This editorial refers to ‘Stroke in paroxysmal atrial fibrillation: report from the Stockholm Cohort of Atrial Fibrillation’†, by L. Friberg et al. on page 967
Atrial fibrillation (AF) is accompanied by substantial morbidity1 and is increasing in both incidence and prevalence.2,3 Stroke is the chief hazard from AF, and is five times more likely among individuals with AF than among those without the condition.4 Moreover, AF-related strokes are associated with an ∼50% increased odds of disability and a 60% increased odds of death at 3 months compared with strokes of other aetiologies.5 The need for effective therapies that reduce morbidity from AF is underscored by the presence of an increasingly ageing population, particularly because the elderly are at increased risk for AF-related complications such as stroke.6
Although several stroke risk stratification schemes exist, which facilitate personalized thrombo-embolism prophylaxis for individuals with AF,1 the underprescription of thrombo-embolism prophylaxis represents an established barrier to care.7–9 The current AF classification scheme endorsed by the American College of Cardiology, American Heart Association, and European Society of Cardiology does not explicitly take stroke risk into account.1 Rather, the AF classification scheme emphasizes rhythm-based patterns of disease. AF is classified as paroxysmal if it self-terminates within 1 week, persistent if it continues beyond this period and is not self-terminating, or permanent if attempts to terminate the rhythm fail or no attempts are made.
Friberg et al. have now attempted to discern whether the incidence of stroke in AF differs according to AF pattern.10 The investigators performed a retrospective, observational analysis among patients diagnosed with AF at a single hospital or primary care centre in the vicinity of Stockholm, Sweden. AF status was ascertained by chart review and patterns were classified in accordance with existing consensus guidelines, although definitions were altered so that subjects who were cardioverted were not included among those classified as having paroxysmal disease. AF classifications were based on review of medical records from subjects' encounters at the hospital and primary care centre. Those with persistent AF were excluded from the analysis. Stroke was ascertained by the National Register of Hospital Discharges, and medication administration was based on the last recorded follow-up.
The study sample consisted of 855 subjects with paroxysmal AF and 1126 with permanent AF. After a follow-up of ∼3 years, 77 strokes occurred among those with paroxysmal AF, and 116 among those with permanent AF. The primary finding was that the incidence of ischaemic stroke was similar between those with paroxysmal AF and those with permanent AF (incidence rate 26 vs. 29 per 1000 patient-years, P = 0.54). The hazard ratio (HR) for ischaemic stroke was similar for paroxysmal and permanent AF, even after adjusting for established stroke risk factors and warfarin use [HR 1.1, 95% confidence interval (CI) 0.78–1.56]. Moreover, the investigators observed an ∼2-fold increase in the standardized incidence of ischaemic stroke for both paroxysmal and permanent AF as compared with the general population. Although the authors also assessed the incidence and hazard of haemorrhagic stroke, the analysis was underpowered to detect true differences, as only 23 subjects experienced a haemorrhagic stroke in the entire sample. As expected, warfarin use at last follow-up was associated with a substantially diminished incidence of stroke (HR 0.44, 95% CI 0.30–0.65) relative to those who were not taking warfarin.
As acknowledged by the authors, retrospective analyses have limitations. Among the drawbacks of such a study design is the potential for misclassification of the pattern of AF or the type of stroke. For example, in the study of Friberg et al., many of those classified as having paroxysmal AF on the basis of medical encounters actually may have had more chronic forms of AF, particularly if they were asymptomatic with AF and did not seek medical attention, or if they sought medical care at other facilities. This misclassification would be likely to mask a true difference in stroke rates between paroxysmal and permanent AF. Another important limitation of this retrospective analysis is that treatments and other confounders that affect stroke risk were not randomly allocated between the paroxysmal and permanent AF groups. Although adjustment for thrombo-embolism prophylaxis may minimize the impact of such confounding, other unmeasured confounders similarly may be imbalanced and therefore can substantially bias the results.
