in wild-type, bim, and lck-bcl-x tg mice—the latter being functionally wild type in the bone marrow—dropped significantly upon IR of animals with a single dose of 1.75 Gy, but, in contrast to thymic cellularity, failed to recover prior to subsequent irradiation (Fig. 3A). The percentages and numbers of LSK cells in puma−/− mice did not change, or, in the case of p53−/− mice, even transiently increased, possibly due to impaired p21 activation, demonstrating resistance of these cells to IR-induced apoptosis (Fig. 3B; Supplemental Fig. 4B,C). Consistent with their relative radio resistance, LT-HSC numbers declined more slowly than MPP numbers in wild-type or bim−/−, but were less affected in puma+/− mice (data not shown), or were unaffected in puma−/− or p53−/− mice (Fig. 3C,D; Supplemental Fig. 4D,E). Apoptosis resistance of Puma- or p53-deficient HSCs was cell-autonomous, as demonstrated on FACS-sorted stem cells subjected to IR ex vivo (Supplemental Fig. 5A). Moreover, loss of Puma preserved the clonal fitness of irradiated LT-HSCs, as shown by their better performance in a competitive reconstitution assay using bone marrow from Ly5.1+ wild-type and Ly5.2+ wild-type or puma−/− mice harvested 24 h after irradiation (Supplemental Fig. 5B). These findings are well in line with the reported role for puma in IR-triggered stem/progenitor cell apoptosis (Wu et al. 2005; Shao et al. 2010; Yu et al. 2010). One study also speculated that IR-driven tumor formation may be reduced in puma−/− mice, but experimental proof was not provided, and all relevant controls died from bone marrow failure or gastrointestinal syndrome shortly after high-dose IR (Yu et al. 2010).
Figure 3.
Loss of Puma prevents stem cell apoptosis upon IR. (A) Cellularity of both femora and tibiae was assessed in mice of the indicated genotypes after exposure to a single dose of IR. (B–D) Cell counting and staining with cell surface marker-specific antibodies were used to identify and enumerate the different stem cell populations. Bars represent mean ± SEM of three to six animals per genotype and time point from three independent experiments. (*) LSK cell numbers were significantly different between wild-type or bim−/− versus puma−/− (P < 0.0002) or p53−/− mice (P < 0.0014) at all time points analyzed after IR; MPP numbers were different between wild-type or bim−/− versus puma−/− (P < 0.04) or p53−/− mice (P < 0.035) at days 1, 3, and 7 after IR; LT-HSC numbers were different between wild-type or bim−/− versus puma−/− (P < 0.038) or p53−/− mice (P < 0.003) at day 7 after IR. (§) In untreated mice, LT-HSC numbers were different between bim−/− and all other genotypes (P < 0.05).
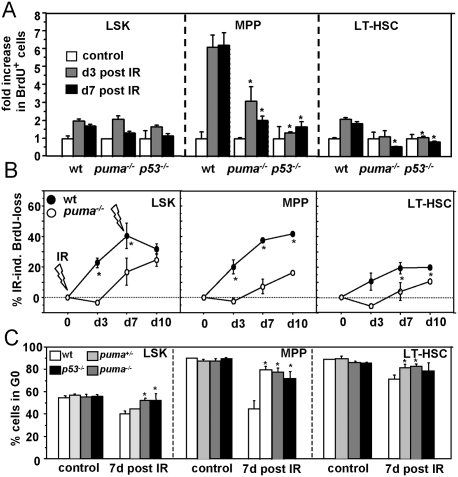
Consistent with a strong demand for compensatory proliferation and HSC mobilization due to apoptotic cell loss of differentiated leukocytes, surviving MPPs showed an approximately sevenfold increase in S-phase activity in BrdU-labeling experiments at days 3 and 7 after IR, compared with untreated controls (P < 0.03), while LT-HSCs displayed an approximately twofold increase (Fig. 4A; Supplemental Fig. 6). In the absence of Puma or p53, however, S-phase activity was significantly less increased (P < 0.04). Noteworthy, this “snapshot analysis” failed to reveal proliferation differences in the LSK population; however, long-term BrdU loading of HSC and subsequent pulse-chase analysis (Wilson et al. 2008) of animals exposed to one or two cycles of IR confirmed the strongly reduced proliferative response—indicated by reduced loss of BrdU label—in puma−/− mice (P < 0.01) in all stem/progenitor cell populations analyzed (Fig. 4B; Supplemental Fig. 7). Ki67 staining and flow cytometric analysis of stem/progenitor cell subsets before and after IR confirmed a significant increase (P < 0.03) in stem cell mobilization out of G0 in wild-type mice compared with puma+/−, puma−/−, or p53−/− animals (Fig. 4C; Supplemental Fig. 8).
