Abstract
Interactions between environmental and biological factors affect the vector competence of Culex pipiens quinquefasciatus for West Nile virus. Three age cohorts from two Cx. p. quinquefasciatus colonies were fed blood containing a low- or high-virus dose, and each group was held at two different extrinsic incubation temperatures (EIT) for 13 days. The colonies differed in the way that they responded to the effects of the environment on vector competence. The effects of mosquito age on aspects of vector competence were dependent on the EIT and dose, and they changed depending on the colony. Complex interactions must be considered in laboratory studies of vector competence, because the extent of the genetic and environmental variation controlling vector competence in nature is largely unknown. Differences in the environmental (EIT and dose) and biological (mosquito age and colony) effects from previous studies of Cx. p. quinquefasciatus vector competence for St. Louis encephalitis virus are discussed.
Introduction
The relationships between vector competence and extrinsic incubation temperature (EIT), virus dose, and mosquito age have been observed in several arbovirus-vector systems.1–6 Although it is well-established that different environmental or biological factors influence mosquito vector competence for arboviruses, there has been little work to determine how various biological and environmental factors interact with one another to influence vector competence. Culex pipiens quinquefasciatus Say vector competence for St. Louis encephalitis virus (SLEV) is dependent on complex interactions between the biological factors of age and mosquito population and the environmental factors of EIT and virus dose.6 West Nile virus (WNV; family Flaviviridae: genus Flavivirus) is maintained in an enzootic cycle involving ornithophilic mosquitoes and avian populations, and it threatens humans whenever large numbers of infectious mosquitoes exhibiting opportunistic feeding coincide with amplification hosts and human populations.7 Culex p. quinquefasciatus feeds on both avian and mammalian hosts,8–10 has been shown to be a competent vector of WNV in the laboratory,3,11,12 has been found in nature to be infected with WNV,13,14 and is considered a prominent WNV vector in the United States.
Here, we characterize the effects of environmental and biological factors on Cx. p. quinquefasciatus vector competence for WNV using four phenotypes: susceptibility to infection, disseminated infection, body virus titer, and leg virus titer. These factors were shown to influence Cx. p. quinquefasciatus vector competence for SLEV.6 Female mosquitoes with no infection of the body show a midgut infection barrier (MIB), whereas females with an infected body and no dissemination to the legs show a midgut escape barrier (MEB).15 We characterized virus titers in the body and legs as a quantitative measure of the ability of the virus to replicate in mosquito tissues. It is likely that mosquitoes with high virus titers are more efficient vectors, because the salivary gland barrier for WNV infection is dose-dependent in Cx. p. pipiens L.,16 Cx. tarsalis Coquillet,17 and Cx. p. quinquefasciatus.18
The degree of intra- and interpopulation variability in interactions between environmental and biological factors has not been explored. However, this variability must be characterized to understand vector competence in nature and the impact of vector competence on the epidemiology of arbovirus cycles. Previous studies with SLEV and Cx. p. quinquefasciatus showed variations in the relationship between susceptibility to infection and disseminated infection caused by environmental and biological interactions that lead to caution concerning interpretations of single-factor experiments.6 Here, we characterize if there are differences in environmental and biological responses in Cx. p. quinquefasciatus with a related virus, WNV.
Large sample sizes are essential for statistical precision to distinguish differences between treatment groups when testing vector competence.19 We use a priori power analyses to determine the sample sizes needed to detect significant differences in vector competence between treatments (GPower; http://www.psycho.uni-duesseldorf.de/aap/projects/gpower/).
Materials and Methods
Mosquitoes and virus.
The two Cx. p. quinquefasciatus colonies, originating from two regions of Florida, used in this study and their rearing are described in detail elsewhere.6 The respective colonies originated in 1995 and 2007, and they will be referred to as the 1995 colony and 2007 colony.
The WN-FL03p2-3 strain of WNV (passaged four times in Vero cells and one time in baby hamster kidney cells) that we used was isolated from a pool of Cx. nigripalpus Theobald mosquitoes from Indian River County, Florida in 2003 (Doumbouya A, unpublished data).
Mosquito infection.
The methods used are described elsewhere.6 Briefly, over 200 mosquitoes were fed on each meal for each colony, age, dose, and EIT to achieve a minimum of 50 mosquitoes per treatment for analysis. Five whole mosquitoes were frozen immediately after feeding from each treatment to assess the amount of WNV in the mosquito homogenate. As reported previously,6 the 2007 colony had a lower rate of blood feeding compared with the 1995 colony, and therefore, the 2007 colony was fed using cotton pledgets to increase the sample size. Vector competence experiments for each colony were not conducted simultaneously. The 1995 colony was fed using membrane feeders in a separate experiment. The differences in feeding preferences may reflect other differences in the colonies, and forcing both colonies to feed using the same method would have introduced other biases of which females did or did not feed. We chose to use the methods that gave us the best feeding rates for each colony. Mosquitoes aged 3–4 days post-eclosion (dpe), 7–8 dpe, and 11–12 dpe were respectively classified as young, middle-aged, and old mosquitoes.
Blood meal preparation.
To provide two consistent different blood meal doses for each age group, a previously frozen WNV stock was diluted for each experiment by mixing with citrated bovine blood before mosquito blood feeding to create blood meals containing approximately 6 logs plaque-forming units (pfu)/mL WNV titer, called the low dose, compared with approximately 6.7 logs pfu/mL, called the high dose. Then, two aliquots of 0.1 mL of infected blood were each added to 0.9 mL BA-1 diluent20 and held at −80°C until processing to determine blood meal titer.
Blood meal and mosquito processing.
We used a 13-day incubation period at 25°C or 28°C6 as the time interval required for Cx. p. quinquefasciatus to become infected after a blood meal, and we processed the mosquitoes as previously described.6 Mosquitoes that survived the incubation period were killed by freezing. Aseptic techniques were used to separate bodies and legs, and samples were placed separately into 0.9 mL BA-1 diluent containing two beads and stored at −80°C until processing. Samples were thawed, homogenized, and centrifuged before further processing.6
Virus assays.
Viral RNA was extracted as previously described6 and evaluated using quantitative real-time TaqMan reverse transcription–polymerase chain reaction (qRT-PCR) with methods described elsewhere.21 Virus found in the body but not the legs represented a non-disseminated infection limited to the midgut. Virus in both the body and legs was considered a disseminated infection. The infection rate was the percentage of all mosquitoes tested having infected bodies. The dissemination rate was the percentage of mosquitoes with infected bodies that also had infected legs.
