Abstract
The state of Veracruz, Mexico, is a well-recognized endemic region for Chagas disease, but the geographic distribution of the disease and its magnitude are still poorly documented. We evaluated the seroprevalence of Trypanosoma cruzi infection in the sanitary jurisdictions of Cordoba and Cosamaloapan in central Veracruz. A total of 654 serum samples from 19 rural localities were tested by using four tests: two enzyme-linked immunosorbent assays, an indirect immunofluorescent, and Western blotting. Overall, 110 (16.8%) of 654 samples were positive for T. cruzi by ≥ 2 tests (95% confidence interval = 14.2–19.9%). The municipality of Tezonapa in the jurisdiction of Cordoba was identified as a potential hyperendemic region with seroprevalence rates ≤ 45% in young children. No cases were detected in the jurisdiction of Cosamaloapan. Further studies should help clarify T. cruzi transmission dynamics in Tezonapa. The magnitude of T. cruzi infection rate in this region calls for the urgent implementation of extensive epidemiologic surveillance and control programs.
Introduction
Chagas disease, or American trypanosomiasis, is caused by the protozoan parasite Trypanosoma cruzi. This disease represents a major public health problem in Latin America, where the World Health Organization estimates that approximately 60 million persons are at risk of infection and 9–12 million are infected.1 In Mexico, Chagas disease is endemic in various regions, but it remains underreported. Thus, limited information is available on the epidemiology of the disease.2,3
The state of Veracruz, Mexico, is one of the regions where the endemicity of T. cruzi infection is well established. It is located in the east-central part of Mexico and has a total population of 7 million persons; it is the third largest state of the country. It is divided into 11 sanitary jurisdictions. Several species of Triatomines have been observed in Veracruz, including some with a high vectorial capacity, such as Triatoma dimidiata or T. pallidepennis.4 Also, the presence of T. cruzi infection has been documented in various human populations.
According to a national seroprevalence survey in the late 1980s, Veracruz was one of the states in Mexico with the highest seroprevalence in the general population (≤ 3.0%).5 Trypansoma cruzi infection has also been documented in blood donors in the sanitary jurisdiction of Orizaba, in central Veracruz,6 and cases of severe chronic chagasic cardiomyopathy have been observed in the jurisdiction of Poza-Rica in northern Veracruz.7 Other studies of the distribution of infection in different sanitary jurisdictions of Veracruz indicated that T. cruzi infection may be absent from the jurisdictions of Coatzacoalcos, Orizaba, and Martinez de la Torre; the highest seroprevalences were observed in the northern jurisdictions of Tuxpan and Panuco (2.8% and 1.6%, respectively) and the central jurisdiction of Cordoba (1.3%).8 Furthermore, detection of T. cruzi infection in children less than 18 years of age (1.8–5.2%) suggested the presence of active transmission of the parasite in these same jurisdictions,4 and anecdotal triatomine collections in the jurisdiction of Cordoba led us to suspect a high endemicity of Chagas disease in this area. The objective of our study was to update and refine seroepidemiologic data in central Veracruz.
Materials and Methods
Study area.
The study was conducted in the municipalities of Tezonapa and Amatlan in the sanitary jurisdiction of Cordoba, and Tierra Blanca, in the jurisdiction of Cosamaloapan, in central Veracruz (18°36′N, 96°41′W) (Figure 1). A total of 19 rural localities were included: Caxapa, Colonia Agricola, El Otate, Laguna Chica, El Mirador, El Suspiro, Las Josefinas, La Joya, La Luna, Paraíso La Reforma, Rancho Nuevo, Raya Caracol, and San Agustín del Palmar from the municipality of Tezonapa; El Moral from the municipality of Amatlan; and Marquez Galindo, Paso Hachote, Paso Magueyito, Tamarindo and Vincente Guerrero from the municipality of Tierra Blanca (Table 1). These villages are at altitudes of 80–700 meters and are located at the junction of the coastal plains of Veracruz on the east and mountains of the Trans-Mexican volcanic belt on the west.
Figure 1.
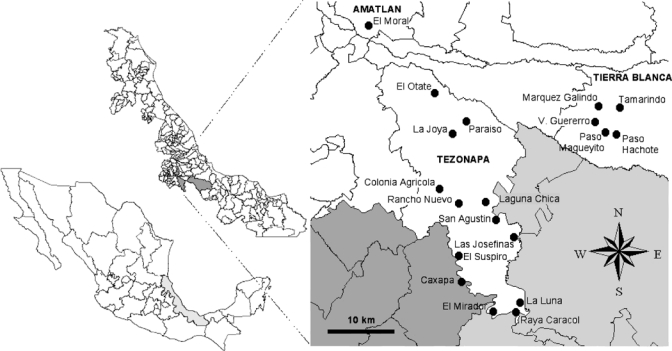
Mexico (bottom left), the state of Veracruz (center), and the study area (inset). White area corresponds to the state of Veracruz, light gray to the state of Oaxaca, and dark gray to the state of Puebla, with lines delimitating the respective municipalities, including Amatlan, Tezonapa, and Tierra Blanca. Black circles indicate the position of the indicated villages.
