Abstract
Globally, sexually transmitted infections (STIs) represent a significant source of morbidity and disproportionately impact the health of women and children. The number of randomized controlled trials testing interventions to prevent STIs has dramatically increased over time. To assess their impact, the authors conducted a systematic review of interventions to prevent sexual transmission or acquisition of STIs other than human immunodeficiency virus, published in the English-language, peer-reviewed literature through December 2009. Ninety-three papers reporting data from 74 randomized controlled trials evaluating 75 STI prevention interventions were identified. Eight intervention modalities were used: behavioral interventions (36% of interventions), vaginal microbicides (16%), vaccines (16%), treatment (11%), partner services (9%), physical barriers (5%), male circumcision (5%), and multicomponent (1%). Overall, 59% of interventions demonstrated efficacy in preventing infection with at least 1 STI. Treatment interventions and vaccines for viral STIs showed the most consistently positive effects. Male circumcision protected against viral STIs and possibly trichomoniasis. Almost two-thirds of behavioral interventions were effective, but the magnitude of effects ranged broadly. Partner services yielded similarly mixed results. In contrast, vaginal microbicides and physical barrier methods demonstrated few positive effects. Future STI prevention efforts should focus on enhancing adherence within interventions, integrating new technologies, ensuring sustainable behavior change, and conducting implementation research.
Keywords: anti-infective agents, circumcision, male, contraception, barrier, primary prevention, sexually transmitted diseases, vaccines
INTRODUCTION
The last 2 decades have marked a major “research transition,” with dramatic growth in emphasis on intervention research in many areas of health, including prevention of human immunodeficiency virus (HIV) and other sexually transmitted infections (STIs). As a result, while earlier STI intervention trials largely focused on treatment, this period saw a surge in well-designed trials of prevention interventions. From 1995 to 2008, funding for STI-related clinical trials provided by the US National Institutes of Health increased nearly 300% (C. Deal, STD Research Branch, National Institute of Allergy and Infectious Diseases, personal communication, 2009). There was an accompanying 7-fold increase in the number of published late-phase randomized controlled trials (RCTs) to prevent STIs and HIV infection undertaken annually between 1990 and 2004. In recognition of the need to standardize reporting of RCT results, the Consolidated Standards of Reporting Trials (CONSORT) statement was published in 1996 (1). The following year, the Food and Drug Administration Modernization Act of 1997 (2) directed the National Institutes of Health to create a publicly available clearinghouse for information on clinical trials to provide patients, clinicians, and scientists with information about new and ongoing clinical trials and to accelerate the dissemination of results to the public. This clearinghouse, www.ClinicalTrials.gov, was launched in 2000 and today provides freely accessible, standardized information regarding more than 80,000 trials in 170 countries around the world (3).
Clearly, the increased institutional and public support for intervention research has driven major advances in STI prevention, the need for which is echoed by a growing emphasis on the elimination of health disparities so inherent in the epidemiology of STIs (4). Despite these advances, the need for effective STI prevention interventions remains acute. However, RCTs, which are considered the “gold standard” to demonstrate intervention efficacy and estimate its magnitude, are increasingly costly undertakings in terms of time and money. This is particularly true for RCTs of preventive interventions because, in contrast to therapeutic interventions, even in high-risk populations, disease exposure cannot be guaranteed. Thus, generally large numbers of participants must be enrolled and followed for extended periods of time.
To optimize future STI prevention research efforts, we looked to the past for lessons learned. In this review, we sought to systematically summarize the results of all published STI prevention RCTs. We highlighted the range and diversity of prevention interventions that have been evaluated, qualitatively compared the effectiveness of these interventions both within and between broadly defined categories of intervention modalities, and identified intervention and trial design characteristics that were associated with significant intervention effects.
MATERIALS AND METHODS
Search methodology
We conducted a systematic review of all late-phase (IIb or III) RCTs of interventions to prevent sexual transmission or acquisition of STIs other than HIV, published in the English-language, peer-reviewed literature through December 2009. For studies published through 2003, we reevaluated and considered for inclusion the 42 RCTs evaluated by Manhart and Holmes (5). We performed a new search to identify studies published in the peer-reviewed literature between January 2004 and December 2009. To do so, we searched PubMed using the following terms: “randomized, randomised, or controlled trial” plus “STD,” “STI,” “Neisseria gonorrhoeae,” “gonorrhea,” “Chlamydia trachomatis,” “chlamydia,” “Trichomonas vaginalis,” “trichomonas,” “Treponema pallidum,” “syphilis,” “herpes simplex virus,” “genital HSV,” “human papillomavirus,” “HPV,” “Haemophilus ducreyi,” or “chancroid.”
One author (C. M. W.) screened the titles of all reports captured by the search and eliminated those that were clearly either unrelated topically (e.g., trials to reduce cardiovascular disease risks) or observational in nature (e.g., cross-sectional studies of STI risk factors). Abstracts of the remaining reports were independently evaluated by all 3 authors to determine relevance for the present review. Reports deemed irrelevant by unanimous agreement were excluded. Full texts of the remaining papers, including those evaluated previously by Manhart and Holmes (5), were assessed by all authors to determine final inclusion.
Inclusion and exclusion criteria
We included studies that evaluated the effectiveness of interventions to prevent the transmission or acquisition of prospectively evaluated, laboratory-confirmed STIs with a controlled design, using randomized assignment to the intervention or control condition. We excluded trials with HIV as the only endpoint and those designed to prevent STI-related pregnancy, puerperal and neonatal morbidity, or other STI-related complications. Thus, 3 trials included in the previous review (5) were excluded here (6–8). A fourth trial that was presented at a scientific conference but not subsequently published in the intervening 6 years was also excluded (9).
Data extraction
From all included papers, we extracted bibliographic information, contextual information (e.g., study years, geographic settings, target populations), descriptions of the intervention and control conditions, and the level at which randomization occurred (i.e., individuals, groups, or communities). (This information is described in Web Table 1 posted on the Journal’s website (http://aje.oxfordjournals.org/).) We categorized the interventions into the following 8 types: 1) behavioral interventions, 2) physical barrier methods, 3) vaginal microbicides, 4) male circumcision, 5) partner services, 6) treatment, 7) vaccines and passive immunization, and 8) multicomponent interventions. To facilitate comparisons between categories of intervention modalities, we tallied the number of each that demonstrated positive, adverse, and no effects on STI risk. We extracted the measure of effect of each intervention on the incidence or prevalence of laboratory-documented STIs, noting the sample size included in each analysis (rather than the total number enrolled, which was only extracted if the sample size analyzed was not provided in the paper and could not be enumerated) (Web Table 1). When multiple intervention effect estimates were presented, we presented adjusted rather than crude estimates. We also selected estimates based on intention-to-treat analyses rather than per-protocol analyses. When STI outcomes were assessed at multiple time points, we abstracted data reflecting the last available time point.
Many trials assessed infection with multiple STI pathogens at follow-up and frequently reported several STI-specific effect estimates in addition to a single overall estimate based on a composite STI outcome. We abstracted the overall measure of effect when these data were available and not different from the individual STI-specific estimates. Although clinically and laboratory-confirmed complications such as pelvic inflammatory disease, genital herpes disease, and human papillomavirus (HPV)-related external anogenital, vaginal, or cervical lesions are also important endpoints, the focus of this review was on interventions designed to prevent infection with STI pathogens, rather than STI-related complications. Therefore, we assessed only laboratory-confirmed infection.
Finally, we abstracted subgroup-specific effect estimates only when prespecified subgroup analyses identified statistically significant effects that overall analyses did not reveal. Using reported incidence or prevalence data and STATA, version 10.0, statistical software (StataCorp LP, College Station, Texas), we calculated point estimates and 95% confidence intervals when not calculated by the authors, and necessary data were presented in the text. We also converted reported vaccine efficacy estimates to incidence rate ratios using a standard formula to further facilitate comparison among studies (incidence rate ratio = 1 – [vaccine efficacy/100%]) (10). On the basis of the abstracted or calculated measures of effect, we rated each intervention as having a positive, adverse, or no effect on infection with STI pathogens. The last of these (no effect) we termed “flat” RCTs.
Quality assessment
To assess the quality of the trials, we extracted or calculated follow-up rates (defined as the proportion of enrolled subjects included in the analysis of effect) and adherence to the assigned intervention or control conditions. We also noted if STI risk behaviors in the intervention and control arms decreased during the study, compared with behaviors reported at baseline, assessing reports of unprotected intercourse, numbers of partners in a specified interval, and nonmonogamy. For this qualitative assessment, changes in STI risk did not need to be statistically significant. For studies that involved more than a single follow-up assessment of STI point prevalence, we noted whether the analysis took into account the potential for differential person-time or STI testing frequency. For example, we noted if the person-time contributed was incorporated into the analysis by calculating incidence rates or using survival analysis methods, whether correlated data methods such as generalized estimating equations were used when appropriate, and whether testing frequency was factored into the analysis to account for differences in opportunity to detect STI outcomes.
RESULTS
Ninety-three papers reporting data from 74 randomized controlled trials evaluating 75 interventions to prevent STIs were identified (11–103) (Figure 1). Behavioral interventions were the most common intervention modality, with 27 (36%) of the 75 interventions falling into this category (Table 1). Trials of vaginal microbicides and vaccines were the next most common, with 12 (16%) reports apiece. Eight (11%) trials evaluated treatment to prevent STI acquisition or transmission, while 7 (9%) evaluated partner services. The effects of physical barrier methods (i.e., diaphragms, female condoms, or choices of male condoms) and male circumcision in preventing STIs were evaluated in 4 (5%) RCTs each, and 1 published trial evaluated a multicomponent intervention. Overall, 44 of the 75 interventions (59%) significantly reduced risk of infection with at least 1 STI pathogen. Only 4 (5%) interventions were associated with increased risk of STIs (17, 53, 54, 103), 1 of which increased STI risk only among a subgroup of male study participants but not overall (17).
Figure 1.
