Abstract
Aims
Fabry disease is a rare X-linked deficiency of α-galactosidase A (αgal), which causes glycosphingolipid accumulation. This study analysed the cardiovascular manifestations of a cohort of Fabry patients, and sought to define relationships between disease severity, αgal activity, and cardiac abnormalities.
Methods and results
We prospectively analysed Fabry patients (139 subjects: 92 males and 47 females) undergoing screening for potential enzyme replacement therapy. Baseline echocardiograms, electrocardiograms, and exams were obtained as part of two multinational clinical trials. Cardiovascular symptoms were present in 60.4%. By echocardiography, the mean left ventricular mass index (LVMI) was increased at 165.5 ± 66.9 g/m2, and 84.8% of patients displayed concentric left ventricular hypertrophy (LVH). Electrocardiographic LVH was present in >50% of adult subjects. In females, log-corrected plasma αgal activity was inversely associated with LVMI (r = −0.45, P < 0.040). Males with extremely low αgal activity and renal disease displayed the most LVH and cardiac symptoms, but LVH was prevalent even in females <20 years old.
Conclusion
Concentric LVH was the predominant cardiac pathology seen in patients with Fabry disease, and was prevalent in both genders by the third decade of life. Left ventricular mass index was inversely correlated with αgal activity, but was prevalent even in younger females.
Keywords: Cardiomyopathy, Echocardiography, Genetics, Hypertrophy, Fabry, α-Galactosidase
Introduction
Fabry disease is a lysosomal storage disorder due to a rare X-linked recessive mutation in the gene encoding the enzyme α-galactosidase A (αgal), although carrier (heterozygous) females may also be affected to varying degrees because of random X-chromosomal inactivation.1 The progressive deposition of glycosphingolipids can lead to early death due to infiltrative and occlusive disease of the heart, kidney, and brain. Although presenting symptoms may be extracardiac, mortality due to myocardial infarction, arrhythmias, stroke, and renal dysfunction is common.1–3
Previous studies addressing the cardiac manifestations of Fabry disease have relied on historical registry data2–5 or been restricted to highly selected populations6–8 and may not accurately represent the entire spectrum or incidence of cardiac disease. Expression of the disease varies: hemizygous males with low residual enzyme activity often display severe manifestations, whereas heterozygous females may range from mild, late-onset to severely affected phenotypes. Systolic and diastolic dysfunction, hypertrophic and restrictive cardiomyopathies, and valvular and conduction system abnormalities have been variably ascribed to Fabry disease.1–8 We prospectively examined the clinical characteristics with respect to echocardiograms and αgal activity of a large multinational cohort of Fabry disease patients enrolled in either a Phase IV trial designed to evaluate enzyme replacement therapy (ERT) in patients with advanced disease9 or a screening study designed to identify potential subjects for this trial.
Methods
Study population
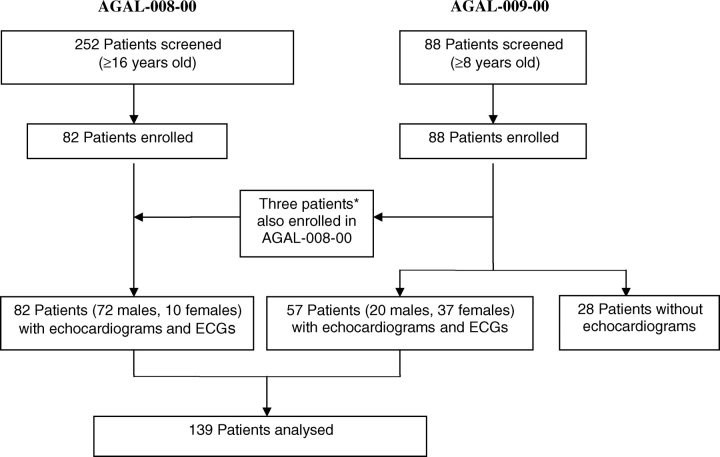
Figure 1 is a flowchart of the 139 subjects analysed in the present report, comprising 82 participants from the AGAL-008-00 study and 57 from the AGAL-009-00 study. AGAL-008-00 was a multinational, placebo-controlled Phase IV trial of ERT (Fabrazyme®, agalsidase beta) in Fabry patients with moderately advanced renal disease. AGAL-009-00 was a pre-screening non-interventional study to characterize and identify potential subjects for the Phase IV trial. Between December 2000 and March 2003, physicians experienced in treating Fabry disease screened patients for one or both studies at 38 sites in North America, Europe, Australia, and Israel. All participants provided informed consent in accordance with their institutions’ Institutional Review Board or Independent Ethics Committee, and study conduct was in accordance with the Declaration of Helsinki.
Figure 1.
Diagram of Fabry cohorts for cardiovascular analysis. *AGAL-008-00 and AGAL-009-00 studies enrolled concurrently at some centres. Three subjects who were enrolled in both studies were analysed solely as AGAL-008-00 subjects.
For AGAL-008-00, 82 patients (72 males and 10 females) were enrolled. The main inclusion criteria included: (i) ≥16 years old, (ii) a current diagnosis of Fabry disease with no prior treatment with recombinant human αgal, (iii) a clinical presentation consistent with Fabry disease, (iv) documented αgal activity <1.5 nmol/h/mL plasma or <4 nmol/h/mg in leucocytes, and (v) mild-to-moderate renal disease, defined as a serum creatinine (Cr) of 1.2–3.0 mg/dL or an estimated Cr clearance <80 mL/min, if Cr was <1.2 mg/dL. All 82 patients had echocardiograms and electrocardiograms (ECGs) performed as baseline assessment. On the basis of historical values obtained at screening, the mean ± standard deviation (SD) plasma αgal activity was 1.0 ± 0.57 nmol/h/mL for 45 of the patients, and the mean ± SD leucocyte αgal activity was 2.2 ± 1.52 nmol/h/mg for the other 37 patients.
