Abstract
Demographics and health practices of 2,232 pregnant women in rural northeastern Ghana and characteristics of their 2,279 newborns were analyzed to determine benefits associated with intermittent preventive treatment (IPTp), antenatal care, and/or bed net use during pregnancy. More than half reported bed net use, 90% reported at least two antenatal care visits, and > 82% took at least one IPTp dose of sulfadoxine-pyrimethamine. Most used a bed net and IPTp (45%) or IPTp alone (38%). Low birth weight (< 2,500 grams) characterized 18.3% of the newborns and was significantly associated with female sex, Nankam ethnicity, first-born status, and multiple births. Among newborns of primigravidae, IPTp was associated with a significantly greater birth weight, significantly fewer low birth weight newborns, improved hemoglobin levels, and less anemia. Babies of multigravidae derived no benefit to birth weight or hemoglobin level from single or multiple doses of sulfadoxine-pyrimethamine during pregnancy. No differences or benefits were seen when a bed net was the only protective factor.
Introduction
The risk and severity of malaria illness among young children and pregnant women in the Kassena Nankana District (KND) in northeastern Ghana may be changing as a result of national strategies that include artesunate combination treatment, intermittent preventive treatment during pregnancy (IPTp) with sulfadoxine-pyrimethamine (S/P), free antenatal care (ANC) and postnatal care, provision and wide use of insecticide-treated bed nets (ITNs), and intensified health education. Ghana readily adopted and began implementation of malaria intervention policies recommended by the World Health Organization (WHO) that target the most vulnerable groups.1 Targeting of women and young children for ITNs began in 1999 through antenatal clinics and routine vaccination programs after an important Ghanaian study showed that bed net use was associated with a 17% reduction in all-cause mortality of young children.2 Artesunate-amodiaquine became the standard first-line treatment for uncomplicated malaria, whether confirmed, or suspected, in 2004 and S/P-based IPTp was introduced in 2005.1,3 Indoor residual spraying of houses with insecticide is being evaluated in parts of northeastern Ghana where the malaria transmission period is sharply limited by seasonal rainfall.
The extent and impact of these relatively new factors, seen as practices during pregnancy, and manifested in the outcomes of the respective pregnancies, was evaluated in a large cohort of pregnant women and their newborns. This work was nested within a larger, five-year birth cohort study designed to provide scientific and logistical guidance to malaria vaccine researchers. The main objectives of this five-year prospective study are to measure the incidence of malaria vaccine trial endpoints (mild malaria, severe malaria, severe anemia, and malaria death) and parasite variation in the face of these recent national policy changes, and to acquire sufficient samples to facilitate analyses of host susceptibility, immune response, and malaria parasite variation.
Before the study, we anticipated that increased IPTp coverage, ANC clinic attendance, bed net use, and place of residence would factor importantly into children's outcomes and that primigravid mothers and firstborn, because of their unique vulnerability to malaria, would be key indicators of the success of prevention strategies and health progress in the district. Although the larger study will generate biological samples, laboratory studies, and data well into the future, this initial report describes baseline demographic, ethnographic, and epidemiologic characteristics of the mothers and newborns with special attention given to malaria prevention and antenatal care during pregnancy in the households and communities. Information from this current, focused profile is an essential, intrinsically valuable starting point and may be of more immediate and practical use if it serves to reinforce and/or improve the performance of local health managers and providers.
Materials and Methods
Study site.
The Kassena Nankana District in the Upper East Region of Ghana has an area of 1,673 km2 and is located at 10°53′ 42′′N, 1°5′ 38′′W. It is composed of Guinea savannah grassland punctuated by sparse woods and granite outcrops. Rainfall is seasonal, occurring from May through November, averages 1,365 mm/year, and provides one growing season. The Tono Dam and irrigation project impounds water from a catchment area of 650 km2 in the northern part of KND and enables perennial farming of rice, fruit, and vegetables in 2,490 hectares of lowland below the dam. Census figures from January 2009 showed that the KND population is 147,676, with ~10% living in the central town of Navrongo and the remaining 90% mainly engaged in subsistence level farming of millet. Residents of KND are principally from three ethnic groups, Kassem, Nankam, Buli; all patrilineal with strong male dominance. There are 77 primary and 40 secondary schools, but 68% of the population over 15 years of age is reported to be illiterate. Infant mortality is 68 deaths/1,000 live births, mortality for children less than five years of age is 106/1,000 live births, and life expectancy is 52.6 years.4
Malaria and severe anemia are among the leading causes of illness in young children. Malaria transmission is sharply defined by rainfall over most of KND, but is higher and perennial within the irrigated sectors and is markedly reduced within the town center of Navrongo.5,6 Medical care is provided through a district hospital, 9 health centers located in smaller towns, and up to 20 community health compounds where ANC, treatment of minor ailments, and immunizations are provided. Medical care is free for all children less than five years of age and for pregnant and nursing women.
Design.
This study was a prospective cohort study in which pregnant women and their live newborn children were enrolled during the course of one year (March 2006–March 2007). Within hours of delivery each pre-enrolled infant was evaluated, vital signs were recorded, and a small sample of blood (cord blood, if the child was delivered at a health center or hospital; heel prick blood if the child was delivered at home) was obtained for malaria screening. Data collection began with a detailed enrollment questionnaire administered to the mother, which was later amended with records of the birth, physical examination, and blood test results. Infants were thereafter followed-up by using mainly passive surveillance for 60 months, with details of all outpatient and inpatient illnesses, deaths, and emigrations recorded. Although many women may have been using long-lasting ITNs during pregnancy, this quality of the bed nets could not be determined with any accuracy and we used the more generic term, bed net in our interviews and in the context of this report.
Ethical considerations.
This study was reviewed and approved by scientific and ethical review boards of the Navrongo Health Research Centre, the Noguchi Memorial Institute for Medical Research, the National Institute for Allergy and Infectious Diseases, and the Naval Medical Research Center. It was conducted in accordance with regulations governing the protection of human subjects in medical research.
A two-stage consent process was used. First, the study was explained to elders, leaders, and members of the community at scheduled durbars during which questions and discussions were encouraged. After these meetings, signed, informed consent was obtained from all pregnant women who wished to enroll their newborns.
Sample size.
Based on a population > 140,000, an estimated 4,000 births each year, and available incidence measures of clinical malaria, severe malaria, and death caused by malaria, a birth cohort of 2,000 children was selected. This sample size, with its potential for > 400 cases of severe malaria during the 6–30-month period of vulnerability was judged sufficient to detect genetic associations with malaria susceptibility or resistance.
Malaria screening and hemoglobin testing.
Blood smears, beginning with cord or heel prick blood samples, and continuing through each occasion of illness and at the twice a year active surveillance points, were stained with Giemsa and examined by a skilled microscopist. Hemoglobin (Hb) testing at delivery was performed from cord blood or after delivery from heel prick by HemoCue (HemoCue AB, Ängelholm, Sweden) instruments calibrated daily with standards. All children were weighed within hours or days of birth and considered to be of low birth weight (LBW) if they weighed < 2,500 grams. Fetal anemia was defined as an Hb level < 12.5 g/dL at birth and an Hb level < 11.0 g/dL was considered to represent severe neonatal anemia.
Data management and analysis.
Survey and laboratory data were captured onto structured forms that were edited, checked and corrected before double entry into an electronic database. Data were sufficiently refined to enable stratified analyses according to 1) six sectors of residence (town, irrigated, north, south, east, and west); 2) mothers reproductive status (primigravida, multigravida); 3) four categories of malaria susceptibility/protection during pregnancy (no bed net or IPTp, bed net only, ITP only, bed net and IPTp); 4) four levels of IPTp coverage (0, 1, 2,and ≥ 3 doses); 5) four ethno-linguistic groups (Nankam, Kasem, Buli, and non-local other); and 6) two seasons of malaria transmission (high = July–December and low = January–June). Data analysis was conduced by using Epi Info version 6.04 (ENSP-Epiconcept-InVS Corp.) and Stata statistical software version 7.0 (Stata Corp., College Station, TX). Statistical comparison was based on univariate analyses and used the Pearson chi-square test or Fisher's exact test for discrete variables and the independent t-test for continuous variables. Two-tailed means and/or risk ratios (RRs) are presented with 95% confidence intervals (CIs). Statistical significance was based on a P value < 0.05.