Table 1.
Demographic details of studied villages and samples, Mexico
| Municipality | Village | Population | No. sampled | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | M | F | < 18 Years of age | Total | M | F | < 18 Years of age | ||
| Colonia Agrícola M. A. Muñoz | 412 | 198 | 214 | 188 | 7 | 2 | 5 | 3 | |
| El Mirador | 222 | 118 | 104 | 99 | 18 | 3 | 15 | 9 | |
| El Otate | 239 | 126 | 113 | 117 | 13 | 1 | 12 | 0 | |
| El Suspiro | 437 | 196 | 241 | 174 | 86 | 19 | 67 | 27 | |
| La Luna | 70 | 31 | 39 | 38 | 18 | 3 | 15 | 10 | |
| Laguna Chica | 1,755 | 848 | 907 | 728 | 3 | 1 | 2 | 2 | |
| Tezonapa | Las Josefinas | 656 | 307 | 349 | 259 | 51 | 16 | 34 | 19 |
| Paraíso la Reforma | 1,945 | 916 | 1,029 | 754 | 40 | 11 | 29 | 12 | |
| Rancho Nuevo | 994 | 485 | 509 | 410 | 35 | 20 | 15 | 15 | |
| Raya Caracol | 477 | 224 | 253 | 228 | 27 | 12 | 16 | 8 | |
| San Agustin del Palmar | 1,140 | 529 | 611 | 422 | 8 | 2 | 6 | 3 | |
| La Joya | 254 | 124 | 130 | 108 | 29 | 10 | 19 | 13 | |
| Caxapa | 1,801 | 893 | 908 | 804 | 52 | 25 | 27 | 26 | |
| Amatlán de los Reyes | El Moral | 208 | 98 | 110 | 62 | 12 | 1 | 11 | 1 |
| Paso Magueyito | 332 | 165 | 167 | 128 | 80 | 26 | 54 | 33 | |
| Vincente Guerrero | 150 | 72 | 78 | 62 | 89 | 31 | 57 | 33 | |
| Tierra Blanca | Marquez Galindo | 347 | 167 | 180 | 147 | 33 | 11 | 22 | 17 |
| Paso Achote | 85 | 39 | 46 | 23 | 31 | 11 | 20 | 3 | |
| Tamarindo | 301 | 140 | 161 | 117 | 22 | 4 | 18 | 2 | |
| Total | 11,825 | 5,676 | 6,149 | 4,868 | 654 | 209 | 444 | 236 | |
Sample collection and diagnosis.
In each village, general information on Chagas disease and the project was provided to households by research personnel during open meetings organized in the rural medical units through the Instituto Mexicano del Seguro Social–Oportunidades Social Program. Interested participants were given an appointment for their family at the medical unit for providing blood samples.
The day of the appointment, written informed consent was obtained from each volunteer, and research personnel collected blood samples in vacutainers tubes. Serum was separated by centrifugation at 1,200 × g for 10 minutes and samples were stored at –70°C until used. A total of 654 serum samples were collected (Table 1) and analyzed for T. cruzi infection by using four different tests: two enzyme-linked immunosorbent assays (ELISAs) based on crude parasite extracts and a recombinant protein, respectively; an indirect immunofluorescent (IIF) test; and Western blot analysis as described.6 Because it has been suggested that using tests based on local antigens could increase the sensitivity of the diagnosis,9 we used both the Y reference strain and the local H1 strain. Cases were considered positive if ≥ 2 tests showed a positive result. A short questionnaire on knowledge of triatomines and general clinical signs was also given to participants when blood samples were obtained.
Enzyme-linked immunosorbent assays.
As described, we used T. cruzi crude extracts and a recombinant protein for ELISAs.6 For the crude extract, parasites (epimastigotes) of the Y and H1 strains were cultured in liver infusion tryptose medium supplemented with 10% fetal calf serum. Because results obtained with the Y and the H1 strains as antigens were identical, only data corresponding to the H1 strain are presented. Briefly, logarithmic phase parasites were harvested by centrifugation at 1,000 × g for 10 minutes at 4°C. The parasite pellet was suspended in 500 μL of phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, and 1.4 mM KH2PO4, pH 7.4) and lysed by cycles of freezing (–70°C) and thawing (25°C). The solution was centrifuged at 10,000 × g for 20 minutes at 4°C. The resulting supernatant (extract) was used as crude antigen extract. Protein concentration was determined by the Bradford method.
