Abstract
This study assessed adrenal function in patients with paracoccididioidomycosis who had been treated to determine a possible connection between high antibody titers and adrenal dysfunction attributable to persistence of the fungus in adrenal gland. Adrenal gland function was studied in 28 previously treated patients, 2 (7.1%) of whom were shown to have adrenal insufficiency and 7 (259%) who showed a below normal response to stimuli by adrenocorticotropic hormone. Paracoccidioides brasiliensis was detected in the adrenal gland from one of the patients with adrenal insufficiency. Although the study failed to demonstrate a significant difference between high antibody titers and low cortisol levels, the proportion of adrenal insufficiency detected and the subnormal response to adrenocorticotropic hormone confirmed that adrenal damage is an important sequela of paracoccidioidomycosis. Studies with a larger number of patients should be conducted to confirm the hypothesis of persistence of P. brasiliensis in adrenal gland after therapy.
Introduction
Paracoccidiodomycosis (PCM) is a systemic endemic disease caused by the thermally dimorphic fungus Paracoccidioides brasiliensis; it is geographically restricted to Latin America where it is considered a prevalent mycosis.1,2 Primary lung involvement leads to damage of multiple organs, including skin, mucous membranes, lymph nodes, adrenal glands, and central nervous system.3 Adrenal insufficiency is a frequent complication of PCM; prevalence rates range from 2.9% to 48.2%, depending on the method used to diagnose the disorder.4–6 The presence of P. brasiliensis in the adrenal gland has been reported, specifically in autopsy cases (85–90%),6,7 and more recently, in patients who have been treated for the disease.8
We have noted that certain patients with the mycosis show persistently elevated antibody titers even after completion of an adequate treatment course, which suggests a continuous antigenic stimulus, probably caused by persistence of the fungus in the body. The present study evaluated the status of the adrenal gland function after completion of antifungal therapy and prolonged follow-up periods; further efforts were made to define the possible relationship among antibody titers against P. brasiliensis, basal cortisol levels, and post-ACTH stimulation results.
Patients and Methods
This cross-sectional study included patients with PCM who had been treated earlier at the Corporación para Investigaciones Biológicas in Medellín, Colombia, and who have had no previous history of adrenal insufficiency not concomitant tuberculosis. At time of the present study, all patients underwent a thorough clinical evaluation to identify possible manifestations of adrenal insufficiency or foci of activity caused by the causative fungus.
All patients were bled after fasting at 8:00 am for measurement of antibody titers by using complement fixation and basal cortisol levels by using electrochimioluminiscence. Patients were then administered 250 μg of adrenocorticotropic hormone ACTH (Synacthen® aqueous; Endocrine Sciencies, Tarzana, CA) in a two-hour infusion. Measurements of serum cortisol levels were repeated an hour after the infusion had been completed.
Those patients who were diagnosed with adrenal insufficiency (see definition below) were subjected to abdominal computerized tomography with emphasis on adrenal glands by using the multislice computer tomograph Somaton Emotion 6 (Siemens, Munich, Germany).
Patients were assigned to one of two groups according to the results of complement fixation, those with low titers (< 1:32) and those with higher titers (≥ 1:32).9 Adrenal insufficiency was defined when basal cortisol or cortisol after ACTH stimulation measurements gave the following results: a basal cortisol < 5 μg/dL or a cortisol post-ACTH stimulation < 18 μg/dL. A subnormal response to ACTH stimulation was considered when the cortisol levels increased < 16.5 μg/dL from baseline values, as defined by Colombo and others.10
All results were kept in a database created in Microsoft Excel® (Microsoft, Redmond, WA) for this purpose and were processed using the SPSS software version 15.0® (SPSS Inc., Chicago, IL). Quantitative data are presented as the mean and standard deviation and qualitative data as absolute frequencies and percentages. The Student's t-test was used to compare quantitative variables. Results are presented as mean difference and 95% confidence intervals. A P value < 0.05 was considered statistically significant. Normality of the quantitative variables was checked by using the Shapiro-Wilk test.
This study was reviewed and approved by the Ethics Committees of the Universidad Pontificia Bolivariana and the Corporación para Investigaciones Biológicas in Medellín, Colombia. The corresponding work was subjected to the principles in the Helsinki Declaration. All patients freely authorized their participation in the study by signing an informed consent form prior to undertaking any activity belonging to the proposed research study.