For AGAL-009-00, 88 patients were screened and enrolled. The main inclusion criteria were: (i) ≥8 years old, (ii) a current diagnosis of Fabry disease with no prior treatment with recombinant human αgal, and (iii) a clinical presentation consistent with Fabry disease. Three patients were subsequently enrolled in AGAL-008-00 and are evaluated as part of that study cohort. Of the remaining patients, 57 (20 males and 37 females) had echocardiograms and ECGs performed as part of the screening and were included in this analyses. Thirty-seven patients (65%) were classified as ‘confirmed’ during the study: 35 patients (all 20 males and 15 females) had a confirmatory genotype and/or enzyme activity criteria (αgal activity level ≤2.4 nmol/h/mL in plasma or <46 nmol/h/mg in leucocytes); two females had a confirmatory genotype with no recorded αgal data; and 20 females had a clinical diagnosis of Fabry disease with plasma αgal levels of 2.5–15.0 nmol/h/mL. Of these 20, at least 18 underwent were subsequently confirmed to have a familial Fabry mutation after study conclusion (W.R.W. et al., personal communication); genotype information on the remaining two females is unavailable. Thus, for the overall cohort (Figure 1), >98% had a genotype and/or αgal activity consistent with Fabry disease.
Echocardiography
Study protocols for both AGAL-008-00 and AGAL-009-00 specified that echocardiograms with standard machines (per individual site preference, settings optimized for endocardial definition) be performed within 28 days of screening. Measurements were made from 2D images by a Level III-certified echocardiologist blinded to all clinical data, using the mean of three cardiac cycles and conventions of the American Society of Echocardiography.10 Segmental wall thickness was assessed by tracings of the endocardial and epicardial circumferences of basal short-axis images at end-diastole, using the Wyatt convention.11
Left ventricular volumes at end-diastole (LVEDV) and end-systole (LVESV) were determined by modified 2D Simpson's formula. Stroke volume (SV) was calculated as [LVEDV−LVESV], and left ventricular ejection fraction (EF) as [SV/LVEDV]. Left ventricular mass was calculated10 and indexed to body surface area to obtain the left ventricular mass index (LVMI). Relative wall thickness (RWT), or eccentricity, was calculated as [(IVS + PWT)/LVEDD].
Clinical data
At screening, serum Cr was measured and estimated glomerular filtration rate (GFR) was calculated by the Modification of Diet in Renal Disease Study Group equation12 [186 × (serum Cr in mg/dL)−1.154 × (age in year)−0.203 × (0.742, for females) × (1.212, if patient ethnicity is African-American). Each patient underwent a review of systems including the presence of symptoms potentially associated with Fabry disease, a cardiovascular assessment, and ECG analysis as listed in Table 2.13
Table 2.
Clinical signs and symptoms in Fabry cohorts combined from AGAL-009-00 and AGAL-008-00
| Clinical signs and symptoms | Number (%) of patients |
M vs. F, P-value | ||
|---|---|---|---|---|
| All, 139 (100) | Male, 92 (66.2) | Female, 47 (33.8) | ||
| Cardiovascular (any of the following)a | 84 (60.4%) | 61 (66.3%) | 23 (48.9%) | <0.048 |
| Dyspnoea | 23 (16.5%) | 15 (16.3%) | 8 (17.0%) | <0.914 |
| Angina | 15 (10.8%) | 8 (8.7%) | 1 (2.1%) | <0.137 |
| Chest pain | 9 (6.5%) | 12 (13.0%) | 3 (6.4%) | <0.231 |
| Oedema | 39 (28.1%) | 35 (38.0%) | 4 (8.5%) | <0.021 |
| Hypertension | 43 (30.9%) | 27 (29.3%) | 16 (34.0%) | <0.571 |
| Hypotension | 4 (2.9%) | 3 (3.3%) | 1 (2.1%) | <0.071 |
| Murmur | 28 (20.1%) | 22 (23.9%) | 6 (12.8%) | <0.121 |
| ECG abnormalities (any of the following)a | 88 (63.3%) | 72 (78.3%) | 16 (34.0%) | <0.001 |
| Bradycardia | 23 (16.5%) | 20 (21.7%) | 3 (6.4%) | <0.021 |
| Conduction abnormality | 26 (18.7%) | 21 (22.8%) | 5 (10.6%) | <0.001 |
| PR < 120 ms | 3 (2.1%) | 3 (3.3%) | 0 (0%) | <0.211 |
| PR > 200 ms | 5 (3.6%) | 3 (3.3%) | 2 (4.3%) | <0.766 |
| RBBB | 8 (5.7%) | 8 (8.7%) | 0 (0%) | <0.037 |
| LBBB | 1 (0.7%) | 1 (1.1%) | 0 (0%) | <0.473 |
| Other (IRBB, LAHB) | 9 (6.5%) | 9 (9.8%) | 0 (0%) | <0.027 |
| LVH (for ≥35 years old., n = 105, 67 M and 38 F) | 53 (50.5%) | 47 (70.1%) | 6 (15.8%) | <0.001 |
| RVH | 3 (2.2%) | 1 (2.1%) | 2 (2.2%) | <0.986 |
| Dermatologicalb | 116 (83.4%) | 83 (90.2%) | 33 (70.2%) | <0.003 |
| Neuralb | 91 (65.5%) | 58 (63.0%) | 33 (70.2%) | <0.400 |
| HEENTb | 87 (62.6%) | 57 (62.0%) | 30 (63.8%) | <0.829 |
| GIb | 79 (56.8%) | 60 (65.2%) | 19 (40.4%) | <0.005 |
| Renalb | 75 (53.9%) | 59 (64.1%) | 16 (34.0%) | <0.001 |
| Musculoskeletalb | 55 (39.5%) | 42 (45.7%) | 13 (27.7%) | <0.040 |
| Hypercholesterolaemia | 25 (18.0%) | 17 (18.5%) | 8 (17.1%) | <0.832 |
aHypertension (SBP ≥ 140 or DBP ≥ 90), hypotension (SBP < 90), conduction abnormality (any of the following: short or long PR interval, right bundle branch block, left bundle branch block, other intraventricular conduction delay), left ventricular hypertrophy (LVH), and right ventricular hypertrophy (RVH); LVH criteria (Sokolow–Lyon) were used to examine only subjects ≥35 years old.
bNon-cardiovascular review of systems is as follows: Dermatologic (angiokeratomas, hypohydrosis, anhydrosis), HEENT (corneal or lens abnormalities, headache, hypacousia, vertigo), GI (abdominal pain, diarrhoea), Renal (hematuria, proteinuria), Neural (acroparesthesia), Musculoskeletal (arthralgia, myalgia, pain).