A priori power analyses22,23 (GPower; http://www.psycho.uniduesseldorf.de/aap/projects/gpower/) using Cohen's small (w = 0.1), medium (w = 0.3), and large (w = 0.5) effect size estimates are described elsewhere.6 These analyses were based on χ2 analyses that examined differences in infection and dissemination rates between treatments. Sample sizes to detect differences with 99% power were ≥ 589 mosquitoes to detect a small to medium (w = 0.2) effect of age, EIT, dose, and colony (P = 0.05; degrees of freedom (df) = 3) on infection rate, and ≥ 262 mosquitoes would be required if only a medium (w = 0.3) effect size was expected.6 The sample sizes that we used allowed us to detect small to medium effect sizes in infection rates. Sample sizes (and therefore, power to detect small differences) for dissemination rates were expected to be lower, because we did not expect 100% infection rates.
Statistical analysis.
The statistical analyses used here are described elsewhere.6 A χ2 test (P < 0.05) was used to analyze infection and dissemination rates with respect to mosquito age in different treatments. Separate analyses of variance (ANOVA) examined virus titers from whole bodies of freshly fed mosquitoes as well as body and leg titers of virus-positive mosquitoes at the end of the incubation period. If significance (P < 0.05) was observed in an analysis, then a Duncan multiple-comparison test24 was used to determine the means that were significantly different. To assess the difference between infection and dissemination, we used a generalized linear mixed model (GLMM) to determine significant differences (P < 0.05) in the likelihood of virus occurrence (1 = infection, 0 = no infection) in mosquito bodies (infection) or legs (dissemination) between treatments that included fixed (colony, age, EIT, and dose) and random (individual mosquito) effects. Mixed model population estimates of the logit [log of the odds ratio (i.e., probability of infection/probability of no infection)] were calculated, and estimates for some treatment effects on colonies and types of body part infection were graphed. Treatment factors, including infection of different body parts, were combined for these graphs to illustrate particular interactions (body part was a factor in our analyses). Details of the GLMM procedure used to analyze the probability of virus in bodies or legs are described elsewhere.6
Results
Virus titer of blood meals and homogenate of freshly fed mosquitoes.
Mosquitoes were fed blood containing a high dose (mean ± standard error: 6.7 ± 0.04 logs pfu WNV/mL) or low dose (6.0 ± 0.02 logs pfu WNV/mL). The titers of WNV were determined in the homogenate of five freshly fed fully engorged mosquitoes (Table 1). Age, dose, and colony significantly affected viral titers in the homogenates of freshly fed mosquitoes (Tables 1 and 2). Significant two- and three-way interactions indicate a complex relationship between colony, age, and dose in the amount of virus. Virus imbibed by females in each colony responded differently to changes in age and dose fed. Blood fed females from both colonies contained less viruses when fed the low dose at all ages compared with the high dose. At the low dose, middle-aged mosquitoes in the 2007 colony had significantly lower virus titers compared with all other ages at both doses. At the high dose, young 1995 colony mosquitoes contained significantly less WNV than middle-aged and old mosquitoes of the 2007 colony. These differences between ages that changed depending on the dose and colony are shown by the significant three-way interaction (Table 2).
Table 1.
The mean titers (logs plaque-forming units WNV/mL) ± standard errors of five whole bodies of freshly fed Cx. p. quinquefasciatus fed blood meals containing a low or high dose of WNV at different ages
| Mosquito age | No. tested | Dose* | ||
|---|---|---|---|---|
| Low | High | |||
| 1995 Colony | ||||
| Young | 5 | 4.3 ± 0.02c | 4.8 ± 0.1b | |
| Middle-aged | 5 | 4.3 ± 0.02c | 5.2 ± 0.01ab | |
| Old | 5 | 4.3 ± 0.03c | 4.9 ± 0.2ab | |
| 2007 Colony | ||||
| Young | 5 | 3.9 ± 0.2c | 5.1 ± 0.1ab | |
| Middle-aged | 5 | 2.8 ± 0.3d | 5.3 ± 0.1a | |
| Old | 5 | 4.2 ± 0.1c | 5.3 ± 0.02a | |
Treatment groups with the same letter in the table are not significantly different by means comparisons.
Table 2.
Results of analysis of variance (PROC ANOVA) of effects of age, dose, and colony on WNV body titers of freshly fed Cx. p. quinquefasciatus
| Effect | F | df; numerator; denominator | P |
|---|---|---|---|
| Age | 3.58 | 2; 48 | 0.036 |
| Dose | 232.94 | 1; 48 | < 0.0001 |
| Colony | 6.09 | 1; 48 | 0.017 |
| Age × dose | 12.88 | 2; 48 | < 0.0001 |
| Dose × colony | 33.56 | 1; 48 | < 0.0001 |
| Age × colony | 10.54 | 2; 48 | 0.0002 |
| Age × dose × colony | 4.98 | 2; 48 | 0.011 |
χ2 test of effects of mosquito age on infection and dissemination rates.
The age of the 1995 colony mosquitoes did not affect WNV infection rates at the low dose at either 25°C (Table 3) or 28°C (Table 4) (25°C: χ2 = 3.23, df = 2, P = 0.199; 28°C: χ2 = 2.97, df = 2, P = 0.226). Dissemination rates also did not differ between ages at the low dose at either EIT (25°C: χ2 = 1.83, df = 2, P = 0.401; 28°C: χ2 = 2.97, df = 2, P = 0.226) (Tables 3 and 4). However, infection rates did not differ between ages in 1995 colony mosquitoes exposed to the high dose of WNV at 28°C, whereas middle-aged mosquitoes had the lowest rate of infection at 25°C (25°C: χ2 = 14.74, df = 2, P = 0.001; 28°C: χ2 = 4.96, df = 2, P = 0.084). Dissemination rates in middle-aged 1995 colony mosquitoes were significantly higher than the young and old mosquitoes at the high dose and at 25°C (χ2 = 19.24, df = 2, P < 0.0001) but not at 28°C (χ2 = 3.49, df = 2, P = 0.175) (Tables 3 and 4).
Table 3.