Results
Twenty-eight patients with previously treated PCM were included in this study. All were males, with a median age of 55.3 years (range = 25–76 years), the mean ± SD time since PCM diagnosis and entry into the study was 13 ± 9.22 years (Table 1).
Table 1.
Baseline characteristics of 28 patients with paracoccidioidomycosis and evaluation of adrenal function
| Characteristic | Value |
|---|---|
| Age, years (range) | 55.3 (25–76) |
| Male:female ratio | 28:0 |
| Occupation, no. (%) | |
| Farmer | 11 (39.3) |
| Mason | 4 (14.3) |
| Several occupations | 3 (10.7) |
| Retired | 3 (10.7) |
| Unemployed | 2 (7.1) |
| Others* | 5 (17.9) |
| Coomorbilities, no. (%) | |
| Chronic obstructive pulmonary disease | 4 (14.3) |
| Smoking | 24 (85.7) |
| High blood pressure | 4 (14.3) |
| Others† | 5 (17.9) |
Watchman, charcoal burner, carpenter, salesman, and driver.
Skin cancer, infection with human immunodeficiency virus, diabetes mellitus, cardiac failure and hypothyroidism.
According to clinical presentation of the disease, 17 (60.7%) patients had the chronic multifocal form, 6 (21.4%) had the sub-acute form, and 5 (17.9%) had the chronic unifocal form. The most frequent sites of involvement at diagnosis were the lungs (85.7%), followed by mucous membranes (64.3%), lymph nodes (32.1%), skin (28.6%), liver (7.1%), and spleen (7.1%). The characteristics of the organs involved by the fungus in the group studied are summarized in Table 2.
Table 2.
Characteristics of 28 patients with paracoccidoidomycosis and evaluation of adrenal function
| Characteristic | Value |
|---|---|
| Form of the mycosis, no. (%) | |
| Chronic unifocal | 17 (60.7) |
| Chronic multifocal | 6 (21.4) |
| Subacute | 5 (17.9) |
| Organs affected, no. (%) | |
| Lungs | 24 (85.7) |
| Mucosae | 18 (64.3) |
| Lymph nodes | 9 (32.1) |
| Skin | 8 (28.6) |
| Liver | 2 (7.1) |
| Spleen | 2 (7.1) |
| Time since diagnosis, years (SD) | 13.32 (9.22) |
| Type of antifungal drug, no. (%) | |
| Itraconazole | 18 (64.3) |
| Ketoconazole | 4 (14.3) |
| Saperconazole | 1 (3.6) |
| Fluconazole | 1 (3.6) |
| Ketoconazole/posaconazole | 1 (3.6) |
| Itraconazole/ketoconazole | 2 (7.1) |
| Amphothericin B/trimethoprim and sulfamethoxazole | 1 (3.6) |
| Duration of treatment, months (SD) | 7.8 (3.6) |
Treatment consisted of the following azolic compounds: itraconazole was given to 18 (64.3%) patients, ketoconazole was given to 4 (14.3%), and other azoles were given to 2 (7.1%) patients; the remaining 4 (14.3%) received antifungal combinations. The mean ± SD duration of treatment was 7.8 ± 3.6 months.
In relation to signs and symptoms of adrenal insufficiency at the time of clinical evaluation for this study, asthenia was reported in three patients, anorexia and adynamia in four, nausea and vomiting in one, and weight loss in two; no patient reported diarrhea. One patient had low blood pressures and 2 patients had orthostatism. Three of the patients had hyperpigmentation of the skin and mucous membranes. A total of 7 of the 28 patients (25%) had signs of adrenal damage.
At time of the initial PCM diagnosis, antibody titers, as measured by complement fixation, were available in the records for 26 (92.9%) of the 28 patients studied and in 27 (96.4%) of 28 at end of antifungal therapy. At diagnosis, 20 (76.9%) patients showed high antibody titers (≥ 1:32), in 11 (40.7%) of whom they persisted at equal titers at end of therapy. At the time of the present evaluation post-therapy, 3 (10.7%) of such patients had persistently high titers (≥ 1:32).