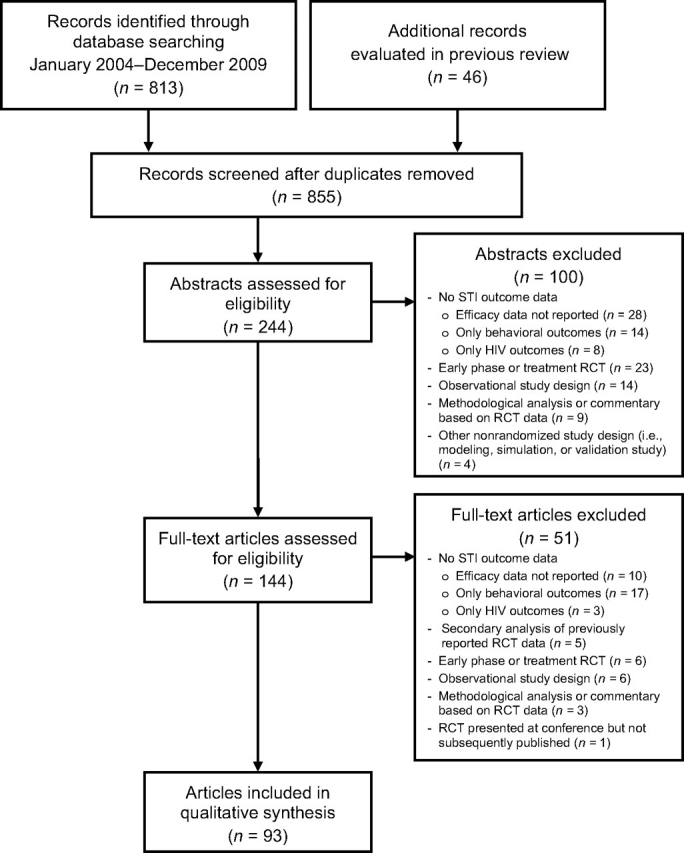
Selection of studies included in this systematic review. The 46 additional records evaluated in previous review were from the article by Manhart and Holmes (J Infect Dis. 2005;191(suppl 1):S7–S24 (5)). HIV, human immunodeficiency virus; RCT, randomized controlled trial; STI, sexually transmitted infection.
Table 1.
Results of 74 Randomized Controlled Trials Evaluating 75 Interventions to Prevent Sexually Transmitted Infections
| STI Prevention Effectivenessa | |||||||
| Type of Intervention | Positive Effect on STI Risk | Adverse Effect on STI Risk | No Effect on STI Risk | Total, no. | |||
| No. | % | No. | % | No. | % | ||
| Behavioral interventions | 17 | 63 | 1b | 6 | 9 | 33 | 27 |
| Physical barrier methods | 4 | 100 | 4 | ||||
| Vaginal microbicides | 3 | 25 | 2 | 17 | 7 | 58 | 12 |
| Male circumcision | 3 | 75 | 1 | 25 | 4 | ||
| Partner services | 4 | 57 | 3 | 43 | 7 | ||
| Treatment | 7 | 88 | 1 | 13 | 8 | ||
| Vaccines and passive immunization | 10 | 83 | 2 | 17 | 12 | ||
| Multicomponent interventions | 1 | 100 | 1 | ||||
| Total | 44 | 59 | 4b | 5 | 27 | 36 | 75 |
Abbreviation: STI, sexually transmitted infection.
Positive effect: intervention significantly reduced the risk of laboratory-confirmed STI in the intervention arm compared with the control arm; adverse effect: intervention significantly increased the risk of laboratory-confirmed STI in the intervention arm compared with the control arm; no effect: intervention showed no significant effect (positive or adverse) and, thus, the null hypothesis could not be rejected. Row percentages are displayed.
A statistically significant adverse effect for a behavioral intervention was reported among a subgroup of 1,783 men, but there was no effect overall (Sex Transm Dis. 2005;32(2):130–138 (17)). Results for this trial are tallied under adverse effect only.
Behavioral interventions
Two-thirds of the 27 published RCTs of behavioral interventions (11–30, 32–40) demonstrated significant effects, with 17 (63%) showing positive results and 1 showing increased risk for STIs in subgroup analyses (Table 1). All behavioral interventions included risk reduction counseling, and slightly more than half (56%) included a skills-building component (e.g., condom use, negotiation, or communication skills). Several trials implemented computerized risk reduction counseling; 3 incorporated condom provision, and 2 used videos. Only 1 trial tested motivational interviewing. Two additional trials were recently completed but publication is pending: the Project Eban trial, an intervention for HIV-serodiscordant African-American couples (104), and the Community Popular Opinion Leaders (C-POL) trial (105).
There was a wide range of effect estimates for the positive trials (9%–83% reduction in risk). The greatest effect was against a combined endpoint of gonorrhea and/or chlamydial infection (48%–83% reduction). Protection against herpes simplex virus type 2 (HSV-2) was somewhat lower (33%–35%), but only 2 studies reported on this; combination endpoints of several STIs had the broadest range of effect (9%–68% reduction). Only 1 behavioral intervention was associated with increased risk of STIs: Men in the Project RESPECT-2 trial who received risk reduction counseling with rapid HIV testing were more likely to acquire gonorrhea, chlamydia, or trichomoniasis than men who received risk reduction counseling accompanied by standard HIV testing (17).
Although only half of the behavioral interventions (52%) were delivered in small-group settings, they were more often successful than interventions delivered one-on-one (79% vs. 42%), and interventions that included skills building were more often effective than those that did not (73% vs. 50%). In most behavioral trials (67%), the control group received some type of risk reduction counseling, and those in which the comparison group received risk reduction counseling were less often effective than trials where the comparison group received only general health promotion (50% vs. 89%). Only 5 of the behavioral trials were conducted in settings outside the United States (21, 24, 30, 34, 35).
High follow-up and adherence rates were strongly correlated with efficacy in the behavioral RCTs. Of the 17 trials yielding positive results, 13 had follow-up rates of ≥75%, and 12 had adherence rates of similar magnitude. In contrast, of the 11 flat behavioral RCTs, follow-up and adherence rates of <75% were common (Table 2). Risk-taking behavior decreased in most trials and in both arms when control-arm risk behavior was reported. The notable exception was the Stepping Stones program, where risk behavior did not change in either arm. Despite this, the trial showed a significant decrease in HSV-2 incidence. The follow-up time varied (often 6–12 months), and none of the behavioral trials assessed sustainability of behavior change. The use of analyses accounting for differential person-time or testing frequency increased over time and was incorporated in the majority of studies (12 of 17 studies or 71%).
Table 2.
Characteristics of 74 Randomized Controlled Trials Evaluating 75 STI Prevention Interventions
| First Author, Year (Reference No.) | Follow-up Rate, %a | Adherence, %b | Decreased Risk-taking Behavior During the Studyc | Accounting by Analysis for Differential Person-Time or Testing Frequency | Effect on STI Riskd | ||
| Intervention | Control | Intervention | Control | ||||
| Behavioral Interventions | |||||||
| Boyer, 1997 (11) | 72 | 48 | NR | Yes | Yes | No | No effect |
| Branson, 1998 (12) | 72 | 47e | NR | Yes | Yes | No | No effect |
| NIMH Multisite HIV Prevention Trial Group, 1998 (13) | 70 | 63f | NR | Yes | Yes | NA | No effect |
| Kamb, 1998 (14) and Gottlieb, 2004 (15) | 81 | 85 | 85 | Yes | Yes | Yes | Positive |
| Metcalf, 2005 (16) | 69 | 68 | 73 | Transient | NR | NR | No effect |
| Metcalf, 2005 (17) | 87 | 99 | 69 | Transient | NR | Yes | Adverse |
| Shain, 1999 (18) and 2002 (19) | 89 | 75 | NR | Yes | Yes | No | Positive |
| Shain, 2004 (20) | >90 | 86g | NR | NR | NR | Yes | Positive |
| VCT Efficacy Study Group, 2000 (21) | 73 | NR | NR | Yes | Yes | NA | No effect |
| Hobfoll, 2002 (22) | 77 | NR | NR | Yes | Yes | NA | Positive |
| Baker, 2003 (23) | 73 | NR | NR | Yes | Yes | No | No effect |
| Kamali, 2003 (24) | >70 | NR | NR | Yes | Yes | Yes | Positive |
| DiClemente, 2004 (25) | 90 | 95 | 94 | Yes | Yes | Yes | Positive |
| Wingood, 2004 (26) | 93 | 95 | 98 | Yes | Yes | Yes | Positive |
| Downs, 2004 (27) | 86 | NR | NR | Yes | Yes | NA | No effect |
| Artz, 2005 (28) | 84 | NR | 100 | Yes | Yes | Yes | No effect |
| Boyer, 2005 (29) | 38 | 85 | 86 | NR | NR | Yes | Positive |
| Feldblum, 2005 (30) | 90 | NR | NR | Yes | Yes | NA | Positive |
| Jemmott, 2005 (32) | 82 | 100 | 100 | Yes | No | NA | Positive |
| Jemmott, 2007 (33) | 85 | 100 | 100 | Yes | Yes | Yes | Positive |
| Jewkes, 2008 (34) | 80 | No | No | Yes | Positive | ||
| Men | 61h | 68h | |||||
| Women | 59h | 64h | |||||
| Patterson, 2008 (35) | 82 | 100 | 100 | Yes | Yes | NA | Positive |
| Peipert, 2008 (36) | 93 | NR | 100 | NR | NR | Yes | Positive |
| Warner, 2008 (37) | NA | 76i | 100i | NR | NR | Yes | Positive |
| Crosby, 2009 (38) | 100 | 100 | 100 | Yes | Yes | No | Positive |
| Grimley, 2009 (39) | 66 | 100 | 100 | Yes | Yes | NA | Positive |
| Marion, 2009 (40) | 53 | 48 | 58 | Yes | Yes | Yes | Positive |
| Physical Barrier Methods | |||||||
| Fontanet, 1998 (41) | 92 | NR | NR | Yes | No effect | ||
| Female condoms | 12j | ||||||
| Male or female condoms | 97j | 98j | |||||
| Feldblum, 2001 (42) | 91 | Yes | Yes | NA | No effect | ||
| Female condoms | 7k | ||||||
| Male condoms | 22k | 24k | |||||
| Steiner, 2006 (43) | 86 | 56l | 54l | Yes | Yes | Yes | No effect |
| Ramjee, 2008 (44) and Sawaya, 2008 (45) | 98 | NR | NR | Yes | No effect | ||
| Diaphragm/gel | 51m | ||||||
| Male condoms | 77m | 87m | |||||
| Vaginal Microbicides | |||||||
| Cutler, 1977 (46) | 43 | NR | NR | NR | NR | Yes | No effect |
| Rendon, 1980 (47) | 56 | NR | NR | NR | NR | Yes | No effect |
| Rosenberg, 1987 (48) | NR | NR | NR | NR | NR | Yes | Positive |
| Louv, 1988 (49) | 78 | NR | NR | NR | NR | Yes | Positive |
| Niruthisard, 1992 (50) | 76 | 47n | 48n | NR | NR | Yes | No effect |
| Kreiss, 1992 (51) | 84 | 81o | 90o | Yes | Yes | Yes | Positive |
| Roddy, 1998 (52) | 91 | 87p | 84p | Yes | Yes | Yes | No effect |
| Richardson, 2001 (53) | 94 | 75o | 80o | NR | NR | Yes | Adverse |
| Roddy, 2002 (54) | 99 | 76p | NA | No | No | Yes | Adverse |
| Van Damme, 2002 (55) | 86 | 82p | 82p | NR | NR | Yes | No effect |
| Van Damme, 2008 (56) | 98 | 87p | 87p | NR | NR | Yes | No effect |
| Halpern, 2008 (57) | 70 | 76q | 80q | Yes | Yes | Yes | No effect |
| Male Circumcision | |||||||
| Mattson, 2008 (58) and Mehta, 2009 (59) | 95 | NA | NA | Yes | Yes | Yes | No effect |