For the recombinant MBP::TcHSP70 protein, the TcHsp70 cDNA insert (GeneBank accession no. AY576621) was cloned into the Eco RI site of the expression vector pMAL-C2 (New England BioLabs, Ipswich, MA) resulting in the plasmid pMAL-Hsp70. The plasmid was used to transform Escherichia coli DH5-a, and the fusion protein MBP::TcHSP70 was induced and purified according to the manufacturer's instructions. Polystyrene plates (Costar Corporation, Cambridge, MA) were coated with recombinant protein (2 mg/mL) or T. cruzi crude antigen extracts (2 mg/mL) in carbonate buffer, pH 9.6, and incubated overnight at 4°C. Unbound antigen was discarded and plates were blocked with 200 μL of PBS containing 5% non-fat milk for 2 hours at 37°C. Plates were incubated with 50 μL of serum samples (1:200 dilution); each plate also included positive and negative control serum samples.
Further washing steps were conducted and a peroxidase-labeled goat anti-human IgG antibody (Pierce, Rockford, IL) was added at a 1:5,000 dilution in PBS/0.05% Tween 20 and incubated for 1 hour at room temperature. After eight washes, 100 μL of 2,2,-azino-bis (3-ethylbenzthiazoline)-6-sulphonic acid (Zymed, South San Francisco, CA) was added as substrate and the reaction was allowed to proceed for 20 minutes at room temperature. The reaction was stopped with 2% sulfuric acid, and absorbance was read at 415 nm with an ELISA microplate reader (Multiscan MS; Labsystems, Vantaa, Finland).
Indirect immunofluorescent assay.
Trypanosoma cruzi epimastigotes from the H1 strain (1 × 106 parasites) were placed on glass slides, blocked with a goat serum (diluted 1:50 in PBS). Slides were incubated with human serum diluted 1:50 in PBS in a moist chamber at room temperature for 30 minutes and washed for 5 minutes in PBS. Positive and negative serum samples were used as controls. Fluorescein-labeled anti-human IgG (Pierce) was added to each slide and incubated at room temperature for 30 minutes. Slides were then washed in PBS, coverslips were added, and slides examined at 400× magnification by fluorescent microscopy. Results were determined on the basis of fluorescence of the organisms in each sample.
Western blotting analysis.
Western blot was carried out as described.6 Briefly, crude antigen extract from the Y strain was separated by electrophoresis in a 10% sodium dodecyl sulfate–polyacrylamide gel and electroblotted onto a nitrocellulose membrane (Bio-Rad, Hercules, CA) at 80 volts a 4°C for 1 hour. The membrane was blocked with 5% (weight/volume) solution of nonfat milk powder and washed with TBST buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.05% Tween 20). The nitrocellulose membrane was cut into strips that were individually incubated (2 hours at 37°C) with 1 mL of human serum diluted 1:100 in TBST/2% skim milk. Control strips were incubated with positive and negative serum samples. Each strip was washed three times with TBST and subsequently incubated with alkaline phosphatase–labeled goat anti-human IgG (Pierce). The strips were then washed as above and the immune complexes were developed with nitrotetrazolium blue and 5-bromo-4-chloro-3-indolyl phosphate. The reaction was stopped with water. Positive, negative, and secondary antibody controls were included in each experiment.
Data analysis.
All proportion data are presented with their 95% confidence intervals (CIs). Proportion data were compared by using Fisher's exact tests, and the kappa index was calculated when applicable. The relationship between age and seroprevalence rate was assessed by chi-square test and by regression analysis. All villages were georeferenced and a spatial database of serologic results was created in ArcView 3.2 (Environmental Systems Research Institute, Redlands, CA) to produce seroprevalence maps. A Google Earth satellite image was used as a background map layer and for a general representation of land cover. Additional spatial data on altitude, vegetation type/land use, soil humidity, average rainfall, and average temperature were obtained from the Instituto Nacional de Estadistica y Geografía (INEGI) and used to extract environmental information corresponding to the studied villages. The relationships between environmental and serologic data were assessed by using Fisher's exact test.
Results
A total of 654 serum samples were collected from mostly women (61%). Age of participants ranged from 1 to 92 years. All 654 samples were tested for antibodies against T. cruzi. A total of 110 samples with ≥ 2 positive test results, corresponding to an overall seroprevalence of 16.8% (95% CI = 14.2–19.9%) (Table 2). One hundred five samples had positive results for three tests, and 44 had positive results for all four tests. Only one sample had a positive result for only one test (IIF). The agreement between the two ELISAs and the IIF was excellent (extract ELISA versus recombinant ELISA, κ = 0.99 ± 0.005; extract ELISA versus IIF, κ = 0.96 ± 0.01; and recombinant ELISA versus IIF, κ = 0.95 ± 0.02). The agreement was much lower with the Western blotting assay, which showed limited sensitivity because it confirmed only 44 of the 110 samples that had positive results for the tests (extract ELISA versus Western blotting, κ = 0.56 ± 0.05 (Table 2).
Table 2.
Summary of serologic test results, Mexico*
| Test | No. positive/no. tested |
|---|---|
| Extract ELISA | 106/652 |
| Recombinant ELISA | 110/654 |
| Indirect immunofluorescent test | 105/654 |
| Western blotting | 46/654 |
| Two tests | 110/654 |
| Three tests | 105/654 |
| Four tests | 44/654 |
ELISA = enzyme-linked immunosorbent assay.