The mean titers (logs plaque-forming units WNV/mL) ± standard errors, infection rates, and dissemination rates for Cx. p. quinquefasciatus fed blood meals containing a low or high dose at different ages and tested after a 13-day incubation period at 25°C
| Mosquito age | No. tested | No. infected (%)* | No. disseminated (%)* | Body titer† | Leg titer† |
|---|---|---|---|---|---|
| 1995 Colony—low dose | |||||
| Young | 76 | 31 (41)a | 2 (6)a | 4.2 ± 0.1hijk | 2.2 ± 0.9e |
| Middle-aged | 109 | 44 (40)a | 3 (7)a | 3.8 ± 0.1jk | 2.7 ± 0.2abcde |
| Old | 88 | 26 (30)a | 0 (0)a | 4.2 ± 0.1hijk | – |
| 1995 Colony—high dose | |||||
| Young | 101 | 76 (75)a | 11 (14)b | 4.3 ± 0.1hijk | 3.2 ± 0.3abcde |
| Middle-aged | 109 | 64 (59)b | 24 (38)a | 5.3 ± 0.1abcd | 3.0 ± 0.2abcde |
| Old | 98 | 81 (83)a | 8 (10)b | 5.1 ± 0.1cde | 3.9 ± 0.7abc |
| 2007 Colony—low dose | |||||
| Young | 100 | 57 (57)b | 1 (2)b | 4.9 ± 0.2def | 3.8abcd |
| Middle-aged | 100 | 69 (69)b | 18 (26)a | 4.6 ± 0.1efgh | 2.9 ± 0.3abcde |
| Old | 76 | 57 (75)a | 7 (12)b | 4.9 ± 0.2def | 3.8 ± 0.4abcd |
| 2007 Colony—high dose | |||||
| Young | 49 | 22 (45)a | 3 (14)a | 3.7 ± 0.2lk | 2.3 ± 0.1de |
| Middle-aged | 50 | 28 (56)a | 3 (11)a | 3.3 ± 0.3l | 2.5 ± 0.7cde |
| Old | 49 | 14 (29)b | 4 (29)a | 4.0 ± 0.4ijk | 2.5 ± 0.2cde |
Table 4.
The mean titers (logs plaque-forming units WNV/mL) ± standard errors, infection rates, and dissemination rates for Cx. p. quinquefasciatus fed blood meals containing a low or high dose at different ages and tested after a 13-day incubation period at 28°C
| Mosquito age | No. tested | No. infected (%)* | No. disseminated (%)* | Body titer† | Leg titer† |
|---|---|---|---|---|---|
| 1995 Colony—low dose | |||||
| Young | 100 | 56 (56)a | 16 (29)a | 4.2 ± 0.1hijk | 3.3 ± 0.2abcde |
| Middle-aged | 82 | 53 (65)a | 24 (45)a | 4.4 ± 0.1ghij | 2.8 ± 0.2abcde |
| Old | 81 | 41 (51)a | 11 (27)a | 4.4 ± 0.1ghij | 3.0 ± 0.3abcde |
| 1995 Colony—high dose | |||||
| Young | 95 | 77 (81)a | 49 (64)a | 5.0 ± 0.2def | 3.2 ± 0.2abcde |
| Middle-aged | 92 | 79 (86)a | 61 (77)a | 5.8 ± 0.1a | 4.2 ± 0.2a |
| Old | 89 | 65 (73)a | 44 (68)a | 5.7 ± 0.1ab | 3.9 ± 0.2abcd |
| 2007 Colony—low dose | |||||
| Young | 116 | 75 (65)b | 41 (55)a | 5.4 ± 0.2abcd | 3.8 ± 0.2abcd |
| Middle-aged | 74 | 23 (31)c | 8 (35)a | 5.3 ± 0.3abcd | 4.2 ± 0.4ab |
| Old | 130 | 128 (98)a | 54 (42)a | 5.6 ± 0.1abc | 3.1 ± 0.1abcde |
| 2007 Colony—high dose | |||||
| Young | 49 | 34 (69)b | 27 (79)a | 4.2 ± 0.2hijk | 2.8 ± 0.3abcde |
| Middle-aged | 49 | 43 (88)a | 34 (79)a | 5.0 ± 0.2def | 3.0 ± 0.2abcde |
| Old | 50 | 20 (40)c | 15 (75)a | 4.9 ± 0.4defg | 2.7 ± 0.3abcde |
The 2007 colony mosquitoes exposed to the low WNV dose showed significant differences in infection rates between ages at either EIT (25°C: χ2 = 6.77, df = 2, P = 0.034; 28°C: χ2 = 106.33, df = 2, P < 0.0001) with old mosquitoes showing higher infection rates than young and middle-aged mosquitoes at both EITs (Tables 3 and 4). Infection rates in mosquitoes receiving the low dose increased with age at the low EIT; however, a non-linear relationship was observed at the high EIT, because middle-aged mosquitoes had significantly fewer infections compared with the two other age groups. Dissemination rates at low dose were also significantly different between ages for 2007 colony mosquitoes at 25°C (χ2 = 13.05, df = 2, P = 0.002) with middle-aged mosquitoes having the highest dissemination rate, but there were no significant differences between ages for mosquitoes held at 28°C (χ2 = 4.17, df = 2, P = 0.124) (Tables 3 and 4). Mosquitoes from the 2007 colony fed the high virus dose showed significant differences in rates of infection between ages when held at either EIT (25°C: χ2 = 7.67, df = 2, P = 0.022; 28°C: χ2 = 25.47, df = 2, P < 0.0001); young and middle-aged mosquitoes had higher rates than old mosquitoes at both EITs (Tables 3 and 4). At the high dose, dissemination rates in the 2007 colony did not differ between the ages at either EIT (25°C: χ2 = 1.64, df = 2, P = 0.442; 28°C: χ2 = 0.168, df = 2, P = 0.919) (Tables 3 and 4).
Effects of environmental and biological factors on body and leg titers.
Body titer at the end of the incubation period was significantly affected by age and EIT but not by dose or colony (Table 5). Significant two-way interactions were observed for age × dose, colony × dose, dose × EIT, and EIT × colony but not colony × age or age × EIT (Table 5). The effects of these interactions can be seen in the differences between means of body titers across treatment groups. For example, body titer differs between ages at the high dose (for both colonies and EITs) but does not differ between ages at the low dose (Tables 3 and 4).
Table 5.
Results of analysis of variance (PROC ANOVA) of effects of age, dose, EIT, and colony on WNV body and leg titer after a 13-day incubation period
| Effect | Body titer of WNV per infected mosquito | Leg titer of WNV per infected mosquito | ||||
|---|---|---|---|---|---|---|
| F | df; numerator;denominator | P | F | df; numerator;denominator | P | |
| Age | 8.93 | 2; 1; 257 | 0.0001* | 0.18 | 2; 434 | 0.839 |
| Dose | 0.25 | 1; 1; 257 | 0.614 | 0.59 | 1; 434 | 0.443 |
| EIT | 69.56 | 1; 1; 257 | < 0.0001* | 2.57 | 1; 434 | 0.110 |
| Colony | 0.30 | 1; 1; 257 | 0.582 | 0.28 | 1; 434 | 0.597 |
| Age × dose | 10.09 | 2; 1; 257 | < 0.0001* | 0.36 | 2; 434 | 0.701 |
| Age × EIT | 2.24 | 2; 1; 257 | 0.107 | 2.56 | 2; 434 | 0.078 |
| Age × colony | 2.60 | 2; 1; 257 | 0.075 | 1.57 | 2; 434 | 0.209 |
| Dose × EIT | 8.05 | 1; 1;257 | 0.005* | 0.13 | 1; 434 | 0.719 |
| Dose × colony | 182.91 | 1; 1; 257 | < 0.0001* | 10.50 | 1; 434 | 0.001 |
| EIT × colony | 7.38 | 1; 1; 257 | 0.007* | 0.01 | 1; 434 | 0.919 |
| Colony × dose × EIT | 0.01 | 1; 1; 257 | 0.921 | 0.01 | 1; 434 | 0.927 |
| Age × dose × EIT | 0.35 | 2; 1; 257 | 0.705 | 0.40 | 2; 434 | 0.672 |
| Age × dose × colony | 2.80 | 2; 1; 257 | 0.062 | 0.19 | 2; 434 | 0.828 |
| Age × EIT × colony | 1.85 | 2; 1; 257 | 0.163 | 0.17 | 2; 434 | 0.841 |
| Age × dose × EIT × colony | 3.00 | 2; 1; 257 | 0.050 | 2.91 | 2; 434 | 0.089 |
Significant values.