| Sobngwi-Tambekou, 2009 (60) and Auvert, 2009 (61) | NR | NA | NA | NR | NR | NA | Positive |
| Tobian, 2009 (62) | 92 | NA | NA | No | No | Yes | Positive |
| Gray, 2009 (63) | 95 | NA | NA | Yes | Yes | NA | Positive |
| Partner Services | |||||||
| Lyng, 1981 (64) | 89 | NR | NR | NR | NR | NA | Positive |
| Schillinger, 2003 (65) | 81 | NR | NR | No | No effect | ||
| Patients with 1 partner | 82r | 75r | |||||
| Patients with >1 partner | 47r | 25r | |||||
| Golden, 2005 (66) | 68 | 61s | 49s | NR | NR | NA | Positive |
| Kissinger, 2005 (67) | 30 | 70t | 49t | NR | NR | NA | Positive |
| Kissinger, 2006 (68) | 81 | 82t | 88t | NR | NR | NA | No effect |
| Cameron, 2009 (69) | 65 | 32u | 34u | NR | NR | Yes | No effect |
| Wilson, 2009 (70) | 86 | NR | NR | Yes | Yes | NA | Positive |
| Treatment | |||||||
| Harrison, 1979 (71) | 75 | 100 | 100 | NR | NR | NA | Positive |
| Wawer, 1999 (72) | 77 | >90v | >90v | Yes | Yes | NA | Positive |
| Kaul, 2004 (73) | 89 | 92w | 92w | Yes | Yes | Yes | Positive |
| McClelland, 2008 (74) | 98 | 92x | 92x | NR | NR | Yes | No effect |
| Schwebke, 2007 (75) | 85 | NR | NR | NR | NR | Yes | Positive |
| Corey, 2004 (76) | 78 | 70y | 70y | NR | NR | Yes | Positive |
| Mayaud 1997 (78) | 71 | NR | NR | No | No | NA | Positive |
| Kamali, 2003 (24) | >70 | NR | NR | Yes | Yes | Yes | Positive |
| Vaccines and Passive Immunization | |||||||
| Szmuness, 1981 (80) | >85 | 95 | 92 | NR | NR | Yes | Positive |
| Francis, 1982 (81) | >84 | 87 | 84 | NR | NR | Yes | Positive |
| Coutinho, 1983 (82) | 96 | 97 | 98 | NR | NR | Yes | Positive |
| Piazza, 1997 (83) | 98 | 100 | 100 | NR | NR | Yes | Positive |
| Corey, 1999 (84) | NR | 76 | 78 | Yes | Yes | Yes | No effect |
| Stanberry, 2002 (85) | >81 | 91 | 91 | NR | NR | Yes | No effect |
| Koutsky, 2002 (86) | 81 | NR | NR | NR | NR | Yes | Positive |
| Harper, 2006 (88) | 85 | 93 | 93 | NR | NR | Yes | Positive |
| Paavonen, 2009 (91) | >90 | 92 | 92 | NR | NR | Yes | Positive |
| Villa, 2006 (93) | 98 | 92 | 95 | NR | NR | Yes | Positive |
| Munoz, 2009 (100) | >96 | 97 | 97 | NR | NR | Yes | Positive |
| Wheeler, 2009 (101) | 97 | 97 | 97 | NR | NR | Yes | Positive |
| Multicomponent Interventions | |||||||
| Ross, 2007 (103) | 73 | NR | NR | Yes | NR | NA | Adverse |
Abbreviations: HIV, human immunodeficiency virus; HPV, human papillomavirus; NA, not applicable; NIMH, National Institute of Mental Health; NR, not reported; PDPT, patient-delivered partner therapy; STI, sexually transmitted infection; VCT, Voluntary HIV-1 Counselling and Testing.
The “follow-up rate” refers to the proportion of enrolled subjects included in the analysis of effect, except for 2 studies that included all randomized subjects in the analysis of effect, irrespective of attendance at study visits (16, 17), and 1 study that evaluated gonorrhea and chlamydia among 98% of randomized subjects, while new-type HPV was evaluated among 75% of subjects from Zimbabwe (45).
For behavioral interventions, “adherence” refers to attendance at all intervention sessions, unless otherwise noted. For vaccine studies, “adherence” refers to receiving all required doses, unless otherwise noted.
Compared with behaviors reported at baseline. The behaviors assessed included reports of the following: not being abstinent, having unprotected intercourse (anal or vaginal), inconsistent condom use or no condom use at last sex, greater condom failure rates, greater number of partners in an interval, nonmonogamy, and douching after sex. Change not necessarily statistically significant.
Positive effect: intervention significantly reduced the risk of ≥1 laboratory-confirmed STIs in the intervention arm compared with the control arm; adverse effect: intervention significantly increased the risk of ≥1 laboratory-confirmed STIs in the intervention arm compared with the control arm; no effect: intervention showed no significant effect (positive or adverse) and, thus, the null hypothesis could not be rejected.
Attended 4 or 5 intervention sessions (of 5 total).
Attended 6 or 7 intervention sessions (of 7 total).
The proportion attending all 3 scheduled risk reduction counseling sessions; only 37% attended any of the 5 optional monthly support group sessions.
The proportion of men and women attending ≥75% of the 17 intervention sessions or completing the single control session.
The proportion in the intervention arm who reported viewing “most” or “all” of the intervention video and identifying at least of 1 of 5 target prevention messages; all individuals in the control arm experienced the “standard” waiting room environment.
The proportion of sex acts involving female condoms or involving male or female condoms.
The proportion of women reporting “consistent” use of female or male condoms.
The proportion of men reporting always using male condoms during the study.
The proportion who reported 100% diaphragm/gel use or using male condoms at least two-thirds of the time.
The proportion who were “ >75% compliant.”
The number of days that the assigned product was used divided by the total number of days that intercourse was reported.
The number of sex acts that the assigned product was used (with or without a condom) divided by the total number of sex acts reported.
The number of vaginal sex acts that the gel was used divided by the total number of vaginal sex acts reported at month 12.
The proportion of patients who reported that their partner was treated among those with only 1 partner, or who reported that all partners were treated among those with >1 partner.
The proportion with “all partners ‘very likely’ to have been treated.”
The proportion of patients successfully reinterviewed who reported giving PDPT to partner (intervention arm) or told partner to get treated (control arm).
The proportion of total partners tested and/or treated based on the number of PDPT slips returned (intervention arm) or verification in clinic and laboratory databases (control arm).
The proportion of enrolled residents who received treatment, calculated overall and not by study arm.
The proportion who received monthly directly observed therapy within 2 weeks of the scheduled follow-up visit; the adherence rate was calculated overall and not by study arm.
The proportion who received monthly directly observed therapy within 2 weeks of the scheduled follow-up visit.
The proportion who reported taking at least 95% of the prescribed doses, calculated overall and not by study arm.
Physical barrier methods
None of the 4 RCTs of physical barrier methods demonstrated reduced risk of STI acquisition, when compared with use of standard male condoms with or without risk reduction information and education (41–45). All of these trials were conducted in low- and middle-income countries, and the follow-up rates were good (75%–98%) (Table 2). However, reported adherence rates and metrics for assessing adherence varied widely across studies. Uptake of female condoms, in particular, was low; only 7% of Kenyan women reported “consistent” use (42), and Thai sex workers reported using them for just 12% of sex acts (41). Self-reported adherence to diaphragms and male condom interventions was higher: 51% of Zimbabwean women randomized to use diaphragms (45) and 56% of Jamaican men randomized to a choice of male condoms (43) reported always using these methods. Overall, participants in each arm of these 4 trials reported similar rates of any physical barrier method use; male condoms (provided in both arms of these trials) were the most frequently used form of physical barrier protection.
Vaginal microbicides
Results of microbicide trials to prevent STIs other than HIV have been mixed. Ten trials of nonoxynol-9 have been reported, testing various dosages and formulations (46–55). Each trial assessed the impact on gonococcal infection, with 3 demonstrating marked reductions in risk (74% and 60% among female sex workers in Thailand (48) and Kenya (51), respectively, and 25% among female sexually transmitted disease (STD) clinic attendees in the United States (49)). Despite these early successes, 2 subsequent trials of nonoxynol-9 gel demonstrated significant 50%–80% increases in risk of gonococcal infections (53, 54). Of the remaining 5 nonoxynol-9 trials, 1 demonstrated a nonsignificant 25% reduction in risk of gonorrhea and/or chlamydia while, among women using the vaginal insert for >75% of coital acts, risk was reduced by 40% (50). Two additional studies suggested small, nonsignificant increases in risk (52, 55), and 2 yielded flat results with respect to preventing gonococcal infection (46, 47). Seven trials evaluated the impact of nonoxynol-9 on C. trachomatis infection alone; only 1 trial among female STD clinic attendees in the United States demonstrated a statistically significant reduction in risk (21%) (49). The impact of nonoxynol-9 on risk of T. vaginalis or T. pallidum infection was assessed in only 1 study, with no effect demonstrated (53).