Figure 4.
Loss of Puma prevents compensatory proliferation of stem cells upon DNA damage. (A) To compare the rates of proliferation in different stem cell populations, the mice of the indicated genotpyes were exposed to IR, and, on days 3 and 7, were injected i.p. with a single dose of BrdU. Four hours later, mice were sacrificed, and the percentage of BrdU+ cells was assessed by flow cytometry in the individual stem cell subsets or total bone marrow. The relative increase in proliferation (BrdU+ cells) in relation to untreated controls was calculated by using the following equation: (IR-induced proliferation percent − spontaneous proliferation percent)/(100 − spontaneous proliferation percent). Bars represent fold induction of BrdU+ cycling cells as mean ± SEM of three to four animals per genotype and three independent experiments. (*) Significant differences in BrdU uptake were observed between wild-type and puma−/− or p53−/− MPP (P < 0.03) and LT-HSC (P < 0.01) subsets at days 3 or 7 post-IR. (B) Mice were fed BrdU in drinking water for 12 d, yielding between 70% and 90% of labeling efficiency in both genotypes alike. Then mice were exposed to 1.75 Gy IR at days 0 and 7. The percentage of BrdU+ stem cell subsets was assessed by flow cytometry. IR-induced loss of BrdU as an indirect measure of proliferation was calculated by subtracting the percentage of BrdU+ cells of mice pre-exposed to IR from the percentage of BrdU+ cells in control animals. Symbols represent mean ± SEM of three to four animals per genotype and time point. Spontaneous BrdU loss was not different between genotypes (P > 0.78). (*) BrdU loss was significantly different at all time points after IR in MPP (P < 0.014) and LSK (P < 0.026) cells at days 3 and 7, and in LT-HSCs (P < 0.014) at days 7 and 10. (C) Cell cycle analysis using Ki67 was performed in control mice or mice exposed to IR 7 d before. The percentage of cells in G0 is plotted. Bars represent mean ± SEM of three animals per genotype. (*) The percentage of stem/progenitor cells in G0 was significantly higher in puma+/−, puma−/−, and p53−/− when compared with wild-type mice (P < 0.04).
Replication stress in stem/progenitor cells is critical for IR-induced tumor formation
Our data show that, besides depleting thymocytes and mature lymphocytes (data not shown), IR also effectively kills MPP and LSK cells in the bone marrow, forcing surviving LT-HSCs out of dormancy. Hence, loss of Puma may delay tumorigenesis by limiting the compensatory proliferation of stem/progenitor cells that might trigger a replication stress-associated DNA damage response, counterselection, and subsequent genomic instability. Consistently, we observed increasing signs of replication stress-associated DNA damage, as revealed by γH2AX foci formation, in wild-type but not puma−/− or p53−/− stem cells isolated 6 wk after the fourth cycle of irradiation (Fig. 5A,B; Supplemental Fig. 9). This suggests that IR-induced thymic lymphomas arise in wild-type mice presumably due to counterselection against an activated DNA damage response (e.g., by loss of p53) and all of its fatal consequences, as suggested recently (Marusyk et al. 2010). This selection pressure is reduced in puma−/− mice, translating into delayed tumor formation (Fig. 1C,D). Animals lacking p53 show accelerated tumor formation despite the absence of replication stress, and this can be accounted for by uncontained genomic instability exacerbated by IR damage. Consistently, thymic lymphomas from p53−/− and p53 mutant knock-in mice show frequent aneuploidy (Liu et al. 2004; Morales et al. 2006).
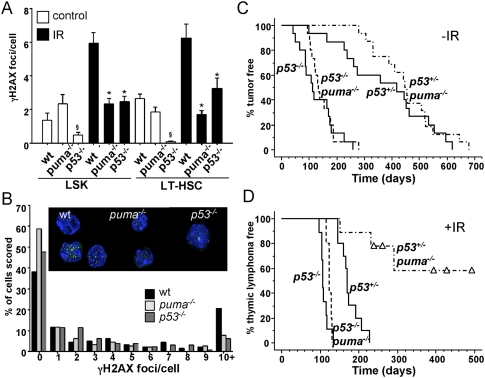
Figure 5.