Leg titers were less variable than body titers and not as influenced as body titer by these conditions (Table 5). Only the dose × colony interaction was significant, indicating that the differences between the colonies in leg titer were dependent on the virus dose. Leg titers in the 1995 colony increased with dose, whereas the leg titers in the 2007 colony decreased (Tables 3 and 4). Leg titers were similar between the other treatment groups (Tables 3 and 4).
GLMM for effects of environmental and biological factors on probability of infection and dissemination.
Table 6 shows the GLMM results testing significant effects on the probability of infection and dissemination. The main effects of age, dose, EIT, and body part significantly affected the probability of body infection (infection) or leg infection (dissemination). Body and leg infection also responded differently to two-way interactions of age × body part, dose × body part, and EIT × body part, showing that the differences between body and leg infections were not the same between ages, doses, and EITs. Significant two-way interactions of age × dose and dose × colony show that the probability of body infection and leg infection between ages was not the same between the doses and that these differences were dependent on the colony. Figure 1 shows the increased probability of infection in bodies and the age × body part interaction. The probability of infection differs with age more in leg infection than in body infection, and the shapes of the curves differ. The colony and colony × body part, age × EIT, age × colony, dose × EIT, and colony × EIT interactions were not significant. This shows that the differences in body part probability of infection (infection and dissemination) were the same in both colonies. Differences in body and leg probability of infection between EITs were the same at both doses and the same between colonies (Table 6). The three-way interactions of age × dose × colony, age × dose × EIT, and age × EIT × colony were significant, illustrating the complexity of the effects (i.e., two-way interactions of dose × colony, dose × EIT, and EIT × colony were not the same for different ages) (Table 6). Figure 2 illustrates some of these interactions, and it shows that colony differences in the probability of infection were not the same and were dependent on age and dose. Here, treatment groups and infection in different body parts are combined to show particular effects and interactions. The age groups in the 2007 colony differed in their response to different doses, whereas the differences between ages in the 1995 colony were not significant (Figure 2). Old mosquitoes in the 2007 colony showed a decline in the probability of infection with increasing dose, unlike the other age classes and the 1995 colony. This trend is evident in the infection rate (body infection) for all ages of 2007 colony mosquitoes at 25°C, but it is more pronounced in old mosquitoes at 25°C and was observed only in the old mosquitoes at 28°C. Some four-way interactions were also significant, showing further complexity (i.e., the three-way interaction of age × dose × EIT was different between body infection and leg infection) (Table 6).
Table 6.
Results of general linear mixed model (PROC GLIMMIX) for the effects of age, dose, EIT, and colony on presence or absence of WNV body or leg infection after a 13-day incubation period
| Type III tests of fixed effects | |||
|---|---|---|---|
| Effect | F | df; numerator; denominator | P |
| Age | 6.13 | 2; 3; 543 | 0.0022* |
| Dose | 47.30 | 1; 3; 986 | < 0.0001* |
| EIT | 168.82 | 1; 3; 732 | < 0.0001* |
| Colony | 1.67 | 1; 3; 986 | 0.197 |
| Body part | 496.31 | 1; 3; 986 | < 0.0001* |
| Age × dose | 9.23 | 2; 3; 986 | 0.0001* |
| Age × EIT | 2.37 | 2; 3; 425 | 0.094 |
| Age × colony | 1.84 | 2; 2; 842 | 0.158 |
| Age × body part | 7.76 | 2; 3; 986 | 0.0004* |
| Dose × EIT | 2.95 | 1; 3; 986 | 0.086 |
| Dose × colony | 71.90 | 1; 2; 863 | < 0.0001* |
| Dose × body part | 16.75 | 1; 3; 986 | < 0.0001* |
| EIT × colony | 0.34 | 1; 3; 986 | 0.563 |
| EIT × body part | 59.51 | 1; 3; 986 | < 0.0001* |
| Colony × body part | 1.78 | 1; 3; 986 | 0.182 |
| Colony × dose × EIT | 12.84 | 1; 2; 947 | 0.0003* |
| Age × dose × EIT | 12.64 | 2; 3; 762 | < 0.0001* |
| Age × dose × colony | 29.11 | 2; 2; 443 | < 0.0001* |
| Age × dose × body part | 1.11 | 2; 3; 986 | 0.329 |
| Age × EIT × colony | 5.73 | 2; 2; 824 | 0.003* |
| Age × EIT × body part | 3.40 | 2; 3; 986 | 0.034* |
| Age × colony × body part | 1.42 | 2; 3; 986 | 0.241 |
| Dose × EIT × body part | 0.27 | 1; 3; 986 | 0.601 |
| Dose × colony × body part | 1.31 | 1; 3; 986 | 0.252 |
| EIT × colony × body part | 0.72 | 1; 3; 986 | 0.397 |
| Age × dose × EIT × colony | 5.57 | 2; 2; 333 | 0.004* |
| Age × dose × EIT × body part | 3.12 | 2; 3; 986 | 0.045* |
Significant values.
Figure 1.
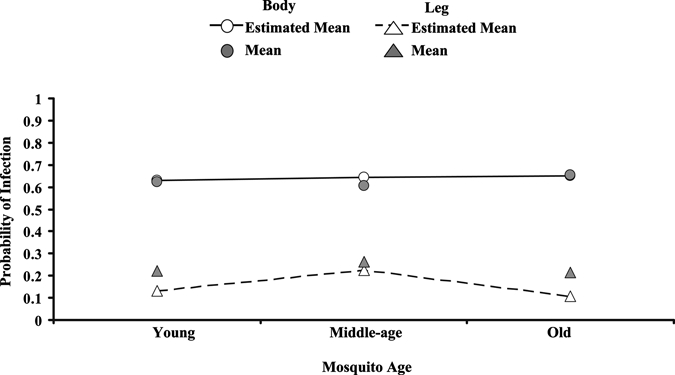
Logit estimates from generalized linear mixed model showing the relationship between the occurrence of body or leg infection between different ages of mosquitoes (age × body part; F = 7.76, df = 2; 3,986, P = 0.0004). The estimated mean probability of infection (model-based estimate; see text for details) and the mean probability of infection (calculated from the data for the appropriate treatment group combined) are plotted for different body parts (body = circle; leg = triangle). The probability of leg infections differs between ages, but body infections are more consistent between ages.
