Abstract
Selecting suitable anti-malarial treatment represents one of the best tools for reducing morbidity and mortality caused by this disease. Sexual and asexual parasite dynamics were thus evaluated in patients involved in antimalarial drug efficacy studies by using combined treatment with and without artemisinin derivatives for treating uncomplicated acute Plasmodium falciparum malaria in Antioquia, Colombia. All treatment doses were supervised and administered according to patients' weight; sexual and asexual parasitemia were evaluated during 28- or 42-days follow-up in 468 patients. Artemisinin-based combination therapy showed greater parasiticidal ability, showing a mean asexual parasitemia survival rate of one day and mean gametocyte survival rate of 1–2 days. Sexual and asexual parasitemias were eliminated more quickly and effectively in the group receiving artemisinin-based combination therapy. Adding 45 mg of primaquine to treatment with artesunate and mefloquine reduced gametocyte and asexual parasite survival by one day.
Introduction
Malaria is considered to be the third most prevalent infectious disease in the world; only respiratory diseases and diarrheal diseases surpass it regarding frequency. It is the first disease in importance among debilitating diseases and represents one of the world's main public health problems.1,2 Malaria produces a greater negative impact on communities' economic development in the tropics and sub-tropics,1 places where the poorest and most vulnerable populations are concentrated. The World Health Organization (WHO) has estimated that 300–500 million new clinical cases occur each year, 90% of them in Africa that involve 1.5–2.7 million deaths being reported, most of them being among children less than five years of age and pregnant women.2,3
Most American countries have adopted the WHO overall control strategy called “Roll Back Malaria,” which is mainly based on opportune and effective anti-malarial treatment as being the best means for reducing morbidity and mortality caused by malaria. Combined therapy has been considered to be the main strategy for controlling plasmodial resistance to anti-malarial drugs,4,5 especially artemisinin-based combination therapy (ACT). In addition to the ability of a drug to eliminate asexual parasites, its efficacy in eliminating gametocytes when selecting the most effective treatment must be considered. The former characteristic is responsible for symptoms and the latter for transmission. The safety of particular drugs, adherence to treatment, and cost/efficacy ratio must also be taken into account.
During malaria parasite development, a small percentage of asexual parasites develop into sexual parasites (gametocytes) over a 10–12-day period. Five morphologically different stages are recognized; the first four stages (1–4) remain sequestrated in the micro-circulation for seven days and the first three stages are susceptible to circulating schizonticides. Artemisinin-derived drugs can affect stage 4. The fifth stage can remain in peripheral blood for up to 56 days and is resistant to all anti-malarial drugs except for 8-aminoquinolines (e.g., primaquine [PQ]).6–8 This stage can become the focus for infection during this time.9 Inhibiting asexual proliferation by inducing immunologic stress and the efficacy of a drug represent two of the factors that can induce gametogenesis.8 Asexual parasitemia persistence determines the type of therapeutic response when evaluating the efficacy of a drug.2,10 Thus, selecting a combined treatment that could most rapidly eliminate gametocytes and asexual parasites would be one of the most important aspects to bear in mind when selecting drugs.
We analyzed the dynamics of gametocytemia and asexual parasitemia. Our study deals with some combined regimens (with and without artemisinin derivatives) for treating patients with acute uncomplicated Plasmodium falciparum malaria who are naturally exposed to malaria, and longitudinal follow-up in two malaria-endemic regions of Colombia.
Materials and Methods
Sexual and asexual parasitemia was analyzed in patients with uncomplicated P. falciparum malaria who had been included in different studies of the therapeutic efficacy of antimalarial drugs in Colombia. Five therapeutic regimens were evaluated within the context of the Amazon Network for the Surveillance of Antimalarial Drug Resistance (RAVREDA). These five regimens were chloroquine (CQ) plus sulfadoxine-pyremethamine (SP) (CQ + SP), amodiaquine (AQ) plus SP (AQ + SP), mefloquine (MQ) plus SP (MQ + SP), artesunate (AS) + SP (AS + SP), and AS + AQ. Two others, AS + MQ and AS + MQ + PQ, had been used in a pilot study.
Study participants.
The reference population consisted of patients having had fever during the last 48 hours who had voluntarily consulted the malaria diagnosis service and had been diagnosed as having malaria by blood smear examination. Men and non-pregnant women (confirmed by rapid test) having parasitemias of 250–50,000 asexual parasites/μL were included. All were more than one year of age and lived permanently in the study site. None had severe comorbidity or severe malaria (according to WHO criteria) or showed clinical or parasitologic danger signs for increasing the possibility of malarial complications. All committed themselves to complying with controls during the next 1–28 days or 1–42 days.
Exclusion criteria during follow-up consisted of having another associated disease requiring additional treatment, having mixed P. vivax plus P. falciparum infection, being hypersensitive to anti-malarial drugs, having had an adverse serious event, moving to another site different from that where the study was conducted, voluntary withdrawal from the study, and third-party administration of anti-malarial drugs.
Study areas.