Bold values indicate P ≤ 0.05.
Statistical analyses
Statistical analyses were performed with STATA 9.1. Data are expressed as mean ± SD. Differences between genders were analysed using the unpaired Student t-test with unequal variance (continuous variables with normal distribution), Mann–Whitney U test (continuous variables with skewed distribution), and Pearson χ2 test (dichotomous variables). Least square linear regression analysis was performed to assess bivariate correlations. Multivariate analysis was utilized to correct for all factors identified as significant influences by univariate testing. Differences were considered statistically significant for two-tailed P-values <0.05.
Results
Demographics and key clinical characteristics of Fabry cohorts
Table 1 summarizes the demographics of the cohort, stratified by gender. The combined study cohort consisted of 139 unique patients, ranging from 13 to 75 years old (mean age 43.1 years) and approximately two-thirds were male. When compared with the females, the males displayed onset of symptoms at an earlier age, lower αgal levels, and higher serum Cr. And 30% of the total study population was hypertensive at the time of enrollment.
Table 1.
Demographics of Fabry cohorts combined from AGAL-009-00 and AGAL-008-00
| Parameter | All patients | Male | Female | M vs. F, P-value |
|---|---|---|---|---|
| Number of patients (%) | 139 (100%) | 92 (66.2%) | 47 (33.8%) | |
| Age (year), mean ± SD (range) | 43.1±12.6 (13.1–75.2) | 41.9 ± 12.1 (13.1–75.2) | 45.4 ± 13.3 (13.4–71.9) | <0.129 |
| Age at onset of Sxa, mean ± SD | 13.8 ± 11.9 | 12.1 ± 11.6 | 17.3 ± 12.0 | < 0.017 |
| Age at Dx, mean ± SD | 29.8 ± 17.6 | 30.1 ± 15.9 | 29.0 ± 20.9 | <0.871 |
| Disease durationb, mean ± SD | 28.2 ± 13.7 | 29.0 ± 12.7 | 26.5 ± 15.7 | <0.387 |
| Ethnicity, n (%) | ||||
| Caucasian | 126 (90.7%) | 84 (91.3%) | 42 (89.4%) | <0.710 |
| Non-caucasian | 13 (9.3%) | 8 (8.7%) | 5 (10.6%) | |
| αGal activityc, mean ± SD (range) n | ||||
| Plasma (nmol/h/mL) | 2.10 ± 2.09 (0–15.0) 97 | 1.18 ± 0.95 (0–6.7) 54 | 3.26 ± 2.53 (0.7–15.0) 43 | <0.001 |
| Leucocyte (nmol/h/mg) | 2.18 ± 1.54 (0–4.0) 40 | 2.10 ± 1.53 (0–4.0) 38 | 3.70 ± 0.46 (3.6–3.8) 2 | <0.020 |
| Haemoglobin (g/dL), mean ± SD | 1.40 ± 0.89 | 1.63 ± 0.51 | 0.92 ± 0.46 | <0.001 |
| Estimated GFR (mL/min), mean ± SD | 71.1 ± 36.5 | 64.5 ± 37.1 | 84.4 ± 31.4 | 0.683 |
| Mean BP (mm Hg), mean ± SD | 109 ± 14 | 109±13 | 108 ± 15 | <0.531 |
| ACE-I or ARB use, n (%) | 37 (26.6%) | 25 (27.2%) | 12 (25.5%) | <0.836 |
aPatient age on date of first reported Fabry symptoms listed in Table 2.
bTime from first onset of symptoms to patient age at screening.
cLevels of αgal activity at time of screening are based on historical documentation of either plasma or leucocyte activity; reference ranges for individual historical screening sites may vary.
Bold values indicate P ≤ 0.05.
Signs and symptoms associated with Fabry disease and decreased α-galactosidase A activity
As shown in Table 2, a majority (60.4%) of the Fabry cohort had a history of abnormal cardiovascular signs and symptoms: hypertension and oedema were most prevalent, followed by murmur, dyspnoea, and angina. A history of hypotension (P < 0.04) or any ECG abnormality (P < 0.001) listed in Table 2 was significantly associated with decreased αgal activity. ECG (Sokolow–Lyon) criteria for left ventricular hypertrophy (LVH) were found in 50.5% of the subjects aged ≥35 years old. Left ventricular hypertrophy by ECG criteria showed a trend (P < 0.06) for association with decreased αgal levels after adjustment for mean blood pressure, serum Cr, and estimated GFR.
Table 2 also shows that the most prevalent non-cardiac signs of Fabry disease were dermatological, followed by acroparesthesias, oculosensory dysfunction, gastrointestinal, and renal symptoms. Only two patients had cardiovascular manifestations without any other signs of Fabry disease. The presence of any single dermatological or renal signs in Table 2 was significantly associated with low αgal levels (P < 0.01 for both).
Echocardiographic characteristics and left ventricular geometry of the Fabry cohorts
In the majority of patients, mean LV volumes and diameters, SV, and LV EF were within normal limits compared with reference values from normal standards (Table 3).10 Nine of the 138 (6.5%) subjects had impaired LV systolic function (EF < 55%); all nine were males and most had mild global hypokinesis. Only two patients, one with severe global hypokinesis and one with an apical aneurysm, were noted to have a dilated cardiomyopathy. Approximately one-quarter of the overall population had echocardiographic evidence of right ventricular hypertrophy.