In addition to this equivocal evidence of efficacy, in several trials, women assigned to nonoxynol-9 experienced symptoms of genital irritation, lesions, or ulcers more frequently than did women in the control arm (50–52). Moreover, increases in risk of HIV acquisition were observed among nonoxynol-9 users in 2 RCTs (51, 55). Risk of STI acquisition and occurrence of adverse events were not correlated with nonoxynol-9 dosages and concentrations. Mode of nonoxynol-9 delivery also had little effect, although both trials of nonoxynol-9 sponges demonstrated significant protection against STIs.
More recently, 2 trials of cellulose sulfate gel were completed (56, 57), both yielding flat results. Despite a trend toward reduced risk of chlamydial infection in 1 study and reduced risk of gonorrhea and/or chlamydia in the other by 20%–30%, both studies were stopped early after a marginally significant increase in risk of HIV was noted in 1 trial (56). Two additional products, BufferGel (ReProtect, Inc., Baltimore, Maryland) and PRO 2000 Gel (Endo Pharmaceuticals, Inc., Newark, New Jersey), were recently evaluated in a 4-arm RCT (compared with placebo gel or normal behavior) among 3,000 women in the United States and Africa. Although results from this trial have not yet been published, investigators reported no impact on gonorrhea, chlamydia, trichomoniasis, syphilis, or HSV-2 (106). PRO 2000 Gel STI efficacy data from the Microbicides Development Programme 301 trial are pending (107).
Overall, significant protective effects of vaginal microbicides were more likely to be detected when male condoms were not provided to women in the control arm. Significant protective effects were observed in only 1 of the 8 trials in which male condoms were provided to all study participants (51), compared with 2 of the 4 trials that did not provide male condoms in the comparison arm (46–49).
Follow-up rates in the microbicide trials that detected significant effects were generally better than those in the flat trials (Table 2). Both trials detecting significant increases in the risk of gonorrhea achieved extremely high follow-up rates (94%–99%) (53, 54); follow-up ranged between 78% and 84% in trials that demonstrated significant reductions in STI risk (49, 51), while flat trials reported 43%–98% follow-up (median, 76%). Adherence rates (typically reported as the proportion of sex acts or sexually active days during which a woman used the product) ranged between 47% and 87% for the 8 trials that reported this information (50–57) but were not reported for 2 of the 3 trials that demonstrated statistically significant reductions in STI risk. Only 2 of the published vaginal microbicide trials were conducted among women in the United States; more than half of the international trials targeted female sex workers.
Male circumcision
Four trials have evaluated male circumcision as an STI prevention intervention; 3 evaluated STI endpoints among male subjects randomized to either immediate or delayed circumcision (58–62). The fourth assessed the impact of STI risk among wives of men randomized to the procedure (63). Male circumcision appears to provide significant protection against viral STIs. In addition to the 50%–60% reduction in HIV acquisition consistently demonstrated (108–110), HPV prevalence was reduced by about one-third (61, 62), and HSV-2 incidence was reduced by 28% (62) in the trials that assessed these endpoints.
The impact of male circumcision on curable STIs was mixed. Circumcision reduced T. vaginalis acquisition by almost half among wives of men who were circumcised in Uganda (63) and among circumcised men, themselves, in South Africa (60), although the latter was of borderline significance. However, prevention of T. vaginalis infection was not demonstrated among circumcised men in the Kenyan RCT (58, 59). Mixed results were also observed for chlamydial infection. Although a borderline significant reduction in chlamydia of >40% was observed among circumcised men in South Africa (60), the procedure did not protect men in the Kenyan RCT (58, 59). Neither trial that assessed gonorrhea observed a protective effect of circumcision (58–60), nor did the 1 trial that reported syphilis results (62), which had limited power to evaluate this endpoint (111).
The male circumcision trials were all conducted in sub-Saharan Africa and were powered primarily to evaluate the efficacy of the procedure in preventing HIV. Follow-up rates were over 90% in the 3 trials that reported this information, and adherence was not an issue for this surgical intervention (Table 2).
Partner services
Efforts to reduce risk of STI reinfection by facilitating partner treatment or referral have been successful in industrialized countries, where all 7 trials of partner services were conducted. Six RCTs evaluated patient-delivered partner therapy (PDPT) (64–69), with half demonstrating statistically significant protective effects against reinfection of the index patient. Two of these trials demonstrated 25%–62% reductions in gonorrhea and/or chlamydia reinfection (66, 67). Although follow-up rates in these successful trials were <70% (Table 2), raising the possibility of bias due to differential losses to follow-up, this strategy clearly resulted in marked decreases in risk of STI reinfection among persons committed to the process. The 79% reduction in risk of trichomoniasis reinfection among Danish women is also noteworthy (64) because standard partner notification is typically not done in US STD clinics when T. vaginalis is detected. A more recent PDPT trial to prevent reinfection with T. vaginalis among women attending US STD clinics did not demonstrate a significant benefit, even though reported follow-up rates and intervention adherence were high (81% and 82%, respectively) (68).
One PDPT trial to prevent chlamydia reinfection among young women in the United States yielded a marginally significant reduction in risk (20%) (65), whereas another among young women in the United Kingdom did not (69). These disparate findings might be explained, in part, by differences in study populations and/or follow-up or adherence rates, which were somewhat lower in the United Kingdom study (65% and 32%, respectively) (69). The extent to which the impact of PDPT is reproducible in low- or middle-income countries has not yet been evaluated.
Finally, Wilson et al. (70) evaluated a program to enhance the partner notification skills of the index patient. This intervention resulted in a 53% decrease in risk of gonorrhea and/or chlamydia reinfection among index patients, consistent with results of the successful PDPT trials.
Adherence metrics and rates ranged widely for these trials. However, overall, adherence in the intervention arms was relatively lower than in other types of interventions but similar to adherence to standard patient-based, partner-referral methods. Notably, women comprised the index patient group in the 3 partner services RCTs that yielded flat results. In contrast, only 1 of the successful trials enrolled women exclusively, 1 enrolled men, and 2 enrolled both men and women, suggesting that women with STIs detected may have more difficulty convincing their male sex partners to take treatment.
Treatment
STI treatment is highly effective in the prevention of secondary infection. All but 1 (74) of the 8 published RCTs of a variety of STI treatment strategies to prevent STI transmission or acquisition demonstrated statistically significant reductions in prevalence or incidence (24, 71–78). Half of the trials evaluated periodic presumptive treatment (72–75), 2 examined enhanced syndromic management (24, 77, 78), and 1 tested postexposure prophylaxis (71) to prevent curable STIs. One additional trial investigated the efficacy of suppressive therapy to prevent HSV-2 transmission in discordant couples (76).
These treatment approaches resulted in 30%–60% reductions in risk of curable STIs and 50% reduction in herpes risk. Gonorrhea incidence fell >50% with periodic presumptive treatment, syndromic management, and postexposure prophylaxis in 3 of the 4 trials that evaluated this outcome (24, 71, 73). Chlamydia incidence declined >60% in 1 trial of periodic presumptive treatment among female sex workers (73) but was not reduced significantly in another RCT using this strategy (72) or with syndromic management (24). Periodic presumptive treatment consistently lowered the incidence of trichomoniasis by 40%–45% in all 3 of the trials reporting on this endpoint (72–74), but this was not statistically significant in one RCT (74), possibly because of monthly screening and treatment for gonorrhea, cervicitis, and trichomoniasis among women in both study arms. Indeed, this was the only trial in this category that did not demonstrate efficacy in preventing any STI. Treatment also reduced risk of genital ulcer disease. Active syphilis declined 20%–40% in both of the syndromic management trials (24, 77, 78) and in 1 of the 2 periodic presumptive treatment trials (72) that evaluated this outcome. The other periodic presumptive treatment trial (73) used a regimen that would not be expected to be effective treatment for syphilis (1 g of azithromycin). Suppressive therapy for herpes halved HSV-2 transmission in discordant couples (76). Finally, in the only RCT conducted in the United States, among women with asymptomatic bacterial vaginosis, metronidazole followed by periodic presumptive treatment resulted in a 30% reduction in incidence of gonorrhea, chlamydia, trichomoniasis, pelvic inflammatory disease, and/or HSV-2 (75).
Each of these trials achieved high follow-up rates (>70%), and adherence was >80% in 4 of the 5 RCTs reporting these rates (Table 2). Risk behaviors decreased in the control groups of 3 of the 4 trials that provided these data.
Vaccines and passive immunization
As for other infectious diseases, vaccines for STIs are one of the most effective preventive interventions available. To date, active and passive immunization approaches for 4 non-HIV STIs have been evaluated in late-phase trials. All of the published RCTs of vaccines to prevent hepatitis B and HPV have demonstrated strong, significant, and sustained protective effects (>80% reduction in new infections) (79–82, 86–93, 100, 101). Both the bivalent and quadrivalent HPV vaccines also appear to confer significant cross-protection against infection with selected nonvaccine, oncogenic HPV types (91, 101). Bimonthly administration of polyvalent hepatitis C immune serum globulin provided significant protection in discordant couples (83). In contrast, neither of the 2 candidate HSV-2 vaccines evaluated to date conferred significant protection against infection among high-risk adults, including partners of HSV-2-positive individuals (84) or among HSV-2-negative adults with or without history of HSV-1 (85). However, the glycoprotein D subunit HSV-2 vaccine significantly reduced development of genital herpes disease almost 75% among subgroups of women who were seronegative for HSV-1 and HSV-2 at enrollment and seroconverted (85). A similar effect was not observed among HSV-1 and HSV-2 naïve men. The HPV vaccines have also demonstrated significant protection against disease endpoints including >95% reduction in external anogenital lesions (e.g., condyloma) (94, 96, 98), cervical intraepithelial neoplasia (88, 91, 94–96, 98, 101, 102), cervical adenocarcinomas in situ (96, 98, 101, 102), vulvar intraepithelial neoplasia (97, 98), and vaginal intraepithelial neoplasia (97, 98). These 12 RCTs, the majority of which were conducted in the United States and other high-income countries, consistently achieved among the highest follow-up (>80%) and adherence (>75%) rates of all of the STI RCTs published to date (Table 2). Although all reported analysis methods that accounted for differential person-time or testing frequency, only 1 trial (84) provided information on changes in risk behaviors (risk behaviors were reduced in the control and intervention arms).