The study was conducted in two malaria-endemic areas in the Antioquia Department of Colombia: Turbo in Urabá (8°5′42″N, 76°44′123″W) and El Bagre in the lower Cauca valley (7°35′25″N, 74°48′27″W). These two regions historically contain 90% of malaria cases in the Antioquia Department. Turbo is located in the Gulf of Uraba on the Caribbean Sea on the border with Panama and in the Darien jungle; it has a warm climate. Economic activity is based on banana crops and cattle farming. The lower Cauca region is located on the north bank of the Cauca River and has a warm, humid climate. The prevailing economic activity is gold lode and alluvial mining. Both sites show a marked influx of rural dwellers from the Cordoba Department and of African descent from the Choco Department.
Similarity has previously been found for these regions regarding age, ethnic group, sex, and parasitemia of the participants. Our research has shown that previous malarial episodes and symptoms were more frequent in persons from El Bagre than from Turbo.11 The malaria annual parasite index was historically higher in the lower Cauca area than in Urabá (73.3 versus 41.5 average episodes per 1,000 inhabitants during 1998–2006).12 A homogeneous distribution of merozoite surface protein 1, merozoite surface protein 2, and glutamate-rich protein was observed in each region.13 However, some differences were found regarding frequencies of molecular resistance markers. Briefly, the 86Tyr P. falciparum multidrug resistance polymorphism associated with African and Asian strains was only found in Turbo.14 A mutant P. falciparium chloroquine resistance transporter haplotype (SMET) has been reported in Colombia, which has never been previously reported in South America.15
Therapeutic regimens.
Four sulfa-combination treatments (SCT) (CQ + SP, AQ + SP, MQ + SP, and AS + SP) were sequentially evaluated at both locations from February 2002 through March 2006. The AS + AQ regimen was evaluated at Turbo from April 2005 through November 2006. The AS + MQ and AS + MQ + PQ regimens were evaluated at Turbo from November 2006 through November 2007. Patients were randomly assigned to these regimens by using an EpiInfo (Centers for Disease Control and Prevention, Atlanta, GA) random number selection function. Supplying the patients with the drugs was entirely supervised and monitored by one of the investigators during a half-hour period; if vomiting occurred, then the dose was supplied again with water. Each drug dose was given according to each patient's weight. A total 25 mg/kg dose for CQ and AQ was provided during the first three days, 25 mg/kg for sulfadoxine and 1.25 mg/kg for pyrimethamine (both in a single dose on the first day). Mefloquine was provided in a single 15 mg/kg dose per day for 3 days, AS was provided in three doses at 12 mg/kg/dose, and PQ was provided in a single 0.75 mg/kg dose on day 28 or day 42, except for the AS + MQ + PQ regimen provided on the first day of treatment. Patients were followed-up by a medical research team that conducted an active search in their homes between days 3 and 42. This search consisted of making a clinical evaluation and obtaining thick and thin blood smears from peripheral blood. A one-day variation per control day was accepted during follow-up (days 7–42).
Sample size.
Sample size was calculated using 95% confidence level and 80% statistical test power. Expected failure percentage between treatments was 18% for a 42 patient sample size for each of the five treatment regimens and for both regions (Turbo and El Bagre). Sample size was increased to 50 patients (a 20% increase) to compensate for losses and withdrawals during the follow-up period.
Enrollment and follow-up blood smears.
Initial diagnosis and parasite count were made using thick and thin blood smears (day 0) prepared with peripheral blood, stained with Field's stain and Giemsa, respectively, and viewed in 100× microscopic fields according to guidelines of the Pan American Health Organization.16 Parasite density was calculated by counting the number of asexual parasites against 200 leukocytes in the thick blood film by using a hand tally counter. If less than 10 parasites were found before reaching 200 leukocytes, then counting was continued until 500 leukocytes were counted. Parasite density (expressed as the number of asexual parasites per microliter) was calculated by dividing the number of asexual parasites by the number of leukocytes counted and then multiplying by an assumed leukocyte density of 8,000 leukocytes/μL. Suitable parasitemia for enrollment required at least 1 parasite for every 12–16 leukocytes, which corresponded to approximately 500 asexual parasites/μL. A thick blood smear was diagnosed as being negative when no parasites were found in 300 microscopic fields. All positive slides and 10% of the negative slides were sent to the National Institutes of Health of Colombia for respective quality control by a second, blinded microscopist. All diagnoses were concordant. Some discrepant results were found regarding low parasitemia counts; a third count was thus made in these cases. The same technique was used for establishing parasite counts on each of the subsequent blood film examinations on days 1, 2, 3, 7, 14, 21, and 28, and on days 35 and 42 for those regimens that included MQ with artemisinin derivatives. Gametocytes were counted as described above for asexual parasites. Therapeutic response was defined in accordance with WHO criteria2: early treatment failure, late clinical failure, late parasitologic failure, and suitable clinical and parasitologic response.
Statistical analysis.
