Abstract
Rocky Mountain spotted fever (RMSF), a potentially fatal tick-borne infection caused by Rickettsia rickettsii, is considered a notifiable condition in the United States. During 2000 to 2007, the annual reported incidence of RMSF increased from 1.7 to 7 cases per million persons from 2000 to 2007, the highest rate ever recorded. American Indians had a significantly higher incidence than other race groups. Children 5–9 years of age appeared at highest risk for fatal outcome. Enzyme-linked immunosorbent assays became more widely available beginning in 2004 and were used to diagnose 38% of cases during 2005–2007. The proportion of cases classified as confirmed RMSF decreased from 15% in 2000 to 4% in 2007. Concomitantly, case fatality decreased from 2.2% to 0.3%. The decreasing proportion of confirmed cases and cases with fatal outcome suggests that changes in diagnostic and surveillance practices may be influencing the observed increase in reported incidence rates.
Introduction
Rocky Mountain spotted fever (RMSF) is a tick-borne disease caused by the intracellular bacterium Rickettsia rickettsii.1,2 Rocky Mountain spotted fever has long been considered one of the most severe tick-borne rickettsial infections, with pre-antibiotic case-fatality rates reported as high as 65–80% in some case series1–4;contemporary estimates from 1981 to 1998 placed modern case-fatality rates at around 3% of reported cases.5,6 The disease causes fever, headache, abdominal pain, and rash in a majority of patients, and may also lead to complications such as encephalitis, acute respiratory distress syndrome, and coagulopathies.1,2 Although most antibiotics are characteristically ineffective against R. rickettsii, tetracycylines offer excellent clinical results. However, a delay in administration of doxycycline, the recommended drug of choice, has been shown to increase the likelihood of fatal outcome.7,8
Because of its potential for severe outcome, RMSF is considered a notifiable condition in the United States. Surveillance for RMSF is a passive system that has provided important information on epidemiologic trends of infection.9 Assessment of historical reports of case counts indicated some fluidity in annual case counts, with the years 1981–1998 marking a period of overall decline in the incidence and number of reported RMSF cases.5,6,10 In the most recently published national summary, an increase in RMSF incidence was observed, from an all-time low of 1.4 cases per million in 1998 to 3.8 cases per million in 2002.10 To determine if RMSF reports and incidence have continued to increase and to characterize the epidemiology of cases, we examined RMSF cases reported during 2000 through 2007.
Methods
National surveillance systems.
Rocky Mountain spotted fever is a nationally notifiable disease within the National Notifiable Diseases Surveillance System.11 The U.S. Centers for Disease Control and Prevention (CDC) receives electronic reports of RMSF cases from state health departments through the National Electronic Telecommunications System for Surveillance (NETSS); these data are used to calculate national incidence and basic demographics (such as gender, race, and age).12 Because NETSS does not collect patient outcome or laboratory data, a second supplemental system based on manually submitted case reports forms (CRFs) is also used; CRF data are used to determine case fatality and hospitalization ratios, and to report diagnostic tests used. Both systems rely on physicians to appropriately recognize and report RMSF to state health departments. In addition, both systems rely on the appropriate application of a national case definition, specifically the classification of confirmed cases and probable cases.13,14 Two different case definitions were in effect during the studied period; the case definition was changed in 2004 to permit use of enzyme-linked immunosorbent assays (ELISA) for diagnosis of probable cases, and to permit use of commercial laboratory cutoffs for determination of positive test results.13,14
Analytic and statistical methods.
Confirmed and probable RMSF cases reported to NETSS from 2000 through 2007 were used to calculate incidence rates by region, state, county, sex, age, and race. Population estimates for years 2000–2007 were obtained from the U.S. Census Bureau,15 and U.S. regions followed census classifications. Rocky Mountain spotted fever was not considered a notifiable disease during some years in Maine (2003–2007), Washington (2003–2004, 2006–2007), Alaska (2005–2007), and Hawaii (2006–2007); however, when collected, reports from those states were analyzed. The year of RMSF illness onset instead of reporting year was used for analyses; therefore, the number of cases presented here may differ from reports of the annual number of cases published in MMWR annual summaries. Incidence rates were expressed as the number of RSMF cases per million corresponding U.S. population. Confirmed and probable RMSF cases reported by CRFs were used to calculate the proportion of hospitalizations and case fatality rate, and to examine diagnostic test usage. Reports missing information were excluded from that segment of the analysis. Poisson regression analysis was used to compare groups. Winter onset cases were compared by region using the χ2 test; EpiInfo was used for statistical calculations.16
Results
NETSS data.
Overall incidence.
Through NETSS, 11,531 RMSF cases were reported with an onset date January 1, 2000 through December 31, 2007. Among those with a recorded case status in NETSS, 19.6% were confirmed and 80.4% were probable cases. The percent of cases meeting a confirmed case definition decreased during the study period to 9.7% of cases in 2007 (Figure 1).
Figure 1.
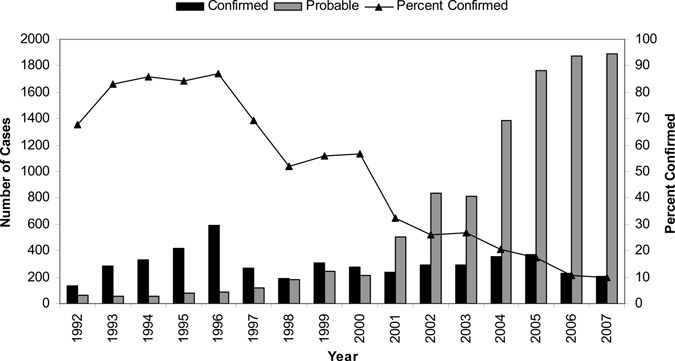
Rocky Mountain spotted fever cases by case classification status, 1992–2007, United States (determined by National Electronic Telecommunications System for Surveillance).
The lowest number of reported cases was 490 in 2000 and the highest was 2,133 in 2005. National incidence increased during 2000 through 2007, from 1.7 in 2000 to a peak of 7.2 cases per million persons in 2005 with 7.0 cases per million reported in both 2006 and 2007 (Figure 2). The average incidence for the first half of the reporting period (2000–2003) was 3.0 cases per million, whereas the average annual incidence for the latter half of the reporting period (2004–2007) was 6.8 cases per million.