There was no significant sex bias in infection rates, although men tended to be slightly more infected than women (19.3% in men versus 16.2% in women; P = 0.37, by Fisher's exact test). The questionnaire also indicated that significantly more seropositive participants (78.5%, 95% CI = 69.8–85.2%) were able to identify triatomine bugs, compared with the seronegative persons (66.0%, 95% CI = 61.7–70.0% P = 0.007, by Fisher's exact test). Similarly, 71.6% (95% CI = 62.4–79.2%) of seropositive participants reported having seen triatomines inside or around their house compared with 48.5% (95% CI = 44.2–52.7%) of seronegative persons (P < 0.0001, by Fisher's exact test). None of the clinical signs investigated, including fever, headache, myalgia, vomiting, or chest pain, was associated with seropositivity, except tachycardia, which was more frequent in seropositive participants (29.1% versus 20.6%; P = 0.041, by Fisher's exact test).
Analysis of the geographic distribution of T. cruzi infection indicated that no case was detected in any of the five villages from the municipality of Tierra Blanca; infection was concentrated in 11 of 13 villages from the municipality of Tezonapa and in the village of El Moral in Amatlán (Figure 2), with an overall seropositivity of 28.9% (95% CI = 24.6–33.7%). The highest infection rates were observed in the villages of Caxapa (61.5%, 95% CI = 47.9–73.5%) and Las Josefinas (40%, 95% CI = 27.6–53.9%). This important difference between the municipalities of Tezonapa and Tierra Blanca could be attributed to environmental factors such as vegetation type/land use and soil humidity, and possibly to the geographic separation of the two areas by mountains (Figure 2). The absence of T. cruzi infection in Tierra Blanca was strongly associated with the lack of forest and more humid soils, and the high infection rates in Tezonapa were associated with the presence of evergreen tropical forest (P = 0.002, by Fisher's exact test) and drier soils (P = 0.002, by Fisher's exact test). Conversely, altitude, average rainfall, and temperature did not vary significantly among villages and were not associated with differences in seroprevalence rates. Also, a greater proportion of inhabitants from Tezonapa knew triatomines compared with those from Tierra Blanca (79.4% versus 48.5%; P < 0.001, by Fisher's exact test), and a greater proportion of them reported seeing triatomines inside or around their house (71.8% versus 22.5%; P < 0.001, by Fisher's exact test).
Figure 2.
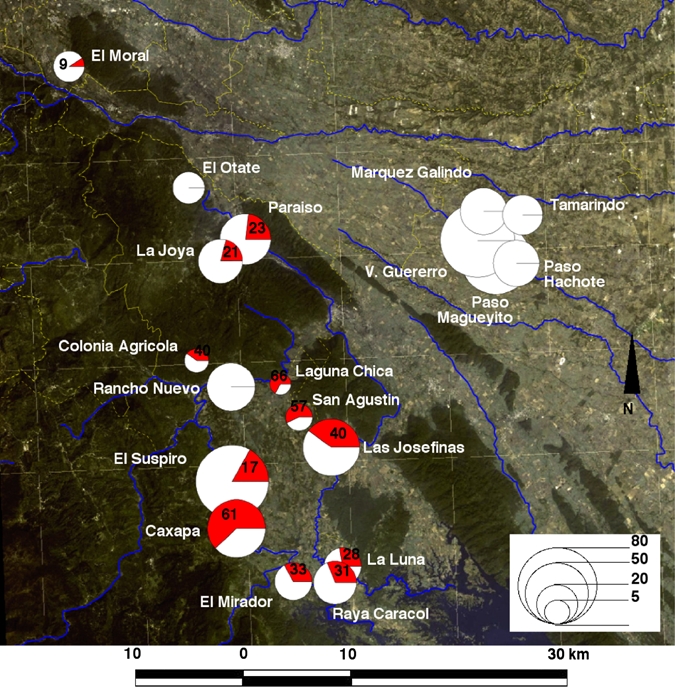
Geographic distribution of Trypanosoma cruzi infection in central Veracruz, Mexico. Circles are proportional to the number of serum samples analyzed in each village as indicated on the bottom right scale. Red areas in the pie charts represent the proportion of T. cruzi-seropositive patients, which is also indicated for villages with seropositive patients. Background image shows a satellite image of the area with blues lines indicating rivers and yellow lines indicating boundaries of the municipalities. This figure appears in color at www.ajtmh.org.