Figure 2.
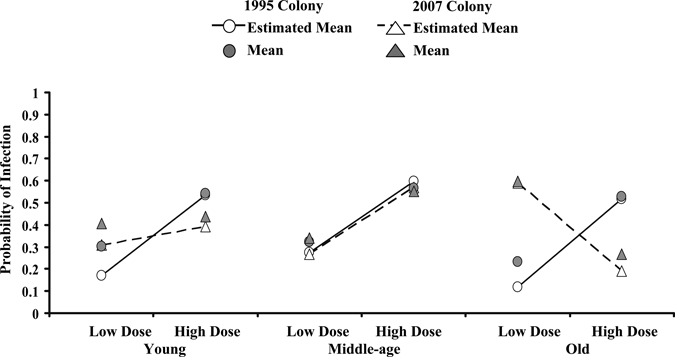
Logit estimates from generalized linear mixed model showing the relationship between the occurrence of body and leg infection between different ages, doses, and colonies (age × dose × colony; F = 29.11, df = 2; 2,443, P < 0.0001). The probability of infection and the estimated mean probability of infection are plotted for different colonies (1995 colony = circle; 2007 colony = triangle). Note that the infections of different body parts are combined for this analysis. Colonies show different infection responses because of age and dose. Means and estimated means are as in Figure 1.
Sample sizes for experiments using mosquitoes of different ages, different EITs, and different doses of WNV (N = 693 for the 1995 colony; N = 570 for the 2007 colony) were within the sample-size estimations using a priori power analyses for infection rates; therefore, we were able to detect small to medium effect sizes with 100% power. Because of the low rates of dissemination, our sample sizes for the experiments (N = 253 for the 1995 colony; N = 215 for the 2007 colony) could detect medium to large effect sizes (w = 0.4) in dissemination rates with 100% power. It is possible that larger sample sizes in the 1995 colony compared with the 2007 colony and in infections versus disseminated infections could have affected the outcome of some statistical comparisons; however, as indicated earlier, power analyses show that analyses were appropriate for detection of small to large effect sizes.
Discussion
The interactions between the environmental (EIT and dose) and biological (age and colony) factors observed in our studies have important implications for interpreting all vector competence studies. Although laboratory vector competence studies can provide comparative information between populations, such comparisons are useful only for the tested biological material and only under the environmental conditions of the test. The information may be of little use to make comparisons with or between field populations under the influence of different environmental and biological factors.
The major contribution to our observed differences in titers for freshly fed mosquito samples was viral dose. At the low dose, freshly fed middle-aged 2007 colony mosquitoes contained a significantly lower virus titer in their bodies, whereas at the high dose, young 1995 colony mosquitoes showed the lowest titers. We did not observe any effects of these initial differences in freshly fed mosquitoes on our observations. Middle-aged 2007 colony mosquitoes given a low dose and young 1995 colony mosquitoes given a high dose did not show consistently lower infection, dissemination, or titer effects. In addition to initial viral dose, another potential source of variation in the study was the use of membrane feeders to infect 1995 colony mosquitoes and pledgets to infect 2007 colony mosquitoes, and thus, caution is warranted when interpreting colony comparisons. Although the different feeding methods did not result in consistent differences between the two colonies in the amount of virus contained in freshly fed mosquitoes, the different feeding methods may influence some of the observed colony differences in vector competence. The colonies also differ in many other ways that could influence these observations. The colonies differed in their geographic origins and likely differed in genetic variation and time maintained as colonies, and additionally, they differed in their blood-feeding behavior observed in one preferring membrane feeders, the other pledgets. The cause of the differences observed in their vector competence could be because of these and many other unknown differences between them. Like the great majority of populations and colonies used in these types of experiments, there are a host of unknown and undefined differences in the mosquitoes. Whatever the cause(s) of the differences in the colonies, these differences caused complex interactions in the effects of biological and environmental factors on vector competence.
Although there were treatment differences and interactions between biological and environmental factors, we also observed cases where factors did not have significant effects on some vector competence phenotypes in one or both colonies. The lack of any effects of some biological and environmental factors on dissemination and/or the absence of significant interactions between the factors provides the possibility to apply observations about vector competence across these factors. For example, in this study, leg titers could be assessed in each colony using mosquitoes of any age group tested and at either EIT tested here. However, care would have to be taken in the dose used and the origin of the mosquitoes, because of the dose and colony effects on leg titer. Caution must be exercised in generalization of any such observations, because interactions may vary due to environmental or biological factors under other laboratory or field conditions.
The vector competence of the two colonies was affected differently by the biological and environmental factors, consistent with the well known observation of between population variations in vector competence. However, here it is important to note that the vector competence of the colonies in different treatments was dependent on other treatments and that this dependence was not the same for the two colonies.
Ideally, studies should be designed to have more than two levels in a particular treatment to evaluate the shape of the curves. However, there is a tradeoff in the number of factors and sample size needed to increase the power of statistical analyses. Hence, our experimental design only allowed for 2–3 levels in each treatment because of our large sample sizes and investigation of several factors. This allowed us to focus on interpreting interactions between factors, rather than within factors.
The 2007 colony was the more competent WNV vector at the low WNV dose at either EIT, having both higher infection and dissemination rates compared with the 1995 colony. This trend was most evident at 25°C, because these vector competence relationships at 28°C were sometimes different between ages. The MIB (affects infection rate) of the 2007 colony was less efficient, resulting in higher infection rates, at low-virus dose than the MIB of the 1995 colony, but this reversed when the colony was provided the high dose; this was consistent for both EITs. The MEB (affects dissemination rate) of the 2007 colony was also less efficient than the MEB of the 1995 colony at the low dose. These observations illustrate that comparing vector competence phenotypes between mosquito populations is dependent on biological and environmental conditions, and relative efficiencies may change because of these conditions. This may also reflect differences in the immune response to viral infection25,26 that were affected by the viral dose.
The effect of age on the MIB was dependent on the colony, dose, and EIT in complex ways. The age of the 1995 colony mosquitoes did not influence infection rates at low doses and both EITs, but at high doses, middle-aged females were more susceptible at 28°C and least susceptible at 25°C. The 2007 colony showed differences in the effect of age and its relationship to other environmental factors compared with the 1995 colony. The 2007 colony showed age effects at the low virus dose where old females had higher infection rates than young females at both EITs and also, at the high dose at 25°C. However, young and middle-aged females in the 2007 colony were the most susceptible at high dose and 28°C.