Multicomponent interventions
Multicomponent STI prevention trials evaluate the joint effects of several prevention interventions. To date, only 1 such trial including STI outcomes has been published, although 2 other trials have recently been completed. In Tanzania, MEMA kwa Vijana (“Good Things for Young People,” an adolescent sexual and reproductive health program working in schools, health facilities, and communities in the Mwanza Region) compared a 4-component intervention (provision of peer risk reduction counseling, youth-friendly clinical STI services, family planning, and STI case management) with a 2-component intervention (provision of family planning and STI case management) (103). There were no reductions in STIs and, in fact, an increase in gonorrhea risk for adolescent girls, concentrated among those who received only 1 year of the intervention. The number of adolescent boys testing positive for gonorrhea was too small for reliable estimates, and adjusted data were not presented. Results from the Regai Dzive Shiri Project in Zimbabwe and those from the PREVEN trial in Peru are pending (112, 113).
DISCUSSION
In the past 30 years, data evaluating 75 STI prevention interventions have been published in the peer-reviewed literature, with >75% emerging in the last decade. In the 6 years since the last review (5), 33 new trials have been published, and the results of several more are currently under review. Almost 60% of all STI prevention RCTs demonstrated a positive effect, and very few reported increased STI risk. Overall, analytical methods were strong and the quality of the trials was high, with few exceptions.
Treatment interventions for all STIs and vaccines for viral STIs showed the most promising, consistently positive effects of the greatest magnitude. Male circumcision protected against viral STIs, although the magnitude of the effect was more limited than that demonstrated with STI treatment or vaccines. Circumcision may also reduce risk of trichomoniasis among men and their female partners and possibly risk of chlamydia among men; however, these data were less consistent. Behavioral interventions were effective in almost two-thirds of RCTs evaluating these approaches, but the magnitude of these effects ranged broadly, and little is known about their long-term impact (e.g., sustainability of behavior change), especially outside of the rarified and relatively supportive environment of RCTs. Partner services RCTs yielded roughly similar results in terms of the proportion of positive trials and the range and magnitude of effects. In contrast, existing RCTs of vaginal microbicides and physical barrier methods demonstrated few or no significant positive effects with respect to preventing STIs. Three issues were consistently important in the outcome of these STI prevention trials: biology of the pathogen, intervention adherence, and provision of STI prevention services to the control group.
The biology of the target pathogen(s), specifically, the pathogenesis, infectivity, and duration of natural infection, clearly influenced the effects of biomedical and behavioral interventions. For example, male circumcision was consistently associated with decreased risk for HSV-2 and HPV but not for gonorrhea or chlamydia, which colonize the male urethra rather than the foreskin and make less use of dendritic cells in their pathogenesis than do HIV and other viral STIs. C. trachomatis vaccines have not been developed, partly because of the challenge of eliciting a robust immune response to these intracellular bacteria. In contrast, trials of behavioral interventions, partner services, and treatment have shown promising results with respect to reducing risk of bacterial STIs by decreasing infectivity, duration of infection, and/or rates of partner change.
Intervention adherence is also clearly important. Male circumcision and vaccines, which require minimal adherence, were uniformly effective against viral STIs. In contrast, partner services, which require patients to provide their partners with medication (e.g., PDPT), demonstrated mixed results. STD clinic patients with newly detected STIs typically have difficulty contacting their partners, which probably contributed to the low adherence rates in these trials. Use of female condoms showed no effect at all, likely because they were rarely used. Adherence was frequently not reported in behavioral trials, and lower adherence to multisession behavioral interventions may explain some of the mixed results in these trials. In treatment studies, participants were often highly motivated to adhere to the provided therapies because of symptoms or perceived risk of STI. Although much work has been done in the field of adherence research for antiretroviral therapies for HIV infection, much remains to be learned about how to encourage adherence to other preventive interventions.
The ethical imperative to provide control groups with prevention services that, in practice, may exceed the local standard of care is a well-recognized challenge to RCTs of STI prevention interventions. An intervention must be extremely powerful to demonstrate benefit above and beyond control activities that also reduce risk for STIs. For example, behavioral trials using a health promotion control condition were more often effective than trials in which the control group received risk reduction counseling and/or condom promotion. Similarly, provision of male condoms to all study participants in trials of physical barrier methods may have impeded the detection of significant protective effects of female condoms or diaphragms. Despite these constraints, the proportion of STI prevention intervention trials delivering flat results (36%) was strikingly lower than the 85% of “flat” HIV prevention interventions evaluated in a recent review (114). Indeed, this differential may be due, in part, to the greater tendency to enhance prevention in control groups participating in HIV prevention RCTs compared with STI prevention RCTs.
Other factors may have contributed to the difference in the proportion of flat HIV and STI prevention RCTs, and recognition of these factors may improve future STI and HIV prevention research efforts. STI intervention trials are often better powered than HIV intervention trials, because the incidence of most STIs generally exceeds that of HIV although power was rarely reported; thus, we did not assess it here. Additionally, STI trials frequently evaluated infection with multiple pathogens, providing more opportunities for detection of study endpoints. Finally, HIV prevention trials often require many years of planning, and the local incidence can change dramatically, affecting power, whereas STI incidence is typically subject to less severe fluctuations over time.
The larger proportion of effective STI prevention interventions (59%) relative to HIV prevention interventions (13%) (114) may be explained in part by the populations targeted. Many of the effective STI behavioral intervention RCTs were conducted among high-risk subpopulations (e.g., STD clinic attendees and minority populations in the United States), primarily assessed bacterial STI transmission that is typically concentrated in a discrete subpopulation, and accordingly used targeted intervention messages for these distinct segments of the population. In contrast, HIV behavioral intervention trials were often conducted in settings where the HIV epidemic was more generalized, and broader messages and interventions, which may be more diluted, were tested.
Some important similarities to the HIV prevention trials are also worth noting. The likelihood of flat results in trials of STI prevention interventions differed by intervention modality, which was also observed among HIV intervention trials. As noted, the percentage of flat STI prevention RCTs was greater for intervention modalities for which adherence was important or provision of a diluted or modified form of the intervention to the comparison group occurred (i.e., behavioral interventions, partner services, microbicides, and physical barrier methods), ranging from 33% to 100% of the trials in these categories. In contrast, only 13%–25% of RCTs of STI treatment, vaccines, and male circumcision yielded flat results. The control groups in these RCTs did not receive diluted forms of the interventions and, with the exception of 1 trial of daily suppressive therapy (76), none of these trials depended heavily on adherence to ensure adequate intervention “dosing.” Finally, fully half of STI intervention trials were conducted in high-income countries, whereas this was true of only 5% of HIV intervention trials. Existing infrastructure and lower poverty levels in high-income countries may facilitate intervention effectiveness, and there may be less enhancement of risk reduction services in comparison groups in these settings.
We have summarized findings from all late-phase RCTs of STI prevention interventions in the published literature. A review of this scope was challenging, in part because of the widely varying trial designs, evaluation and analysis methods, and presentations of study findings in the studies we assessed. Therefore, quantitative summary estimates were not appropriate (115). These results are limited by publication bias; negative and flat trials are much less likely to be published than positive trials. However, the recent large financial investments in many STI prevention trials may have increased the likelihood of publication of null results. Additionally, information on participation, follow-up, and adherence rates, which is essential to evaluate data quality and generalizability, was not always clearly reported. Confidence intervals, which allow readers to better gauge the stability of estimates, were also not uniformly presented. Most reports did not include projected incidences of study outcomes, which would help to determine if a flat result were due to a true lack of effect or simply to inadequate study power. Finally, clear descriptions of the study setting and control and intervention conditions, which could facilitate comparisons across studies of similar intervention types and might help to explain discrepant results, were sometimes lacking.
Despite these limitations, tremendous progress has been made in STI prevention. Biomedical interventions including treatment, vaccines, and male circumcision are highly effective against selected STIs. Behavioral interventions have also shown promise, although the duration of effect is still largely unknown. The extent to which findings from RCTs of individual STI prevention approaches are generalizable to nonstudy settings and the feasibility of population-level scale-up of these individual interventions are also unclear. Implementation research should continue to assess these carefully.
It has long been recognized that there will be few “magic bullets” for STI prevention, and successful efforts will likely require sustained, multipronged approaches. The impact of multicomponent interventions has been assessed in only 3 RCTs to date (only 1 in the published literature), and significant protective effects have not yet been shown. Despite the disappointing results of the initial multicomponent trials, these represent only the first generation of such interventions. Several multicomponent HIV prevention interventions are currently being investigated under the National Institutes of Health Methods for Prevention Packages Program (MP3) initiative, and similar efforts for STI prevention are needed.
The majority of RCTs of non-HIV STI prevention interventions conducted to date have demonstrated efficacy, providing strong support for a range of intervention modalities. The next generation of STI prevention RCTs should build on this foundation by harnessing advances in areas ranging from genomics, proteomics, and immunology to social network analysis and information technology to evaluate innovative biomedical and behavioral approaches. Our review suggests that focused efforts to improve adherence within RCTs and prevention programs should be a high priority. This should include development of improved definitions, metrics, and analysis methods, as well as approaches to augment adherence, itself. Second, a reassessment of what is ethically and scientifically appropriate to provide to control groups in late-phase STI prevention RCTs is also needed, especially if enhanced prevention services are not sustainable and may compromise the ability to detect modest, but real, intervention effects. Third, although many behavioral interventions have been effective in the short term, the data are limited in terms of long-term effectiveness in real-world settings. Longer-term behavioral intervention trials will be needed to determine the extent to which behavior change is sustained and to identify reasons for failure to implement and/or maintain safe behaviors. Fourth, building on past successes, future efforts should focus on implementation research to identify the most efficient way to scale up successful interventions. Finally, more focused research and development of new strategies and assessment metrics that can be used to evaluate packages of partially successful approaches, woven together in synergistic ways, will prove invaluable.
Supplementary Material
Acknowledgments
Author affiliations: Center for AIDS and STD, University of Washington, Seattle, Washington (Catherine M. Wetmore, Lisa E. Manhart); Department of Global Health, University of Washington, Seattle, Washington (Catherine M. Wetmore, Judith N. Wasserheit); and Department of Medicine, University of Washington, Seattle, Washington (Judith N. Wasserheit).
The work was supported by the National Institute of Allergy and Infectious Diseases, through the University of Washington Center for AIDS Research (grant P30 AI27757) and through additional support (grant R01 AI072728). Dr. Wetmore was funded by the University of Washington STD/AIDS Research Training Fellowship grant from the National Institute of Allergy and Infectious Diseases (grant T32 AI07140).