Figure 2.
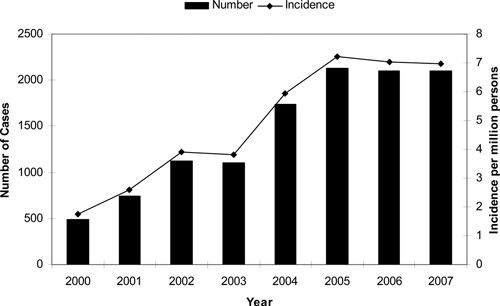
Rocky Mountain spotted fever cases and incidence (per millions persons), 2000–2007, United States (reported through the National Electronic Telecommunications System for Surveillance).
Cases were reported from 46 states and the District of Columbia during the study period. Five states—North Carolina, Oklahoma, Arkansas, Tennessee, and Missouri—accounted for 64% of all RMSF cases (Figure 3). The West North Central, South Atlantic, East and West South Central census regions all had incidence rates above the national average incidence (Table 1). With the exception of the Pacific and Middle Atlantic regions, incidence in all regions increased more than 200% from the first half (2000–2003) to the second half (2004–2007) of the reporting period (Table 1).
Figure 3.
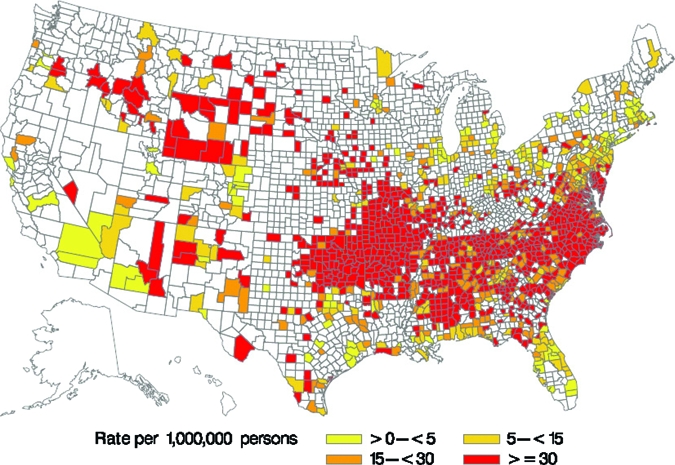
Rocky Mountain spotted fever incidence by county, 2000–2007, United States (reported through the National Electronic Telecommunications System for Surveillance).
Table 1.
Rocky Mountain spotted fever case reports and incidence (per million persons) by state and geographic region, 2000–2007, United States (National Electronic Telecommunications System for Surveillance)
| Region | 2000–2007 | 2000–2003 | 2004–2007 | Percent change 2000–2003 vs. 2004–2007 | |
|---|---|---|---|---|---|
| n | Incidence | Incidence | Incidence | ||
| New England | 95 | 0.84 | 0.46 | 1.21 | + 260% |
| Connecticut | 6 | 0.22 | 0.22 | 0.22 | |
| Maine* | 1 | 0.10 | 0.00 | 0.19 | |
| Massachusetts | 58 | 1.13 | 0.62 | 1.63 | |
| New Hampshire | 4 | 0.39 | 0.20 | 0.58 | |
| Rhode Island | 25 | 2.94 | 1.41 | 4.47 | |
| Vermont | 1 | 0.20 | 0.00 | 0.40 | |
| Middle Atlantic | 489 | 1.52 | 1.11 | 1.93 | + 174.4% |
| New Jersey | 170 | 2.48 | 1.56 | 3.39 | |
| New York | 127 | 0.83 | 0.48 | 1.16 | |
| Pennsylvania | 192 | 1.95 | 1.77 | 2.12 | |
| East North Central | 298 | 0.81 | 0.53 | 1.10 | + 207.0% |
| Illinois | 124 | 1.23 | 0.68 | 1.77 | |
| Indiana | 30 | 0.60 | 0.45 | 0.76 | |
| Michigan | 30 | 0.37 | 0.30 | 0.45 | |
| Ohio | 107 | 1.17 | 0.86 | 1.48 | |
| Wisconsin | 7 | 0.16 | 0.00 | 0.32 | |
| West North | 1,146 | 7.30 | 3.79 | 10.74 | + 283.7% |
| Central | 40 | 1.70 | 0.77 | 2.62 | |
| Iowa | 22 | 1.01 | 0.37 | 1.64 | |
| Kansas | 21 | 0.52 | 0.20 | 0.83 | |
| Minnesota | 963 | 21.00 | 11.09 | 30.64 | |
| Missouri | 74 | 5.32 | 2.18 | 8.40 | |
| Nebraska | 2 | 0.39 | 0.39 | 0.39 | |
| North Dakota | 24 | 3.88 | 3.29 | 4.46 | |
| South Dakota | |||||
| South Atlantic | 5,704 | 13.01 | 8.05 | 17.67 | + 219.5% |
| Delaware | 67 | 10.19 | 4.69 | 15.40 | |
| Dist. Columbia | 10 | 2.15 | 1.73 | 2.57 | |
| Florida | 130 | 0.95 | 0.80 | 1.08 | |
| Georgia | 389 | 5.49 | 3.30 | 7.52 | |
| Maryland | 514 | 11.69 | 9.57 | 13.74 | |
| North Carolina | 3,581 | 52.61 | 29.70 | 74.16 | |
| South Carolina | 445 | 13.28 | 12.49 | 14.03 | |
| Virginia | 529 | 8.93 | 4.29 | 13.35 | |
| West Virginia | 39 | 2.70 | 1.67 | 3.74 | |
| East South Central | 1525 | 10.94 | 6.81 | 14.95 | + 219.5% |
| Alabama | 381 | 10.55 | 3.92 | 17.03 | |
| Kentucky | 28 | 0.85 | 0.86 | 0.84 | |
| Mississippi | 156 | 6.77 | 7.18 | 6.38 | |
| Tennessee | 960 | 20.33 | 13.07 | 27.29 | |
| West South | 2,063 | 7.81 | 4.92 | 10.56 | + 214.6% |
| Central | 802 | 36.57 | 23.27 | 49.44 | |
| Arkansas | 25 | 0.70 | 0.28 | 1.14 | |
| Louisiana | 1,064 | 37.85 | 24.68 | 50.71 | |
| Oklahoma | 172 | 0.96 | 0.40 | 1.49 | |
| Texas | |||||
| Mountain | 190 | 1.20 | 0.62 | 1.74 | + 278.6% |
| Arizona | 51 | 1.11 | 0.09 | 2.02 | |
| Colorado | 22 | 0.60 | 0.34 | 0.85 | |
| Idaho | 29 | 2.61 | 0.75 | 4.33 | |
| Montana | 14 | 1.89 | 1.93 | 1.86 | |
| Nevada | 7 | 0.38 | 0.82 | 0.00 | |
| New Mexico | 24 | 1.59 | 0.54 | 2.60 | |
| Utah | 6 | 0.31 | 0.54 | 0.10 | |
| Wyoming | 37 | 9.19 | 6.05 | 12.23 | |
| Pacific | 21 | 0.06 | 0.06 | 0.05 | – |
| Alaska* | 0 | – | – | – | |
| California | 7 | 0.02 | 0.04 | 0.01 | |
| Hawaii* | 0 | – | – | – | |
| Oregon | 14 | 0.49 | 0.43 | 0.55 | |
| Washington* | 0 | – | – | – | |
| Total | 11,531 | 4.94 | 3.02 | 6.79 | 224.6% |
RMSF not considered a notifiable disease for all study years.