Data was collected by completing a form and compiling this information in a database created using EpiInfo 6.04 software (Centers for Disease Control and Prevention). Data were analyzed and plotted by using SPSS version 10.0 software (SPSS Inc., Chicago, IL). Quantitative variables were summarized and presented as median and quartiles, and qualitative variables were given in terms of absolute and relative frequencies. The Kolmogorov-Smirnov and Shapiro-Wilk tests were used to assess normality regarding age, weight, parasitemia, and gametocytemia for each treatment. Sexual and asexual parasites dynamics were analyzed in three ways: 1) the percentage of patients having parasites per day (ring forms or gametocytes), 2) average parasite count per day, and 3) parasite survival rate.
The difference in proportions test was used for comparing the percentage of patients having gametocytes on each follow-up day, and the Kruskal-Wallis test or two-way analysis of variance (ANOVA) were used for comparing sexual and asexual parasitemias, age and weight regarding treatments, follow-up days, and sex. Spearman's rho correlation coefficient was used for evaluating the relationship between asexual parasitemia and gametocytes and both of these factors were compared with age, weight, disease evolution days, and the number of malarial episodes. Relative risk (RR) was used for measuring the causal association of having gametocytes during follow-up for each treatment. The Kaplan-Meier survival analysis (KMSA) test was used for determining sexual and asexual parasite clearance time in each treatment. Log-rank or Breslow tests were used for comparing parasite survival curves according to treatment. Each patient who did not totally eliminate parasites during follow-up, or when a patient became lost during follow-up, was considered to be an uncensored case for this survival analysis; a censored case was defined as when sexual or asexual parasites disappeared during follow-up.
Ethical aspects.
Informed consent was obtained from the patients who agreed to participate in the study. The study was reviewed and approved by the Ethics Committee of the Universidad de Antioquia.
Results
Baseline conditions.
A total of 468 persons 1–80 years of age (mean ± SD age = 26 ± 16 years, median age = 22 years) were included in the study; 50% were 14–37 years of age and 64.5% were male. The mean ± SD number of malarial episodes occurring during a person's life was 1.9 ± 1.6, and the mean ± SD time to disease progression was 5.5 ± 4.5 days; 68.5% of the sample had no history of malaria.
Age, weight, gametocytemia, and parasitemia characteristics were similar between treatment groups, and no statistically significant differences were observed (Table 1). There were no statistically significant differences in baseline conditions between the two locations for the five treatment regimens evaluated.
Table 1.
Baseline characteristics of patients with Plasmodium falciparum regarding seven treatment regimens, Colombia
| Characteristic | No. | Age (years) | Weight (kg) | Asexual parasites/μL | Gametocytes/μL | ||||
|---|---|---|---|---|---|---|---|---|---|
| P*50 | P(25–75) | P50 | P(25–75) | P50 | P(25–75) | P50 | P(25–75) | ||
| CQ and SP | 64 | 17.5 | 7.5–36.8 | 51.5 | 32.3–62.0 | 2,860 | 1,190–7,700 | 0 | 0–0 |
| AQ and SP | 91 | 21.0 | 14.0–30.8 | 57.6 | 42.4–63.6 | 4,860 | 2,280–12,330 | 0 | 0–0 |
| MQ and SP | 92 | 23.0 | 13.0–37.0 | 58.0 | 40.1–67.2 | 4,310 | 1,550–10,285 | 0 | 0–0 |
| AS and SP | 98 | 22.0 | 15.0–41.0 | 58.3 | 45.0–66.0 | 5,110 | 2,198–13,025 | 0 | 0–0 |
| AS and AQ | 52 | 30.0 | 15.5–38.0 | 59.0 | 46.4–70.0 | 7,120 | 2,750–17,095 | 0 | 0–0 |
| AS and MQ | 25 | 20.0 | 14.0–41.1 | 50.3 | 40.5–62.5 | 3,280 | 1,220–7,140 | 0 | 0–20 |
| AS, MQ, and PQ | 25 | 23.8 | 12.4–37.1 | 57.2 | 28.7–67.0 | 3,120 | 940–5,440 | 0 | 0–0 |
| P value for K-S | 0.00001 | 0.00001 | 0.00001 | 0.00001 | |||||
| P value for K-W | 0.2030 | 0.1740 | 0.0600 | 0.3680 | |||||
P = percentile; CQ = chloroquine; SP = sulfadoxine-pyrimethamine; AQ = amodiaquine; MQ = mefloquine; AS = artesunate; PQ = primaquine; K-S Kolmogorov-Smirnov test, K-W = Kruskal-Wallis test.
Treatment and follow-up.
Therapeutic efficacy according to analysis per protocol is shown in Table 2. The CQ + SP treatment had a suitable clinical and parasitologic response rate of 83%. The therapeutic efficacy rates were 98% for AQ + SP and AS + SP, 99% for MQ + SP, and 100% for AS + AQ, AS + MQ, and AS + MQ + PQ. A total of 4.5% of the patients dropped out of the study during follow-up, mostly because patients could not be located or they had moved to another place.
Table 2.