Replication stress-associated DNA damage in wild-type HSCs after IR and restoration of thymic lymphoma development in puma−/− mice by concomitant loss of p53. (A) Quantification of DNA damage foci in LSK cells and LT-HSCs derived from mice of the indicated genotypes. Six weeks after the fourth cycle of IR, stem cells were sorted from the bone marrow onto poly-L-lysine-coated coverslips and stained for γH2AX. (B) Quantification of the percentage of LT-HSCs containing a given number of γH2AX foci. (Insert) Immunofluorescence detection of γH2AX foci in LT-HSCs and LSK cells derived from wild-type, puma−/−, and p53−/− mice 6 wk after the last dose of irradiation. (Green) γH2AX; (blue) DAPI. Images were acquired with a Leica confocal scanning microscope, and the numbers of foci per cell were quantified by using the CellProfiler software, measuring an average of 100 cells per sample from four to six mice per genotype. (*) IR wild type versus puma−/− or p53−/− (P < 0.001). LT-HSCs from untreated p53−/− mice show reduced foci number (P < 0.05). (B) Assessment of foci number per cell, in relation to the total number of cells analyzed. Cohorts of p53+/− and p53−/− mice proficient or deficient for puma were monitored for spontaneous tumorigenesis and tumor formation triggered by fractionated IR. (C,D) Kaplan-Meier analysis of tumor-free survival of untreated p53+/− (n = 15), p53−/− (n = 15), p53+/−puma−/− (n = 16), and p53−/−puma−/− (n = 15) mice (C), or thymic lymphoma-free survival of irradiated p53+/− (n = 10), p53−/− (n = 9), p53+/−puma−/− (n = 9), and p53−/−puma−/− (n = 5) mice (D). Mean survival in days ± SE: 176 ± 8 for p53+/− versus 307 ± 37 for p53+/−puma−/−; P < 0.0001.
We hypothesized that loss of genomic stability after IR dominates over the protective effect exerted by absent replication stress, caused by the lack of Puma-mediated apoptosis. To test this, we investigated tumor formation in puma−/− mice also lacking one or both alleles of p53. Spontaneous tumorigenesis caused by loss of one or both alleles of p53 was not altered (P > 0.36) on a Puma-deficient background (Fig. 5C). Notably, p53−/−puma−/− mice developed IR-induced thymic lymphomas as rapidly as p53−/− mice (Fig. 5D), consistent with our hypothesis. However, in contrast to puma−/− mice, puma−/− mice lacking one allele of p53 developed thymic lymphomas again (three out of nine), but also a range of other malignancies, while p53+/− mice, able to induce puma, developed exclusively thymic lymphomas (Fig. 5D; Supplemental Table1; Supplemental Fig. 3). Overall, the immunophenotypes of thymic lymphomas arising were similar, suggesting comparable pathology in the genotypes tested (Supplemental Fig. 3C). As reported before (Kemp et al. 1994), all p53+/− thymic lymphomas had lost their wild-type p53 allele, documented by increased levels of ARF protein (Supplemental Fig. 10; Eischen et al. 1999). In the absence of Puma, loss of the second p53 allele was still observed in most lymphomas tested, but disease onset occurred with clearly delayed kinetics (Fig. 5C,D; Supplemental Fig. 10). This demonstrates that stem cell quiescence due to impaired apoptosis of differentiated leukocytes and reduced repopulation demand lowers the impetus for loss of heterozygosity (LOH) of p53, and thereby impairs IR-induced thymic lymphomagenesis. Hence, it would be highly interesting to test for Puma expression levels in Li-Fraumeni syndrome patients in relation to the onset of malignant disease.
In conclusion, our results demonstrate that maintaining stem/progenitor cell numbers upon DNA damage is required to secure their genomic integrity, and is critical for tumor suppression. Furthermore, our observations support the concept that repeated attrition and regeneration of tissues can contribute to tumor formation, and, during anti-cancer therapy, facilitates the rise of treatment-resistant cancer cells that frequently show increased genomic instability (Allan and Travis 2005). In contrast to related mechanisms discussed in the pathology of liver or gastrointestinal cancer, inflammation appears to be not essential here, as evidenced by normal IR-induced lymphomagenesis in myd88 knockout mice (Michalak et al. 2010).
Finally, while inhibition of Puma may be used to minimize anti-cancer therapy-induced aplasia, mimicking its function by application of “BH3 mimetics” (Labi et al. 2008)—alone or in combination with curative or palliative anti-cancer treatment regimens––may actually increase the risk for developing secondary malignancies; e.g., by killing expanding nonmalignant lymphocytes or their progenitors.
Materials and methods
Tumorigenesis
Mice were subjected to four weekly doses of IR with 1.75 Gy from the age of 4 wk (±2 d) in a linear accelerator. For the induction of fibrosarcomas, mice were injected i.m. with 1 mg of 3-MC in sesame oil. As a control, mice were injected with vehicle alone.