Table 3.
Echocardiographic characteristics of Fabry cohorts (AGAL-009-00 and AGAL-008-00)
| All patients | Male | Female | M vs. F, P-value | |
|---|---|---|---|---|
| Number (%) of patients | 139a | 92a (66.2%) | 47 (33.8%) | |
| Heat rate (b.p.m.), mean ± SD | 66.7 ± 12.9 | 65.4 ± 12.3 | 69.5 ± 13.7 | <0.132 |
| LVEDV (mL), mean ± SDa | 96.1 ± 26.19 | 105.4 ± 25.5 | 78.5 ± 16.8 | <0.001 |
| LVESV (mL), mean ± SDa | 33.7 ± 16.3 | 38.5 ± 17.2 | 24.5 ± 8.9 | <0.001 |
| SV (mL), mean ± SDa | 66.1 ± 8.19 | 66.9 ± 14.8 | 53.9 ± 10.9 | <0.001 |
| EF (%), mean ± SDa | 62.4 ± 14.9 | 64.4 ± 8.4 | 69.3 ± 6.9 | <0.001 |
| Pts with EF < 55%a | 9 (6.7%) | 9 (9.8%) | 0 (0%) | <0.027 |
| LVEDD (cm), mean ± SD | 4.39 ± 0.54 | 4.54 ± 0.524 | 4.12 ± 0.484 | <0.001 |
| LVESD (cm), mean ± SD | 2.80 ± 0.48 | 2.89 ± 0.520 | 2.62 ± 0.344 | <0.001 |
| IVS (cm)a, mean ± SD | 1.43 ± 0.39 | 1.49 ± 0.41 | 1.33 ± 0.34 | <0.024 |
| PWT (cm)a, mean ± SD | 1.40 ± 0.30 | 1.42 ± 0.30 | 1.35 ± 0.30 | <0.236 |
| Mean radial WT (cm)a, mean ± SD | 1.45 ± 0.31 | 1.49 ± 0.31 | 1.38 ± 0.30 | <0.052 |
| Relative WTa (eccentricity), mean ± SD | 0.65 ± 0.18 | 0.65 ± 0.18 | 0.67 ± 0.19 | <0.642 |
| LV mass (g)a, mean ± SD | 303.4±131.2 | 332.7 ± 139.1 | 248.5 ± 94.0 | <0.001 |
| LVMI (g/m2)a, mean ± SD | 165.5 ± 66.9 | 178.7 ± 70.1 | 140.3 ± 52.5 | <0.001 |
| RVH, n (%) | 35 (25.9%) | 20 (21.7%) | 14 (29.8%) | <0.296 |
| RV hypokinesis, n (%) | 1 (0.7%) | 1 (1.1%) | 0 (0%) | ns |
| LAE (≥mild), n (%) | 56 (40.3%) | 35 (38.0%) | 21 (44.7%) | <0.450 |
| RAE (≥mild), n (%) | 30 (21.6%) | 21 (22.8%) | 9 (19.1%) | <0.618 |
| Bi-atrial enlargement, n (%) | 24 (17.3%) | 15 (16.3%) | 9 (19.1%) | ns |
| MR (≥moderate), n (%) | 15 (11.0%) | 12 (13.5%) | 3 (6.4%) | <0.024 |
| AR (>mild), n (%) | 3 (2.2%) | 3 (3.5%) | 0 (0%) | ns |
| Dilated aortic root (>40 mm diameter), n (%) | 3 (2.1%) | 3 (3.3%) | 0 (0%) | ns |
| TR(>mild), n (%) | 1 (0.7%) | 1 (5.3%) | 0 (0%) | ns |
| PASP (mmHg), n (%) | 31 ± 7.3 | 31 ± 7.1 | 31 ± 7.9 | ns |
| n | (n = 58, AGAL-009 cohort) | 21 | 37 | |
| Mitral E velocity (m/s), n (%) | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.2 | ns |
| Mitral A velocity (m/s), n (%) | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.6 ± 0.3 | |
| E:A ratio, n (%) | 1.9 ± 0.9 | 2.2 ± 0.9 | 1.7 ± 0.8 | |
| TDI E′ velocity (cm/s), n (%) | −11.7±3.6 (n = 6) | −14.0 ± 3.6 | −9.7 ± 2.3 | |
| E/E′ ratio, n (%) | 8.7 ± 2.8 | 10.3 ± 2.1 | 7.1 ± 2.5 | |
LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; SV, stroke volume; EF, ejection fraction; IVS, interventricular septal thickness; PWT, posterior wall thickness; WT, wall thickness; RVH, right ventricular hypertrophy; RV, right ventricular; LAE, left atrial enlargement; RAE, right atrial enlargement; MR, mitral regurgitation; AR, aortic regurgitation; TDI, tissue-Doppler imaging (septal); TR, tricuspid regurgitation; PASP, estimated pulmonary artery systolic pressure; ns, non-significant.
aThe echocardiogram of one male subject was of insufficient quality for accurate 2D measurements.
Bold values indicate P ≤ 0.05.