Treatment response regarding combined antimalarial treatment, Colombia
| Response | CQ and SP* | AQ and SP | MQ and SP | AS and SP | AS and AQ | AS and MQ | AS, MQ, and PQ |
|---|---|---|---|---|---|---|---|
| ETF, no. (%) | 1 (1.6) | 1 (1.1) | 0 | 0 | 0 | 0 | 0 |
| LCF, no. (%) | 10 (15.6) | 1 (1.1) | 0 | 0 | 0 | 0 | 0 |
| LPF, no. (%) | 0 | 0 | 1 (1.1) | 2 (2.0) | 0 | 0 | 0 |
| SCPR, no. (%) | 53 (82.8) | 89 (97.8) | 91 (98.9) | 96 (98.0) | 52 (100) | 25 (100) | 25 (100) |
| Total | 64 | 91 | 92 | 98 | 52 | 25 | 25 |
CQ = chloroquine; SP = sulfadoxine-pyrimethamine; AQ = amodiaquine; MQ = mefloquine; AS = artesunate; PQ = primaquine; ETF = early therapeutic failure; LCF = late clinical failure; LPF = late parasitologic failure; SCPR = suitable clinical and parasitologic response.
Sexual and asexual parasitemia dynamics per location.
Sexual and asexual parasitemia dynamics of each treatment regimen showed no statistically significant differences between the two municipalities (by log-rank test). The sexual and asexual parasitemia survival analysis in Turbo and El Bagre for each treatment regimen, except for AS + AQ, AS + MQ, and AS + MQ + PQ, which were only evaluated in Turbo, are summarized in Table 3. The dynamics of sexual and asexual parasitemias of patients could then be analyzed regardless of where they had been included.
Table 3.
Kaplan-Meier analysis of asexual and sexual parasite survival amongst patients at two locations according to treatment regimen, Colombia
| Treatment | Days with asexual parasites | Days with gametocytes | ||||
|---|---|---|---|---|---|---|
| Turbo | El Bagre | Log-rank test P | Turbo | El Bagre | Log-rank test P | |
| Mean (95% CI)* | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | |||
| CQ and SP | 4 (2–6) | 4 (2–6) | 0.9319 | 19 (17–20) | 19 (18–21) | 0.4875 |
| AQ and SP | 2 (1–2) | 2 (1–3) | 0.3864 | 12 (9–15) | 16 (14–18) | 0.0780 |
| MQ and SP | 3 (1–5) | 2 (1–2) | 0.2336 | 12 (9–16) | 11 (8–14) | 0.3824 |
| AS and SP | 1 (1–1) | 1 (1–1) | 0.5148 | 6 (3–8) | 4 (2–7) | 0.5057 |
CI = confidence interval; Log-rank test = a test for comparing parasite survival between two places; CQ = chloroquine; SP = sulfadoxine-pyrimethamine; AQ = amodiaquine; MQ = mefloquine; AS = artesunate.
Sexual parasitemia dynamics per treatment regimen.
The percentage of patients having gametocytes in each treatment presented a pattern similar to that of mean gametocytemia dynamics in such treatments. The same interpretation could be reached with either of the two graphic analyses (Figure 1A and C) because ACT eliminated gametocytes more rapidly than the other combined treatments. Overall gametocyte prevalence at the time of enrollment was 16.2% (range = 10–24%), and no difference was found in the percentage of patients having gametocytes among the seven treatments. Gametocyte prevalence increased from 18% to 53.6% for each treatment 24 hours after starting it, except for AS + MQ + PQ, which showed a reduction of 25%. The percentage of patients with gametocytes on follow-up days 2, 3, and 7 continued to increase, except for AS + MQ + PQ and AS + MQ, in which it continued to decrease, leading to complete gametocyte disappearance on days 14 and 21 (Figure 1A). The percentage of patients having gametocytes for SCTs showed a between days 7 and 14, and ACT showed a gametocytemia peak between days 1 and 3. The RRs for having gametocytes on follow-up days for each treatment are shown in Table 4. The SCT increased RR for having gametocytes from 2 to 10 times more between days 1 and 14. This increase occurred when they gametocytes began to decrease; However, gametocytes did totally disappeared on day 28 and had a statistically significant causal association between days 2 and 14 (except for AS + SP) (Figure 1A and Table 4).
Figure 1.
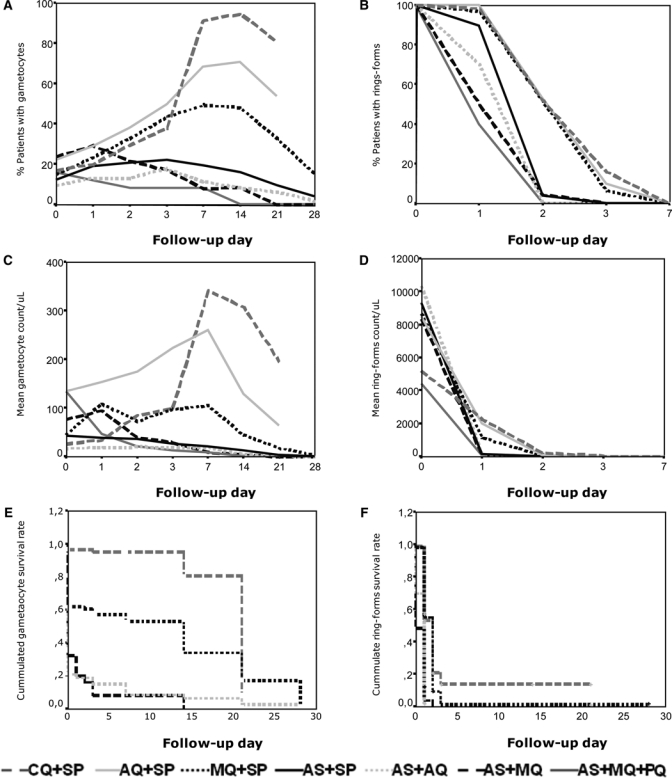
Parasitemia dynamics according to treatment regimen, Colombia. A, Percentage of patients having gametocytes per follow-up day. B, Percentage of patients having ring forms per follow-up day. C, Mean gametocytes per follow-up day. D, Mean ring forms per follow-up day. E, Analysis of gametocyte survival per follow-up day. F, Analysis of ring form survival per follow-up day.