Flow cytometry, BrdU labeling, pH3, and Ki67 staining
HSC populations were defined using a biotin-labeled lineage marker flow cytometry kit (e-Bioscience). BrdU was injected i.p. (1 mg per mouse in 200 μL of saline) 4 h prior to sacrifice, or was administered for 12 d in the drinking water (0.8 mg/mL + 1% glucose). To quantify cells in S phase, single-cell suspensions were surface-stained prior to flow cytometric analysis using the BrdU-APC flow kit (BD Biosciences). Indirect immunofluorescence staining on ethanol-fixed thymocytes (70% in PBS) was performed to quantify mitotic cells using an anti-pH3 (Ser10)-specific antibody (Cell Signaling) and goat anti-rabbit Alexa 488 (Invitrogen). Cells in G0 were quantified by cell surface marker staining, followed by fixation in 4% PFA and permeabilization using 0.05% Triton-X-100, followed by intracellular FITC-Ki67 (BD Biosciences) and 7AAD staining.
Immunofluorescence
Staining of γ-H2AX foci was performed as described (Viale et al. 2009). Images were acquired using a Leica SP5 confocal laser-scanning microscope (Leica Microsystems) with a 63× glycerol immersion objective, and image analysis was conducted using CellProfiler software.
Statistical analysis
Statistical analysis was performed by ANOVA or, for comparison of survival of mice and tumor onset, the log-rank (Mantel-Cox) test. Unpaired Student's t-test was used to compare differences in proliferation and BrdU incorporation, as well as the number of γH2AX foci between genotypes. P-values of <0.05 were considered to be significant.
Acknowledgments
We are grateful to K. Rossi, C. Soratroi, R. Pfeilschifter, and I. Gaggl for technical assistance; P. Lukas and his team for enabling irradiation experiments; G. Böck for cell sorting; S. Korsmeyer for bcl-x tg; and M. Serrano for p53−/− mice. We thank J. Adams and A. Strasser for sharing mice, reagents, and unpublished data. This work was supported by grants from the Austrian Science Fund (FWF): SFB021 to A.V., and P19481-B12 to A.E.; and the TWF to V.L.
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1940210.
Supplemental material is available at http://www.genesdev.org.
References
- Allan JM, Travis LB 2005. Mechanisms of therapy-related carcinogenesis. Nat Rev Cancer 5: 943–955 [DOI] [PubMed] [Google Scholar]
- Brathwaite O, Bayona W, Newcomb EW 1992. p53 mutations in C57BL/6J murine thymic lymphomas induced by γ-irradiation and N-methylnitrosourea. Cancer Res 52: 3791–3795 [PubMed] [Google Scholar]
- Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ 1995. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 377: 552–557 [DOI] [PubMed] [Google Scholar]
- Chao DT, Linette GP, Boise LH, White LS, Thompson CB, Korsmeyer SJ 1995. Bcl-xL and Bcl-2 repress a common pathway of cell death. J Exp Med 182: 821–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egle A, Harris AW, Bouillet P, Cory S 2004. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc Natl Acad Sci 101: 6164–6169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eischen CM, Weber JD, Roussel MF, Sherr CJ, Cleveland JL 1999. Disruption of the ARF–Mdm2–p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev 13: 2658–2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlacher M, Michalak EM, Kelly PN, Labi V, Niederegger H, Coultas L, Adams JM, Strasser A, Villunger A 2005. BH3-only proteins Puma and Bim are rate-limiting for g-radiation- and glucocorticoid-induced apoptosis of lymphoid cells in vivo. Blood 106: 4131–4138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlacher M, Labi V, Manzl C, Bock G, Tzankov A, Hacker G, Michalak E, Strasser A, Villunger A 2006. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J Exp Med 203: 2939–2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenzel A, Grespi F, Chmelewskij W, Villunger A 2009. Bcl2 family proteins in carcinogenesis and the treatment of cancer. Apoptosis 14: 584–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cao I, Garcia-Cao M, Martin-Caballero J, Criado LM, Klatt P, Flores JM, Weill JC, Blasco MA, Serrano M 2002. ‘Super p53’ mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO J 21: 6225–6235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison SP, Jeffers JR, Yang C, Nilsson JA, Hall MA, Rehg JE, Yue W, Yu J, Zhang L, Onciu M, et al. 2008. Selection against PUMA gene expression in Myc-driven B-cell lymphomagenesis. Mol Cell Biol 28: 5391–5402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan HS, Brown MB 1952. A quantitative dose-response study of lymphoid-tumor development in irradiated C57 black mice. J Natl Cancer Inst 13: 185–208 [PubMed] [Google Scholar]