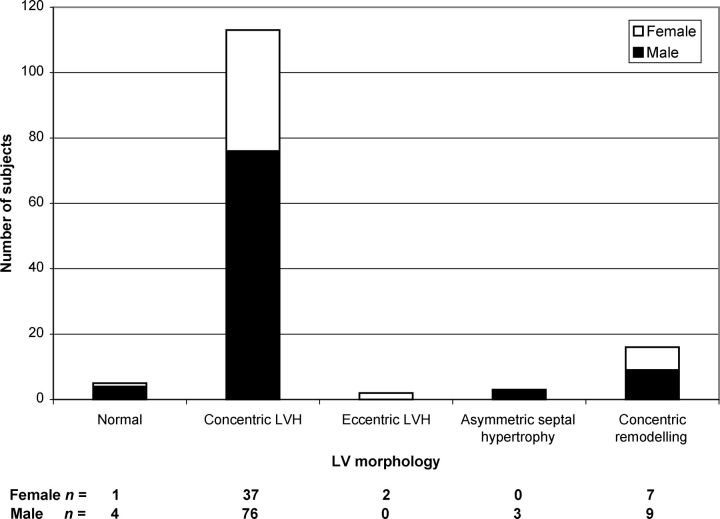
The mean LVMI for the study population is shown in Table 3. Increased LV mass (defined as LVMI > 95 g/m2 for females and >115 g/m2 for males)10 was present in the 118 (84.9%) of the subjects. The mean interventricular septum and posterior wall thicknesses were abnormally increased at 1.43 ± 0.39 and 1.40 ± 0.30 cm, respectively (Table 3 and Figure 2). Of the subjects with increased LVMI, 95.7% had concentric hypertrophy (defined as increased LVMI and RWT > 0.42) and 1.7% had eccentric hypertrophy (i.e. increased LVMI and RWT ≤ 0.42).12 Three males (2.2%) had an asymmetric hypertrophy with a septal-to-posterior wall thickness ratio of ≥1.5; a significant left ventricular outflow tract gradient and mitral systolic anterior motion was noted in one. Sixteen patients (11.5%) had concentric remodelling (i.e. normal LVMI but RWT > 0.42). Only five patients (3.6%) had normal LV mass and geometry, and these subjects were significantly younger (by 14.5 years, P < 0.007) with shorter disease duration (by 12 years, P < 0.038) than those with LVH. The overall distribution of LV geometric patterns is shown in Figure 3, and illustrates that the vast majority (81.3%) of this Fabry population had concentric LVH.
Figure 2.
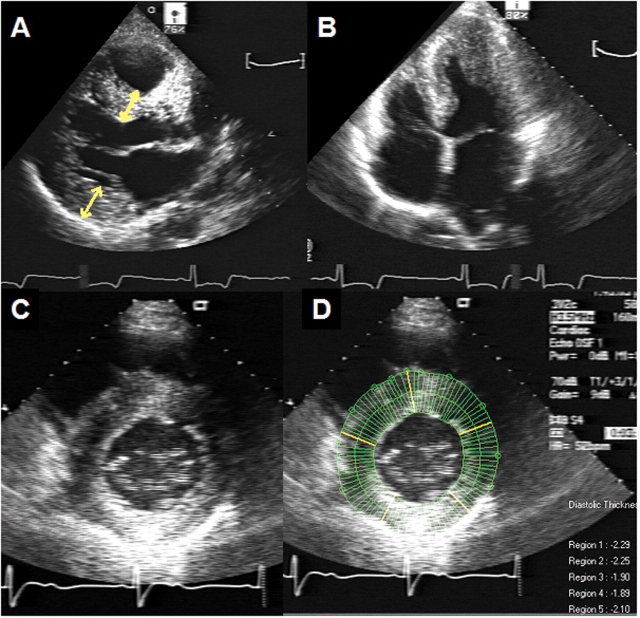
Representative 2D echocardiogram of a 49-year-old male Fabry patient. (A) Parasternal long-axis window at end-diastole. Yellow arrows indicate standard ASE measurements of the interventricular septum and posterior wall at the base. (B) Apical four-chamber window at end-systole. Severe concentric left ventricular hypertrophy (LVH) and preserved ejection fraction is shown. (C) Parasternal short-axis window at end-diastole. (D) Radial chord method utilized to measure regional left ventricular wall thickness.
Figure 3.
Distribution of left ventricular morphological patterns in Fabry cohorts. P = non-significant for males vs. females of each group.
Notably, over one-third of the patients had left atrial enlargement. Mitral leaflet thickening was seen in 50% of the population and was often associated with moderate or severe mitral regurgitation (Table 3). A smaller proportion displayed aortic valve thickening and/or mild aortic insufficiency. There was no clear association of atrial enlargement, valvular abnormalities, or other remaining echocardiographic parameters listed in Table 3 with αgal activity.
Diastolic function was assessed by mitral inflow Doppler in only the AGAL-009 cohort, with tissue-Doppler data limited to an even smaller subset (Table 3). Forty-eight per cent of subjects examined displayed a mitral Doppler pattern suggestive of restrictive disease, with E:A ratios of ≥2.0. Tissue Doppler data were limited to only a small sample of the AGAL-009 patients, with a mean septal E′ velocity of −11.8 ± 3.6 cm/s (normal reference value −13 cm/s for mean age of 43 years old),14 and mean E/E′ ratio of 8.7 ± 2.8. There was a correlation noted between E′ velocity and LVMI (r = −0.91, P < 0.013) in this group, of whom two-third had concentric LVH.
Analysis of the relationship between left ventricular mass index and clinical variables
As increased LV mass was present in almost 85% of the cohort by echocardiography, we investigated the relationships between LVMI, other clinical variables, and plasma αgal activity. In univariate analysis, age, serum Cr, and haemoglobin were directly related to mean wall thickness and LVMI; estimated GFR was inversely correlated with LVMI. Past or present hypertension alone was insufficient to account for differences in LVMI. Only a trend for the use of angiotensin-converting enzyme inhibitors (ACE-Is) or angiotensin II receptor blockers (ARBs) (P < 0.10) was noted for association with LVMI. The presence of LVH by both ECG and transthoracic echocardiogram criteria was associated with lower αgal levels (P < 0.0001) and a positive history of cardiovascular symptoms (P < 0.002).