Nevertheless, this study is an important reminder of prior lessons learned. In 1994, a meta-analysis of randomized trials of antithrombotic therapy reported that stroke risk does not differ according to AF pattern (Table 1).6 Unfortunately, this lesson has not been heeded. Rather, decisions to prescribe thrombo-embolism prophylaxis may be more influenced by perceived rhythm-based patterns of AF than by an individual's stroke risk.7 The underestimation of stroke risk is one factor contributing to the underprescription of thrombo-embolism prophylaxis.11 Friberg and colleagues as well as others previously have reported that individuals with paroxysmal AF are less likely to receive thrombo-embolism prophylaxis than those with more chronic forms of AF regardless of stroke risk.8,9 Similarly, data suggest that there remains a general misconception that pharmacological rhythm control reduces the risk of stroke in individuals with AF.12 While not proven, the logic influencing these practice observations is probably predicated on the notion that individuals who experience less AF (i.e. those with paroxysmal AF) experience less atrial mechanical dysfunction, a factor commonly cited in the pathogenesis of AF-related stroke, and thus a reduced risk of stroke itself.
Table 1.
Association between AF rhythm-based pattern and stroke
| Study | No. with AF | AF types | Stroke risk | Adjustment for stroke risk factors? |
|---|---|---|---|---|
| Sage et al.16 | 140 | Intermittent, chronic | NS | No |
| Roy et al.17 | 254 | Paroxysmal, chronic | NS | No |
| Petersen and Godtfredsen18 | 426 | Paroxysmal, chronic | Chronic >paroxysmal | Yes |
| Treseder et al.19 | 414 | Transient, constant | Constant >transient | No |
| Kopecky et al.20 | 97 | Isolated, recurrent, chronic | NS | No |
| Cabin et al.21 | 272 | Paroxysmal, chronic | NS | No |
| Moulton et al.22 | 265 | Paroxysmal, sustained | NS | No |
| Atrial Fibrillation Investigators6 | 3706 | Paroxysmal, constant | NS | No |
| Levy et al.23 | 756 | Paroxysmal, recent onset, chronic | NS | Yes |
| Hart et al.24 | 2012 | Intermittent, sustained | NS | Yes |
| Hohnloser et al.25 | 6706 | Paroxysmal, sustained | NS | Yes |
| Friberg et al.10 | 1981 | Paroxysmal, permanent | NS | Yes |
NS, not significant.
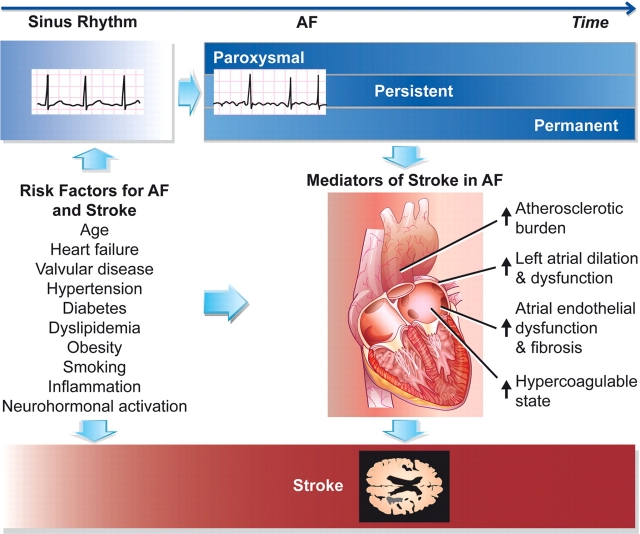
There are several pitfalls with this logic. First, prospective evidence from randomized controlled trials does not support the notion that rhythm control strategies reduce the risk of ischaemic stroke in AF.13 Secondly, ambulatory monitoring reveals that asymptomatic sustained AF occurs more frequently than symptomatic AF among individuals with paroxysmal disease, suggesting that clinical classification of AF on the basis of clinical encounters and occasional electrocardiograms may drastically underestimate the true burden of AF.14 Thirdly, approximately a quarter of strokes in AF are estimated to be non-cardioembolic.15 Thus, the relative contribution of AF duration to stroke risk remains unclear. Currently defined rhythm-based patterns of AF do not distinguish stroke risk (Figure 1). At the present time, clinicians should rely on clinical guidelines that advocate antithrombotic therapy on the basis of established risk factors for stroke and bleeding.1 Risk can be more accurately estimated using validated prediction algorithms.1
Figure 1.