Table 4.
Relative risk of having gametocytes with the different treatment regimens according to follow-up day, Colombia
| Treatment | Day of follow-up | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 7 | 14 | |
| RR (95% CI)* | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| CQ and SP | 1.7 (0.5–5.4) | 3.7 (0.9–14.8) | 4.6 (1.2–17.9) | 11.4 (3.0–43.3) | 10.9 (2.9–41.0) |
| AQ and SP | 2.4 (0.8–7.2) | 4.76 (1.2–18.4) | 5.9 (1.5–22.7) | 8.5 (2.2–32.4) | 1.0 (0.2–6.8) |
| MQ and SP | 2.0 (0.6–6.1) | 4.12 (1.1–16.1) | 5.9 (1.5–22.5) | 6.2 (1.6–23.7) | 5.6 (1.4–21.2) |
| AS and SP | 1.6 (0.5–4.9) | 2.6 (0.6–10.3) | 2.6 (0.7–10.5) | 2.4 (0.6–9.7) | 1.8 (0.4–7.5) |
| AS and AQ | 1.1 (0.3–4.0) | 1.7 (0.4–7.5) | 2.2 (0.5–9.2) | 1.4 (0.3–6.6) | 0.9 (0.25.0) |
| AS and MQ | 2.4 (0.7–8.3) | 2.7 (0.6–12.7) | 2.1 (0.4–10.3) | 1.0 (0.2–6.8) | 1.0 |
| AS, MQ, and PQ† | 1.0 | 1.0 | 1.0 | 1.0 | – |
RR = relative risk; CI = confidence interval; CQ = chloroquine; SP = sulfadoxine-pyrimethamine; AQ = amodiaquine; MQ = mefloquine; AS = artesunate; PQ = primaquine. Value in bold are statistically significant.
Reference treatment with which other treatments were compared.
Mean KMAS results for gametocyte survival time during follow-up are shown in Table 5. Treatment with AS +MQ + PQ, AS + MQ, and AS + AQ led to shorter gametocyte survival, mean time ranging from one to two days, and CQ + SP, AQ + SP, and MQ + SP led to a longer survival mean (11–19 days). The log-rank test was used to compare gametocyte survival curves between treatments. This analysis showed that AS + MQ + PQ differed from the other treatments, except for AS + MQ. Treatment with AS + MQ differed from the other treatments (except for AS + AQ), and SCT did not differ, except for AS + SP, which differed from all other treatments (Figure 1E).
Table 5.
Summary of survival analysis and mean time for sexual and asexual parasites to disappear in treated patients, Colombia
| Treatment | Days with asexual parasites | Days with gametocytes | ||
|---|---|---|---|---|
| Mean (95% CI)*† | Mean (95% CI)‡ | Mean (95% CI)† | Mean (95% CI)‡ | |
| CQ and SP | 4 (2–6)a | 2.4 (1.5–3.3) | 19 (18–20)a | 18.1 (16.7–19.5) |
| AQ and SP | 2 (1–2)a | 1.8 (1.4–2.2) | 14 (13–16)a | 13.6 (11.8–15.4) |
| MQ and SP | 2 (1–3)a | 1.8 (1.2–2.4) | 11 (9–14)a | 10.4 (8.3–12.5) |
| AS and SP | 1 (1–1)b | 0.9 (0.9–1.0) | 5 (3–7)b | 3.7 (2.2–5.2) |
| AS and AQ | 1 (1–1)c | 0.7 (0.6–0.8) | 2 (1–4)cd | 2.1 (0.5–3.7) |
| AS and MQ | 1 (0–1)cd | 0.5 (0.3–0.8) | 2 (0–3)d | 1.6 (0.0–3.2) |
| AS, MQ, and PQ | 0 (0–1)d | 0.4 (0.2–0.6) | 1 (0–1)de | 0.6 (0.0–1.4) |
| Breslow statistic = 203.91, P = 0.00001 | Breslow statistic 173.64, P = 0.00001 | |||
CI = confidence interval; CQ = chloroquine; SP = sulfadoxine-pyrimethamine; AQ = amodiaquine; MQ = mefloquine; AS = artesunate; PQ = primaquine. Superscript letters that are not repeated among the treatments show statistically significant differences by the log-rank test or Breslov test.
Parasite mean survival time obtained by using Kaplan-Meier analysis.