Further stratification of participants from the municipality of Tezonapa indicated a significant difference in infection rate according to age (χ2 = 19.2, degrees of freedom = 6, P = 0.004) (Figure 3). Strikingly, infection rate was significantly higher in children, reaching up to 45.3% (95% CI = 34.5–56.6%) in those 1–10 years of age and lower in adults (16.6–22.9% in those > 20 years of age). Infection rates were significantly correlated with age (r2 = 0.915, P = 0.004, by second-order polynomial regression). The youngest seropositive patient was 2 years of age and the oldest patient was 69 years of age. Infection rate in women of reproductive age (15–45 years of age) was 34.3% (36 of 141, 95% CI = 19.0–33.1%). Cases of household aggregation of T. cruzi infection were detected (siblings, mother–child, father–child), representing up to 25.2% of all cases of T. cruzi infection (27 of 107, 95% CI = 18.0–34.2%).
Figure 3.
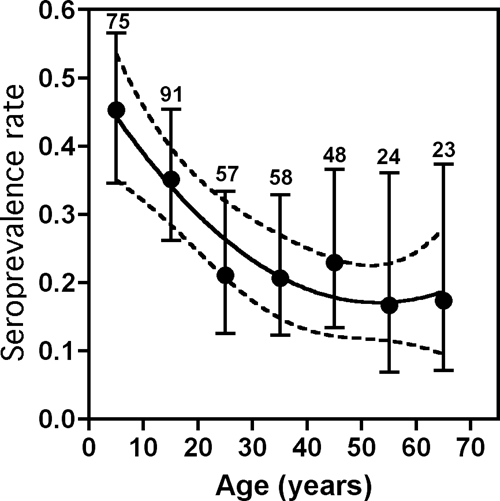
Distribution of Trypanosoma cruzi infection in Tezonapa, Mexico by age groups. The population was stratified according to the indicated age groups and infection rates are shown as mean ± 95% confidence interval. Numbers on the top of the error bars refer to the number of samples for each group. Seroprevalence rates were significantly associated with age (r2 = 0.915, P = 0.004, by second-order polynomial regression), as indicated by the regression line and its 95% confidence area (dotted lines).
Discussion
The purpose of the study was to update epidemiologic information for T. cruzi infection in central Veracruz. Serum samples were tested with four diagnostic tests (two ELISAs [total extract and recombinant protein], IIF, and Western blotting. Concordance among the two ELISAs and the IFF was high, which ensured high reliability of the results. Conversely, Western blotting appeared to have a low sensitivity because it confirmed only a limited proportion of the cases with positive results for other tests. This low performance of Western blotting has been observed in a previous study in the state of Morelos, Mexico, in which only 20% of ELISA-positive cases were confirmed by Western blotting,10 and in a study with samples from blood donors in Orizaba, Veracruz, Mexico.6 This finding may be caused by poor recognition of denatured antigens, and suggests that Western blotting may not be an appropriate method for the confirmation of T. cruzi infection. Nonetheless, the high concordance between tests based on the Y and H1 strains, which belong to different lineages, is in agreement with results of studies that showed excellent performance of tests based on a variety of antigen sources with serum from patients in Mexico.11,12
On the basis of ≥ 2 positive test results, we detected an average seroprevalence of T. cruzi infection of 16.8% (95% CI = 14.2–19.9%) in the study area, which is considerably higher than previous estimates. Seroprevalence rates of 1.3% and 1.8% in the general population and children (< 18 years of age), respectively, have been reported in the jurisdiction of Cordoba, although sample sizes were much smaller and geographic locations were not specified and may have been different.4,8 Interestingly, no cases have been detected in children from the jurisdiction of Cosamaloapan,4 and our data appear to confirm this observation because we did not detect cases in the municipality of Tierra Blanca in the jurisdiction of Cosamaloapan. However, we found a concentration of high seroprevalence rates in the jurisdiction of Cordoba, particularly in the municipality of Tezonapa, which had an average seropositivity of 28.9%. Such a high T. cruzi infection rate would be among the highest rates reported in Mexico3 and suggests the existence of a hyperendemic zone.
We found that T. cruzi infection was strongly associated with ecologic characteristics of the Tezonapa Valley, which is delimited by mountains on the eastern, northern, and western sides and by Laguna Temascal, Oaxaca, on the southern side. Villages in this valley are surrounded by open fields for agriculture and large and numerous patches of evergreen tropical forest, and the soil is dry. These characteristics appear to differ markedly from those in Tierra Blanca, where no cases of T. cruzi infection have been detected, and no patches of tropical forest are present. Villages there are surrounded by fields and pastures, and the soil is more humid. The association of T. cruzi infection with ecologic factors can be interpreted as a strong indicator of vector transmission. This finding is consistent with reports of inhabitants of Tezonapa, most of whom (71.8%) mentioned having seen triatomines inside or around their house. Only a few did so in Tierra Blanca (22.5%), which strongly suggests differences in vectorial exposure between the two municipalities. Several species of triatomines have been reported in Veracruz, the most abundant being T. dimidiata,4 and preliminary bug collections indicate that this species is the main vector in the region (Ramos-Ligonio A et al., unpublished data). House infestation rates of approximately 15% have been reported for the jurisdiction of Cordoba, with a colonization index > 70%,4 which indicates the presence of a small proportion of heavily colonized houses. Nonetheless, no vector control programs have been implemented in the region.