Demographics.
Slightly more males (56.9%) than females were reported with RMSF through NETSS. Most cases during the study period occurred in whites (86.8%), followed by blacks (7.9%), and American Indians (3.9%); fewer cases involved Asian/Pacific Islanders (0.6%), or persons reporting a race category of “Other” (0.8%). Hispanic ethnicity was reported for 4.1% of cases. Race-specific incidence was higher among American Indians (16.8 cases per million population) than those for white (4.4), black (2.6), and Asian/Pacific Islander (0.5) race groups. The overall mean and median age of onset was 46 and 42 years of age, respectively. Incidence increased with age, peaking in the 50–59 and 60–69 age groups (6.9 and 7.0 cases per million, respectively) (Figure 4).
Figure 4.
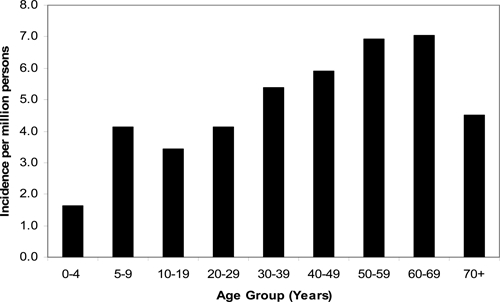
Rocky Mountain spotted fever incidence by age group, 2000–2007, United States (reported through the National Electronic Telecommunications System for Surveillance).
Seasonality.
Among the NETSS cases that indicated an onset month, RMSF peaked in June and July (N = 3429, 38%). Fewer cases (N = 351, 4.1%) reported onset during the colder winter months of December, January, or February. Middle Atlantic states (New York, New Jersey, and Pennsylvania) reported a significantly higher proportion of cases with winter onset than other U.S. regions (9.3% and 3.9%, respectively; P < 0.001).
CRF data.
A total of 7,796 RMSF reports were submitted by CRFs that stated an illness onset during 2000 through 2007. The cases reported through CRFs were similar to NETSS cases in terms of gender (57.4% male), age (mean age 40 years, median 41), and race distribution (86.6% white, 8.4% black, 4.4% American Indian, 0.6% Asian/Pacific Islanders) (Table 2). However, cases reported by CRF were less likely to be classified as confirmed (5.8%) than cases reported through NETSS (19.6%); CRF case classifications are considered more accurate because they are verified by CDC staff on the basis of provided laboratory data, and corrected when necessary.
Table 2.
Rocky Mountain spotted fever demographic profiles and outcome, 2000–2007, United States (National Electronic Telecommunications System for Surveillance [NETSS] and Case Report Forms [CRF])*
| NETSS | CRF | |||
|---|---|---|---|---|
| Number | Percent | Number | Percent | |
| Number of cases | 11,531 | – | 7,796 | – |
| Confirmed | 2,258 | 19.6 | 453 | 5.8 |
| Probable | 9,266 | 80.4 | 7,343 | 94.2 |
| Male | 6,511 | 56.9 | 4,413 | 57.4 |
| Female | 4,939 | 43.1 | 3,275 | 42.6 |
| Race | 8,334 | 88.1 | 6,165 | 86.6 |
| White | ||||
| Black | 762 | 8.1 | 596 | 8.4 |
| American Indian | 377 | 4.0 | 312 | 4.4 |
| Asian/Pacific Islander | 54 | 0.6 | 44 | 0.6 |
| Other race | 69 | 0.8 | – | – |
| Hispanic ethnicity | 338 | 4.1 | 239 | 3.7 |
| Age (years) | 46 | – | 40 | – |
| Mean | 42 | – | 41 | – |
| Median | ||||
| Hospitalized | – | – | 1,768 | 23.4 |
| Not hospitalized | – | – | 5,777 | 76.6 |
| Confirmed cases hospitalized | – | – | 162 | 37.4 |
| Probable cases hospitalized | 1,606 | 22.6 | ||
| Died | – | – | 35 | 0.5 |
| Did not die | – | – | 7,206 | 99.5 |
| Confirmed cases died | – | – | 13 | 3.0 |
| Probable cases died | 22 | 0.3 | ||
Cases for which data not reported were excluded from that part of the analysis, so denominators may not be the same for all categories.
Hospitalizations.