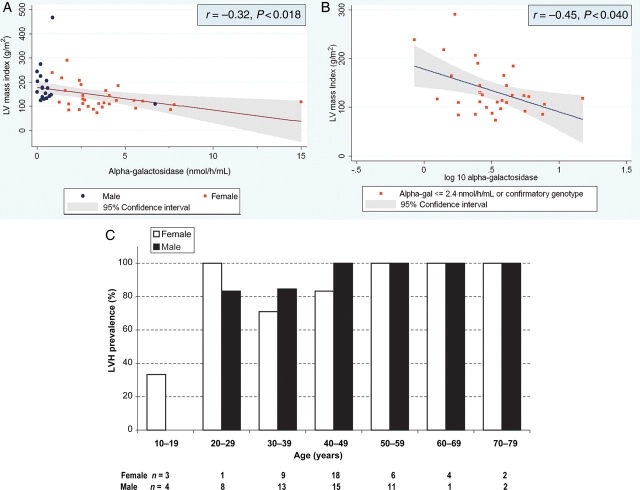
Most patients had αgal activity measured in plasma than in leucocytes (Table 1), so further analyses were conducted for patients with plasma αgal activity. Figure 4A shows that in univariate analysis of these 50 patients (19 males and 31 females), enzyme activity was significantly inversely correlated with LVMI (r = −0.32, P < 0.018). This relationship remained highly significant (P < 0.001) after correction for age, estimated GFR, and anaemia. An even stronger relationship between the logarithm of plasma αgal activity and LVMI in the total population (r = −0.38 and P < 0.006) was observed. However, since all males except one genotypically confirmed subject had plasma αgal activity ≤1.5 nmol/h/mL, the correlation was largely observed in females.
Figure 4.
Plasma α-galactosidase activity vs. left ventricular mass index (LVMI) in (A) males (n=19) and females (n = 31), and (B) females only. Data are shown only for those subjects with αgal activity reported for plasma and measurable LVMI. Subjects with αgal data derived from leucocytes or with activity expressed ambiguously (e.g. ≤1.5 nmol/h/mL) were not included here. Reference ranges for individual historical screening sites may vary. Genotyping information is not currently available for the subject represented by the open square in (B). (C) Age distribution of all subjects with increased LVMI and αgal activity assayed in plasma.
A separate analysis of all females with plasma αgal data, shown in Figure 4B, revealed a significant inverse correlation of LVMI with log-corrected αgal activity (r = −0.45, P < 0.040), after correction for age, estimated GFR, and haemoglobin. In this group, taking into account all significant confounders found by univariate analysis, decreased residual αgal activity was independently associated with increased LVMI.
Figure 4C details the age distribution of male and female Fabry subjects with αgal measured by plasma method. Increased LVMI was noted to be present in two of the four females <30 years old, neither of whom had a history of hypertension or anaemia.
Cardiovascular manifestations of Fabry stratified by gender and α-galactosidase A activity
Fabry-associated characteristics were compared in hemizygous males and heterozygous females as shown in Tables 1–3. Males had lower αgal activity and manifested symptoms at an earlier age. The incidence and extent of disease, as reflected by renal dysfunction, ECG abnormalities (namely intraventricular conduction abnormalities and LVH), and cardiovascular symptoms, was higher in males. Although the prevalence of LVH by echocardiography was similar between genders, the extent of increase in LVMI was significantly higher in males, and only men exhibited left ventricular systolic dysfunction.
Discussion
Although identified a century ago, Fabry disease remains a challenge to diagnose and treat. Registry data suggest that at least 10% of patients may first present with a cardiac event (www.fabryregistry.com),5 and that cardiac disease is a leading cause of mortality in affected females.4,15 Traditional palliative treatments consist of restricted diets and haemodialysis, but ERT is now emerging as potentially effective therapy.16–18
In the present cohort of 139 Fabry patients (representing over 21 different mutations), a high prevalence of cardiovascular manifestations was found. Left ventricular hypertrophy was present in up to 41% by ECG and >84% by echocardiography. The presence of LVH in both studies was associated with higher prevalence of cardiovascular symptoms and lower αgal activity. Although males in the cohort had almost uniformly negligible αgal activity and a higher degree of LVH than females, even females <30 years old were detected to have increased LVMI. A small proportion (6.5%) of males had decreased LV systolic function, and of these an even smaller fraction—both AGAL-008-00 males with paced rhythm and no history of angina—had LV dilatation. One of these males, with a 46-year history of symptoms and moderate renal disease, had an LV aneurysm suggestive of more advanced disease and/or fibrosis. In females, a broader range of residual αgal activity was present, and the severity of cardiac hypertrophy was inversely associated with log-corrected αgal activity. These findings suggest that increased LV mass is not merely a secondary response to age, renal failure, or pressure-overload. Accumulation of glycosphingolipid has been observed microscopically within cardiomyocytes, the microvasculature, and conduction system,6 and may contribute directly to hypertrophy as well as to valve leaflet thickening. However, the glycosphingolipids have been estimated to constitute <1% of cardiac mass;19 hence other mechanisms, speculatively neurohormonal, inflammatory, or vasoreactive in nature, must additionally drive organomegaly. No significant correlation of any diastolic parameters (mitral inflow or tissue-Doppler imaging) with αgal activity was found, although septal E′ velocities were negatively and significantly correlated with LVMI in this small subgroup.
These data provide a large prospective in-depth echocardiographic analysis of a Fabry disease cohort. The study cohort included mostly middle-aged patients with at least mild disease, in which over 60% had cardiovascular manifestations. A high proportion of females with cardiovascular and echocardiographic abnormalities was observed, corroborating registry data.4,5,15,20 Linhart et al.4 retrospectively surveyed a larger cohort of untreated and ERT-treated Fabry patients and found a similar prevalence of cardiac signs and symptoms but a slightly lower overall frequency of echocardiographic LVH in untreated patients. In the recent largest study of Fabry cardiomyopathy to date, Kampmann et al.21 performed a cross-sectional echocardiographic study of 177 males and females Fabry patients and also found a lower baseline prevalence (48.6% of males and 36.4% of females) of echocardiographic LVH, which may be due to the exclusion of hypertensive or ACE-I/ARB-treated subjects. Their study also found that concentric hypertrophy was the most prevalent cardiomyopathy. In the subset of subjects followed longitudinally, age of onset and progressive LVMI increase appeared slower in females. Our current report extends current registry knowledge, by systematically examining detailed echocardiographic characteristics for association with the αgal activity levels and clinical status. Our results suggest that reduced αgal activity contributes to increased LVMI, ECG abnormalities, and clinical symptoms via mechanisms independent of age, hypertension, ACE-I, and ARB use, renal dysfunction, and anaemia.