Arithmetic mean in days taken for parasites to disappear.
Gametocyte level was compared with other independent variables (age, weight, sex, follow-up days, treatment, and therapeutic response). Only the type of treatment (ANOVA F = 13.801, degrees of freedom [df] = 6, P = 0.0001) and follow-up days interacting with treatment were found to have a significant main effect (ANOVA F = 1.6 df = 36, P = 0.013). This finding could explain the gametocyte level in this study (32%). Post-hoc analysis by using the Games–Howell test contrasted gametocytemia with different treatments. It was deduced that gametocytes from patients treated with AQ + SP had a similar pattern to those from patients treated with CQ + SP. These treatments showed patterns different from ACT, and gametocytes in patients treated with MQ + SP had a similar pattern to those treated with AS + MQ and AS + MQ + PQ but a different pattern from those with other treatments.
Asexual parasitemia dynamics per treatment regimen.
Asexual parasitemia dynamics for patients treated with ACT (AS + MQ, AS + MQ + PQ, and AS + AQ) showed that all three treatments eliminated asexual parasitemias before day 1 (Figure 1B and D and Table 5). Treatment with SCT (CQ + SP, AQ + SP, and MQ + SP) eliminated asexual parasitemias between days 1.8 and 2.4.
Mean asexual parasite survival time by KMSA test during follow-up is shown in Table 5. One-day survival with ACT was notable, and contrasted with that for CQ + SP, which had a parasite survival rate of 4 days (range = 2–6 days) in 95% of the patients (Figure 1F).
Asexual parasitemia was analyzed for other independent variables (age, weight, sex, follow-up days, treatment, and therapeutic response). Only follow-up day (ANOVA F = 175.436, df = 6, P = 0.0001) and type of treatment interacting with follow-up days (ANOVA F = 2.635, df = 36, P = 0.0001) had a significant effect. This finding could explain the asexual parasite rate (34.7%). Post-hoc analysis using the Games–Howell test compared asexual parasitemias with different treatments and showed that parasites in patients treated with AQ + SP and MQ + SP differed significantly from those in patients treated with AS + MQ + PQ. Other treatments did not show any significant differences.
There was no statistically significant correlation between sexual and asexual parasitemias, or between these factors with age, weight, days of disease progression, and number of malaria episodes.
Discussion
The percentage loss of persons during follow-up was relatively low (4.5%). This finding was partly because of the active search for patients and the perseverance of the fieldwork team, who had more than seven years experience in the area being studied. The age of persons being evaluated showed a variation of 63% variation; most of them (60%) being of working age (15–50 years of age).
Combined treatments with artemisinin derivatives (especially MQ was added) were 100% effective in eliminating parasitemias on the second day of evaluation. This finding was partly because artemisinin derivatives have a higher parasite reduction rate than the other drugs.5,17 These drugs also show a broad spectrum of action against different blood stages, especially against young parasites, which could have prevented parasites from differentiating into gametocytes. These drugs have shorter half-life time (45 minutes), thereby making them less prone to development of resistance. This finding is in contrast to what was observed with MQ, which has a prolonged half-life time (2–3 weeks).
Artemisinin-based combination therapy reduced gametocyte numbers most rapidly because AS has a recognized gametocytocidal effect against stage 4 parasites. This factor must be taken into account when treating patients with P. falciparum malaria in malaria-endemic areas, Use of this therapy would lead to a reduction in transmission risk because gametocytes may remain in the circulation for up to 56 days. Gametocytes disappeared from all patients treated with AS + MQ by day 21 post-treatment. However, such an effect was more potent when 45 mg of PQ was added, which led to elimination of gametocytes from all patients one week earlier, thus reducing the probability of malaria infection being transmitted throughout the region. This finding was partly the result of the gametocytocidal action of PQ. Although gametocytes disappeared faster in persons treated with ACT, such an impact on transmission should be evaluated in future studies.
The difference between mean gametocyte disappearance time by KMSA test analysis or when using the arithmetic mean is because the first procedure takes censored and non-censored cases into account and the second procedure does not discriminate between these cases (i.e., independently of therapeutic response to treatment). Mean gametocyte survival time ranged from 1 to 5 days for ACT and from 11 to 19 days for SCT. Either analysis could be used to show the differences between ACT and the other treatments, especially for AS + MQ + PQ, AS + MQ and AS + AQ, which resulted in lower mean gametocyte disappearance times (1–2 days), and for AS + MQ + PQ, which resulted in a reduction of gametocytes on the first day treatment was provided.
Unlike Bousema and others,18 who provided PQ after a regimen of AS + MQ was completed (i.e., on the third day), our report shows the greater parasiticidal and gametocytocidal efficacy of ACT when 45 mg of PQ was added to the first dose. This addition reduced gametocyte prevalence during treatment and the risk of transmission to another host. These results are in contrast to those reported by El-Sayed and others19 who concluded that adding PQ did not reduce sexual and asexual parasitemias. These results may be partly explained by the fact that PQ was added to treatment with AS + SP on the fourth day after starting treatment, and treatment was evaluated for asymptomatic patients who had been diagnosed as having submicroscopic parasitemia by polymerase chain reaction. In the current study, PQ was provided on the first day of treatment for symptomatic patients who had sought treatment because of a febrile syndrome. However, these patients were diagnosed by thick blood smears and, because these smears show less sensitivity, this represents a limiting factor when these results are compared with those from other studies.