The overall proportion of hospitalized CRF cases was 23.4%. Confirmed cases had a higher percent of patients hospitalized (37.4%) compared with that for probable cases (22.6%). The annual percent of patients hospitalized decreased during the study period, from a high of 36.4% reported in 2000 to a low of 18.1% reported in 2005 (Figure 5A). A higher proportion of males were hospitalized than females (25.0% and 21.7%, respectively; P < 0.001). The percent of patients hospitalized was highest among 0–4 year olds (36.2%) and 70 + year olds (38.5%) (Figure 5B). By race, blacks were most frequently hospitalized (31.0%), followed by American Indians (24.5%), whites (23.0%), and Asian/Pacific Islanders (22.7%). Specific life-threatening complications, including meningitis/encephalitis (N = 95, 1.7%), renal failure (N = 28, 0.5%), adult respiratory distress syndrome (N = 25 cases, 0.4%), and coagulopathy (N = 11 cases, 0.2%), were reported for a minority of patients.
Figure 5.
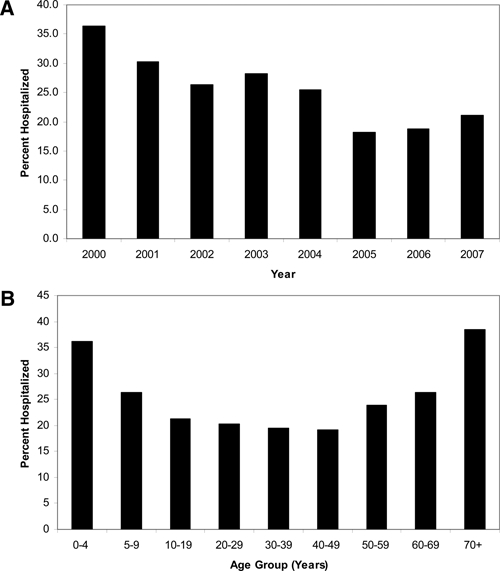
Rocky Mountain spotted fever hospitalizations (A) by year and (B) by age group, 2000–2007, United States (reported through Case Report Forms).
Fatal outcome.
The overall proportion of CRF cases with fatal outcome was 0.5%. Confirmed cases had a higher percentage of patients with fatal outcome (3.0%) compared with probable cases (0.3%). The annual case fatality decreased during 2000 to 2007 (Figure 6A). American Indians experienced the highest reported case fatality (2.2%), followed by whites (0.5%) and African Americans (0.2%). Among cases stratified by age, the highest case fatality was reported among patients aged 5–9 years (2.6%), adults 70 + years (1.3%), and children aged 0–4 years (1.2%) (Figure 6B).
Figure 6.
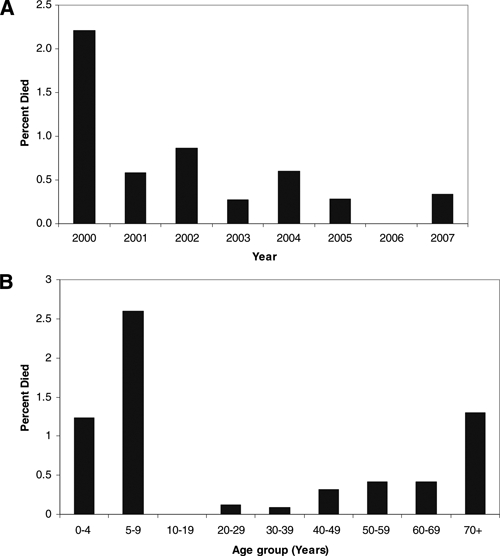
Rocky Mountain spotted fever case fatality (A) by year and (B) age group, 2000–2007, United States (reported through Case Report Forms).
Laboratory testing.
During the study period, the focus of RMSF testing shifted away from indirect immunofluorescent assay (IFA) immunoglobulin (Ig)G assays, concomitant with a 2004 change in the case definition that included qualitative assays (ELISA) for categorization of probable cases. While only 2.8% of cases reported in 2001 were based on ELISA testing, this percentage increased to a mean of 38.3% for 2005–2007 (Figure 7). Although the specific assay used was not always provided, at least 11% of cases reported using assays that detected IgM antibodies. Use of polymerase chain reaction (PCR), culture, or immunochemical stains for RMSF diagnosis was noted for less than 0.5% of reported cases.
Figure 7.
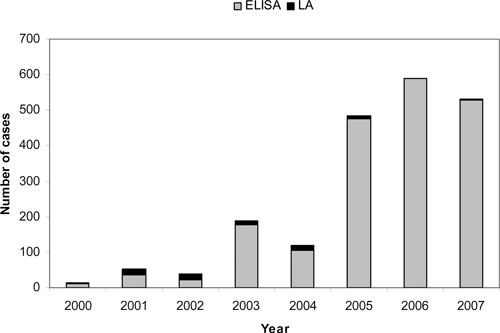
Rocky Mountain spotted fever cases diagnosed by enzyme-linked immunosorbent assays (ELISA) and latex agglutination (LA), 2000–2007, United States (reported through Case Report Forms).
Discussion
The incidence of RMSF reported through national surveillance increased 4-fold during 2000–2007 to approximately 7 cases per million persons annually, the highest annual rate recorded since record-keeping was first established early in the 20th century (Figure 8).5,6,9,10 Along with the unprecedented increase in incidence, a decrease in the percentage of patients with fatal outcome was also observed, to a record-breaking low.5,6,9,10 The recent precipitous decline in reported case fatality is not easily explained by current medical advances, as the prescribed treatment of RMSF, mainly empiric administration of effective antibiotics and provision of supportive care, has not changed in the past 20 years.8
Figure 8.
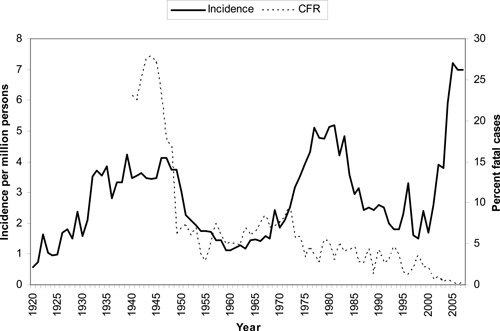
Historical surveillance for Rocky Mountain spotted fever, incidence and case fatality ratios (CFR) based on national surveillance data for the United States, 1920–2007. (From 1980 through 2007, incidence was derived from data reported through the National Electronic Telecommunications System for Surveillance, and percent fatal cases was derived from Case Report Forms).