Results
Overview.
A total of 2,232 women and their 2,279 newborns were enrolled from all six administrative sectors of the district and from 209 of the 301 clusters registered by the Navrongo Demographic Surveillance System. Ethnic composition, place and type of delivery, and the frequency of bed net and/or IPTp use during pregnancy are shown in Figure 1. Most enrollments were from the Nankam ethno-linguistic group (58%), followed by Kasem (34.4%), Buli (3.8%), and non-local, but mainly Ghanaian others (3.7%). Women's median age was 26 years (range = 13–63 years), and primigravidae accounted for 25%. More than half (53.5%) of the women reported using a bed net during pregnancy, 90% reported receiving ANC at least twice (Figure 2), and > 82% received at least one dose of IPTp S/P during their gestation. Most women used a bed net and IPTp during pregnancy (45%), followed by IPTp alone (38%) and bed net alone (8.6%); 8.5% of women professed no use of either a bed net or IPTp. Low birth weight characterized 418 (18.3%) of the 2,279 live births. Mean Hb level of newborns was 14.2 g/dL (95% CI = 14.15–14.35 g/dL). Fetal anemia (Hb level < 12.5 g/dL) characterized 407 (21.5%) of 1,888 tested newborns. Seven percent of newborns were severely anemic with an Hb level < 11.0 g/dL.
Figure 1.
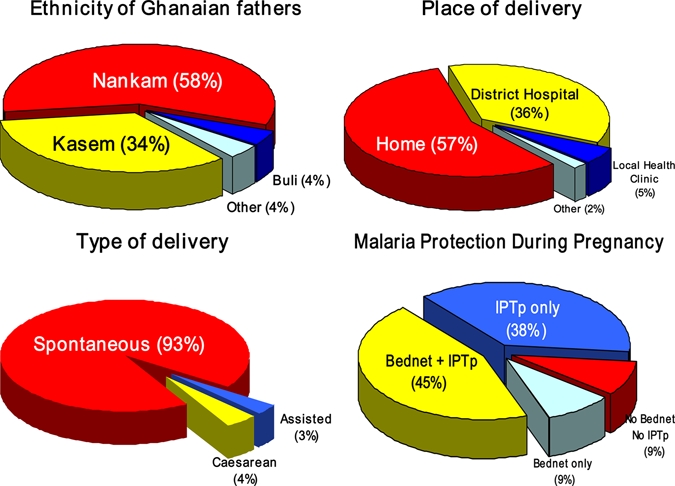
Ethnic composition, place and type of delivery, and malaria protection during pregnancy, Kassena-Nankana District, northeastern Ghana, March 2006–March 2007. This figure appears in color at www.ajtmh.org.
Figure 2.
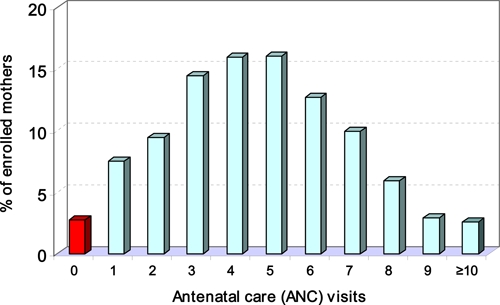
Frequency distribution of antenatal care clinic visits by pregnant women in the Kassena-Nankana District, northeastern Ghana, March 2006–March 2007. This figure appears in color at www.ajtmh.org.
Location effect.
The populous central sector (Navrongo town, population = 13,952) stood out for a number of reasons: highest levels of education, ANC clinic visits, and proportions meeting the IPTp target of 3 S/P doses during pregnancy, and fewest anemic babies (Table 1). Furthermore, these town women reported the fewest prior pregnancies and the lowest number of prior miscarriages or deaths of children, and gave birth to significantly fewer LBW and anemic babies than women in northern, southern, or eastern sectors of the district. In contrast, women from the non-irrigated eastern sector were least educated, reported fewest ANC clinic visits, least bed net use, lowest numbers meeting the IPTp target (three S/P doses), and had the highest proportions of anemic and underweight babies. Reported bed net use during pregnancy was highest in the irrigated sector; significantly higher (P < 0.04) than any other sector, but women residing in the irrigated sector were far more likely to have reported no IPTp S/P use during pregnancy (22% versus 12%, RR = 1.83, 95% CI = 1.43–2.32%, P < 0.0001).
Table 1.
Characteristics of enrolled mothers and newborns by sector of residence in the Kassena Nankana District, northeastern Ghana*
| Characteristic | Central | North | South | East | West | Irrigated |
|---|---|---|---|---|---|---|
| No. (%) enrolled | 251 (11) | 319 (14) | 802 (35) | 523 (23) | 75 (3) | 309 (14) |
| Mother's age, years, mean ± 95% CI | 26.9 ± 0.7 | 26.9 ± 0.7 | 27.6 ± 0.5 | 26.8 ± 0.6 | 26.2 ± 1.1 | 26.2 ± 0.7 |
| Mother's education, years, mean ± 95% CI | 7.5 ± 0.6 | 3.4 ± 0.4 | 2.8 ± 0.3 | 2.1 ± 0.3 | 6.2 ± 1.1 | 3.9 ± 0.4 |
| Reporting no education, % | 15.1 | 45.4 | 55.1 | 61.4 | 25.3 | 37.2 |
| Previous pregnancies, mean ± 95% CI | 2.5 ± 0.2 | 3.3 ± 0.2 | 3.3 ± 0.1 | 3.2 ± 0.2 | 2.8 ± 0.4 | 3.0 ± 0.2 |
| Prior miscarriage/death of child, % | 13.9 | 12.7 | 17.4 | 14.1 | 17.9 | 16.7 |
| ANC clinic visits, mean ± 95% CI | 6.5 ± 0.3 | 4.3 ± 0.2 | 4.5 ± 0.2 | 4.0 ± 0.2 | 5.8 ± 0.6 | 4.5 ± 0.2 |
| S/P doses during pregnancy, mean ± 95% CI | 2.0 ± 0.1 | 1.8 ± 0.1 | 1.8 ± 0.1 | 1.7 ± 0.1 | 2.0 ± 0.2 | 1.7 ± 0.1 |
| Reporting no S/P use, % | 11.5 | 14.4 | 16.8 | 20.1 | 10.7 | 22.0 |
| Meeting IPTp target (3 S/P), % | 45.4 | 29.6 | 31.3 | 29.4 | 37.3 | 29.1 |
| Bed net use during pregnancy, % | 45.8 | 43.4 | 58.8 | 39.8 | 65.0 | 76.7 |
| Cord blood parasitemic, no, (%) | 4 (1.6) | 10 (3.1) | 17 (2.1) | 8 (1.5) | 1 (1.3) | 6 (1.9) |
| Birth weight, kg, mean ± 95% CI | 2.92 ± 0.05 | 2.88 ± 0.05 | 2.90 ± 0.04 | 2.86 ± 0.05 | 2.96 ± 0.09 | 2.90 ± 0.05 |
| Low birth weight (< 2,500 grams), % | 11.0 | 17.0 | 20.0 | 24.0 | 9.0 | 14.0 |
| Cord blood Hb (g/dL), mean ± 95% CI | 14.5 ± 0.2 | 14.1 ± 0.3 | 14.2 ± 0.2 | 14.2 ± 0.2 | 14.0 ± 0.5 | 14.3 ± 0.3 |
| Neonatal anemia (Hb < 11.0 g/dL), % | 2.5 | 6.3 | 8.2 | 8.6 | 5.5 | 6.7 |
| Fetal anemia (Hb < 12.5 g/dL), % | 11.7 | 23.6 | 23.5 | 24.3 | 18.0 | 20.2 |
| All-cause infant mortality by 20–33 months, % | 14 (6) | 21 (7) | 42 (5) | 26 (5) | 3 (4) | 14 (4) |
The urbanized central sector is the town of Navrongo and site of the district hospital. CI = confidence interval; ANC = antenatal care; S/P = sulfadoxine-pyrimethamine; IPTp = intermittent preventive treatment; Hb = hemoglobin.