The mean ± SD cortisol basal level was 13. 45 ± 4.81 μg/dL (range = 1.40–20.05 μg/dL). After ACTH stimulation, the ± SD mean cortisol level increased to 34.36 ± 7.41 μg/dL (range = 22.04–51.68 μg/dL). The mean ± SD increase after stimulation was 20.47 ± 6.35 μg/dL (range = 8.50–34.60 μg/dL). In one patient, no cortisol samples after ACTH stimulation were obtained because of his poor clinical condition. Adrenal insufficiency was diagnosed in 2 (7.14%) patients whose basal cortisol values were 1.4 μg/dL and 4.8 μg/dL, respectively. A total of 7 (25.9%) of the 27 patients showed minor increases (16.5 μg/dL) after ACTH stimulation, with only one having received ketoconazole treatment for PCM. All of them had cortisol values > 18 μg/dL after ACTH administration. Comparison of cortisol values obtained with antibody titers against P. brasiliensis are shown in Tables 3 and 4.
Table 3.
Cortisol levels in relation to antibody titers against Paracoccidoides brasiliensis at end of therapy for paracoccidioidomycosis*
| Cortisol levels, μg/dL (SD) | Antibody titers | |||
|---|---|---|---|---|
| Low (n = 16) | High (n = 11) | P | Mean difference (95% CI) | |
| Basal | 14.56 (3.89) | 11.40 (5.55) | 0.09 | 3.16 (–0.57 to 6.89) |
| Post-ACTH | 35.88 (6.52) | 32.16 (8.82) | 0.22 | 3.72 (–2.49 to 9.94) |
| Mean increase | 21.32 (6.29) | 19.76 (6.62) | 0.55 | 1.56 (–3.78 to 6.90) |
CI = confidence interval; ACTH = adrenocorticotropic hormone.
Table 4.
Cortisol levels in relation to antibody titers against Paracoccidiodes brasiliensis at follow-up visit*
| Cortisol levels, μg/dL (SD) | Antibody titers | |||
|---|---|---|---|---|
| Low (n = 25) | High (n = 3) | P | Mean difference (95% CI) | |
| Basal | 13.20 (4.99) | 15.50 (2.33) | 0.44 | −2.30 (–8.38 to 3.78) |
| Post-ACTH | 33.66 (7.34) | 39.93 (6.55) | 0.23 | −6.26 (–15.44 to 2.91) |
| Mean increase | 19.97 (6.45) | 24.42 (4.37) | 0.26 | −4.45 (–12.41 to 3.50) |
CI = confidence interval; ACTH = adrenocorticotropic hormone.
The two patients with adrenal insufficiency had skin and mucous membranes hyperpigmentation. In addition, one of them had constitutional symptoms and low blood pressure. In this patient, abdominal computed tomography showed adrenal glands with an irregular, nodular appearance, calcifications (Figure 1). The biopsy of one of the glands confirmed the presence of P. brasiliensis in the gland's parenchyma. In the remaining patient, computed tomography of the adrenal glands showed morphologically normal glands but no biopsy specimens were obtained. Both patients had been treated previously with itraconazole for control of their fungal infection.
Figure 1.
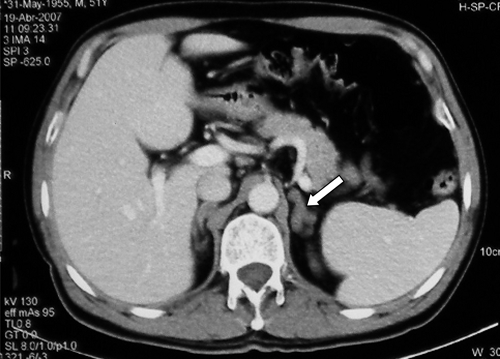
Adrenal gland in a patient with paracoccidioidomycosis and adrenal insufficiency. Note the nodules and calcifications.
Discussion
The adrenal gland tissues are highly susceptible to invasion by multiple pathogens, including P. brasiliensis and other fungi, because of immunosuppression caused by their high steroid concentrations.11–13 Paracoccidioides brasiliensis has special tropism for adrenal tissues, as shown by its visualization in the glands of 90% of the autopsy cases.6,7
Fungal invasion of the adrenal glands results in a chronic granulomatous inflammation of the parenchyma, the degree of which appears to correlate with the extent of adrenal dysfunction.6,12–14 This finding enables a wide spectrum of involvement that extends from the asymptomatic patient, in whom the gland may act as a reservoir for P. brasiliensis, passing through the patient with a subnormal response to ACTH stimulation, and ending with a patient with symptoms accompanied by low basal cortisol levels.