The authors thank Aleta Elliott for administrative support in gathering manuscripts for this review.
Portions of this work were presented at the 18th Meeting of the International Society for STD Research in conjunction with the British Association for Sexual Health and HIV, London, United Kingdom, June 30, 2009.
Conflict of interest: none declared.
Glossary
Abbreviations
- HIV
human immunodeficiency virus
- HPV
human papillomavirus
- HSV-1
herpes simplex virus type 1 (HSV-2 defined similarly)
- PDPT
patient-delivered partner therapy
- RCT
randomized controlled trial
- STD
sexually transmitted disease
- STI
sexually transmitted infection
References
- 1.Begg C, Cho M, Eastwood S, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA. 1996;276(8):637–639. doi: 10.1001/jama.276.8.637. [DOI] [PubMed] [Google Scholar]
- 2.US Food and Drug Administration. Food and Drug Administration Modernization Act of 1997. Public Law 105–115, 105th Congress, section 113. DOC ID f:publ115.105. Rockville, MD: Food and Drug Administration; 1997. ( http://www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/FDAMA/FullTextofFDAMAlaw/default.htm) [Google Scholar]
- 3.US National Institutes of Health. ClinicalTrials.gov. Bethesda, MD: National Library of Medicine; 2000 ( www.ClinicalTrials.gov). (Accessed November 21, 2009) [Google Scholar]
- 4.Hallfors DD, Iritani BJ, Miller WC, et al. Sexual and drug behavior patterns and HIV and STD racial disparities: the need for new directions. Am J Public Health. 2007;97(1):125–132. doi: 10.2105/AJPH.2005.075747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manhart LE, Holmes KK. Randomized controlled trials of individual-level, population-level, and multilevel interventions for preventing sexually transmitted infections: what has worked? J Infect Dis. 2005;191(suppl 1):S7–S24. doi: 10.1086/425275. [DOI] [PubMed] [Google Scholar]
- 6.Gray RH, Wabwire-Mangen F, Kigozi G, et al. Randomized trial of presumptive sexually transmitted disease therapy during pregnancy in Rakai, Uganda. Am J Obstet Gynecol. 2001;185(5):1209–1217. doi: 10.1067/mob.2001.118158. [DOI] [PubMed] [Google Scholar]
- 7.Koblin B, Chesney M, Coates T. Effects of a behavioural intervention to reduce acquisition of HIV infection among men who have sex with men: the EXPLORE randomised controlled study. EXPLORE Study Team. Lancet. 2004;364(9428):41–50. doi: 10.1016/S0140-6736(04)16588-4. [DOI] [PubMed] [Google Scholar]
- 8.Scholes D, Stergachis A, Heidrich FE, et al. Prevention of pelvic inflammatory disease by screening for cervical chlamydial infection. N Engl J Med. 1996;334(21):1362–1366. doi: 10.1056/NEJM199605233342103. [DOI] [PubMed] [Google Scholar]
- 9.Labbe AC, Dzokoto A, Khonde N. A randomised placebo-controlled trial of routine monthly antibiotics against gonococcal and chlamydial infections among female sex workers in Ghana and Benin: intention-to-treat analysis. Presented at the 15th Biennial Congress of the International Society for Sexually Transmitted Diseases Research (ISSTDR), Ottawa, Canada, July 27, 2003–July 30, 2007. [Google Scholar]
- 10.Koepsell T, Weiss N. Epidemiologic Methods: Studying the Occurrence of Illness. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 11.Boyer CB, Barrett DC, Peterman TA, et al. Sexually transmitted disease (STD) and HIV risk in heterosexual adults attending a public STD clinic: evaluation of a randomized controlled behavioral risk-reduction intervention trial. AIDS. 1997;11(3):359–367. doi: 10.1097/00002030-199703110-00014. [DOI] [PubMed] [Google Scholar]
- 12.Branson BM, Peterman TA, Cannon RO, et al. Group counseling to prevent sexually transmitted disease and HIV: a randomized controlled trial. Sex Transm Dis. 1998;25(10):553–560. doi: 10.1097/00007435-199811000-00011. [DOI] [PubMed] [Google Scholar]
- 13.The NIMH. Multisite HIV Prevention Trial: reducing HIV sexual risk behavior. The National Institute of Mental Health (NIMH) Multisite HIV Prevention Trial Group. Science. 1998;280(5371):1889–1894. doi: 10.1126/science.280.5371.1889. [DOI] [PubMed] [Google Scholar]
- 14.Kamb ML, Fishbein M, Douglas JM, Jr, et al. Efficacy of risk-reduction counseling to prevent human immunodeficiency virus and sexually transmitted diseases: a randomized controlled trial. Project RESPECT Study Group. JAMA. 1998;280(13):1161–1167. doi: 10.1001/jama.280.13.1161. [DOI] [PubMed] [Google Scholar]
- 15.Gottlieb SL, Douglas JM, Jr, Foster M, et al. Incidence of herpes simplex virus type 2 infection in 5 sexually transmitted disease (STD) clinics and the effect of HIV/STD risk-reduction counseling. J Infect Dis. 2004;190(6):1059–1067. doi: 10.1086/423323. [DOI] [PubMed] [Google Scholar]
- 16.Metcalf CA, Malotte CK, Douglas JM, Jr, et al. Efficacy of a booster counseling session 6 months after HIV testing and counseling: a randomized, controlled trial (RESPECT-2) Sex Transm Dis. 2005;32(2):123–129. doi: 10.1097/01.olq.0000151420.92624.c0. [DOI] [PubMed] [Google Scholar]
- 17.Metcalf CA, Douglas JM, Jr, Malotte CK, et al. Relative efficacy of prevention counseling with rapid and standard HIV testing: a randomized, controlled trial (RESPECT-2) Sex Transm Dis. 2005;32(2):130–138. doi: 10.1097/01.olq.0000151421.97004.c0. [DOI] [PubMed] [Google Scholar]
- 18.Shain RN, Piper JM, Newton ER, et al. A randomized, controlled trial of a behavioral intervention to prevent sexually transmitted disease among minority women. N Engl J Med. 1999;340(2):93–100. doi: 10.1056/NEJM199901143400203. [DOI] [PubMed] [Google Scholar]
- 19.Shain RN, Perdue ST, Piper JM, et al. Behaviors changed by intervention are associated with reduced STD recurrence: the importance of context in measurement. Sex Transm Dis. 2002;29(9):520–529. doi: 10.1097/00007435-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Shain RN, Piper JM, Holden AE, et al. Prevention of gonorrhea and Chlamydia through behavioral intervention: results of a two-year controlled randomized trial in minority women. Sex Transm Dis. 2004;31(7):401–408. doi: 10.1097/01.olq.0000135301.97350.84. [DOI] [PubMed] [Google Scholar]
- 21.Efficacy of voluntary HIV-1 counselling and testing in individuals and couples in Kenya, Tanzania, and Trinidad: a randomised trial. The Voluntary HIV-1 Counseling and Testing Efficacy Study Group. Lancet. 2000;356(9224):103–112. [PubMed] [Google Scholar]
- 22.Hobfoll SE, Jackson AP, Lavin J, et al. Effects and generalizability of communally oriented HIV-AIDS prevention versus general health promotion groups for single, inner-city women in urban clinics. J Consult Clin Psychol. 2002;70(4):950–960. doi: 10.1037//0022-006x.70.4.950. [DOI] [PubMed] [Google Scholar]
- 23.Baker SA, Beadnell B, Stoner S, et al. Skills training versus health education to prevent STDs/HIV in heterosexual women: a randomized controlled trial utilizing biological outcomes. AIDS Educ Prev. 2003;15(1):1–14. doi: 10.1521/aeap.15.1.1.23845. [DOI] [PubMed] [Google Scholar]
- 24.Kamali A, Quigley M, Nakiyingi J, et al. Syndromic management of sexually-transmitted infections and behaviour change interventions on transmission of HIV-1 in rural Uganda: a community randomised trial. Lancet. 2003;361(9358):645–652. doi: 10.1016/s0140-6736(03)12598-6. [DOI] [PubMed] [Google Scholar]
- 25.DiClemente RJ, Wingood GM, Harrington KF, et al. Efficacy of an HIV prevention intervention for African American adolescent girls: a randomized controlled trial. JAMA. 2004;292(2):171–179. doi: 10.1001/jama.292.2.171. [DOI] [PubMed] [Google Scholar]
- 26.Wingood GM, DiClemente RJ, Mikhail I, et al. A randomized controlled trial to reduce HIV transmission risk behaviors and sexually transmitted diseases among women living with HIV: the WiLLOW Program. J Acquir Immune Defic Syndr. 2004;37(suppl 2):S58–S67. doi: 10.1097/01.qai.0000140603.57478.a9. [DOI] [PubMed] [Google Scholar]
- 27.Downs JS, Murray PJ, Bruine de Bruin W, et al. Interactive video behavioral intervention to reduce adolescent females’ STD risk: a randomized controlled trial. Soc Sci Med. 2004;59(8):1561–1572. doi: 10.1016/j.socscimed.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 28.Artz L, Macaluso M, Meinzen-Derr J, et al. A randomized trial of clinician-delivered interventions promoting barrier contraception for sexually transmitted disease prevention. Sex Transm Dis. 2005;32(11):672–679. doi: 10.1097/01.olq.0000175404.18098.dd. [DOI] [PubMed] [Google Scholar]
- 29.Boyer CB, Shafer MA, Shaffer RA, et al. Evaluation of a cognitive-behavioral, group, randomized controlled intervention trial to prevent sexually transmitted infections and unintended pregnancies in young women. Prev Med. 2005;40(4):420–431. doi: 10.1016/j.ypmed.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Feldblum PJ, Hatzell T, Van Damme K, et al. Results of a randomised trial of male condom promotion among Madagascar sex workers. Sex Transm Infect. 2005;81(2):166–173. doi: 10.1136/sti.2004.010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoke TH, Feldblum PJ, Damme KV, et al. Randomised controlled trial of alternative male and female condom promotion strategies targeting sex workers in Madagascar. Sex Transm Infect. 2007;83(6):448–453. doi: 10.1136/sti.2006.024612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jemmott JB, III, Jemmott LS, Braverman PK, et al. HIV/STD risk reduction interventions for African American and Latino adolescent girls at an adolescent medicine clinic: a randomized controlled trial. Arch Pediatr Adolesc Med. 2005;159(5):440–449. doi: 10.1001/archpedi.159.5.440. [DOI] [PubMed] [Google Scholar]