Ethnic groups.
Analysis stratified by father's ethnicity (Table 2) showed that Nankam were predominant in the eastern (99%) and southern (84%) sectors and Kasem were predominant in the northern (94%), central (65%), and western (88%) sectors. Buli and other, non-local ethnic households collectively accounted for < 8% of the total population enrolled, but Bulis accounted for 13% of the irrigated sector, and non-local others accounted for 25% of enrollments in the central town sector. Nankam ethnicity was associated in the mothers with significantly older age, least education, fewest ANC clinic visits, and least IPTp. Among newborns, Nankam ethnicity was associated with significantly more LBW deliveries by primigravid (32.4%) and multigravid (18%) mothers. Mean birth weight and Hb level were comparable among the four ethno-linguistic groups but fetal anemia was more frequent in Nankam (23%) and Kasem (21%) newborns than in Buli (14%) or other (15%) newborns.
Table 2.
Characteristics of enrolled mothers and newborns by father's ethno-linguistic affiliation in the Kassena Nankana District, northeastern Ghana*
| Characteristic | Kasem | Nankam | Buli | Other |
|---|---|---|---|---|
| No. (%) households enrolled | 784 (34.4) | 1,324 (58.1) | 87 (3.8) | 84 (3.7) |
| Mother's age, years, mean ± 95% CI | 26.65 ± 0.46 | 27.41 ± 0.37 | 26.16 ± 1.38 | 25.73 ± 1.20 |
| Mother's education, years, mean ± 95% CI | 4.94 ± 0.31 | 2.42 ± 0.19 | 3.21 ± 0.78 | 7.54 ± 0.97 |
| Reporting no education, % | 31.1 | 58.5 | 51.7 | 11.9 |
| First-time pregnancies, % | 27.4 | 20.8 | 32.2 | 40.5 |
| Previous pregnancies, mean ± 95% CI | 3.70 ± 0.13 | 3.88 ± 0.10 | 3.81 ± 0.37 | 2.94 ± 0.30 |
| Previous death/miscarriage, % | 12.6 | 16.6 | 25.4 | 16.0 |
| ANC clinic visits during pregnancy, mean ± 95% CI | 4.99 ± 0.18 | 4.23 ± 0.12 | 5.52 ± 0.49 | 5.99 ± 0.57 |
| S/P doses during pregnancy, mean ± 95% CI | 1.83 ± 0.07 | 1.73 ± 0.06 | 1.71 ± 0.27 | 1.96 ± 0.23 |
| Reporting no S/P treatment, % | 14.4 | 18.3 | 28.7 | 13.1 |
| Meeting IPTp target, % | 33.5 | 30.1 | 39.1 | 41.7 |
| Bed net use during pregnancy, % | 54.6 | 52.3 | 73.6 | 42.9 |
| Pre-term births, % | 0.5 | 0.8 | 1.1 | 0.0 |
| Birth weight, kg, mean ± 95% CI | 2.89 ± 0.03 | 2.88 ± 0.03 | 2.88 ± 0.09 | 2.91 ± 0.07 |
| Low birth weight (< 2,500 grams), % | 15.9 | 21.0 | 11.5 | 5.9 |
| Low birth weight primigravid only, % | 23.3 | 32.4 | 17.9 | 5.9 |
| Low birth weight multigravid only, % | 13.2 | 18.0 | 8.5 | 6.0 |
| Cord blood Hb (g/dL), mean ± 95% CI | 14.15 ± 0.16 | 14.27 ± 0.14 | 14.63 ± 0.51 | 14.46 ± 0.50 |
| Severe neonatal anemia (Hb < 11.0 g/dL), % | 5.6 | 8.0 | 6.9 | 5.0 |
| Fetal anemia (Hb < 12.5 g/dL), % | 21.1 | 22.9 | 13.9 | 15.0 |
| All-cause infant mortality by 20–33 months, % | 6.4 | 4.8 | 3.4 | 3.6 |
Other comprises non-local, but mainly Ghanaian ethno-linguistic groups; CI = confidence interval; ANC = antenatal care; S/P = sulfadoxine-pyrimethamine; IPTp = intermittent preventive treatment; Hb = hemoglobin.
The typically higher proportion of LBW babies born to primigravidae was clearly apparent in Nankam (32% primigravidae versus 18% multigravidae), Kasem (23% primgravidae versus 13% multigravidae), and Buli (18% primigravidae versus 8.5% multigravidae) households, but not seen in those classed other (5.9% primigravidae versus 6.0% multigravidae). Despite reporting the lowest bed net use during pregnancy, women from these other households had the highest levels of education (mean ± SD = 7.5 ± 1.0 years), ANC clinic attendance (6.0 ± 0.6 visits), and IPTp adherence (42% took ≥ 3 S/P doses). Disproportionately higher numbers of primigravidae (and firstborns) made up enrollments of other (40.5%) and Buli (32.2%) compared with Nankam (20.8%) and Kasem (27.4%).
Seasonal effect.
Women who gave birth during the low-transmission season for malaria (January–June) gestated over a longer period of high malaria risk than those who gave birth during the high-transmission season for malaria (July–December) (4.8 versus 4.0 high-transmission months.). ANC clinic visits, mean S/P doses/woman, and proportion meeting the IPTp target were all greater (P < 0.10) in those who gestated during the high-transmission season and delivered during the low-transmission season. Among babies born during the low-transmission season, the mean Hb level was significantly lower (14.0 g/dL versus 14.4 g/dL; P < 0.0001) and fetal anemia was more prevalent (24.3% versus 19.3%; P = 0.016). No difference was seen in mean birth weight or proportion of LBW infants, but cord blood parasitemia was more prevalent during the high-transmission season (3.1% versus 0.8%; P < 0.001). Cord blood parasitemia was detected in 2.1% of deliveries, present in all 6 sectors, and found in 10 of the 12 enrollment months, but clustered in time; 34% occurring in August 2007 births. The higher prevalence of cord blood parasitemias in primigravida (2.4%) and LBW (3.1%) was notable (P < 0.12) but not statistically significant.
Birth weight, Hb, and anemia.
Low birth weight (< 2,500 grams, 18.3%) was associated with female babies (20.3% [234 of 1,154] versus 16.5% [185 of 1,124]; RR = 1.23, 95% CI = 1.0–1.5, P = 0.02), and strongly associated with Nankam ethnicity (21% [278 of 1,324] versus 15% [140 of 955]; RR = 1.43, 95% CI = 1.2–1.7, P < 0.0001), firstborns (26% [146 of 551] versus 16% [272 of 1,727]; RR = 1.6, 95% CI = 1.4–1.9, P < 0.0001), and multiple births (63% [61 of 97] versus 16% [357 of 2,182]; RR = 3.9, 95% CI = 3.2–4.6, P < 0.0001). Relative to normal birthweight babies, mothers of LBW babies were significantly younger and reported significantly fewer ANC clinic visits during their pregnancy, but there was no association with bed net use or IPTp. Near equal proportions of underweight girls and boys were born to first-time mothers (25.4% boys versus 27.5% girls), but among multigravidae, most LBW infants were girls (18.8% girls versus 13.5% boys; P = 0.01) (Table 3). By 20–33 months of age, there was a significantly higher all-cause death rate among LBW infants (10.0% [42 of 418] versus 4.2% [78 of 1,861]; RR = 2.5, 95% CI = 1.7–3.8, P < 0.0001). Mean Hb levels of normal and LBW babies were comparable, but severe neonatal anemia was associated with LBW (10% [32 of 319] versus 6% [100 of 1,569]; P = 0.02). Fetal anemia was significantly more prevalent in multigravid births (23.4% [330 of 1,410] versus 16.1% [77 of 478]; RR = 1.4, 95% CI = 1.2–1.8, P = 0.001) but not associated with sex (22% versus 21%) or LBW (24% versus 21%). We identified 77 children, predominantly Nankam (57 of 77) and female (49 of 77), who had LBW and fetal anemia.