Although T. dimidiata can be found in a wide diversity of habitats,13 it seems to be more frequently associated with woodland/forest areas than with agricultural land,14 which may explain the difference between Tezonapa and Tierra Blanca. As in previous studies,15,16 further analysis of additional factors should enable identification of a greater number of environmental determinants of entomologic and T. cruzi infection risk in this region. Based on a population of 52,000 persons, our seroprevalence data indicate that there may be > 10,000 T. cruzi-infected patients in Tezonapa.
Analysis of various sub-population groups identified other important features of the epidemiology of T. cruzi infection. First, there was no sex bias in infection in this region because men and women were equally infected. More importantly, we observed a strong association with age, with infection rates in younger children > 45% in the municipality of Tezonapa. This finding is clear evidence of active and ongoing T. cruzi transmission in this area. Such alarming data should warrant rapid implementation of Chagas disease control programs. Such high infection rates in children have been reported previously in the Chaco province, Argentina, and in Bolivia,17–20 where Chagas disease control has proven to be challenging.21
The observation that children were more than twice as likely to be infected than persons in older age groups was rather unexpected because it is contrary to most other studies, which show an increase in infection rates with age.22–24 Such an increase is usually attributed to the cumulative exposure to infected vectors, which increases with age. A high infection rate in children may result from congenital transmission and/or high vectorial exposure. Women of reproductive age in Tezonapa had a seroprevalence of T. cruzi infection of 34.3%, which suggests that congenital transmission may contribute significantly to the elevated infection rate in children. On the basis of a vertical transmission rate ≤ 10%,25 we may expect up to 3.4% of newborns to be congenitally infected, and possibly more in case of family clustering.26 Based on a total of 1,000 birth per year, this would correspond to approximately 35 infected newborns per year in the municipality of Tezonapa. Again, this finding would be much higher than what has been reported in northern Veracruz, where no cases of congenital transmission were detected despite a seroprevalence of T. cruzi infection in pregnant women of 3.5%.27
Importantly, identification of a large proportion of cases of family aggregation (27% of seropositive persons) suggests that some households are more affected than others. However, this finding may be caused by an infected mother transmitting T. cruzi to several of her children, or to a family living in a heavily infested/colonized house, or both. Although our data do not enable discrimination between these scenarios, the high seroprevalence observed suggests that both processes may be occurring in the region.
It is unclear why children would be at greater risk of vectorial transmission than adults in this population, but differences in daily activities or sleeping habits leading to differences in vector exposure may account for this observation. Alternatively, lower seroprevalence in adults may be caused by a higher death rate of seropositive persons or conversely to a loss of seroreactivity after spontaneous cure of the infection.28 This finding may also be caused by a potential selection bias because participants were mostly women and children. Additional studies should help clarify these issues.
In conclusion, we reported an average seroprevalence of T. cruzi infection of 16.8% in central Veracruz and identified the municipality of Tezonapa as a potential hyperendemic region, with seroprevalence rates ≤ 45% in young children. Such a high transmission rate is likely associated with congenital and vector transmission, and further studies should help identify the respective contribution of both processes. Also, the magnitude of T. cruzi infection rate in this region calls for the urgent implementation of extensive epidemiologic surveillance activities and Chagas disease control programs.
Acknowledgments
We thank Justino Rodriguez, Q. I. Leticia Robles, and Programa Instituto Mexicano del Seguro Social–Oportunidades for technical assistance.
Footnotes
Financial support: This study was supported by grant 2007-C01-67927 from Fondos Mixtos–Consejo Nacional de Ciencia y Tecnología Conacyt, Mexico and grant DGI-2006 from the Universidad Veracruzana.
Authors' addresses: Angel Ramos-Ligonio, Aracely López-Monteon, and Daniel Guzmán-Gómez, LADISER Inmunología y Biología Molecular, Facultad de Ciencias Químicas, Universidad Veracruzana, Orizaba, Veracruz, Mexico, E-mails: angramos@uv.mx, aralopez@uv.mx, and daguzman@uv.mx. José Luis Rosales-Encina, Laboratorio de Biología Molecular, Departamento de Infectómica y Patogénesis Molecular, Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional–Instituto Politécnico Nacional de los Estados Unidos Mexicanos, Col. San Pedro Zacatenco, Delegación Gustavo A. Madero, Mexico City, Mexico, E-mail: rosales@cinvestav.mx. Yairh Limón-Flores, Laboratorio de Parasitología, Centro de Investigaciones Regionales Dr. Hideyo Noguchi, Universidad Autónoma de Yucatán, Merida, Yucatan, Mexico, E-mail: oliver@uady.mx. Eric Dumonteil, Laboratorio de Parasitología, Centro de Investigaciones Regionales Dr. Hideyo Noguchi, Universidad Autónoma de Yucatán, Merida, Yucatan, Mexico and Department of Tropical Medicine, School of Public Health and Tropical Medicine, Tulane University, New Orleans, LA, E-mail: edumonte@tulane.edu.