- 33.Jemmott LS, Jemmott JB, III, O'Leary A. Effects on sexual risk behavior and STD rate of brief HIV/STD prevention interventions for African American women in primary care settings. Am J Public Health. 2007;97(6):1034–1040. doi: 10.2105/AJPH.2003.020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jewkes R, Nduna M, Levin J, et al. Impact of Stepping Stones on incidence of HIV and HSV-2 and sexual behaviour in rural South Africa: cluster randomised controlled trial. BMJ. 2008;337:391–395. doi: 10.1136/bmj.a506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patterson TL, Mausbach B, Lozada R, et al. Efficacy of a brief behavioral intervention to promote condom use among female sex workers in Tijuana and Ciudad Juarez, Mexico. Am J Public Health. 2008;98(11):2051–2057. doi: 10.2105/AJPH.2007.130096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peipert JF, Redding CA, Blume JD, et al. Tailored intervention to increase dual-contraceptive method use: a randomized trial to reduce unintended pregnancies and sexually transmitted infections. Am J Obstet Gynecol. 2008;198(6):630.e1–630.e8. doi: 10.1016/j.ajog.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warner L, Klausner JD, Rietmeijer CA, et al. Effect of a brief video intervention on incident infection among patients attending sexually transmitted disease clinics. PLoS Med. 2008;5(6) doi: 10.1371/journal.pmed.0050135. e135. (doi:10.1371/journal.pmed.0050135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crosby R, DiClemente RJ, Charnigo R, et al. A brief, clinic-based, safer sex intervention for heterosexual African American men newly diagnosed with an STD: a randomized controlled trial. Am J Public Health. 2009;99(suppl 1):S96–S103. doi: 10.2105/AJPH.2007.123893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grimley DM, Hook EW., III A 15-minute interactive, computerized condom use intervention with biological endpoints. Sex Transm Dis. 2009;36(2):73–78. doi: 10.1097/OLQ.0b013e31818eea81. [DOI] [PubMed] [Google Scholar]
- 40.Marion LN, Finnegan L, Campbell RT, et al. The Well Woman Program: a community-based randomized trial to prevent sexually transmitted infections in low-income African American women. Res Nurs Health. 2009;32(3):274–285. doi: 10.1002/nur.20326. [DOI] [PubMed] [Google Scholar]
- 41.Fontanet AL, Saba J, Chandelying V, et al. Protection against sexually transmitted diseases by granting sex workers in Thailand the choice of using the male or female condom: results from a randomized controlled trial. AIDS. 1998;12(14):1851–1859. doi: 10.1097/00002030-199814000-00017. [DOI] [PubMed] [Google Scholar]
- 42.Feldblum PJ, Kuyoh MA, Bwayo JJ, et al. Female condom introduction and sexually transmitted infection prevalence: results of a community intervention trial in Kenya. AIDS. 2001;15(8):1037–1044. doi: 10.1097/00002030-200105250-00012. [DOI] [PubMed] [Google Scholar]
- 43.Steiner MJ, Hylton-Kong T, Figueroa JP, et al. Does a choice of condoms impact sexually transmitted infection incidence? A randomized, controlled trial. Sex Transm Dis. 2006;33(1):31–35. doi: 10.1097/01.olq.0000187200.07639.c6. [DOI] [PubMed] [Google Scholar]
- 44.Ramjee G, van der Straten A, Chipato T, et al. The diaphragm and lubricant gel for prevention of cervical sexually transmitted infections: results of a randomized controlled trial. PLoS One. 2008;3(10) doi: 10.1371/journal.pone.0003488. e3488. (doi:10.1371/journal.pone.0003488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sawaya GF, Chirenje MZ, Magure MT, et al. Effect of diaphragm and lubricant gel provision on human papillomavirus infection among women provided with condoms: a randomized controlled trial. Obstet Gynecol. 2008;112(5):990–997. doi: 10.1097/AOG.0b013e318189a8a4. [DOI] [PubMed] [Google Scholar]
- 46.Cutler JC, Singh B, Uri C, et al. Vaginal contraceptive as prophylaxis against gonorrhea and other sexually transmissible diseases. Adv Plan Parent. 1977;12:45–56. [Google Scholar]
- 47.Rendon AL, Covarrubias J, McCarney KE, et al. A controlled comparative study of phenylmercuric acetate, nonoxynol-9 and placebo vaginal suppositories as prophylactic agents against gonorrhea. Curr Ther Res. 1980;27:780–783. [Google Scholar]
- 48.Rosenberg MJ, Rojanapithayakorn W, Feldblum PJ, et al. Effect of the contraceptive sponge on chlamydial infection, gonorrhea, and candidiasis. A comparative clinical trial. JAMA. 1987;257(17):2308–2312. [PubMed] [Google Scholar]
- 49.Louv WC, Austin H, Alexander WJ, et al. A clinical trial of nonoxynol-9 for preventing gonococcal and chlamydial infections. J Infect Dis. 1988;158(3):518–523. doi: 10.1093/infdis/158.3.518. [DOI] [PubMed] [Google Scholar]
- 50.Niruthisard S, Roddy RE, Chutivongse S. Use of nonoxynol-9 and reduction in rate of gonococcal and chlamydial cervical infections. Lancet. 1992;339(8806):1371–1375. doi: 10.1016/0140-6736(92)91195-e. [DOI] [PubMed] [Google Scholar]
- 51.Kreiss J, Ngugi E, Holmes K, et al. Efficacy of nonoxynol 9 contraceptive sponge use in preventing heterosexual acquisition of HIV in Nairobi prostitutes. JAMA. 1992;268(4):477–482. [PubMed] [Google Scholar]
- 52.Roddy RE, Zekeng L, Ryan KA, et al. A controlled trial of nonoxynol 9 film to reduce male-to-female transmission of sexually transmitted diseases. N Engl J Med. 1998;339(8):504–510. doi: 10.1056/NEJM199808203390803. [DOI] [PubMed] [Google Scholar]
- 53.Richardson BA, Lavreys L, Martin HL, Jr, et al. Evaluation of a low-dose nonoxynol-9 gel for the prevention of sexually transmitted diseases: a randomized clinical trial. Sex Transm Dis. 2001;28(7):394–400. doi: 10.1097/00007435-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 54.Roddy RE, Zekeng L, Ryan KA, et al. Effect of nonoxynol-9 gel on urogenital gonorrhea and chlamydial infection: a randomized controlled trial. JAMA. 2002;287(9):1117–1122. doi: 10.1001/jama.287.9.1117. [DOI] [PubMed] [Google Scholar]
- 55.Van Damme L, Ramjee G, Alary M, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360(9338):971–977. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 56.Van Damme L, Govinden R, Mirembe FM, et al. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N Engl J Med. 2008;359(5):463–472. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- 57.Halpern V, Ogunsola F, Obunge O, et al. Effectiveness of cellulose sulfate vaginal gel for the prevention of HIV infection: results of a phase III trial in Nigeria. PLoS One. 2008;3(11) doi: 10.1371/journal.pone.0003784. e3784. (doi:10.1371/journal.pone.0003784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mattson CL, Campbell RT, Bailey RC, et al. Risk compensation is not associated with male circumcision in Kisumu, Kenya: a multi-faceted assessment of men enrolled in a randomized controlled trial. PLoS One. 2008;3(6) doi: 10.1371/journal.pone.0002443. e2443. (doi:10.1371/journal.pone.0002443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mehta SD, Moses S, Agot K, et al. Adult male circumcision does not reduce the risk of incident Neisseria gonorrhoeae, Chlamydia trachomatis, or Trichomonas vaginalis infection: results from a randomized, controlled trial in Kenya. J Infect Dis. 2009;200(3):370–378. doi: 10.1086/600074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sobngwi-Tambekou J, Taljaard D, Nieuwoudt M, et al. Male circumcision and Neisseria gonorrhoeae, Chlamydia trachomatis and Trichomonas vaginalis: observations after a randomised controlled trial for HIV prevention. Sex Transm Infect. 2009;85(2):116–120. doi: 10.1136/sti.2008.032334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Auvert B, Sobngwi-Tambekou J, Cutler E, et al. Effect of male circumcision on the prevalence of high-risk human papillomavirus in young men: results of a randomized controlled trial conducted in Orange Farm, South Africa. J Infect Dis. 2009;199(1):14–19. doi: 10.1086/595566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tobian AA, Serwadda D, Quinn TC, et al. Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Engl J Med. 2009;360(13):1298–1309. doi: 10.1056/NEJMoa0802556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gray RH, Kigozi G, Serwadda D, et al. The effects of male circumcision on female partners’ genital tract symptoms and vaginal infections in a randomized trial in Rakai, Uganda. Am J Obstet Gynecol. 2009;200(1):42e.1–42e.7. doi: 10.1016/j.ajog.2008.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lyng J, Christensen J. A double-blind study of the value of treatment with a single dose tinidazole of partners to females with trichomoniasis. Acta Obstet Gynecol Scand. 1981;60(2):199–201. [PubMed] [Google Scholar]
- 65.Schillinger JA, Kissinger P, Calvet H, et al. Patient-delivered partner treatment with azithromycin to prevent repeated Chlamydia trachomatis infection among women: a randomized, controlled trial. Sex Transm Dis. 2003;30(1):49–56. doi: 10.1097/00007435-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 66.Golden MR, Whittington WL, Handsfield HH, et al. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N Engl J Med. 2005;352(7):676–685. doi: 10.1056/NEJMoa041681. [DOI] [PubMed] [Google Scholar]
- 67.Kissinger P, Mohammed H, Richardson-Alston G, et al. Patient-delivered partner treatment for male urethritis: a randomized, controlled trial. Clin Infect Dis. 2005;41(5):623–629. doi: 10.1086/432476. [DOI] [PubMed] [Google Scholar]
- 68.Kissinger P, Schmidt N, Mohammed H, et al. Patient-delivered partner treatment for Trichomonas vaginalis infection: a randomized controlled trial. Sex Transm Dis. 2006;33(7):445–450. doi: 10.1097/01.olq.0000204511.84485.4c. [DOI] [PubMed] [Google Scholar]
- 69.Cameron ST, Glasier A, Scott G, et al. Novel interventions to reduce re-infection in women with chlamydia: a randomized controlled trial. Hum Reprod. 2009;24(4):888–895. doi: 10.1093/humrep/den475. [DOI] [PubMed] [Google Scholar]
- 70.Wilson TE, Hogben M, Malka ES, et al. A randomized controlled trial for reducing risks for sexually transmitted infections through enhanced patient-based partner notification. Am J Public Health. 2009;99(suppl 1):S104–S110. doi: 10.2105/AJPH.2007.112128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harrison WO, Hooper RR, Wiesner PJ, et al. A trial of minocycline given after exposure to prevent gonorrhea. N Engl J Med. 1979;300(19):1074–1078. doi: 10.1056/NEJM197905103001903. [DOI] [PubMed] [Google Scholar]