- Kemp CJ, Wheldon T, Balmain A 1994. p53-deficient mice are extremely susceptible to radiation-induced tumorigenesis. Nat Genet 8: 66–69 [DOI] [PubMed] [Google Scholar]
- Kominami R, Niwa O 2006. Radiation carcinogenesis in mouse thymic lymphomas. Cancer Sci 97: 575–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labi V, Grespi F, Baumgartner F, Villunger A 2008. Targeting the Bcl-2-regulated apoptosis pathway by BH3 mimetics: A breakthrough in anticancer therapy? Cell Death Differ 15: 977–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao M-J, Van Dyke T 1999. Critical role for Atm in suppressing V(D)J recombination-driven thymic lymphoma. Genes & Dev 13: 1246–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Parant JM, Lang G, Chau P, Chavez-Reyes A, El-Naggar AK, Multani A, Chang S, Lozano G 2004. Chromosome stability, in the absence of apoptosis, is critical for suppression of tumorigenesis in Trp53 mutant mice. Nat Genet 36: 63–68 [DOI] [PubMed] [Google Scholar]
- Macleod KF, Sherry N, Hannon G, Beach D, Tokino T, Kinzler K, Vogelstein B, Jacks T 1995. p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev 9: 935–944 [DOI] [PubMed] [Google Scholar]
- Marusyk A, Porter CC, Zaberezhnyy V, DeGregori J 2010. Irradiation selects for p53-deficient hematopoietic progenitors. PLoS Biol 8: e1000324 doi: 10.1371/journal.pbio.1000324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak EM, Villunger A, Adams JM, Strasser A 2008. In several cell types tumor suppressor p53 induces apoptosis largely via Puma but Noxa can contribute. Cell Death Differ 15: 1019–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak EM, Jansen ES, Happo L, Cragg MS, Tai L, Smyth GK, Strasser A, Adams JM, Scott CL 2009. Puma and to a lesser extent Noxa are suppressors of Myc-induced lymphomagenesis. Cell Death Differ 16: 684–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak EM, Vandenberg CJ, Delbridge AR, Wu L, Scott CL, Adams JM, Strasser A 2010. Apoptosis-promoted tumorigenesis: γ-Irradiation-induced thymic lymphomagenesis requires Puma-driven leukocyte death. Genes Dev (this issue). doi: 10.1101/gad.1940110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales JC, Franco S, Murphy MM, Bassing CH, Mills KD, Adams MM, Walsh NC, Manis JP, Rassidakis GZ, Alt FW, et al. 2006. 53BP1 and p53 synergize to suppress genomic instability and lymphomagenesis. Proc Natl Acad Sci 103: 3310–3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Jamieson CH, Weissman IL 2008. Stems cells and the pathways to aging and cancer. Cell 132: 681–696 [DOI] [PubMed] [Google Scholar]
- Shao L, Sun Y, Zhang Z, Feng W, Gao Y, Cai Z, Wang ZZ, Look AT, Wu WS 2010. Deletion of proapoptotic Puma selectively protects hematopoietic stem and progenitor cells against high-dose radiation. Blood 115: 4707–4714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viale A, De Franco F, Orleth A, Cambiaghi V, Giuliani V, Bossi D, Ronchini C, Ronzoni S, Muradore I, Monestiroli S, et al. 2009. Cell-cycle restriction limits DNA damage and maintains self-renewal of leukaemia stem cells. Nature 457: 51–56 [DOI] [PubMed] [Google Scholar]
- Vousden KH, Lane DP 2007. p53 in health and disease. Nat Rev Mol Cell Biol 8: 275–283 [DOI] [PubMed] [Google Scholar]
- Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, et al. 2008. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135: 1118–1129 [DOI] [PubMed] [Google Scholar]
- Wu WS, Heinrichs S, Xu D, Garrison SP, Zambetti GP, Adams JM, Look AT 2005. Slug antagonizes p53-mediated apoptosis of hematopoietic progenitors by repressing puma. Cell 123: 641–653 [DOI] [PubMed] [Google Scholar]
- Yu H, Shen H, Yuan Y, Xufeng R, Hu X, Garrison SP, Zhang L, Yu J, Zambetti G, Cheng T 2010. Deletion of Puma protects hematopoietic stem cells and confers long-term survival in response to high-dose γ-irradiation. Blood 115: 3472–3480 [DOI] [PMC free article] [PubMed] [Google Scholar]