References
- 1.World Health Organization . Reporte Sobre la Enfermedad de Chagas. 17–20 de Abril de 2005, Actualizado en Julio de 2007, Buenos Aires, Argentina. Geneva: World Health Organization; 2007. [Google Scholar]
- 2.Dumonteil E. Update on Chagas' disease in Mexico. Salud Publica Mex. 1999;41:322–327. doi: 10.1590/s0036-36341999000400010. [DOI] [PubMed] [Google Scholar]
- 3.Cruz-Reyes A, Pickering-Lopez JM. Chagas disease in Mexico: an analysis of geographical distribution during the past 76 years: a review. Mem Inst Oswaldo Cruz. 2006;101:345–354. doi: 10.1590/s0074-02762006000400001. [DOI] [PubMed] [Google Scholar]
- 4.Salazar PM, Rojas G, Bucio M, Cabrera M, Garcia G, Ruiz A, Guevara Y, Tapia R. Seroprevalencia de anticuerpos contra Trypanosoma cruzi y su asociacion con factores de riesgo en menores de 18 anos de Veracruz, Mexico. Rev Panam Salud Publica. 2007;22:75–82. doi: 10.1590/s1020-49892007000700001. [DOI] [PubMed] [Google Scholar]
- 5.Velasco Castrejon O, Valdespino JL, Tapia Conyer R, Salvatierra B, Guzman Bracho C, Magos C, Llausas A, Gutierrez G, Sepulveda J. Seroepidemiologia de la enfermedad de Chagas en Mexico. Salud Publica Mex. 1992;34:186–196. [PubMed] [Google Scholar]
- 6.Ramos-Ligonio A, Ramirez-Sanchez ME, Gonzalez-Hernandez JC, Rosales-Encina JL, Lopez-Monteon A. Prevalencia de anticuerpos contra Trypanosoma cruzi en donadores de sanger del IMSS, Orizaba, Veracruz, Mexico. Salud Publica Mex. 2006;48:13–21. doi: 10.1590/s0036-36342006000100004. [DOI] [PubMed] [Google Scholar]
- 7.Olivera-Mar A, Hernandez-Vicencio C, Camacho-Marie M, Hernandez-Becerril N, Monteon-Padilla VM, Vallejo M, Reyes PA. Chronic chagasic cardiomyopathy at the Hospital General de Zona no. 24 IMSS. Poza Rica, Veracruz [in Spanish] Arch Cardiol Mex. 2006;76:269–276. [PubMed] [Google Scholar]
- 8.Segura EL, Escobar-Mesa A. Epidemiología de la enfermedad de Chagas en el estado de Veracruz. Salud Publica Mex. 2005;47:201–208. doi: 10.1590/s0036-36342005000300003. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez B, Monteon V, Reyes PA, Espinoza B. Standardization of micro-enzymes-linked immunosorbent assay (ELISA) and western blot for detection of Trypanosoma cruzi antibodies using extracts from Mexican strains as antigens. Arch Med Res. 2001;32:382–388. doi: 10.1016/s0188-4409(01)00303-4. [DOI] [PubMed] [Google Scholar]
- 10.Rangel-Flores H, Sanchez B, Mendoza-Duarte J, Barnabe C, Breniere FS, Ramos C, Espinoza B. Serologic and parasitologic demonstration of Trypanosoma cruzi infections in an urban area of central Mexico: correlation with electrocardiographic alterations. Am J Trop Med Hyg. 2001;65:887–895. doi: 10.4269/ajtmh.2001.65.887. [DOI] [PubMed] [Google Scholar]
- 11.Monteon VM, Ramos A, Reyes PA. Reactivity of sera from Chagas patients to extracts of Mexican Trypanosoma cruzi isolates [in Spanish] Rev Biol Trop. 1993;41:861–865. [PubMed] [Google Scholar]
- 12.Luquetti AO, Espinoza B, Martinez I, Hernandez-Becerril N, Ponce C, Ponce E, Reyes PA, Hernandez O, Lopez R, Monteon V. Performance levels of four Latin American laboratories for the serodiagnosis of Chagas disease in Mexican sera samples. Mem Inst Oswaldo Cruz. 2009;104:797–800. doi: 10.1590/s0074-02762009000500023. [DOI] [PubMed] [Google Scholar]
- 13.Dorn PL, Monroy C, Curtis A. Triatoma dimidiata (Latreille, 1811): a review of its diversity across its geographic range and the relationship among populations. Infect Genet Evol. 2007;7:343–352. doi: 10.1016/j.meegid.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Ibarra-Cerdena CN, Sanchez-Cordero V, Townsend Peterson A, Ramsey JM. Ecology of North American Triatominae. Acta Trop. 2009;110:178–186. doi: 10.1016/j.actatropica.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Dumonteil E, Gourbière S. Prediction of Triatoma dimidiata vector abundance and infection rate: a risk map for Trypanosoma cruzi natural transmission in the Yucatan peninsula of Mexico. Am J Trop Med Hyg. 2004;70:514–519. [PubMed] [Google Scholar]