- 72.Wawer MJ, Sewankambo NK, Serwadda D, et al. Control of sexually transmitted diseases for AIDS prevention in Uganda: a randomised community trial. Rakai Project Study Group. Lancet. 1999;353(9152):525–535. doi: 10.1016/s0140-6736(98)06439-3. [DOI] [PubMed] [Google Scholar]
- 73.Kaul R, Kimani J, Nagelkerke NJ, et al. Monthly antibiotic chemoprophylaxis and incidence of sexually transmitted infections and HIV-1 infection in Kenyan sex workers: a randomized controlled trial. JAMA. 2004;291(21):2555–2562. doi: 10.1001/jama.291.21.2555. [DOI] [PubMed] [Google Scholar]
- 74.McClelland RS, Richardson BA, Hassan WM, et al. Improvement of vaginal health for Kenyan women at risk for acquisition of human immunodeficiency virus type 1: results of a randomized trial. J Infect Dis. 2008;197(10):1361–1368. doi: 10.1086/587490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schwebke JR, Desmond R. A randomized trial of metronidazole in asymptomatic bacterial vaginosis to prevent the acquisition of sexually transmitted diseases. Am J Obstet Gynecol. 2007;196(6):517.e1–517e.6. doi: 10.1016/j.ajog.2007.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Corey L, Wald A, Patel R, et al. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med. 2004;350(1):11–20. doi: 10.1056/NEJMoa035144. [DOI] [PubMed] [Google Scholar]
- 77.Grosskurth H, Mosha F, Todd J, et al. Impact of improved treatment of sexually transmitted diseases on HIV infection in rural Tanzania: randomised controlled trial. Lancet. 1995;346(8974):530–536. doi: 10.1016/s0140-6736(95)91380-7. [DOI] [PubMed] [Google Scholar]
- 78.Mayaud P, Mosha F, Todd J, et al. Improved treatment services significantly reduce the prevalence of sexually transmitted diseases in rural Tanzania: results of a randomized controlled trial. AIDS. 1997;11(15):1873–1880. doi: 10.1097/00002030-199715000-00013. [DOI] [PubMed] [Google Scholar]
- 79.Szmuness W, Stevens CE, Harley EJ, et al. Hepatitis B vaccine: demonstration of efficacy in a controlled clinical trial in a high-risk population in the United States. N Engl J Med. 1980;303(15):833–841. doi: 10.1056/NEJM198010093031501. [DOI] [PubMed] [Google Scholar]
- 80.Szmuness W, Stevens CE, Zang EA, et al. A controlled clinical trial of the efficacy of the hepatitis B vaccine (Heptavax B): a final report. Hepatology. 1981;1(5):377–385. doi: 10.1002/hep.1840010502. [DOI] [PubMed] [Google Scholar]
- 81.Francis DP, Hadler SC, Thompson SE, et al. The prevention of hepatitis B with vaccine. Report of the Centers for Disease Control Multi-Center Efficacy Trial among homosexual men. Ann Intern Med. 1982;97(3):362–366. doi: 10.7326/0003-4819-97-3-362. [DOI] [PubMed] [Google Scholar]
- 82.Coutinho RA, Lelie N, Albrecht-Van Lent P, et al. Efficacy of a heat inactivated hepatitis B vaccine in male homosexuals: outcome of a placebo controlled double blind trial. Br Med J (Clin Res Ed) 1983;286(6374):1305–1308. doi: 10.1136/bmj.286.6374.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Piazza M, Sagliocca L, Tosone G, et al. Sexual transmission of the hepatitis C virus and efficacy of prophylaxis with intramuscular immune serum globulin. A randomized controlled trial. Arch Intern Med. 1997;157(14):1537–1544. [PubMed] [Google Scholar]
- 84.Corey L, Langenberg AG, Ashley R, et al. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. Chiron HSV Vaccine Study Group. JAMA. 1999;282(4):331–340. doi: 10.1001/jama.282.4.331. [DOI] [PubMed] [Google Scholar]
- 85.Stanberry LR, Spruance SL, Cunningham AL, et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med. 2002;347(21):1652–1661. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 86.Koutsky LA, Ault KA, Wheeler CM, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347(21):1645–1651. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 87.Harper DM, Franco EL, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364(9447):1757–1765. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 88.Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367(9518):1247–1255. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 89.Romanowski B, de Borba PC, Naud PS, et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. GlaxoSmithKline Vaccine HPV-007 Study Group. Lancet. 2009;374(9706):1975–1985. doi: 10.1016/S0140-6736(09)61567-1. [DOI] [PubMed] [Google Scholar]
- 90.Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369(9580):2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 91.Paavonen J, Naud P, Salmerón J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 92.Villa LL, Costa RL, Petta CA, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6(5):271–278. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 93.Villa LL, Costa RL, Petta CA, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer. 2006;95(11):1459–1466. doi: 10.1038/sj.bjc.6603469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 95.FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 96.FUTURE II Study Group. Prophylactic efficacy of a quadrivalent human papillomavirus (HPV) vaccine in women with virological evidence of HPV infection. J Infect Dis. 2007;196(10):1438–1446. doi: 10.1086/522864. [DOI] [PubMed] [Google Scholar]
- 97.Joura EA, Leodolter S, Hernandez-Avila M, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369(9574):1693–1702. doi: 10.1016/S0140-6736(07)60777-6. [DOI] [PubMed] [Google Scholar]
- 98.Barr E, Gause CK, Bautista OM, et al. Impact of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, 18) L1 virus-like particle vaccine in a sexually active population of North American women. Am J Obstet Gynecol. 2008;198(3):261.e1–261.e11. doi: 10.1016/j.ajog.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 99.Tay EH, Garland S, Tang G, et al. Clinical trial experience with prophylactic HPV 6/11/16/18 VLP vaccine in young women from the Asia-Pacific region. Int J Gynaecol Obstet. 2008;102(3):275–283. doi: 10.1016/j.ijgo.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 100.Muñoz N, Manalastas R, Jr, Pitisuttithum P, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24–45 years: a randomised, double-blind trial. Lancet. 2009;373(9679):1949–1957. doi: 10.1016/S0140-6736(09)60691-7. [DOI] [PubMed] [Google Scholar]
- 101.Wheeler CM, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in sexually active women aged 16–26 years. J Infect Dis. 2009;199(7):936–944. doi: 10.1086/597309. [DOI] [PubMed] [Google Scholar]
- 102.Brown DR, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J Infect Dis. 2009;199(7):926–935. doi: 10.1086/597307. [DOI] [PubMed] [Google Scholar]
- 103.Ross DA, Changalucha J, Obasi AI, et al. Biological and behavioural impact of an adolescent sexual health intervention in Tanzania: a community-randomized trial. AIDS. 2007;21(14):1943–1955. doi: 10.1097/QAD.0b013e3282ed3cf5. [DOI] [PubMed] [Google Scholar]
- 104.NIMH Multisite HIV/STD Prevention Trial for African American Couples Group. Eban HIV/STD risk reduction intervention: conceptual basis and procedures. J Acquir Immune Defic Syndr. 2008;49(suppl 1):S15–S27. doi: 10.1097/QAI.0b013e318184255d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.NIMH Collaborative HIV/STD Prevention Trial Group. Methodological overview of a five-country community-level HIV/sexually transmitted disease prevention trial. AIDS. 2007;21(suppl 2):S3–S18. doi: 10.1097/01.aids.0000266453.18644.27. [DOI] [PubMed] [Google Scholar]
- 106.Karim SA, Coletti A, Richardson BA. Safety and effectiveness of vaginal microbicides BufferGel and PRO 2000 gel for the prevention of HIV infection in women. Presented at the 16th Conference on Retroviruses and Opportunistic Infections (CROI), Montreal, Canada, February 8, 2009–February 11, 2009. [Google Scholar]
- 107.Microbicides Development Programme. HIV ‘Prevention’ gel PRO 2000 Proven Ineffective. London, United Kingdom: UK Medical Research Council and Imperial College London; 2009. [Google Scholar]
- 108.Auvert B, Taljaard D, Lagarde E, et al. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2(11) doi: 10.1371/journal.pmed.0020298. e298. (doi:10.1371/journal.pmed.0020298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369(9562):643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 110.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369(9562):657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 111.Golden MR, Wasserheit JN. Prevention of viral sexually transmitted infections—foreskin at the forefront. N Engl J Med. 2009;360(13):1349–1351. doi: 10.1056/NEJMe0900762. [DOI] [PubMed] [Google Scholar]
- 112.Cowan FM, Pascoe SJ, Langhaug LF, et al. The Regai Dzive Shiri Project: a cluster randomised controlled trial to determine the effectiveness of a multi-component community-based HIV prevention intervention for rural youth in Zimbabwe—study design and baseline results. Trop Med Int Health. 2008;13(10):1235–1244. doi: 10.1111/j.1365-3156.2008.02137.x. [DOI] [PubMed] [Google Scholar]
- 113.Garcia PJ, Holmes KK, Garnett GP. The PREVEN Project: urban community randomized trial of a multicomponent intervention for prevention of STI in Latin America. Presented at the 18th Meeting of the International Society for STD Research (ISSTDR) in Conjunction With the British Association for Sexual Health and HIV (BASHH), London, United Kingdom, June 30, 2009. [Google Scholar]
- 114.Padian NS, McCoy SI, Balkus JE, et al. Weighing the gold in the gold standard: challenges in HIV prevention research. AIDS. 2010;24(5):621–635. doi: 10.1097/QAD.0b013e328337798a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Petitti DB. Meta-Analysis, Decision Analysis, and Cost-Effectiveness Analysis: Methods for Quantitative Synthesis in Medicine. New York, NY: Oxford University Press; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