Risk of stroke in AF. Patterns of recurrent AF may be classified as paroxysmal, persistent, or permanent. A hypothetical paradigm is displayed in which the probability of a given pattern of AF varies over the lifecourse of AF, with darker blue shading indicating a higher probability corresponding to a given pattern. Shared risk factors for incident AF and stroke are indicated, as are several mediators of stroke once a patient develops AF. The risk of stroke, displayed in red at the bottom of the figure, is greater once in AF as compared with sinus rhythm, and is generally similar across paroxysmal, persistent, and permanent patterns of AF.
What then, is the value of the currently endorsed AF pattern-based classification scheme? In research, classification of individuals based on patterns of AF has been difficult. AF is characteristically transient, and therefore conventional methods for monitoring AF rhythm are bound to result in misclassification of the AF pattern. Clinically, these distinctions represent convenient proxies that identify the prevalence of co-morbidities commonly associated with each separate pattern of AF. However, the independent role of these patterns for distinguishing the response to various therapies, prediction of morbidity, and prediction of survival is uncertain. Moreover, it remains unclear whether these distinctions merely represent different stages of AF or separate biological subtypes of disease.
Our understanding of AF pathogenesis has grown substantially in the past several years, with new insights into the genetic, molecular, and electrophysiological mediators of this disease. This knowledge presents an opportunity to re-examine the classification of AF in order to determine whether convenient distinctions that effectively summarize both pathogenic and clinical factors are possible. In the meantime, clinicians should recognize that currently defined AF patterns are not useful for approximation of stroke risk.
Conflict of interest: none declared.
References
- 1.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–e354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 3.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 5.Lamassa M, Di Carlo A, Pracucci G, Basile AM, Trefoloni G, Vanni P, Spolveri S, Baruffi MC, Landini G, Ghetti A, Wolfe CD, Inzitari D. Characteristics, outcome, and care of stroke associated with atrial fibrillation in Europe: data from a multicenter multinational hospital-based registry (The European Community Stroke Project) Stroke. 2001;32:392–398. doi: 10.1161/01.str.32.2.392. [DOI] [PubMed] [Google Scholar]
- 6.Atrial Fibrillation Investigators. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154:1449–1457. [PubMed] [Google Scholar]
- 7.Nieuwlaat R, Capucci A, Lip GY, Olsson SB, Prins MH, Nieman FH, Lopez-Sendon J, Vardas PE, Aliot E, Santini M, Crijns HJ. Antithrombotic treatment in real-life atrial fibrillation patients: a report from the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2006;27:3018–3026. doi: 10.1093/eurheartj/ehl015. [DOI] [PubMed] [Google Scholar]
- 8.Friberg L, Hammar N, Ringh M, Pettersson H, Rosenqvist M. Stroke prophylaxis in atrial fibrillation: who gets it and who does not? Report from the Stockholm Cohort-study on Atrial Fibrillation (SCAF-study) Eur Heart J. 2006;27:1954–1964. doi: 10.1093/eurheartj/ehl146. [DOI] [PubMed] [Google Scholar]
- 9.Waldo AL, Becker RC, Tapson VF, Colgan KJ. Hospitalized patients with atrial fibrillation and a high risk of stroke are not being provided with adequate anticoagulation. J Am Coll Cardiol. 2005;46:1729–1736. doi: 10.1016/j.jacc.2005.06.077. [DOI] [PubMed] [Google Scholar]