It should be taken into account that 8-aminoquinolines, such as PQ, are the only drugs that show gametocytocidal activity for mature stages of gametocytes. However, their use could be limited because of their ability to induce hemolysis in patients with glucose-6-phosphate dehydrogenase deficiency.20–24
Sulfa-combination treatments (CQ + SP, AQ + SP, and MQ + SP) were more delayed in eliminating asexual parasitemia by day 3 and most increased gametocyte percentage and number. Gametocytes continued increasing up to day 14 when they started to become reduced but they had not totally disappeared by day 28. These results are consistent with those of Stepniewska and others.25 Such asexual parasite patterns could have been caused by the selective effect of SP on mature trophozoite and schizont stages, which could have significantly influenced the clinical and parasitologic response of a patient. Thus, the disappearance of asexual parasites would have been much slower. The risk of having gametocytes during follow-up was shown in this study with SCT. Such results were also reported by Price and others,10 who concluded that treatments that slowly eliminated asexual parasite load showed a greater risk of having gametocytes during follow-up. This finding could have been caused by the number of parasites that became detached from the capillaries and became visible in peripheral blood, with a large number of these gametocytes being in stages 1–3, which are not infective.23 These results are consistent with those of those of Barnes and White,8 who concluded that SP stimulates gametogenesis. Treatment with AS + SP showed an intermediate response pattern regarding the SCT pattern and that of other ACTs.
Use of ACT simultaneously with 45 mg of PQ could reduce absolute costs of treating a patient with P. falciparum malaria by reducing the number of visits to diagnosis centers or health establishments, thus ensuring rapid recovery of the patient and a lower risk of infection. Further studies are required to evaluate the cost/benefit ratio of this new therapeutic alternative.
In conclusion, ACT eliminated asexual and sexual parasitemias more rapidly and effectively than SP regimens. Addition of 45 mg of PQ to the AS + MQ treatment regimen reduced gametocyte survival by one day. When the AS + MQ + PQ regimen was compared with ACT, sexual parasitemias decreased by 2–4 days and 10–18 days when compared with SCT. Further studies are needed for evaluating the effect of PQ, AS, and AS + PQ on gametocyte viability.
Acknowledgments
We thank the Malaria Group at the Universidad de Antioquia; Armando Galeano (Antioquia Department District Health Office); the Pan-American Health Organization; the local hospitals in the towns of Turbo and El Bagre; Felipe Sanin, Mary Luz López, Tania Álvarez and Patricia Rocha, and Rosa Angela Tamayo for their support, and Jason Garry for translating the manuscript.
Footnotes
Authors' address: Gonzalo Álvarez, Alberto Tobón, Juan-Gabriel Piñeros, Alexandra Ríos, and Silvia Blair, Malaria Group, Universidad de Antioquia, Medellín, Colombia.
References
- 1.WHO-UNICEF . Roll Back Malaria World Malaria Report. Geneva: World Health Organization; 2005. WHO-UNICEF. [Google Scholar]
- 2.WHO . Assessment and Monitoring of Antimalarial Drug Efficacy for the Treatment of Uncomplicated falciparum Malaria. Geneva: World Health Organization, WHO/CDS/CSR/RMD document; 2003. Geneva. [Google Scholar]
- 3.Suh KN, Kain KC, Keystone JS. Malaria. CMAJ. 170. 2004:1693–1702. doi: 10.1503/cmaj.1030418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kremsner PG, Krishna S. Antimalarial combinations. Lancet. 2004;364:285–294. doi: 10.1016/S0140-6736(04)16680-4. [DOI] [PubMed] [Google Scholar]
- 5.Orjuela P, Gonzalez I, Osorio L. Combination therapy as a strategy to prevent antimalarial drug resistance [in Spanish] Biomedica (Bogota) 2004;24:423–437. [PubMed] [Google Scholar]
- 6.Talman AM, Domarle O, McKenzie FE, Ariey F, Robert V. Gametocytogenesis: the puberty of Plasmodium falciparum. Malar J. 2004;3:24. doi: 10.1186/1475-2875-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arango E, Alvarez T, Carmona J, Blair S. Gametocyte levels in response to differing malaria treatments in two municipalities of Colombia [in Spanish] Biomedica (Bogota) 2004;24:79–88. [PubMed] [Google Scholar]
- 8.Barnes KI, White NJ. Population biology and antimalarial resistance: The transmission of antimalarial drug resistance in Plasmodium falciparum. Acta Trop. 2005;94:230–240. doi: 10.1016/j.actatropica.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Fujioka H, Aikawa M. In: Malaria: Molecular and Clinical Aspects. Wahlgren M, Perlmann P, editors. Amsterdam: Harwood Academic; 1999. pp. 21–36. (The malaria parasite and its life-cycle). [Google Scholar]
- 10.Price R, Nosten F, Simpson JA, Luxemburger C, Phaipun L, ter Kuile F, van Vugt M, Chongsuphajaisiddhi T, White NJ. Risk factors for gametocyte carriage in uncomplicated falciparum malaria. Am J Trop Med Hyg. 1999;60:1019–1023. doi: 10.4269/ajtmh.1999.60.1019. [DOI] [PubMed] [Google Scholar]
- 11.Carmona-Fonseca J. La malaria en Colombia, Antioquia y las zonas de Urabá y Bajo Cauca: panorama para interpretar la falla terapéutica antimalárica. Parte 2. Iatreia. 2004;17:34–53. [Google Scholar]
- 12.Dirección Seccional de Salud de Antioquia Eventos en Salud Pública. Serie Infeccosas, 1998–2007. http://www.dssa.gov.co/index.php/estadisticas/eventos-de-salud-publica Available at. Accessed February 26, 2010.