Table 3.
Comparative analysis of enrolled mothers and newborns stratified by birth weight (< 2,500 grams vs. ≥ 2,500 grams) in the Kassena Nankana District, northeastern Ghana*
| Characteristic | Birth weight < 2,500 grams | Birth weight > 2,500 grams | P |
|---|---|---|---|
| No. enrolled (%) | 418/2,279 (18.3) | 1,861/2,279 (81.7) | |
| Male:female ratio | 0.79 (185:233) | 1.01 (939:922) | 0.02 |
| Primigravid births, % | 34.9 | 21.8 | < 0.0001 |
| Multiple births, % | 14.6 | 1.9 | < 0.0001 |
| Mother' age, years, mean ± 95% CI | 25.62 ± 0.66 | 27.36 ± 0.30 | < 0.0001 |
| Mother's education, years, mean ± 95% CI | 3.23 ± 0.38 | 3.57 ± 0.19 | NS |
| Bed net use during pregnancy, % | 50.2 | 54.3 | NS |
| ANC clinic visits, mean ± 95% CI | 4.22 ± 0.22 | 4.69 ± 0.11 | < 0.0001 |
| S/P doses during pregnancy, mean ± 95% CI | 1.74 ± 0.11 | 1.78 ± 0.05 | NS |
| Reporting no S/P use, % | 18.2 | 16.9 | NS |
| Meeting IPTp target (3 S/P) % | 28.9 | 32.7 | NS |
| Birth weight, kg, mean ± 95% CI | 2.18 ± 0.02 | 3.05 ± 0.02 | < 0.0001 |
| Cord blood Hb (g/dL), Mean ± 95% CI | 14.37 ± 0.28 | 14.22 ± 0.11 | NS |
| Severe neonatal anemia (Hb < 11.0 g/dL) (%) | 32/319 (10) | 100/1,569 (6.4) | 0.02 |
| Fetal anemia (Hb < 12.5 g/dL) (%) | 77/319 (24.1) | 330/1,569 (21.0) | NS |
| All-cause infant mortality by 20–33 months (%) | 10.0 | 4.2 | < 0.0001 |
CI = confidence interval; NS = not significant; ANC = antenatal care; S/P = sulfadoxine-pyrimethamine; IPTp = intermittent preventive treatment; Hb = hemoglobin.
Mother's reproductive status.
Primigravidae averaged nine years younger than multigravidae but had significantly more education, more frequent ANC clinic visits, and better IPTp coverage (Table 4). Despite the significantly lower birth weights of infants born to first-time mothers, these children had healthier Hb profiles and significantly less fetal anemia (16% [77 of 478] versus 23% [111 of 1,410]; RR = 0.69, 95% CI = 0.55–0.86, P = 0.001). Mean birth weights and Hb level did not show an upward increment with increased S/P doses in children of primigravid mothers, but % LBW and % fetal anemia showed a downward increment, especially in the larger group of firstborn children whose mothers used bed nets (Figure 3A and B).
Table 4.
Comparative analysis of enrolled mothers and newborns stratified by mother's reproductive status (primigravid vs. multigravid) in the Kassena Nankana District, northeastern Ghana*
| Characteristic | Primigravid | Multigravid | P |
|---|---|---|---|
| No (%) enrolled | 552 (24.2) | 1727 (75.8) | |
| Mother's age, years, mean ± 95% CI | 20.48 ± 0.25 | 29.13 ± 0.29 | < 0.0001 |
| Mother's education, years, mean ± 95% CI | 5.86 ± 0.36 | 2.76 ± 0.18 | < 0.0001 |
| ANC clinic visits, mean ± 95% CI | 4.96 ± 0.20 | 4.49 ± 0.11 | < 0.0001 |
| S/P doses during pregnancy, mean ± 95% CI | 1.85 ± 0.09 | 1.75 ± 0.05 | < 0.0001 |
| Receiving no S/P (%) | 86/551 (16) | 305/1,727 (18) | NS |
| Meeting IPTp target (3 S/P doses) (%) | 193/551 (35) | 538/1,727 (31) | 0.09 |
| Bed net use during pregnancy (%) | 229/552 (41) | 991/1,727 (57) | < 0.0001 |
| Birth weight, kg, mean ± 95% CI | 2.72 ± 0.04 | 2.94 ± 0.02 | < 0.0001 |
| Low birth weight (< 2,500 grams) (%) | 146/551 (26) | 272/1727 (16) | < 0.0001 |
| Cord blood Hb (g/dL), mean ± 95% CI | 14.40 ± 0.19 | 14.20 ± 0.12 | 0.10 |
| Neonatal anemia (Hb < 11.0 g/dL) (%) | 21/478 (4) | 111/1410 (8) | 0.009 |
| Fetal anemia (Hb < 12.5 g/dL) (%) | 77/478 (16.1) | 330/1410 (23.4) | 0.001 |
| Cord blood parasitemia (%) | 13/549 (2) | 34/1709 (2) | NS |
| All-cause infant death by 20–33 months (%) | 36/552 (6.5) | 84/1727 (4.9) | NS |
CI = confidence interval; ANC = antenatal care; S/P = sulfadoxine-pyrimethamine; NS = not significant; IPTp = intermittent preventive treatment; Hb = hemoglobin.
Figure 3.
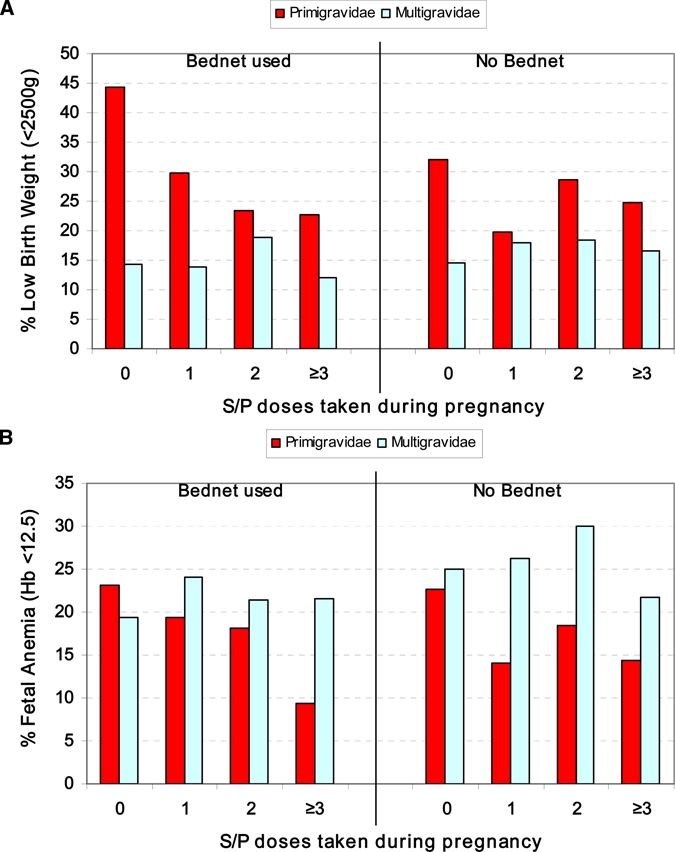
Comparison of pregnancy outcome for primigravidae and multigravidae, stratified by bed net group, at different levels of intermittent preventive treatment coverage for A, frequency of low birth weight and B, fetal anemia in newborns, Kassena-Nankana District, northeastern Ghana, March 2006–March 2007. This figure appears in color at www.ajtmh.org.
Intermittent preventive treatment during pregnancy.