Symptomatic adrenal insufficiency is observed in 3–10% of PCM patients,11,12 with this problem being observed in a larger number of patients (14–21%) through an insufficient cortisol increase after ACTH stimulation, even in those patients that have received treatment for the fungal infection.15 Similar data were found in this study, namely, 7.1% for adrenal insufficiency and 25.9% for subnormal response after ACTH stimulation.
Despite the fact that a subnormal response to ACTH stimulation does not carry physiological implications, the varying degrees of adrenal involvement found (symptomatic adrenal insufficiency, subnormal response to ACTH stimulation, and damage detected only by autopsy studies), suggest a wide range of adrenal abnormalities, an important proportion of which had no clinical manifestations. Faiçal and others demonstrated the presence of P. brasiliensis in one patient with previously treated PCM in whom adrenal insufficiency developed several years after completion of antifungal therapy.8 This finding suggests that the adrenal glands could act as a reservoir for the fungus, thus facilitating relapses; progressive destruction of the gland with subsequent development of insufficiency may also occur.
The persistence of high antibody titers against P. brasiliensis by the end of treatment and during post-therapy follow-up periods is a frequent finding in patients with PCM.16 This finding suggests the persistence of the fungus in the body, as shown by the 40.7% of the patients in whom high antibody titers persisted not only up to the end of treatment, but also in 10.7% of them at time of their last follow-up visit (mean observation time = 13.32 years). However, an association between elevated antibody titers and low cortisol levels could not be established. Nonetheless, this study demonstrated the presence of P. brasiliensis in one patient with symptomatic adrenal insufficiency eight years after completion of an otherwise effective therapy; this patient was included in a previous report.17
The inclusion in this series of patients with prior use of ketoconazole, a known reversible inhibitor of 21-αhydroxylase, could have complicated the results of this study. However, none of the patients with adrenal insufficiency had previously received this azole and only one patient with previous use of this medication showed subnormal response after the administration of ACTH.
This study showed that in PCM patients who had been previously treated, the prevalence of adrenal insufficiency was 7.1% with the subnormal response to ACTH stimulation being higher (25.9%). These figures confirm not only the high frequency of damage caused by the fungus to the adrenal glands but its persistence throughout the years after completion of therapy. Although it was not possible to establish an association between high antibody titers against P. brasiliensis and low cortisol levels, subsequent studies involving more patients should be conducted to clarify a possible relationship between these observations. Follow-up studies should be conducted in an attempt to assess if in, due time, the subnormal response to ACTH would predict progress towards adrenal insufficiency.
Acknowledgments
We thank the patients and the personnel of the Medical Mycology Diagnostic team of the Corporación para Investigaciones Biológicas for participating in the study. The study was presented as an abstract during the Tenth International Congress on Paracoccidioidomycosis, August 7–10, 2008, Medellín, Colombia.
Footnotes
Financial support: This study was supported by Corporación para Investigaciones Biológicas, Centro de Investigación para el Desarrollo y la Innovación from the Universidad Pontificia Bolivariana and Hospital Pablo Tobón Uribe.
Authors' addresses: Angela M. Tobón, Carlos A. Agudelo, and Angela Restrepo, Corporación para Investigaciones Biológicas, Medellín, Colombia, E-mails: atobon@cib.org.co, carlosagudelo@yahoo.com, and arestrepo@cib.org.co. Carlos A. Restrepo and Carlos A. Villa, Universidad Pontificia Bolivariana, School of Health Sciences, Medellín, Colombia, E-mails: restrepocastro@gmail.com and andres801@une.net.co. William Quiceno, Hospital Pablo Tobón Uribe, Medellín, Colombia, E-mail: williamdejquiceno@hotmail.com. Santiago Estrada, Laboratorio Clínico Congregación Mariana, Medellín, Colombia, E-mail: sestrada@congregacionmariana.org.co.