The causes of past historical fluctuations in RMSF incidence and case fatality have been widely speculated. A 1950s low in case reports has been suggested to have resulted from a void in surveillance activities after the death of a prominent RMSF researcher who had spurred disease surveillance efforts, and an increase in the 1970s was speculated to have been impacted by the evolution of new RMSF diagnostic tests.9 The observed contemporary changes should also be subject to close scrutiny.
Since 2000, several important changes have occurred that may have influenced public health surveillance activities for RMSF. A new CRF data collection tool was introduced in 2001, and its availability was widely advertised to state health departments.8 Two other less severe tick-borne rickettsial diseases, ehrlichiosis and anaplasmosis, became reportable around the same time, and states were encouraged to use the same CRF reporting system that was being used for RMSF.8 These factors may have stimulated interest in tick-borne disease surveillance at the physician and state level and led to improved detection of tick-borne illnesses. The publication of a document in 2006 providing national guidelines for the diagnosis, treatment, and management of tick-borne rickettsial diseases, including RMSF, ehrlichiosis, and anaplasmosis, may have influenced physician recognition of these diseases and increased diagnosis and reporting practices.8 An influx of federal monies to state programs to support public health preparedness (from $42 million in 2000, to an annual mean of $734 million from 2004–2007) may have further strengthened state capacities to conduct surveillance for previously neglected diseases, such as tick-borne illnesses [CDC, unpublished data]. Another factor that may have further contributed to the observed changes in incidence was the adoption of a new RMSF surveillance case definition 2004, to reflect an increasing trend among physicians to request ELISA tests for diagnosis of RMSF.13,14 Although this case definition change was necessary to support changing clinical practices, this change resulted in the inclusion of probable cases with less supporting data than under past case definitions.
The number of reported cases meeting a confirmed case definition has remained stable during the last decade, but the overall percentage of confirmed cases has decreased (Figure 7). In our study, confirmed cases differed from probable cases in several important ways; case fatality among confirmed cases was 10 times that of probable cases and was also more likely to be hospitalized. Although the methods applied to confirmation of RMSF cases (i.e., testing of paired sera, culture, PCR, or immunohistochemistry (IHC) of biopsy and autopsy specimens) have remained consistent over time, the use of newer ELISA-based methods became more prominent during the study period, and most of the increase in RMSF reports can be attributed to an increase in the number of probable cases that were diagnosed with single serologic assays.
The use of single serologic tests for diagnosing acute rickettsial infections is questionable. Background seroprevalence for antibodies to R. rickettsii in the southeastern United States can be as high as 20% among healthy adults (CDC, unpublished data), and 10–12% in children.17 Other studies examining background seroprevalence in northern states or among geographically widespread military personnel suggest a seroprevalence of 4% to 6%.18,19 The diagnosis of RMSF based on a single serologic test, as predominantly occurred during the current study period, may result in the erroneous diagnosis in non-RMSF cases with historical titers from past exposures or with nonspecific cross-reactive IgM class antibodies. The reported sensitivity and specificity of single IgM tests have been reported as low as 23% and 77%, respectively, when compared with testing of IgG in paired sera, for the diagnosis of rickettsial infections in endemic areas.20
The prevalence of anti-R. rickettsii antibodies appears to increase with age,18,19 likely as a function of increased risk of exposure to either R. rickettsii or other rickettsial organisms over a lifetime, the effect of historical titers may be higher among adults. In the current study, children had a lower incidence rate compared with adults, but had the highest risk of fatal outcome, in contrast to prior studies that showed the highest incidence among children and placing older adults at greater risk for fatal outcome.5,6 The incidence and risk for fatal outcome in children 0–9 years of age has actually remained fairly consistent over the past 15 years, ranging from 2 to 4 cases per million persons and 1–3% with a fatal outcome.6,10 In contrast, incidence and case fatality among older age groups has changed dramatically. Given that background antibodies to RMSF increase with age,18,19 this observation may suggest that single serologic tests have greater positive predictive value among children, and that single-titer data from this age group more accurately reflect true RMSF cases.
Another factor that may influence increased risk for fatal outcome among younger age groups is continued reluctance among healthcare providers to prescribe doxycycline to children. The performance of the recommended dose and duration of doxycycline is significantly superior when compared with alternative therapies,7 and has been shown to have no appreciable impact on dental staining.21 Reviews of healthcare providers in the southern United States have shown that 50–75% would prescribe an antibiotic other than doxycycline when presented with a child with suspect RMSF [CDC, unpublished data].22 This continued practice may contribute to the continued higher proportion of RMSF cases with fatal outcome observed among children, and physicians should always prescribe doxycycline as the primary antibiotic of choice for the treatment of suspect RMSF in children.23
This study expanded a previously reported trend that American Indians experience disproportionate RMSF incidence and case fatality compared with other race groups.24 During 2000–2007, American Indians experienced a burden of RMSF almost four times that of whites, and experienced a 4-fold greater risk of fatal outcome. An emerging focus of RMSF associated with Rhipicephalus sanguineus (the brown dog tick) was identified among American Indians in Arizona beginning in 2002 and continuing throughout the study period, and this outbreak has contributed to the increasing incidence within this race group.25 It is clear that this group deserves renewed focus to identify reasons for this disparity and areas of appropriate intervention.24–27
We found that RMSF onset followed expected and previously reported seasonal trends,5,6,10 with the majority of cases reporting illness onset during summer months when peak tick activity is expected. Only 4% of RMSF cases reported onset during winter months. A recent report had suggested that 15% of U.S. RMSF cases in 2006 occurred in December and January, and interpreted this as possible evidence of non-Rickettsia rickettsii infections being incorrectly reported as RMSF.28 However, that report used date of publication in the MMWR as a surrogate for onset date. These dates do not correlate well because of lags in reporting times from state health departments.29 In our study, only the Middle Atlantic region (New York, Pennsylvania, and New Jersey) reported a significantly higher rate of winter onset RMSF than other parts of the United States, despite having a low overall incidence of RMSF. This finding could reflect infection with and serologic cross-reactivity to other urban spotted fever group rickettsial infections known to be endemic in this region, such as Rickettsia akari (rickettsialpox) and for which confirmed cases are recognized year-round.30
In addition to R. akari, several other spotted fever group rickettsiae are known to cause human illness occurrence in the United States, including Rickettsia parkeri, Rickettsia massiliae, and Rickettsia spp. 364D.31–38 There is also evidence that Rickettsia amblyommii, a tick-borne spotted fever group Rickettsia previously considered nonpathogenic, may cause mild illness and be misdiagnosed as RMSF on common serologic assays.39 Geographic variations in severity have been noted, and a focus of unusually mild illness has been noted in North Carolina where R. amblyommii is prevalent.40 A recent letter to Lancet suggested that up to one-third of U.S. cases diagnosed as RMSF might actually be caused by R. parkeri infection.34 This supposition was based on testing of a relatively small set of specimens (N = 15) and may not be broadly representative of the U.S. situation34; however, it nonetheless highlights that current U.S. systems for RMSF surveillance are not necessarily specific for R. rickettsii. Extensive antigenic cross-reactivity occurs among organisms within the genus Rickettsia,41–43 and the high percentage of cases reported in the United States that are diagnosed solely by antigenic detection methods means that case reports lack specificity. In fact, the disease historically thought of as “Rocky Mountain spotted fever” may be more correctly viewed as a constellation of infections caused by various species within the genus Rickettsia, with possible wide variations in clinical spectrum, disease severity, geographic distributions, and potential for fatal outcomes.44–47 Beginning in 2010, the name of the reporting category will be changed to “Spotted Fever Rickettsioses (including RMSF).”48 This description more accurately defines the spectrum of agents that may be causing disease, but will not change surveillance methods.