- 13.Montoya L, Maestre A, Carmona J, Lopes D, Do Rosario V, Blair S. Plasmodium falciparum: diversity studies of isolates from two Colombian regions with different endemicity. Exp Parasitol. 2003;104:14–19. doi: 10.1016/s0014-4894(03)00112-7. [DOI] [PubMed] [Google Scholar]
- 14.Montoya P, Tobón A, Blair S, Carmona J, Maestre A. Polymorphisms of the pfmdr1 gene in field samples of Plasmodium falciparum and their association with therapeutic response to antimalarial drugs and severe malaria in Colombia. Biomedica (Bogota) 2007;27:204–215. [PubMed] [Google Scholar]
- 15.Restrepo E, Carmona-Fonseca J, Maestre A. Plasmodium falciparum: high frequency of pfcrt point mutations and emergence of new mutant haplotypes in Colombia. Biomedica (Bogota) 2008;28:523–530. [PubMed] [Google Scholar]
- 16.López-Antuñano FJ. In: Diagnosis de Malaria. López-Antuñano FJ, editor. Washington: Pan-American Health Organization; 1988. pp. 39–50. (Diagnostico microscópico de los parásitos de la malaria en la sangre). [Google Scholar]
- 17.von Seidlein L, Jawara M, Coleman R, Doherty T, Walraven G, Targett G. Parasitemia and gametocytaemia after treatment with chloroquine, pyrimethamine/sulfadoxine, and pyrimethamine/sulfadoxine combined with artesunate in young Gambians with uncomplicated malaria. Trop Med Int Health. 2001;6:92–98. doi: 10.1046/j.1365-3156.2001.00683.x. [DOI] [PubMed] [Google Scholar]
- 18.Bousema JT, Gouagna LC, Drakeley CJ, Meutstege AM, Okech BA, Akim IN, Beier JC, Githure JI, Sauerwein RW. l. Plasmodium falciparum gametocyte carriage in asymptomatic children in western Kenya. Malar J. 2004;3:18. doi: 10.1186/1475-2875-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Sayed B, El-Zaki SE, Babiker H, Gadalla N, Ageep T, Mansour F, Baraka O, Milligan P, Babiker A. A randomized open-label trial of artesunate-sulfadoxine-pyrimethamine with or without primaquine for elimination of sub-microscopic P. falciparum parasitaemia and gametocyte carriage in eastern Sudan. PLoS One. 2007;2:e1311. doi: 10.1371/journal.pone.0001311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carmona-Fonseca J, Alvarez G, Maestre A. Methemoglobinemia and adverse events in Plasmodium vivax malaria patients associated with high doses of primaquine treatment. Am J Trop Med Hyg. 2009;80:188–193. [PubMed] [Google Scholar]
- 21.Carmona-Fonseca J, Alvarez G, Rios A, Vasquez MF. Deficiencia de glucosa 6-fostato deshidrogenasa en hombres sanos y en pacientes maláricos; Turbo (Antioquia, Colombia) Rev Bras Epidemiol. 2008;11:252–265. [Google Scholar]
- 22.Baird JK, Hoffman SL. Primaquine therapy for malaria. Clin Infect Dis. 2004;39:1336–1345. doi: 10.1086/424663. [DOI] [PubMed] [Google Scholar]
- 23.Von Seidlein L, Drakeley C, Greenwood B, Walraven G, Targett G. Risk factors for gametocyte carriage in Gambian children. Am J Trop Med Hyg. 2001;65:523–527. doi: 10.4269/ajtmh.2001.65.523. [DOI] [PubMed] [Google Scholar]
- 24.Beutler E. Glucose-6-phospate dehydrogenase deficiency: diagnosis, clinical and genetic implications. Am J Clin Pathol. 1997;47:303–311. doi: 10.1093/ajcp/47.3.303. [DOI] [PubMed] [Google Scholar]
- 25.Stepniewska K, Price RN, Sutherland CJ, Drakeley CJ, von Seidlein L, Nosten F, White NJ. Plasmodium falciparum gametocyte dynamics in areas of different malaria endemicity. Malar J. 2008;7:249. doi: 10.1186/1475-2875-7-249. [DOI] [PMC free article] [PubMed] [Google Scholar]