References
- 1.Franco M, Mendes RP, Moscardi-Bacchi M, Rezkallah-Iwaso M, Montenegro MR. Paracoccidioidomycosis. Bailliere's Clin Trop Med Commun Dis. 1989;4:185–220. [Google Scholar]
- 2.Restrepo A, Tobón A. In: Principles and Practice of Infectious Diseases. Sixth edition. Mandell GL, Bennett JE, Dolin R, editors. New York: Churchill Livingstone; 2005. pp. 3062–3068. (Paracoccidioides brasiliensis). [Google Scholar]
- 3.Restrepo A, Tobón A, Agudelo C. In: Diagnosis and Treatment of Human Mycoses. Hospenthal DR, Rinaldi MG, editors. Totowa, NJ: Humana Press; 2008. pp. 331–342. (Paracoccidioidomycosis). [Google Scholar]
- 4.Del Negro G, Wajchenberg BL, Pereira VG, Schneider J, Ulhoa-Cintra AB, Assis LM, Sampaio AS. Addison's disease associated with South American blastomycosis. Ann Intern Med. 1961;54:189–197. [Google Scholar]
- 5.Oñate J, Tobón A, Restrepo A. Insuficiencia suprarenal secundaria a paracoccidioidomicosis. Biomedica (Bogota) 2002;22:280–286. [PubMed] [Google Scholar]
- 6.Tendrich M, Wanke B, Del Negro G, Wajchenberg B. In: Paracoccioidomycosis. Franco M, Da Silva C, Restrepo A, Del Negro G, editors. Boca Raton, FL: CRC Boca Raton; 1993. pp. 303–310. (Adrenocortical involvement in paracoccidioidomycosis). [Google Scholar]
- 7.Salfelder K, Doehnert G, Doehnert HR. Paracoccidioidomycosis; anatomic study with complete autopsies. Virchows Arch A Pathol Pathol Anat. 1969;348:51–76. [PubMed] [Google Scholar]
- 8.Faiçal S, Borri ML, Hauache OM, Ajzen S. Addison's disease caused by Paracoccidioides brasiliensis: diagnosis by needle aspiration biopsy of the adrenal gland. AJR Am J Roentgenol. 1996;166:461–462. doi: 10.2214/ajr.166.2.8553971. [DOI] [PubMed] [Google Scholar]
- 9.Gomez BL, Figueroa JI, Hamilton AJ, Diez J, Rojas M, Tobon AM, Hay RJ, Restrepo A. Antigenemia in patients with paracoccidioidomycosis: detection of the 87-kilodalton determinant during and after antifungal therapy. J Clin Microbiol. 1998;36:3309–3316. doi: 10.1128/jcm.36.11.3309-3316.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colombo A, Faiçal S, Kater C. Systematic evaluation of the adrenocortical function in patients with paracoccidioidomycosis. Mycopathologia. 1994;127:89–93. doi: 10.1007/BF01103064. [DOI] [PubMed] [Google Scholar]
- 11.Oelkers W. Adrenal insufficiency. N Engl J Med. 1996;335:1206–1212. doi: 10.1056/NEJM199610173351607. [DOI] [PubMed] [Google Scholar]
- 12.Stewart PM. In: Williams Textbook of Endocrinology. 11th edition. Williams RH, Foster DW, Kronenberg HM, editors. Philadelphia: W.B. Saunders; 2007. pp. 445–504. (The adrenal cortex). [Google Scholar]
- 13.Winqvist O, Rorsman F, Kämpe O. Autoimmune adrenal insufficiency: recognition and management. BioDrugs. 2000;13:107–114. doi: 10.2165/00063030-200013020-00004. [DOI] [PubMed] [Google Scholar]
- 14.Lacaz CS, Porto E, Martins JE, Heins-Vaccari E, de Melo NT. In: Tratado de Micologia Médica Lacaz. Ninth edition. Lacaz C, Porto E, Martins JE, editors. Sao Paulo, Brazil: Sarvier; 2002. pp. 639–729. (Paracoccidioidomicose). [Google Scholar]
- 15.Restrepo-Moreno A. In: Clinical Mycology. Dismukes WE, Pappas PG, Sobel JD, editors. New York: Oxford University Press; 2003. pp. 328–345. (Paracoccidioidomycosis). [Google Scholar]
- 16.Camargo ZP. Serology of paracoccidioidomycosis. Mycopathologia. 2008;165:289–302. doi: 10.1007/s11046-007-9060-5. [DOI] [PubMed] [Google Scholar]
- 17.Agudelo CA, Muñoz C, Ramírez A, Gutierrez J, Vélez S, Pérez JC, Vélez A, Tobón AM, Restrepo A. Identification of Paracoccidioides brasiliensis in adrenal glands biopsies of two patients with paracoccioidomycosis and adrenal insufficiency. Rev Inst Med Trop Sao Paulo. 2009;51:45–48. doi: 10.1590/s0036-46652009000100008. [DOI] [PubMed] [Google Scholar]