Relative to first-time mothers who reported no IPTp during pregnancy (n = 86), one IPTp dose of S/P (n = 114) was associated with a significantly greater mean birth weight (2.8 kg versus 2.6 kg; P = 0.04), significantly fewer LBW newborns (23% versus 37%; RR = 0.6, 95% CI = 0.40–0.95, P = 0.03), an improved Hb profile (14.6 g/dL versus 14.0 g/dL; P = 0.10), and less neonatal anemia (4% versus 8.6%). In babies born to primigravidae, IPTp target dosing alone, without bed net use was associated with significantly improved Hb levels but when combined with bed net use, IPTp target dosing was associated with significantly increased birth weights, significantly fewer LBW deliveries, and notably reduced frequencies of neonatal anemia and fetal anemia (Table 5). Among babies born to multigravid mothers, no significant or even marginal improvements in birth weight, Hb level, or anemia were derived from single or multiple doses of S/P during pregnancy. These differences are even more surprising in light of the fact that professed bed net use/ownership by multigravidae exceeded that of primigravidae in all four IPTp categories (Figure 4).
Table 5.
Effect of S/P-based IPTp target dosing in primigravid mothers and their newborns: analysis and comparison between no bed net and bed net used groups in the Kassena Nankana District, northeastern Ghana*
| Characteristic | Primigravidae, no bed net | Primigravidae, bed net used | ||||
|---|---|---|---|---|---|---|
| No IPTp | IPTp target met | P | No IPTp | IPTp target met | P | |
| Number | 50 | 101 | 36 | 92 | ||
| Mother's age, years, mean ± 95% CI | 20.09 ± 0.8 | 20.87 ± 0.62 | NS | 20.01 ± 0.89 | 20.93 ± 0.64 | NS |
| Mother's education, years, mean ± 95% CI | 4.9 ± 1.08 | 5.99 ± 0.90 | NS | 5.00 ± 1.3 | 6.86 ± 0.94 | 0.02 |
| ANC clinic visits, mean ± 95% CI | 3.38 ± 0.66 | 5.93 ± 0.35 | < 0.001 | 3.50 ± 0.80 | 6.1 ± 0.43 | < 0.001 |
| Pre-term births (%) | 2/50 (4) | 1/101 (1) | NS | 3/36 (8.3) | 1/92 (1.1) | NS |
| Birth weight, kg, mean ± 95% CI | 2.69 ± 0.12 | 2.74 ± 0.09 | NS | 2.55 ± 0.16 | 2.73 ± 0.09 | 0.05 |
| Underweight births (< 2,500 grams) (%) | 16/50 (32) | 25/101 (24.7) | NS | 16/36 (44.4) | 21/92 (22.8) | 0.015 |
| Hb (g/dL) at birth, mean ± 95% CI | 13.72 ± 0.59 | 14.59 ± 0.49 | 0.03 | 14.46 ± 1.01 | 14.42 ± 0.39 | NS |
| Neonatal anemia (Hb < 11.0 g/dL) (%) | 4/44 (9.1) | 5/91 (5.5) | NS | 2/26 (7.7) | 1/86 (1.2) | NS |
| Fetal anemia (Hb < 12.5 g/dL) (%) | 10/44 (22.7) | 13/91 (14.3) | NS | 6/26 (23.1) | 8/86 (9.3) | 0.088 |
| All-cause infant death by 20–33 months) (%) | 4/50 (8) | 4/101 (4) | NS | 2/36 (5.5) | 7/92 (7.6) | NS |
IPTp = intermittent preventive treatment; CI = confidence interval; NS = not significant; Hb = hemoglobin.
Figure 4.
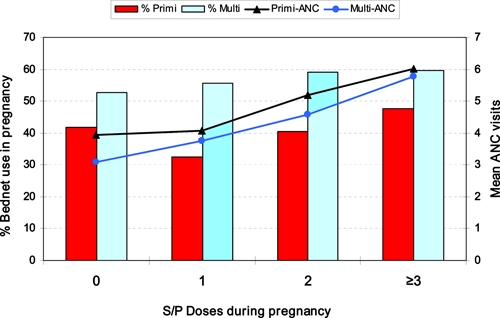
Frequency of bed net use and mean antenatal care visits stratified by mother's reproductive status (primigravid or multigravid) and level of intermittent preventive treatment (IPTp) coverage (IPTp target = three doses of sulfadoxine-pyrimethamine), Kassena-Nankana District, northeastern Ghana, March 2006–March 2007. This figure appears in color at www.ajtmh.org.
Bed net use and effect.
Bed net use during pregnancy was associated with significantly older age, more education, higher ANC clinic attendance, better IPTp coverage, and a history of miscarriage or the death of a child. Among all newborns, mother's use of bed net was associated with significantly reduced neonatal anemia (5.9% [59 of 1,004] versus 8.3% [73 of 884]; P = 0.05), and beneficial trends in mean birth weight and the rate of LBW deliveries. Independently, however, no significant differences were associated with bed net use in 391 women, either primigravidae or multigravidae, who received no IPTp coverage and for whom a bed net was the only protective factor. Education and ANC clinic attendance were highest in women who used a bed net and IPTp. There was no significant difference in education or ANC clinic attendance in mothers who used a bed net alone and those who reported no malaria protection during pregnancy (Figure 5). A point measure of mortality, when the birth cohort ranged from 20.3 to 33.4 months of age, found all-cause deaths highest among infants born to mothers who did not use either a bed net or IPTp and lowest among those who used both a bed net and IPTp (7.7% [15 of 194] versus 4.3% [44 of 1,023]; P = 0.065). This effect was largely attributed to infants of multigravid mothers (85 of 120 deaths) and trended progressively downward with increased S/P use (Figure 6).
Figure 5.
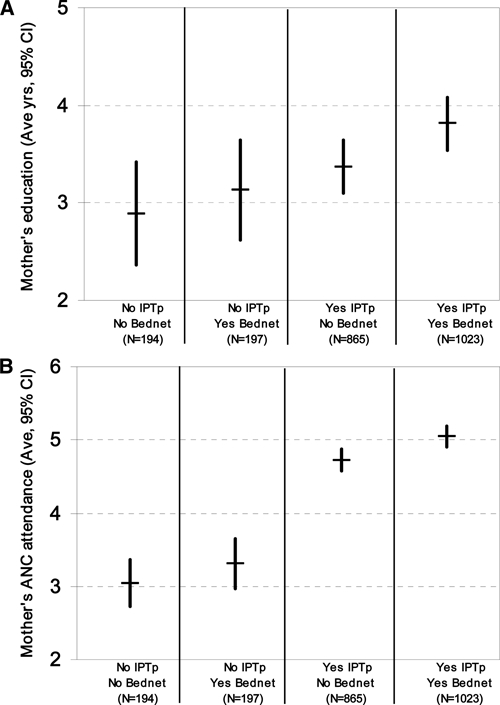
Mean years of A, education and B, antenatal care clinic visits of enrolled women stratified by malaria prevention (bed net use and/or sulfadoxine-pyrimethamine–based intermittent preventive treatment) during pregnancy, Kassena-Nankana District, northeastern Ghana, March 2006–March 2007.
Figure 6.
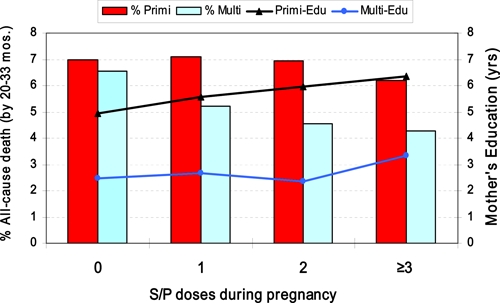
Comparison of mother's education and frequency of all-cause infant death, stratified by reproductive status (primigravid or multigravid) and level of intermittent preventive treatment coverage, Kassena-Nankana District, northeastern Ghana, March 2006–March 2007. A point estimate of all-cause infant mortality was made on October 17, 2008, when enrolled infants ranged in age from 22.3 months to 33.4 months. This figure appears in color at www.ajtmh.org.
Multiple births.