- 16.Bustamante DM, Monroy C, Rodas A, Juarez J, Malone JB. Environmental determinants of the distribution of Chagas disease vectors in southeastern Guatemala. Geospatial Health. 2007;2:199–211. doi: 10.4081/gh.2007.268. [DOI] [PubMed] [Google Scholar]
- 17.Biancardi MA, Conca Moreno M, Torres N, Pepe C, Altcheh J, Freilij H. Seroprevalencia de la enfermedad de Chagas en 17 parajes del “Monte Impenetrable” de la Provincia del Chaco. Medicina (B Aires) 2003;63:125–129. [PubMed] [Google Scholar]
- 18.Medrano-Mercado N, Ugarte-Fernandez R, Butron V, Uber-Busek S, Guerra HL, Araujo-Jorge TC, Correa-Oliveira R. Urban transmission of Chagas disease in Cochabamba, Bolivia. Mem Inst Oswaldo Cruz. 2008;103:423–430. doi: 10.1590/s0074-02762008000500003. [DOI] [PubMed] [Google Scholar]
- 19.Albarracin-Veizaga H, Carvalho ME, Nascimento EM, Rodrigues VL, Casanova C, Barata JM. Chagas disease in an area of recent occupation in Cochabamba, Bolivia. Rev Saude Publica. 1999;33:230–236. doi: 10.1590/S0034-89101999000300003. [DOI] [PubMed] [Google Scholar]
- 20.Bowman NM, Kawai V, Levy MZ, Cornejo del Carpio JG, Cabrera L, Delgado F, Malaga F, Cordova Benzaquen E, Pinedo VV, Steurer F, Seitz AE, Gilman RH, Bern C. Chagas disease transmission in periurban communities of Arequipa, Peru. Clin Infect Dis. 2008;46:1822–1828. doi: 10.1086/588299. [DOI] [PubMed] [Google Scholar]
- 21.Gurtler RE, Kitron U, Cecere MC, Segura EL, Cohen JE. Sustainable vector control and management of Chagas disease in the Gran Chaco, Argentina. Proc Natl Acad Sci USA. 2007;104:16194–16199. doi: 10.1073/pnas.0700863104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montoya R, Dias JC, Coura JR. Chagas disease in a community in southeast Brazil. I. A serologic follow-up study on a vector controlled area. Rev Inst Med Trop Sao Paulo. 2003;45:269–274. doi: 10.1590/s0036-46652003000500006. [DOI] [PubMed] [Google Scholar]
- 23.Mazariego-Arana MA, Monteon VM, Ballinas-Verdugo MA, Hernandez-Becerril N, Alejandre-Aguilar R, Reyes PA. Seroprevalence of human Trypanosoma cruzi infection in different geografic zones of Chiapas, Mexico. Rev Soc Bras Med Trop. 2001;34:453–458. doi: 10.1590/s0037-86822001000500008. [DOI] [PubMed] [Google Scholar]
- 24.Greer GJ, Nix NA, Cordon-Rosales C, Hernandez B, MacVean CM, Powell MR. Seroprevalence of Trypanosoma cruzi infection in three rural communities in Guatemala. Rev Panam Salud Publica. 1999;6:110–116. doi: 10.1590/s1020-49891999000700005. [DOI] [PubMed] [Google Scholar]
- 25.Buekens P, Almendares O, Carlier Y, Dumonteil E, Eberhard M, Gamboa-Leon R, James M, Padilla N, Wesson D, Xiong X. Mother-to-child transmission of Chagas' disease in North America: why don't we do more? Matern Child Health J. 2008;12:283–286. doi: 10.1007/s10995-007-0246-8. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez Negrette O, Mora MC, Basombrio MA. High prevalence of congenital Trypanosoma cruzi infection and family clustering in Salta, Argentina. Pediatrics. 2005;115:e668–e672. doi: 10.1542/peds.2004-1732. [DOI] [PubMed] [Google Scholar]
- 27.Olivera Mar A, Guillen Ortega F, Cruz Vidal S, Hernandez-Becerril N, Perez Galdamez E, Cordova Concepcion G, Reyes PA, Monteon VM. Serological and parasitological screening of Trypanosoma cruzi infection in mothers and newborns living in two Chagasic areas of Mexico. Arch Med Res. 2006;37:774–777. doi: 10.1016/j.arcmed.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Mott KE, Lehman JS, Jr, Hoff R, Morrow RH, Muniz TM, Sherlock I, Draper CC, Pugliese C, Guimaraes AC. The epidemiology and household distribution of seroreactivity to Trypanosoma cruzi in a rural community in northeast Brazil. Am J Trop Med Hyg. 1976;25:552–562. doi: 10.4269/ajtmh.1976.25.552. [DOI] [PubMed] [Google Scholar]