Colony variation and likely variation between natural populations in vector competence can change significantly depending on the environment. Therefore, laboratory comparisons with natural conditions in characterizing vector competence are very difficult to do with assurance. For example, although mosquito age was not important in determining infection in the 1995 colony at low dose and was unaffected by the EIT used here, age was a significant factor in the 2007 colony vector competence where evaluations using young mosquitoes at low dose and 25°C would result in lower infection rates than using older mosquitoes. A vector competence experiment using a range of mosquito ages would likely show different results than another experiment using a specific age and would also be expected to vary between colonies.
The MEB reflected by dissemination rates also showed complex interactions between the effects of biological and environmental factors that differed depending on the colony. The MEB in the 2007 colony was influenced differently by virus dose, EIT, and age compared with the MEB of the 1995 colony. The 1995 colony dissemination rates were not significantly different because of age at the low dose, and only the middle-aged mosquitoes showed significantly higher dissemination rates compared with the other age groups but only at 25°C. However, the 2007 colony showed higher dissemination rates of middle-aged mosquitoes at the low dose, but no age differences at 28°C; this colony had no differences in dissemination rate between ages at the high dose at both EITs. This highlights that there are biological and environmental conditions where the MEB can differ in permissiveness compared with the MIB, and the two phenotypes can respond to the same environments differently. This illustrates the importance of characterizing dissemination rates independently from infection rates, in this case, at 25°C. Studies that report dissemination rate as the number of disseminated mosquitoes relative to the total number of females tested may be of epidemiologic value for testing population dissemination, but it confounds the biological interpretation regarding escape barriers. Indeed, dissemination rates calculated in such a manner would miss populations where the dissemination rate is greater than the infection rate as observed in several age groups in our studies.
Body and leg titers also showed differences in their relationships with the different treatment factors. Females in the 2007 colony tended to have higher body titers than 1995 females for most treatments. Notably, the difference between the colonies was also dependent on EIT and dose, because 1995 colony females had higher body titers compared with 2007 at the high doses at 25°C, again illustrating that the two colonies responded differently depending on environmental factors. Age did not influence leg titer in either colony. Leg titers also showed colony differences in the amount of WNV replication during disseminated infection that was dependent on virus dose. This was evident, because the 1995 colony mosquitoes showed increasing leg titer at the high dose, whereas the 2007 colony had lower leg titers at the high dose compared with low dose, resulting in the significant dose × colony interaction. Our observations are consistent with the hypothesis that virus replication in our colonies was less influenced by the environmental treatments than the MIB and MEB.
The GLMM supported the above analyses, showing that mosquito age, dose, and EIT influenced the probability of a body or leg infection. However, the GLMM was used to assess if body part, body infection (MIB), and disseminated leg infection (MEB) were influenced by the treatment factors in the same manner. The lack of a significant colony × body part interaction showed that the differences between the occurrence of body infection and disseminated leg infection were the same in both colonies. If this relationship is similar between other colonies or populations, it is possible that infection rate (MIB) could predict dissemination rate (MEB) across different colonies or populations. However, the age × body part, dose × body part, and EIT × body part were all significant, illustrating that predicting MEB based on the MIB would depend on the age of the tested population and also, the dose and EIT.
The influence of mosquito age on Cx. p. quinquefasciatus vector competence was dependent on many factors and inconsistent, showing that the vector competence effects of this biological factor are unpredictable under the conditions of our test. Depending on conditions, young, middle-aged, and old mosquitoes could show significantly higher or lower infection rates. Under some conditions (i.e., 1995 colony at low dose) at both EITs, there were no effects because of mosquito age on either infection or dissemination rates. Under other conditions (i.e., 2007 colony at both doses and EITs), there were significant effects because of age. In the 2007 colony, infection rates were sometimes higher in mosquitoes fed the low dose than those fed the high dose. This was unexpected, because it is commonly observed in many vector-pathogen systems that higher doses result in higher infection rates. Cx. tarsalis modulates infection from Western equine encephalitis virus (WEEV) at 32°C,27 and Cx. univittatus shows slower WNV growth at 30°C.28 It may be that some age groups in our 2007 colony are capable of modulating WNV infection at 25°C and 28°C. This primarily occurred under the least permissive conditions of 25°C in all age groups and could be caused by a mosquito immune response occurring under these conditions that interfered with infection rates. Another explanation is that the high dose in 2007 colony mosquitoes resulted in more defective interfering particles (DIPs) that inhibited WNV replication in mosquito tissues so that the low dose mosquitoes showed comparatively higher infection rates. Defective interfering particles are virus particles that are missing part of their genome and therefore, cannot cause infection in host tissues.29,30 The DIPs can prevent normal virus particles from replicating because of competition for cell resources and receptors, and this phenomenon occurs most prevalently in infection studies using high multiplicity of infection (i.e., high dose).29,31 In mosquito cell culture, growth of Japanese encephalitis virus is inhibited by DIPs,30 and this was also a hypothesized reason for decreased dengue virus infection in Aedes aegypti;32 however, this has not yet been explored for mosquitoes and WNV. The lower infection rates at the high dose versus low dose may also be caused by a stronger immune response occurring in 2007 colony mosquitoes that could have been triggered by increased viral replication because of the high dose, although this phenomenon has only been studied in mammalian cells and not mosquito cells infected with WNV.25 This phenomenon also occurred in only old 2007 colony mosquitoes at 28°C, showing that this age of mosquito may have been more prone to DIPs or an immune response. Although there was also an age effect on dissemination rates for the 2007 colony mosquitoes fed a low dose of WNV (at either EIT), the same was not true for mosquitoes fed a high dose of WNV (at either EIT). This indicates that, at the high dose of WNV, age did not affect vector competence in this colony. It is possible that the high dose of WNV overcame dissemination barriers (MEB), regardless of EIT or age. In general, the probability of infection decreases as the dose decreases. The observations here illustrate how little we know about the mechanisms contributing to differences in vector competence and the need for further study on mechanisms and environmental effects on vector competence.
West Nile virus and SLEV are closely related viruses transmitted by the same mosquito species in the United States. A previous study6 used the same colonies and treatments, although the SLEV doses were different from those used here for WNV. Despite this difference, the following are several conclusions comparing the observations for WNV and SLEV. (1) Mosquito age can influence Cx. p. quinquefasciatus vector competence for both WNV and SLEV. (2) The effects of mosquito age change in dynamic ways depending on the type of virus (WNV or SLEV), mosquito colony, virus dose, and EIT. (3) In some mosquito colonies, MIB is related to MEB; however, particularly for WNV, this relationship changes depending on virus dose. (4) An EIT difference of only 3°C had a substantial effect on both SLEV and WNV vector competence.