Multiple births occurred at a high rate in our study population (23/1,000). Mothers of twins were older, overwhelmingly multiparous (98%), less educated, more likely to have experienced a miscarriage or child's death, and took less S/P during pregnancy, but were comparable in ANC clinic visits and pre-term deliveries. Their babies' mean birth weight was significantly lower, with 63% of newborns weighing < 2,500 grams (versus 16% of single births with LBW; P < 0.0001). Mean Hb level of twins was significantly higher (14.8 g/dL versus 14.2 g/dL; P = 0.03), but the frequency of fetal anemia was comparable in the two groups. Twin's death rate, although higher, was not significantly different from that of singletons (8.2% versus 5.3%).
Discussion
Robust sample size, a well-designed enrollment questionnaire, and a well-established demographic surveillance system combined to make this one of the largest indicator surveys conducted in western Africa. Unlike previous studies, which have been based on the control and/or manipulation of specific dependent variables, such as IPTp doses in a statistically defined study population, and usually during a period most likely to result in an effect, our results are taken across the entire year, from a large and diverse cross-section of the population, and therefore reflect real-life practices and program performances. These natural patterns and outcomes, emerging from an “all comer” study enrollment plan, may provide important guidance to health care planners and providers.
Recent survey statistics for the Upper East Region of Ghana, where our study was conducted, and for the same year in which it started, reported that only 51% of pregnant women received at least one dose of IPTp with S/P, and that 79% of women 15–49 years of age were illiterate. It was further reported that despite 92% of women receiving ANC at least once, 58% did not deliver in a health facility, and 10% of newborns weighed < 2,500 grams.4 However, these numbers were based on a sample of only 58 pregnant women drawn from across the entire region. An even more focused survey conducted in KND found that bed net use during pregnancy increased more than two-fold during 2000–2003, but even during the high malaria transmission season, when Anopheles spp. biting rates inside houses averaged 10–20 per person-night, the user rate corresponded poorly with the ownership rate recorded. In both survey years, more than 75% of the nets were treated with insecticide and each was sold at a subsidized price of US $0.56.7 Long-term, extensive ownership of bed nets in the irrigated sectors of KND may relate to a heightened nuisance factor and increased household wealth, or may derive from their provision and cost subsidy by the authority managing the irrigation network.
Multimedia-based campaigns and health education have been encouraging pregnant women to make use of the hospital or community clinics for delivery, and our finding of 55% of home deliveries is significantly improved from the 72% reported in KND during 2000, but unchanged from the 55.4% during 2003.7 Similarly, bed net use during pregnancy, which was reported by 29% of 102 nursing and pregnant women surveyed in 2000, increased to 58% in 2003, but our collective measure of 53.5% during 2006–2007 is also indicative of no improvement since 2003. Consideration should be given to the fact that bed net use during 2006–2007 varied considerably among sectors, ranging from 40% in the eastern sector to 77% in the irrigated sector.
Consistently lower prevalence of parasitemia and anemia among young children living in the central sector led us to hypothesize a protective effect associated with town residence in KND, presumably related to socioeconomic status that enabled purchase of bed nets, access to a hospital, prompt treatment, few suitable Anopheles spp. breeding sites, and waste water conditions that favored non-vector Culex mosquitoes. A recent case–control study of young children in KND determined that severe anemia was strongly associated with not using a bed net, and that significantly fewer cases of severe anemia came from the irrigated sector.8 Our results, which report bed net use during pregnancy by 76% of women living in the irrigated sector is little different from that in 2002, when surveys found bed net use highest in the town and irrigated sectors, and lowest in the eastern sector.5
It is encouraging to see that pregnant women overall averaged 4.6 ANC clinic visits during pregnancy, but it is sobering to realize that the WHO goal of four ANC clinic visits was only met by half the enrolled women. Our graph showing ANC clinic visits ranging from 0 to > 10 is believed to reflect de facto transition of the national ANC program of Ghana based on 13 visits to a new, WHO-based focused antenatal care program of four, focused visits.9 Our result, which showed that LBW deliveries were associated with a highly significant lower mean number of ANC clinic visits, is justification for renewed efforts to educate women in the more depressed eastern sector, and to ensure that education stressing the importance of ANC clinic visits is extended downward, even to girls of primary school age. As little as a one-year increment in the mother's education has been determined to affect a 7–9% decrease in mortality rates of children less than five years of age.10 An encouraging manifestation of improved education among girls in the Upper East Region of Ghana is seen in the fact that younger, first-time mothers had significantly higher ANC clinic attendance than older, multigravid women.
Although women's age was comparable across the six sectors, their education differed markedly, with more education clearly associated with town residence and non-local ethnicity. Overall, pregnant women averaged only 3.5 years of education, but nearly half reported no education. This response, assumed to equate with illiteracy, was highest among Nankam (58%) and Buli (52%) and considerably lower among Kasem (33%) and non-local others (12%). Fortunately, women's education appears to be improving considerably in this district, as shown by the highly significant difference between years of education in younger, first-time mothers and older, multiparous mothers (5.9 years versus 2.8 years; P < 0.0001). However, no education was reported by 25% of these younger primigravidae, and education of girls remains a challenge in this district, as in many parts of the world.
The 2003 Ghana Demographic and Health Survey11 reported no education for 28% of women and the recent Multiple Indicator Cluster Survey4 in Ghana cited a national average for women's literacy of 55.4%, ranging from 40.4% among women 45–49 years of age to 71% among women 15–19 years of age. A more impressive affirmation of the interrelationship between women's education and health was seen in the fact that mother's education and ANC clinic attendance were highest, and significantly more so, among women who used bed nets and IPTp S/P during pregnancy. The overall greater use of bed nets by multigravidae and at each level of IPTp dosing might relate to opportunity, experience, and possibly improved economic status. Because of previous ANC clinic attendance, and program targeting, multigravidae had more chances to learn about benefits of bed nets or receive a free or low-cost bed net during one of their earlier pregnancies. Moreover, the bitter personal experience of infant illness and death would likely make older multigravid women wiser and more appreciative of benefits of bed nets.
More than 82% of pregnant women in KND received at least 1 of the 3 doses of S/P prescribed under the national IPTp policy in Ghana, and 60% received ≥ 2 doses, but the 3-dose target was met by only 32%, primarily those living in a town and having easier access to the hospital for these free treatments. A total of 391 (17%) women reported no S/P use during pregnancy. This finding might be expected among those not attending ANC clinics, but most (85%) attended ANC clinics at least once and should have received a dose of S/P. Because the lowest mean number of doses taken and the lowest proportion meeting the IPTp target were in the rural eastern sector and among Nankam women, efforts should be made to strengthen their respective health centers and to ensure timely, reliable, and sufficient supply of drug for pregnant women. Education of girls, women, husbands, and rural health workers must be delivered in such a way that the benefits of IPTp and the hazards of noncompliance can be clearly and simply understood.
Although improvements can be made, it is nevertheless encouraging to see that IPTp coverage of 60% in KND meets the Roll Back Malaria target set for mid 2005,12 exceeds the 27.5% nationwide level reported for Ghana,4 and far exceeds the 18% average derived from household surveys in 16 countries in Africa.1 With highest reported bed net use of any sector, prompted by near-perennial malaria transmission, we are somewhat perplexed by the low IPTp coverage attained in the irrigated sector, where 22% of women reported no S/P use during pregnancy. Is it possible that women and/or health providers in the irrigated sector believe that bed nets are adequate and are complacent in adopting/promoting IPTp as an additional safeguard? Alternatively, a relatively new healthcare program such as IPTp in the irrigated sector may be more complicated and prone to error if it requires special coordination in dividing cost and labor between government and the entity managing the irrigation network.