This period of increased reporting of RMSF has been accompanied by increases in other tick-borne diseases, including human ehrlichiosis, anaplasmosis, and Lyme disease.29,49 Some researchers have speculated that increases in tick-borne diseases may be fueled by ecologic or climate changes,50,51 and an increase in actual disease is clearly supported by some focal patterns of recent RMSF emergence.25 However, the findings from our study suggest that the increase in reported RMSF incidence further involves a complex interplay of physician awareness, diagnostic practices, and reporting policies. In the future, development of active tick-borne disease surveillance programs in defined, geographic areas may help better assess the true burden of disease caused by R. rickettsii and other tick-borne diseases. In those studies, emphasis should be placed on acquiring appropriate specimens to ensure confirmation of a diagnosis. Until then, monitoring of national RMSF surveillance data will continue to provide the most valuable tool to track and monitor changing trends in RMSF epidemiology, despite its inherent limitations.
Supplementary Material
Acknowledgments
We thank the clinic, hospital, and state health department physicians and staff for their continuing efforts involving detection and reporting of RMSF cases by CRFs and NETSS. We thank Lindsey Pool and Deborah Adams for technical assistance. We thank Alicia Anderson and Chris Paddock for expert review and advice.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the funding agency.
Footnotes
Financial support: There are no disclosures for financial support.
Authors' addresses: John J. Openshaw, David L. Swerdlow, John W. Krebs, Robert C. Holman, Eric Mandel, Alexis Harvey, Dana Haberling, Robert F. Massung, and Jennifer H. McQuiston, Division of Viral and Rickettsial Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: fzh7@cdc.gov.
References
- 1.Helmick CG, Bernard KW, D'Angelo LJ. Rocky Mountain spotted fever: clinical, laboratory, and epidemiological features of 262 cases. J Infect Dis. 1984;150:480–488. doi: 10.1093/infdis/150.4.480. [DOI] [PubMed] [Google Scholar]
- 2.Dantas-Torres F. Rocky Mountain spotted fever. Lancet Infect Dis. 2007;7:724–732. doi: 10.1016/S1473-3099(07)70261-X. [DOI] [PubMed] [Google Scholar]
- 3.Wilson LB, Chowning WM. Studies in pyroplasmosis hominis (“spotted fever” or “tick fever” of the Rocky Mountains. J Infect Dis. 1904;1:31–57. doi: 10.1093/clinids/1.3.540. [DOI] [PubMed] [Google Scholar]
- 4.Rucker WC. Rocky Mountain spotted fever. Public Health Rep. 1912;27:1465–1482. [Google Scholar]
- 5.Dalton MJ, Clarke MJ, Holman RC, Krebs JW, Fishbein DB, Olson JG, Childs JE. National surveillance for Rocky Mountain spotted fever, 1981–1992: epidemiologic summary and evaluation of risk factors for fatal outcome. Am J Trop Med Hyg. 1995;52:405–413. doi: 10.4269/ajtmh.1995.52.405. [DOI] [PubMed] [Google Scholar]
- 6.Treadwell TA, Holman RC, Clarke MJ, Krebs JW, Paddock CD, Childs JE. Rocky Mountain spotted fever in the United States, 1993–1996. Am J Trop Med Hyg. 2000;63:21–26. doi: 10.4269/ajtmh.2000.63.21. [DOI] [PubMed] [Google Scholar]
- 7.Holman RC, Paddock CD, Curns AT, Krebs JW, McQuiston JH, Childs JE. Analysis of risk factors for fatal Rocky Mountain spotted fever: evidence for superiority of tetracyclines for therapy. J Infect Dis. 2001;184:1437–1444. doi: 10.1086/324372. [DOI] [PubMed] [Google Scholar]
- 8.Chapman AS, Bakken JS, Folk SM, Paddock CD, Bloch KC, Krusell A, Sexton DJ, Buckingham SC, Marshall GS, Storch GA, Dasch GA, McQuiston JH, Swerdlow DL, Dumier SJ, Nicholson WL, Walker DH, Eremeeva ME, Ohl CA. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis–United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep. 2006;55:1–27. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5504a1.htm Tickbkorne Rickettsial Diseases Working Group; CDC. Available at. Accessed October 23, 2009. [PubMed] [Google Scholar]
- 9.Childs JE, Paddock CD. Passive surveillance as an instrument to identify risk factors for fatal Rocky Mountain spotted fever: is there more to learn? Am J Trop Med Hyg. 2002;66:450–457. doi: 10.4269/ajtmh.2002.66.450. [DOI] [PubMed] [Google Scholar]
- 10.Chapman AS, Murphy SM, Demma LJ, Holman RC, Curns AT, McQuiston JH, Krebs JW, Swerdlow DL. Rocky Mountain spotted fever in the United States, 1997–2002. Vector Borne Zoonotic Dis. 2006;6:170–178. doi: 10.1089/vbz.2006.6.170. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention . National Notifiable Diseases Surveillance System (NNDSS), 1980–2007. Division of Integrated Surveillance Systems and Services, National Center for Public Health Informatics, Coordinating Center for Health Information and Service; 2009. http://www.cdc.gov/ncphi/disss/nndss/nndsshis.htm Available at. Accessed October 22, 2009. [Google Scholar]
- 12.Centers for Disease Control and Prevention . National Electronic Telecommunications System for Surveillance (NETSS) Division of Integrated Surveillance Systems and Services, National Center for Public Health Informatics, Coordinating Center for Health Information and Service; 2009. http://www.cdc.gov/ncphi/disss/nndss/netss.htm Available at. Accessed October 22, 2009. [Google Scholar]
- 13.Centers for Disease Control and Prevention Rocky Mountain Spotted Fever (Rickettsia rickettsii) (RMSF) 1996 Case Definition. 2009. http://www.cdc.gov/ncphi/disss/nndss/casedef/rocky1996.htm Available at. Accessed October 23, 2009.