Age influenced infection rates for both WNV and SLEV in the 1995 colony fed the high dose, and young and middle-aged mosquitoes generally showed higher infection rates than old mosquitoes. At the low dose, age only had a significant effect on infection rate for SLEV and not WNV in the 1995 colony. In the 2007 colony, however, there was an effect of age for WNV but not for SLEV, showing that age influences vector competence in this colony differently for these two viruses. Differences in dissemination rates between ages showed several distinctions between WNV and SLEV, depending on the colony and conditions.
There were more significant environmental and biological effects and interactions for SLEV body titers compared with WNV. Variation in SLEV replication in Cx. p. quinquefasciatus was more influenced by dose, age, and EIT than was WNV replication. EIT and age had an influence on replication for both viruses. Leg titer for WNV was not significantly affected by any treatment effects, whereas SLEV leg titer showed significant dose and colony effects. This indicates that virus replication and dissemination out of the midgut is caused by different controlling mechanisms for each virus or that similar mechanisms for each virus respond differently under the tested conditions. In general, the treatment factors showed less influence on body and leg titers for WNV-infected versus SLEV-infected mosquitoes. For example, body infection and disseminated leg infection with both SLEV and WNV differed depending on the EIT, age, and virus dose. However, although the differences between infection and dissemination were the same between the two colonies for WNV, there was a significant colony × body part interaction with SLEV.6 Although the WNV observations support the possibility of using MIB to predict MEB in different colonies, the observations with SLEV are not consistent. Without further information on the specific controlling mechanisms for the MIB and the MEB and how these mechanisms are affected by environmental changes, the ability to apply this information to other populations is problematic.
Neither colony had consistently higher infection or dissemination rates for either WNV or SLEV. It is interesting that the observed vector competence differences between the colonies were not as pronounced at the high dose, high EIT for both viruses, suggesting that virus barriers are less pronounced under highly permissive conditions for both viruses.
Natural mosquito populations likely show temporal variation in vector competence because of dynamic environmental and population variation, and this has important epidemiologic implications for arboviral transmission cycles. Our study shows that age, EIT, and dose are all important factors influencing both infection and dissemination rates of Cx. p. quinquefasciatus and that the effects of each factor are influenced by the other factors in complex ways. We have also shown that, although there are similarities between mosquito populations, there are important differences in how specific populations respond to the influence of biological and environmental factors for two different viruses. Therefore, conflicting reports of the effects of an environmental factor when tested one at a time in different species and with different viruses are not surprising. These factors must be considered when evaluating vector competence in laboratory studies to assess the epidemiological importance of the vector in nature. Studies on interactions between various factors in nature may produce very different phenotypes from those observed in the laboratory. The vector competence effects of interactions between several biological and environmental variables for other Cx. p. quinquefasciatus populations, other mosquito species, and other pathogens have hardly been explored. The underlying mechanisms controlling vector competence variation in colonies or natural populations remain largely unknown. However, the complex interactions observed here caused by biological and environmental factors highlight the importance of studies that address both intrinsic and extrinsic factors influencing differential intra- and interspecies vector competence. We are only beginning to address these very complex, important issues.
Biological and environmental effects on the vector competence of Cx. p. quinquefasciatus for WNV were complex, and hence, analyses of the power of experimental designs would be helpful in all vector competence studies to assist in interpreting results.
Acknowledgments
The authors thank Christopher N. Mores for providing BL-3 containment space and technical advice on portions of this project, Jessie Dyer, Krystle E. Greene, and Heather L. Robinson for laboratory assistance, Jonathan F. Day, Sheri L. Anderson, and three anonymous reviewers for critically reviewing earlier versions of the manuscript, Dulce M. Bustamante, Linda J. Young, and James C. Colee for statistical advice, and Donald A. Shroyer for providing material used to establish the 2007 mosquito colony.
Footnotes
Financial support: This research was supported by the National Institute of Health Grant AI-42164 to Cynthia C. Lord and Walter J. Tabachnick. Kendra N. Pesko was supported by a University of Florida Graduate Alumni Fellowship.
Authors' addresses: Stephanie L. Richards, Cynthia C. Lord, and Walter J. Tabachnick, Florida Medical Entomology Laboratory, Department of Entomology and Nematology, University of Florida—IFAS, Vero Beach, FL, E-mails: slrichar@ufl.edu, clord@ufl.edu, and wjt@ufl.edu. Kendra N. Pesko, Department of Pathology, University of New Mexico School of Medicine, Albuquerque, NM, E-mail: KPesko@salud.unm.edu.
References
- 1.Hardy JL, Houk EJ, Kramer LD, Reeves WC. Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Annu Rev Entomol. 1983;28:229–262. doi: 10.1146/annurev.en.28.010183.001305. [DOI] [PubMed] [Google Scholar]
- 2.Mellor PS. Replication of arboviruses in insect vectors. J Comp Pathol. 2000;123:231–247. doi: 10.1053/jcpa.2000.0434. [DOI] [PubMed] [Google Scholar]
- 3.Sardelis MR, Turell MJ, Dohm DJ, O'Guinn ML. Vector competence of selected North American Culex and Coquillettidia mosquitoes for West Nile virus. Infect Dis. 2001;7:1018–1022. doi: 10.3201/eid0706.010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahmood F, Chiles RE, Fang Y, Green EN, Reisen WK. Effects of time after infection, mosquito genotype, and infectious viral dose on the dynamics of Culex tarsalis vector competence for western equine encephalomyelitis virus. J Am Mosq Control Assoc. 2006;22:272–281. doi: 10.2987/8756-971X(2006)22[272:EOTAIM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 5.Styer L, Carey J, Wang J, Scott T. Mosquitoes do senesce: departure from the paradigm of constant mortality. Am J Trop Med Hyg. 2007;76:111–117. [PMC free article] [PubMed] [Google Scholar]