Our figure of 18.3% LBW in this large cross-sectional population greatly exceeds the national average of 9.1% reported in a recent survey.4 However, this reported national average was a survey estimate and not intended to represent true prevalence of LBW in all districts. Reasons stated by the authors for this discrepancy are the intentional census-based weighting of the national survey, a general tendency in clinics to weigh only two of every five newborns, and likely bias in choosing which infants to weigh.4 Our report of 26.5% LBW among primigravidae and 15.7% among multigravidae during 2006–2007, at a time of relatively extensive bed net use, ANC clinic attendance, IPTp target success, and artesunate combination treatment, is puzzling because our findings are not very different from those for 1995–1996 when LBW frequencies for 10 sites in Ghana ranged from 18% to 40% among primigravidae and from 6% to 18% among multigravidae.13
The overall low prevalence of cord blood parasitemia and reduced levels of anemia we detected among births by primigravidae might be consistent with effective prevention and/or reduction of malaria in pregnancy. Cord blood screening by microscopy can grossly underestimate placental malaria that is the cause of intrauterine growth retardation and LBW in newborns. For example, peripheral blood and placental smears identified, respectively, 26% and 29% of Malian women as parasitemic, whereas only 1 (0.01%) of 1,110 cord blood smears from their deliveries showed a parasitemia.14 In one study, cord blood smears were 20-fold less sensitive than placental smears for detecting placental malaria in Tanzanian women,15 but were only 1.5 times less sensitive than placental blood smears in another study.16
It seems remarkable that a single dose of S/P during pregnancy could significantly improve the health of the newborn, and that these effects should only be seen in first-born children. It is no less surprising that this drug, even at multiple doses, should demonstrate a virtual absence of benefit in children born of multigravid mothers. Fortunately, this basic phenomenon has been well demonstrated by trials at numerous locations. Study of IPTp effect in pregnancy and delivery has derived mainly from controlled clinical trials evaluating the recommended WHO IPTp regimens; few investigations have intentionally assessed single-dose S/P despite the likelihood that IPTp programs will fall short of target with many women receiving suboptimal dosing. A placebo-controlled, double-blind trial in Kenya determined that a single dose of S/P had significant impact in reducing severe maternal anemia when given in late pregnancy to primigravidae but no mention was made of benefit to newborns.17,18 Also in western Kenya, under conditions of intense perennial malaria transmission, two doses of IPTp S/P were highly effective in reducing placental malaria in primigravids but monthly dosing of S/P showed no benefit.19
In defense of limited S/P use during pregnancy, plasma IgG, which mediates protection against placental malaria, was reportedly unaffected in primigravidae given single-dose S/P, but was reduced 2–8-fold in those given multiple doses.20 In rural Burkina Faso, where 23% of primigravid women were parasitemic at delivery and 24% of their newborns were LBW, > 1 IPTp dose of S/P was associated with fewer parasitemias, but 2–3 doses were associated with significantly fewer LBW deliveries, and birth weights that increased in direct proportion to IPTp dosing.21 Irrespective of parity, single-dose S/P in pregnant, human immunodeficiency virus-positive, Zambian women was associated with poorer outcomes than two doses for both mother and newborn, but three or more doses yielded no further benefits.22
In reflecting on IPTp dosing recommendations as a balance between scientific evidence and programmatic feasibility, it has been suggested that threshold benefits of IPTp dosing may be largely determined by transmission intensity and ITN use.23 In our context, lower, seasonal malaria transmission in KND and bed net use by more than half the pregnant women may have enabled a typically inferior, single dose of S/P to look good. Despite this outcome, and our inability to show a statistically greater benefit of ≥ 2 S/P doses, we firmly support the programmatic target of 3 IPTp doses currently used in Ghana. Upward trending of fetal/neonatal anemia was seen with increased S/P use during pregnancy by multigravidae, but we found no correlation between S/P dose and Hb level that might support a hypothesis of drug-based folic acid antagonism and suppression of hematopoeisis.
Numerous studies have demonstrated that S/P-based IPTp and bed net use during pregnancy reduce maternal malaria-induced anemia in primigravidae and improve birth weights of babies. Less clear is the effect of IPTp in improving Hb levels of the newborn and reducing fetal/neonatal anemias. Fetal anemia is relatively common in developing nations and maternal iron deficiency is considered to be the main contributor. However, race, sex, hemoglobinopathies, prematurity, birth weight, and disease are additional determinants.24 In our population, primigravid status, increased maternal education, increased attendance at ANC clinics, IPTp target dosing, and gestation during low-transmission months were independently associated with significantly lower frequencies of fetal anemia.
By western standards, the low mean Hb level and high frequency of fetal anemia measured in our study would seem to reflect serious maternal iron deficiency and malaria, but with iron supplement and S/P, both standard components of the ANC clinic visit, we expected to see cord blood Hb levels correlated with ANC clinic visits and S/P doses taken. Because we did observe this correlation, we are uncertain how much to make of these hematologic findings.
Verhoeff and others25 reported fetal anemia in 23% of babies born to Malawian women who received S/P at least twice during pregnancy and iron-folate supplement at each monthly ANC clinic visit but did not, or could not, identify factors contributing to or ameliorating the condition. Brabin and others26 found a high rate of fetal anemia among Malawian newborns, and although gravidity appeared not to be a factor, maternal anemia, pre-term delivery, and birth during the rainy season were independently associated. The frequency of fetal anemia in our population in Ghana was far lower than that recently reported in southeastern Nigeria,27 but comparable to those reported in Malawi under similar patterns of IPTp coverage.25,26 Interestingly, our findings are also comparable to other malarious areas of Africa, Asia, and Papua New Guinea well before the advent of IPTp or bed nets.28 Nevertheless, we are impressed by the progressive decrease of neonatal anemia and fetal anemia associated with increasing IPTp S/P during pregnancy by primigravid mothers in Ghana and posit this effect as an unappreciated benefit of IPTp in first-born children.
In summary, these survey findings and analyses provide a detailed and descriptive profile of actual individual practices during pregnancy, and their outcomes, over an entire year in a community that is beset by intense seasonal malaria. The results further emphasize that primigravid women and their first-born babies are uniquely vulnerable to malaria, must be the special targets of education and services in the form of ANC, IPTp, ITNs, and should be followed-up closely as the primary indicator populations for further progress against malaria. The findings we have reported for primigravid and multigravid mothers and their babies may add modestly to an already large body of research that validates the benefits of S/P-based IPTp. However, there is special programmatic value in seeing measurable, significant impacts against malaria that enhance health and quality of life. The real significance of our report lies in the fact that these are real-life measures of program performance, maternal practice, ethnic differences, and pregnancy outcome. It is hoped that the comparative demographics for the eastern, predominantly Nankam, sector of KND will alert health providers to disparities that exist and to factors that might improve the lives of women and children.
Acknowledgments
We thank the parents and children for participating in the study and the health workers and support personnel of the Navrongo Health Research Centre for their assistance.
Disclaimer: The views of the authors expressed herein do not purport to reflect those of the Ghanaian Ministry of Health, the U.S. Navy, or the U.S. Department of Defense.
Footnotes
Financial support: This study was supported by National Institutes of Health Division of Microbiology and Infectious Diseases Contract #HHSN266200400016C and interagency agreement #Y1-AI-4866.
Authors' addresses: Abraham R. Oduro, Francis Anto, Frank Atuguba, Thomas Anyorigiya, Martin Adjuik, Patrick Ansah, and Abraham Hodgson, Navrongo Health Research Center, Navrongo, Ghana. David J. Fryauff and William O. Rogers, Naval Medical Research Center, Silver Spring, MD, E-mail: david.fryauff@med.navy.mil. Kwadwo A. Koram and Francis Nkrumah, Noguchi Memorial Institute for Medical Research, Accra, Ghana.
References
- 1.World Health Organization . World Malaria Report 2008. Geneva: World Health Organization; 2008. WHO/HTM/GMP/2008.1. [Google Scholar]
- 2.Binka FN, Kubaje A, Adjuik M, Williams LA, Lengeler C, Maude GH, Armah GE, Kajihara B, Adiamah JH, Smith PG. Impact of permethrin impregnated bednets on child mortality in the Kassena-Nankana district, Ghana: a randomized controlled trial. Trop Med Int Health. 1996;1:147–154. doi: 10.1111/j.1365-3156.1996.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . Geneva: 2006. http://www.who.int/countries/gha/news/2006/anti.malaria.drug.policy Available at. [Google Scholar]
- 4.Ghana Multiple Indicator Cluster Survey (GMICS) 2006. http://www.measuredhs.com/pubs/pub_details Available at.