- 14.Centers for Disease Control and Prevention Rocky Mountain Spotted Fever (Rickettsia rickettsii) (RMSF) 2004 Case Definition. 2009. http://www.cdc.gov/ncphi/disss/nndss/casedef/rocky2004.htm Accessed 10/23/2009.
- 15.U.S. Census Bureau State population estimates and demographic components of population change: 2000 to 2006. 2009. http://www.census.gov/popest/datasets.html Available at. Accessed February 1, 2009.
- 16.EpiInfo version 6.0 Statcalc. Centers for Disease Control and Prevention. 2009. http://www.cdc.gov/epiinfo/about.htm Accessed October 22, 2009.
- 17.Marshall GS, Stout GG, Jacobs RF, Schultze GE, Paxton H, Buckingham SC, DeVincenzo JP, Jackson AM, San Joaquin VH, Standaert SM, Woods CR. Antibodies reactive to Rickettsia rickettsii among children living in the southeast and south central regions of the United States. Arch Pediatr Adolesc Med. 2003;157:443–448. doi: 10.1001/archpedi.157.5.443. TICS Group. [DOI] [PubMed] [Google Scholar]
- 18.Hilton E, DeVoti J, Benach JL, Halluska ML, White DJ, Paxton H, Dumler JS. Seroprevalence and seroconversion for tick-borne diseases in a high-risk population in the northeast United States. Am J Med. 1999;106:404–409. doi: 10.1016/s0002-9343(99)00046-7. [DOI] [PubMed] [Google Scholar]
- 19.Graf PCF, Chretien JP, Ung L, Gaydos JC, Richards AL. Prevalence of seropositivity to spotted fever group rickettsiae and Anaplasma phagocytophilum in a large, demographically diverse U.S. sample. CID. 2008;46:70–77. doi: 10.1086/524018. [DOI] [PubMed] [Google Scholar]
- 20.Reller ME, Clemens EG, Woods CW, Dumler JS. Abstract 56, presented at 23rd Meeting of the American Society for Rickettsiology. Hilton Head, SC: 2009. 2009. (Serologic assessment of the burden of rickettsial infections in southern Sri Lanka). Aug 15–18. [Google Scholar]
- 21.Lochary ME, Lockhart PB, Williams WT., Jr Doxycycline and staining of permanent teeth. Pediatr Infect Dis J. 1998;17:429–431. doi: 10.1097/00006454-199805000-00019. [DOI] [PubMed] [Google Scholar]
- 22.O'Reilly M, Paddock C, Elchos B, Goddard J, Childs J, Currie M. Physician knowledge of the diagnosis and management of Rocky Mountain spotted fever: Mississippi, 2002. Ann N Y Acad Sci. 2003;990:295–301. doi: 10.1111/j.1749-6632.2003.tb07379.x. [DOI] [PubMed] [Google Scholar]
- 23.American Academy of Pediatrics . In: Red Book: Report of the Committee on Infectious Diseases. 28th edition. Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Elk Grove Village, IL: American Academy of Pediatrics; 2009. 2009. pp. 573–575. (Rocky Mountain spotted fever). [Google Scholar]
- 24.Holman RC, McQuiston JH, Haberling DL, Cheek JE. Increasing incidence of Rocky Mountain spotted fever among the American Indian population in the United States. Am J Trop Med Hyg. 2009;80:601–605. [PubMed] [Google Scholar]
- 25.Demma LJ, Traeger MS, Nicholson WL, Paddock CD, Blau DM, Eremeeva ME, Dasch GA, Levin ML, Singleton J, Jr, Zaki SR, Cheek JE, Swerdlow DL, McQuiston JH. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N Engl J Med. 2005;11:587–594. doi: 10.1056/NEJMoa050043. [DOI] [PubMed] [Google Scholar]
- 26.Demma LJ, Holman RC, Mikosz CA, Curns AT, Swerdlow DL, Paisano EL, Cheek JE. Rocky Mountain spotted fever hospitalizations among American Indians. Am J Trop Med Hyg. 2006;75:537–541. [PubMed] [Google Scholar]
- 27.McQuiston JH, Holman RC, Groom AV, Kaufman SF, Cheek JE, Childs JE. Incidence of Rocky Mountain spotted fever among American Indians in Oklahoma. Public Health Rep. 2000;115:469–475. doi: 10.1093/phr/115.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raoult D, Parola P. Rocky Mountain spotted fever in the USA: a benign disease or a common diagnostic error? Lancet. 2008;8:587–589. doi: 10.1016/S1473-3099(08)70210-X. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention Summary of notifiable diseases–United States, 2006. MMWR Recomm Rep. 2008;55:1–94. [PubMed] [Google Scholar]
- 30.Paddock CD, Zaki SR, Koss T, Singleton J, Jr, Sumner JW, Comer JA, Eremeeva ME, Dasch GA, Cherry B, Childs JE. Rickettsialpox in New York City: a persistent urban zoonosis. Ann N Y Acad Sci. 2003;990:36–44. doi: 10.1111/j.1749-6632.2003.tb07334.x. [DOI] [PubMed] [Google Scholar]
- 31.Parola P, Labruna MB, Raoult D. Tick-borne rickettsioses in America: unanswered questions and emerging diseases. Curr Infect Dis Rep. 2009;11:40–50. doi: 10.1007/s11908-009-0007-5. [DOI] [PubMed] [Google Scholar]