Study limitations
A limitation of this study is the lack of a control group consisting of individuals without Fabry disease. Additionally, a majority of patients had moderate renal dysfunction in order to be included in AGAL-008-00; consequently, patients with early disease may be under-represented. The identification of Fabry disease can be problematic and may introduce ascertainment bias, particularly in females who are often initially identified by family history.4 Although atypical variants with only cardiac manifestations have been reported,7 only two such subjects were identified here. Because αgal activities for participants in the AGAL studies were based on historical screening data, values could not be normalized to a single reference range. Of note, only two females and less than half of the males had αgal activity measured in leucocytes (which may be more reliable), hence sample size was too small for significant analysis. Finally, tissue-Doppler analysis, which may provide additional information on the diastolic function of Fabry cardiomyopathy,22 was performed only in a limited number of subjects, since many were screened prior to the widespread use of this technique.
Clinical implications
In summary, the prevalence of cardiovascular signs and symptoms, particularly concentric LVH, is high in this cohort of patients with Fabry disease. Left ventricular hypertrophy was already prevalent in subjects of both genders by the second decade of life. Low αgal activity in Fabry patients appears to be independently associated with the development of cardiac hypertrophy. None of these subjects received ERT, which has now been demonstrated to slow progression of a composite endpoint of renal, cardiac, and central nervous system events in the AGAL-008-00 trial,9 and in some cases appears to limit the increase in LV mass and wall thickness.18 Our results confirm and extend findings from recent registry data regarding the high prevalence of cardiac hypertrophy in both Fabry males and females, which increases with age, disease severity, and αgal deficiency and raise the possibility that ERT may need to be instituted early, particularly in females with low αgal activity, in order to significantly alter the course of Fabry cardiomyopathy.
Funding
This work was supported by a research grant from Genzyme Corporation to J.C.W., C.Y.H., H.S., M.B., K.S., S.D.S.; and a GCRC Grant M01-RR00425 to W.R.W.
Conflict of interest: W.R.W. is a consultant to Genzyme, Shire, and Amicus Corporations. S.P. is a consultant to Genzyme, Actelion, and Amicus Corporations. R.A. is an employee of Genzyme Corporation.
The study investigators for AGAL-009-00 were: Gideon Bach, MD, Jerusalem, Israel; David Bodensteiner, MD, Kansas City, KS; Louis Caplan, MD, Boston, MA; Louis Elsas, MD, Miami, FL; Paul Fernhoff, MD, Atlanta, GA; Richard Finkel, MD, Philadelphia, PA; Brian Hutchison, MD, Nedland, Australia; Edwin Kolodny, MD, New York, NY; S.P., MD, San Francisco, CA; David Saltissi, MD, Brisbane, Australia; Prof. David Sillence, Westmead, Australia; K.S., MD, Boston, MA; W.R.W., MD, PhD, Los Angeles, CA.
The study investigators for AGAL-008-00 were: M.B., MD, New York, NY; John A. Barranger, MD, PhD, Pittsburgh, PA; Daniel Bichet, MD, Québec, Canada.; David Bodensteiner, MD, Kansas City, KS; Jan Bultas, MD, Prague, Czech Republic; David Bushinsky, MD, Rochester, NY; Joel Charrow, MD, Chicago, IL; Christine M. Eng, MD, Houston, TX; Richard W. Erbe, MD, Buffalo, NY; Paul Fernhoff, MD, Atlanta, GA; Richard Finkel, MD, Philadelphia, PA; Robert M. Greenstein, MD, Farmington, CT; János Grubits, MD, Sopron, Hungary; Robert J. Hopkin, MD, Cincinnati, OH; Marie McDonald, MD, Durham, NC; S.P., MD, San Francisco, CA; C. Ronald Scott, MD, Seattle, WA; K.S., MD, Boston, MA; Anna Tylki-Szymanska, MD, Warsaw, Poland; Stephen Waldek, MB, BCh, Manchester, UK; David Warnock, MD, Birmingham, AL; Neal Weinreb, MD, Coral Springs, FL; Chester Whitley, PhD, MD, Minneapolis, MN; W.R.W., MD, PhD, Los Angeles, CA; Michael L. West, MD, Halifax, Canada; Philip Wyatt, MD, Toronto, Canada.