- 5.Koram KA, Owusu-Agyei S, Fryauff DJ, Anto F, Hodgson A, Atuguba F, Hoffman SL, Nkrumah FK. Seasonal profiles of malaria infection, anemia, and bednet use among age groups and communities in northern Ghana. Trop Med Int Health. 2003;8:793–802. doi: 10.1046/j.1365-3156.2003.01092.x. [DOI] [PubMed] [Google Scholar]
- 6.Appawu M, Owusu-Agyei S, Dadzie S, Asoala V, Anto F, Koram K, Rogers W, Nkrumah FK, Hoffman SL, Fryauff DJ. Malaria transmission dynamics at a site in northeastern Ghana proposed for testing malaria vaccine. Trop Med Int Health. 2004;9:164–170. doi: 10.1046/j.1365-3156.2003.01162.x. [DOI] [PubMed] [Google Scholar]
- 7.Owusu-Agyei S, Awini E, Anto F, Mensah-Afful T, Adjuik M, Hodgson A, Afari E, Binka F. Assessing malaria control in the Kassena-Nankana district of northern Ghana through repeated surveys using the RBM tools. Malar J. 2007;6:103–110. doi: 10.1186/1475-2875-6-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oduro AR, Koram KA, Rogers W, Atuguba F, Ansah P, Anyorigiya T, Ansah A, Anto F, Mensah N, Hodgson A, Nkrumah F. Severe falciparum malaria in young children of the Kassena-Nankana district of northern Ghana. Malar J. 2007;6:1–7. doi: 10.1186/1475-2875-6-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Population Council Program Brief No. 11. 2008. (Frontiers in Reproductive Health).http://popcouncil.org/frontiers Available at.
- 10.Cleland JG, van Ginneken JK. Maternal education and child survival in developing countries: the search for pathways of influence. Soc Sci Med. 1988;27:1357–1368. doi: 10.1016/0277-9536(88)90201-8. [DOI] [PubMed] [Google Scholar]
- 11.Ghana Demographic and Health Survey (GDHS) 2003. http://measuredhs.com Available at.
- 12.Roll Back Malaria . The African Summit on Roll Back Malaria, Abuja, Nigeria. Geneva: World Health Organization; 2000. WHO/CDS/RBM/200017. [Google Scholar]
- 13.Brabin BJ, Agbaje SOF, Ahmed Y, Briggs ND. A birthweight nomogram for Africa as a malaria-control indicator. Ann Trop Med Parasitol. 1999;93:S43–S57. [PubMed] [Google Scholar]
- 14.Kayentao K, Kodio M, Newman RD, Maiga H, Doumtabe D, Ongoiba A, Coulibaly D, Keita AS, Maiga B, Mungai M, Parise ME, Doumbo O. Comparison of intermittent preventive treatment with chemoprophylaxis for the prevention of malaria during pregnancy in Mali. J Infect Dis. 2005;191:109–116. doi: 10.1086/426400. [DOI] [PubMed] [Google Scholar]
- 15.Kabyemela ER, Fried M, Kurtis JD, Mutabingwa TK, Duffy PE. Fetal responses during placental malaria modify the risk of low birth weight. Infect Immun. 2008;76:1527–1534. doi: 10.1128/IAI.00964-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adebami OJ, Gowa JA, Oyedeji GA, Oyelami OA, Omoniyi-Esan GO. Associations between placental and cord blood malaria infection and fetal malnutrition in an area of malaria holoendemicity. Am J Trop Med Hyg. 2007;77:209–213. [PubMed] [Google Scholar]
- 17.Shulman CE, Graham WJ, Jilo H, Lowe BS, New L, Obiero J, Snow RW, Marsh K. Malaria is an important cause of anaemia in primigravidae: evidence from a district hospital in coastal Kenya. Trans R Soc Trop Med Hyg. 1998;90:535–539. doi: 10.1016/s0035-9203(96)90312-0. [DOI] [PubMed] [Google Scholar]
- 18.Shulman CE. Malaria in pregnancy: its relevance to safe-motherhood programmes. Ann Trop Med Parasitol. 1999;93:S59–S66. doi: 10.1080/00034989957745. [DOI] [PubMed] [Google Scholar]
- 19.Parise ME, Ayisi JG, Nahlen BL, Schultz LJ, Roberts JM, Misore A, Muga R, Oloo AJ, Steketee RW. Efficacy of sulfadoxine-pyrimethamine for prevention of placental malaria in an area of Kenya with a high prevalence of malaria and human immunodeficiency virus infection. Am J Trop Med Hyg. 1998;59:813–822. doi: 10.4269/ajtmh.1998.59.813. [DOI] [PubMed] [Google Scholar]
- 20.Staalsoe T, Shulman CE, Dorman EK, Kawuondo K, Marsh K, Hviid L. Intermittent preventive sulfadoxine-pyrimethamine treatment of primigravidae reduces levels of plasma immunoglobulin G, which protects against pregnancy-associated Plasmodium falciparum malaria. Inf Imm. 2004;72:5027–5030. doi: 10.1128/IAI.72.9.5027-5030.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sirima SB, Cotte AH, Konate A, Moran AC, Asamoa K, Bougouma EC, Diarra A, Ouedraogo A, Parise ME, Newman RD. Malaria prevention during pregnancy: assessing the disease burden one year after implementing a program of intermittent preventive treatment in Koupela District, Burkina Faso. Am J Trop Med Hyg. 2006;75:205–211. [PubMed] [Google Scholar]
- 22.Gill CJ, Macleod WB, Mwanakasale V, Chalwae V, Mwananyanda L, Champo D, Mukwamataba D, Chilengi R, Thea DM, Hamer DH. Inferiority of single-dose sulfadoxine-pyrimethamine intermittent preventive therapy for malaria during pregnancy among HIV-positive Zambian women. J Infect Dis. 2007;196:1577–1584. doi: 10.1086/522137. [DOI] [PubMed] [Google Scholar]
- 23.ter Kuile FO, Steketee RW. Intermittent preventive therapy with sulfadoxine-pyrimethamine during pregnancy: seeking information on optimal dosing frequency. J Infect Dis. 2007;196:1575–1576. doi: 10.1086/522233. [DOI] [PubMed] [Google Scholar]
- 24.de Alarcon PA, Johnson MC, Werner EJ. In: Neonatal Hematology. de Alarcon PA, Werner EJ, editors. Cambridge, United Kingdom: Cambridge University Press; 2005. pp. 40–57. (Erythropoiesis, red cells, and the approach to anemia). [Google Scholar]
- 25.Verhoeff FH, Brabin L, Chimsuku L, Kazembe P, Broadhead RL. Malaria in pregnancy and its consequences for the infant in rural Malawi. Ann Trop Med Parasitol. 1999;93:S25–S33. doi: 10.1080/00034989957718. [DOI] [PubMed] [Google Scholar]
- 26.Brabin BJ, Kalanda BF, Verhoeff FH, Chimsuku LH, Broadhead RL. Risk factors for foetal anaemia in a malarious area of Malawi. Ann Trop Paediatr. 2004;24:311–321. doi: 10.1179/027249304225019136. [DOI] [PubMed] [Google Scholar]
- 27.Uneke CJ, Iyare FE, Sunday-Adeoye H, Ajayi JA. Evaluation of maternal malaria at childbirth using rapid diagnostic test and its relationship with birth weight and fetal hemoglobin levels in Nigeria. Internet J Gynecology and Obstetrics. 2008;10:2–7. [Google Scholar]
- 28.Brabin BJ. Fetal anaemia in malarious areas: its causes and significance. Ann Trop Paediatr. 1992;12:303–310. doi: 10.1080/02724936.1992.11747589. [DOI] [PubMed] [Google Scholar]