- 6.Richards SL, Lord CC, Pesko K, Tabachnick WJ. Environmental and biological factors influence Culex pipiens quinquefasciatus Say (Diptera: Culicidae) vector competence for Saint Louis encephalitis virus. Am J Trop Med Hyg. 2009;81:264–272. [PMC free article] [PubMed] [Google Scholar]
- 7.Godsey MS, Blackmore MS, Panella NA, Burkhalter K, Gottfried K, Halsey LA, Rutledge CR, Langevin SA, Gates R, Lamonte KM, Lambert A, Lanciotti RS, Blackmore AGM, Loyless T, Stark L, Oliveri R, Conti L, Komar N. West Nile virus epizootiology in the southeastern United States, 2001. Vector Borne Zoonotic Dis. 2005;5:82–89. doi: 10.1089/vbz.2005.5.82. [DOI] [PubMed] [Google Scholar]
- 8.Irby WS, Apperson CS. Hosts of mosquitoes in the coastal plain of North Carolina. J Med Entomol. 1988;25:85–93. doi: 10.1093/jmedent/25.2.85. [DOI] [PubMed] [Google Scholar]
- 9.Niebylski ML, Meek CL. Blood-feeding of Culex mosquitoes in an urban environment. J Am Mosq Control Assoc. 1992;8:173–177. [PubMed] [Google Scholar]
- 10.Molaei G, Andreadis TG, Armstrong PM, Bueno R, Jr, Dennett JA, Real SV, Sargent C, Bala A, Randle Y, Guzman H, Travassos da Rosa A, Wuithiranyagool T, Tesh RB. Host feeding pattern of Culex quinquefasciatus (Diptera: Culicidae) and its role in transmission of West Nile virus in Harris County, Texas. Am J Trop Med Hyg. 2007;77:73–81. [PubMed] [Google Scholar]
- 11.Goddard LB, Roth AE, Reisen WK, Scott TW. Vector competence of California mosquitoes for West Nile virus. Infect Dis. 2002;8:1385–1391. doi: 10.3201/eid0812.020536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reisen WK, Fang Y, Martinez VM. Avian host and mosquito (Diptera: Culicidae) vector competence determine the efficiency of West Nile and St. Louis encephalitis virus transmission. J Med Entomol. 2005;42:367–375. doi: 10.1093/jmedent/42.3.367. [DOI] [PubMed] [Google Scholar]
- 13.Rutledge CR, Day JF, Lord CC, Stark LM, Tabachnick WJ. West Nile infection rates in Culex nigripalpus (Diptera: Culicidae) do not reflect transmission rates in Florida. J Med Entomol. 2003;40:253–258. doi: 10.1603/0022-2585-40.3.253. [DOI] [PubMed] [Google Scholar]
- 14.Lillibridge KM, Parsons R, Randle Y, Travassos Da Rosa A, Guzman H, Siirin M, Wuithiranyagool T, Hailey C, Higgs S, Bala A, Pascua R, Meyer T, Vanlandingham D, Tesh R. The 2002 introduction of West Nile virus into Harris County, Texas, an area historically endemic for St. Louis encephalitis. Am J Trop Med Hyg. 2004;70:676–681. [PubMed] [Google Scholar]
- 15.Bosio CF, Fulton RE, Salasek ML, Beaty BJ, Black WC. Quantitative trait loci that control vector competence for dengue-2 virus in the mosquito Aedes aegypti. Genetics. 2000;156:687–698. doi: 10.1093/genetics/156.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiawsirisup S, Platt KB, Evans RB, Rowley WA. A comparison of West Nile virus transmission by Ochlerotatus trivittatus (COQ.), Culex pipens (L.), and Aedes albopictus (Skuse) Vector Borne Zoonotic Dis. 2005;5:40–47. doi: 10.1089/vbz.2005.5.40. [DOI] [PubMed] [Google Scholar]
- 17.Kramer LD, Hardy JL, Presser SB, Houk EJ. Dissemination barriers for western equine encephalomyelitis virus in Culex tarsalis infected after ingestion of low viral doses. Am J Trop Med Hyg. 1981;30:190–197. doi: 10.4269/ajtmh.1981.30.190. [DOI] [PubMed] [Google Scholar]
- 18.Girard YA, Klinger KA, Higgs S. West Nile virus dissemination and tissue tropisms in orally infected Culex pipiens quinquefasciatus. Vector Borne Zoonotic Dis. 2004;4:109–122. doi: 10.1089/1530366041210729. [DOI] [PubMed] [Google Scholar]
- 19.Lord CC, Rutledge CR, Tabachnick WJ. Relationships between host viremia and vector susceptibility for arboviruses. J Med Entomol. 2006;43:623–630. doi: 10.1603/0022-2585(2006)43[623:rbhvav]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, Komar N, Panella NA, Allen BC, Volpe KE, Davis BS, Roehrig JT. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richards SL, Mores CN, Lord CC, Tabachnick WJ. Impact of extrinsic incubation temperature and virus exposure on vector competence of Culex pipiens quinquefasciatus (Diptera: Culicidae) for WNV. Vector Borne Zoonotic Dis. 2007;7:629–636. doi: 10.1089/vbz.2007.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen J. A power primer. Psychol Bull. 1992;111:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 23.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 24.SAS . SAS/STAT User's Guide for Personal Computers, version 8.2. Cary, NC: SAS; 2002. [Google Scholar]
- 25.Frederickson BL, Smith M, Katze MG, Shi P-Y, Gale M. The host response to West Nile virus infection limits viral spread through the activation of the interferon regulatory factor 3 pathway. J Virol. 2004;78:7737–7747. doi: 10.1128/JVI.78.14.7737-7747.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smartt CT, Richards SL, Anderson SL, Erickson JS. West Nile virus infection alters midgut gene expression in Culex pipiens quinquefasciatus. Am J Trop Med Hyg. 2009;81:258–263. [PMC free article] [PubMed] [Google Scholar]
- 27.Kramer LD, Hardy JL, Presser SB. Characterization of modulation of western equine encephalomyelitis virus by Culex tarsalis (Diptera: Culicidae) maintained at 32°C following parenteral infection. J Med Entomol. 1998;35:289–295. doi: 10.1093/jmedent/35.3.289. [DOI] [PubMed] [Google Scholar]
- 28.Reisen WK, Fang Y, Martinez VM. Effects of temperature on the transmission of West Nile virus by Culex tarsalis (Diptera: Culicidae) J Med Entomol. 2006;43:309–317. doi: 10.1603/0022-2585(2006)043[0309:EOTOTT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 29.Brinton MA. Analysis of extracellular West Nile virus particles produced by cell cultures from genetically resistant and susceptible mice indicated enhanced amplification of defective interfering particles by resistant cultures. J Vasc Interv Radiol. 1983;46:860–870. doi: 10.1128/jvi.46.3.860-870.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai K-N, Tsang S-F, Huang C-H, Chang R-Y. Defective interfering RNAs of Japanese encephalitis virus found in mosquito cells and correlation with persistent infection. Virus Res. 2007;124:139–150. doi: 10.1016/j.virusres.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Debnath NC, Tiernery R, Sil BK, Wills MR, Barrett ADT. In vitro homotypic and heterotypic interference by defective interfering particles of West Nile virus. J Gen Virol. 1991;72:2705–2711. doi: 10.1099/0022-1317-72-11-2705. [DOI] [PubMed] [Google Scholar]
- 32.Richardson J, Molina-Cruz A, Salazar MI, Black W. Quantitative analysis of dengue-2 virus RNA during the extrinsic incubation period in individual Aedes aegypti. Am J Trop Med Hyg. 2006;74:132–141. [PubMed] [Google Scholar]