- 32.Eremeeva ME, Bosserman EA, Demma LJ, Zambrano ML, Blau DM, Dasch GA. Isolation and identification of Rickettsia massiliae from Rhipicephalus sanguineus ticks collected in Arizona. Appl Environ Microbiol. 2006;72:5569–5577. doi: 10.1128/AEM.00122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vitale G, Mansuelo S, Rolain JM, Raoult D. Rickettsia massiliae human isolation. Emerg Infect Dis. 2006;12:174–175. doi: 10.3201/eid1201.050850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raoult D, Paddock CD. Rickettsia parkeri infection and other spotted fevers in the United States. N Engl J Med. 2005;353:626–627. doi: 10.1056/NEJM200508113530617. [DOI] [PubMed] [Google Scholar]
- 35.Paddock CD, Sumner JW, Comer JA, Zaki SR, Goldsmith CS, Goddard J, McLellan SL, Tamminga CL, Ohl CA. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;15:805–811. doi: 10.1086/381894. [DOI] [PubMed] [Google Scholar]
- 36.Paddock CD. Rickettsia parkeri as a paradigm for multiple causes of tick-borne spotted fever in the western hemisphere. Ann N Y Acad Sci. 2005;1063:315–326. doi: 10.1196/annals.1355.051. [DOI] [PubMed] [Google Scholar]
- 37.Paddock CD, Finley RW, Wright CS, Robinson HN, Schrodt BJ, Lane CC, Ekenna O, Blass MA, Tamminga CL, Ohl CA, McLellan SL, Goddard J, Holman RC, Openshaw JJ, Sumner JW, Zaki SR, Eremeeva ME. Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin Infect Dis. 2008;47:1188–1196. doi: 10.1086/592254. [DOI] [PubMed] [Google Scholar]
- 38.Shapiro MR, Fritz CL, Tait K, Paddock CD, Nicholson WL, Abramowicz KF, Karpathy SE, Dasch GE, Sumner JW, Adem PV, Scott JJ, Padgett KA, Zaki SR, Eremeeva ME. Rickettsia 364D: a newly recognized cause of eschar-associated illness in California. Clin Infect Dis. 2010;50:541–548. doi: 10.1086/649926. [DOI] [PubMed] [Google Scholar]
- 39.Apperson CS, Engber B, Nicholson WL, Mead DG, Engel J, Yabsley MJ, Dail K, Johnson J, Watson DW. Tickborne diseases in North Carolina: is “Rickettsia amblyommii” a possible cause of rickettsiosis reported as Rocky Mountain spotted fever? Vector Borne Zoonotic Dis. 2008;8:597–606. doi: 10.1089/vbz.2007.0271. [DOI] [PubMed] [Google Scholar]
- 40.Adjemian JZ, Krebs JW, Mandel E, McQuiston J. Spatial clustering by disease severity among reported Rocky Mountain spotted fever cases in the United States, 2001–2005. Am J Trop Med Hyg. 2008;80:72–77. [PubMed] [Google Scholar]
- 41.La Scola B, Raoult D. Laboratory diagnosis of rickettsioses: current approaches to diagnosis of old and new rickettsial diseases. J Clin Microbiol. 1997;35:2715–2727. doi: 10.1128/jcm.35.11.2715-2727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaplan JE, Schonberger LB. The sensitivity of various serologic tests in the diagnosis of Rocky Mountain spotted fever. Am J Trop Med Hyg. 1986;35:840–844. doi: 10.4269/ajtmh.1986.35.840. [DOI] [PubMed] [Google Scholar]
- 43.Kantso B, Svendsen CB, Jorgensen CS, Krogfelt KA. Evaluation of serological tests for the diagnosis of rickettsiosis in Denmark. J Microbiol Methods. 2009;76:285–288. doi: 10.1016/j.mimet.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 44.Walker DH. Rickettsiae and rickettsial infections: the current state of knowledge. Clin Infect Dis. 2007;45:S39–S44. doi: 10.1086/518145. [DOI] [PubMed] [Google Scholar]
- 45.Walker DH, Paddock CD, Dumler JS. Emerging and re-emerging tick-transmitted rickettsial and ehrlichial infections. Med Clin North Am. 2008;92:1345–1361. doi: 10.1016/j.mcna.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Parola P, Labruna MB, Raoult D. Tick-borne rickettsioses in America: unanswered questions and emerging diseases. Curr Infect Dis Rep. 2009;11:40–50. doi: 10.1007/s11908-009-0007-5. [DOI] [PubMed] [Google Scholar]
- 47.Paddock C. The science and fiction of emerging rickettsioses. Ann N Y Acad Sci. 2009;1166:133–143. doi: 10.1111/j.1749-6632.2009.04529.x. [DOI] [PubMed] [Google Scholar]
- 48.Council of State and Territorial Epidemiologists, Public Health Reporting and National Notification for Spotted Fever Rickettsioses (including Rocky Mountain spotted fever) Position Statement 09-ID-16. 2009. http://www.cste.org/ps2009/09-ID-16.pdf Available at. Accessed October 23, 2009.
- 49.Centers for Disease Control and Prevention Summary of notifiable diseases–United States, 2007. MMWR Recomm Rep. 2009;56:1–100. [Google Scholar]
- 50.Brownstein JS, Holford TR, Fish D. Effect of climate change on lyme disease risk in North America. EcoHealth. 2005;2:38–46. doi: 10.1007/s10393-004-0139-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Randolph SE. Dynamics of tick-borne disease systems: minor role of recent climate change. Rev Sci Tech. 2008;27:367–381. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