References
- 1.Desnick RJ, Brady R, Barranger J, Collins AJ, Germain DP, Goldman M, Grabowski G, Packman S, Wilcox WR. Fabry disease, an under-recognized multisystemic disorder: expert recommendations for diagnosis, management, and enzyme replacement therapy. Ann Intern Med. 2003;138:338–346. doi: 10.7326/0003-4819-138-4-200302180-00014. [DOI] [PubMed] [Google Scholar]
- 2.MacDermot KD, Holmes A, Miners AH. Anderson-Fabry disease: clinical manifestations and impact of disease in a cohort of 98 hemizygous males. J Med Genet. 2001;38:750–760. doi: 10.1136/jmg.38.11.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacDermot KD, Holmes A, Miners AH. Anderson-Fabry disease: clinical manifestations and impact of disease in a cohort of 60 obligate carrier females. J Med Genet. 2001;38:769–775. doi: 10.1136/jmg.38.11.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linhart A, Kampmann C, Zamorano JL, Sunder-Plassmann G, Beck M, Mehta A, Elliott PM. Cardiac manifestations of Anderson-Fabry disease: results from the international Fabry outcome survey. Eur Heart J. 2007;28:1228–1235. doi: 10.1093/eurheartj/ehm153. [DOI] [PubMed] [Google Scholar]
- 5.Eng CM, Fletcher J, Wilcox WR, Waldek S, Scott CR, Sillence DO, Breunig F, Germain DP, Nicholls K, Banikazemi M. Fabry disease: baseline medical characteristics of a cohort of 1765 males and females in the Fabry Registry. J Inherit Metab Dis. 2007;30:184–192. doi: 10.1007/s10545-007-0521-2. [DOI] [PubMed] [Google Scholar]
- 6.Linhart A, Palecek T, Bultas J, Ferguson JJ, Hrudova J, Karetová D, Zeman J, Ledvinová J, Poupetova H, Elleder M, Aschermann M. New insights in cardiac structural changes in patients with Fabry's disease. Am Heart J. 2000;139:1101–1108. doi: 10.1067/mhj.2000.105105. [DOI] [PubMed] [Google Scholar]
- 7.von Scheidt W, Eng CM, Fitzmaurice TF, Erdmann E, Hubner G, Olsen EG, Christomanou H, Kandolf R, Bishop DF, Desnick RJ. An atypical variant of Fabry's disease with manifestations confined to the myocardium. N Engl J Med. 1991;324:395–399. doi: 10.1056/NEJM199102073240607. [DOI] [PubMed] [Google Scholar]
- 8.Kampmann C, Baehner F, Whybra C, Martin C, Wiethoff CM, Ries M, Gal A, Beck M. Cardiac manifestations of Anderson-Fabry disease in heterozygous females. J Am Coll Cardiol. 2002;40:1668–1674. doi: 10.1016/s0735-1097(02)02380-x. [DOI] [PubMed] [Google Scholar]
- 9.Banikazemi M, Bultas J, Waldek S, Wilcox WR, Whitley CB, McDonald M, Finkel R, Packman S, Bichet DG, Warnock DG, Desnick RJ for the Fabry Disease Clinical Trial study Group. Agalsidase-beta therapy for advanced Fabry disease. Ann Intern Med. 2007;146:77–86. doi: 10.7326/0003-4819-146-2-200701160-00148. [DOI] [PubMed] [Google Scholar]
- 10.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 111.Wyatt HL, Haendchen RV, Meerbaum S, Corday E. Assessment of quantitative methods for 2-dimensional echocardiography. Am J Cardiol. 1983;52:396–401. doi: 10.1016/0002-9149(83)90146-7. [DOI] [PubMed] [Google Scholar]
- 122.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 13.Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;37:161–186. doi: 10.1016/0002-8703(49)90562-1. [DOI] [PubMed] [Google Scholar]
- 14.Henein M, Lindqvist P, Francis D, Mörner S, Waldenström A, Kazzam E. Tissue Doppler analysis of age-dependency in diastolic ventricular behaviour and filling. Eur Heart J. 2002;23:162–171. doi: 10.1053/euhj.2001.3032. [DOI] [PubMed] [Google Scholar]
- 15.Mehta A, Ricci R, Widmer U, Dehout F, Garcia de Lorenzo A, Kampmann C, Linhart A, Sunder-Plassmann G, Ries M, Beck M. Fabry disease defined: baseline clinical manifestations of 366 patients in the Fabry Outcome Survey. Eur J Clin Invest. 2004;34:236–242. doi: 10.1111/j.1365-2362.2004.01309.x. [DOI] [PubMed] [Google Scholar]
- 16.Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, Waldek S, Caplan L, Linthorst GE, Desnick RJ International Collaborative Fabry Disease Study Group. Safety and efficacy of recombinant human alpha-galactosidase A—replacement therapy in Fabry's disease. N Engl J Med. 2001;345:9–16. doi: 10.1056/NEJM200107053450102. [DOI] [PubMed] [Google Scholar]
- 17.Weidemann F, Breunig F, Beer M, Sandstede J, Turschner O, Voelker W, Ertl G, Knoll A, Wanner C, Strotmann JM. Improvement in cardiac function during enzyme replacement therapy in patients with Fabry disease: a prospective strain rate imaging study. Circulation. 2003;108:1299–1301. doi: 10.1161/01.CIR.0000091253.71282.04. [DOI] [PubMed] [Google Scholar]
- 18.Hughes DA, Eliott PM, Shah J, Zuckerman J, Coghlan G, Brookes J, Mehta AB. Effects of enzyme replacement therapy on the cardiomyopathy of Anderson-Fabry disease: a randomised, double-blind, placebo-controlled clinical trial of agalsidase alfa. Heart. 2008;94:153–158. doi: 10.1136/hrt.2006.104026. [DOI] [PubMed] [Google Scholar]
- 19.Elleder M, Bradova V, Smid F, Budĕsínský M, Harzer K, Kustermann-Kuhn B, Ledvinová J, Bĕlohlávek, Král V, Dorazilová V. Cardiocyte storage and hypertrophy as a sole manifestation of Fabry's disease. Report on a case simulating hypertrophic non-obstructive cardiomyopathy. Virchows Arch A Pathol Anat Histopathol. 1990;417:449–455. doi: 10.1007/BF01606034. [DOI] [PubMed] [Google Scholar]
- 20.Wilcox WR, Oliveira JP, Hopkin RJ, Ortiz A, Banikazemi M, Feldt-Rasmussen U, Sims K, Waldek S, Pastores GM, Lee P, Eng CM, Marodi L, Stanford KE, Breunic F, Wanner C, Warnock DG, Lemay RM, Germain DP. Females with Fabry disease frequently have major organ involvement: Lessons from the Fabry Registry. Mol Gen Met. 2008;93:112–129. doi: 10.1016/j.ymgme.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Kampmann C, Linhart A, Baehner F, Palecek T, Wiethoff CM, Miebach E, Whybra C, Gal A, Bultas J, Beck M. Onset and progression of Anderson-Fabry disease related cardiomyopathy. Int J Cardiol. 2008;130:367–373. doi: 10.1016/j.ijcard.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Pieroni M, Chimenti C, Ricci R, Sale P, Russo MA, Frustaci A. Early detection of Fabry cardiomyopathy by tissue Doppler imaging. Circulation. 2003;107:1978–1984. doi: 10.1161/01.CIR.0000061952.27445.A0. [DOI] [PubMed] [Google Scholar]