- 10.Friberg L, Hammar N, Rosenqvist M. Stroke in paroxysmal atrial fibrillation: report from the Stockholm Cohort of Atrial Fibrillation. Eur Heart J. 2010;31:967–975. doi: 10.1093/eurheartj/ehn599. First published on 6 November 2009. doi:10.1093/eurheartj/ehn599. [DOI] [PubMed] [Google Scholar]
- 11.Bungard TJ, Ghali WA, Teo KK, McAlister FA, Tsuyuki RT. Why do patients with atrial fibrillation not receive warfarin? Arch Intern Med. 2000;160:41–46. doi: 10.1001/archinte.160.1.41. [DOI] [PubMed] [Google Scholar]
- 12.Rowan SB, Bailey DN, Bublitz CE, Anderson RJ. Trends in anticoagulation for atrial fibrillation in the U.S.: an analysis of the national ambulatory medical care survey database. J Am Coll Cardiol. 2007;49:1561–1565. doi: 10.1016/j.jacc.2006.11.045. [DOI] [PubMed] [Google Scholar]
- 13.Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 14.Page RL, Wilkinson WE, Clair WK, McCarthy EA, Pritchett EL. Asymptomatic arrhythmias in patients with symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia. Circulation. 1994;89:224–227. doi: 10.1161/01.cir.89.1.224. [DOI] [PubMed] [Google Scholar]
- 15.Hart RG, Halperin JL, Pearce LA, Anderson DC, Kronmal RA, McBride R, Nasco E, Sherman DG, Talbert RL, Marler JR. Lessons from the Stroke Prevention in Atrial Fibrillation trials. Ann Intern Med. 2003;138:831–838. doi: 10.7326/0003-4819-138-10-200305200-00011. [DOI] [PubMed] [Google Scholar]
- 16.Sage JI, Van Uitert RL. Risk of recurrent stroke in patients with atrial fibrillation and non-valvular heart disease. Stroke. 1983;14:537–540. doi: 10.1161/01.str.14.4.537. [DOI] [PubMed] [Google Scholar]
- 17.Roy D, Marchand E, Gagne P, Chabot M, Cartier R. Usefulness of anticoagulant therapy in the prevention of embolic complications of atrial fibrillation. Am Heart J. 1986;112:1039–1043. doi: 10.1016/0002-8703(86)90318-2. [DOI] [PubMed] [Google Scholar]
- 18.Petersen P, Godtfredsen J. Embolic complications in paroxysmal atrial fibrillation. Stroke. 1986;17:622–626. doi: 10.1161/01.str.17.4.622. [DOI] [PubMed] [Google Scholar]
- 19.Treseder AS, Sastry BS, Thomas TP, Yates MA, Pathy MS. Atrial fibrillation and stroke in elderly hospitalized patients. Age Ageing. 1986;15:89–92. doi: 10.1093/ageing/15.2.89. [DOI] [PubMed] [Google Scholar]
- 20.Kopecky SL, Gersh BJ, McGoon MD, Whisnant JP, Holmes DR, Jr, Ilstrup DM, Frye RL. The natural history of lone atrial fibrillation. A population-based study over three decades. N Engl J Med. 1987;317:669–674. doi: 10.1056/NEJM198709103171104. [DOI] [PubMed] [Google Scholar]
- 21.Cabin HS, Clubb KS, Hall C, Perlmutter RA, Feinstein AR. Risk for systemic embolization of atrial fibrillation without mitral stenosis. Am J Cardiol. 1990;65:1112–1116. doi: 10.1016/0002-9149(90)90323-s. [DOI] [PubMed] [Google Scholar]
- 22.Moulton AW, Singer DE, Haas JS. Risk factors for stroke in patients with nonrheumatic atrial fibrillation: a case–control study. Am J Med. 1991;91:156–161. doi: 10.1016/0002-9343(91)90008-l. [DOI] [PubMed] [Google Scholar]
- 23.Levy S, Maarek M, Coumel P, Guize L, Lekieffre J, Medvedowsky JL, Sebaoun A. Characterization of different subsets of atrial fibrillation in general practice in France: the ALFA study. The College of French Cardiologists. Circulation. 1999;99:3028–3035. doi: 10.1161/01.cir.99.23.3028. [DOI] [PubMed] [Google Scholar]
- 24.Hart RG, Pearce LA, Rothbart RM, McAnulty JH, Asinger RW, Halperin JL. Stroke with intermittent atrial fibrillation: incidence and predictors during aspirin therapy. Stroke Prevention in Atrial Fibrillation Investigators. J Am Coll Cardiol. 2000;35:183–187. doi: 10.1016/s0735-1097(99)00489-1. [DOI] [PubMed] [Google Scholar]
- 25.Hohnloser SH, Pajitnev D, Pogue J, Healey JS, Pfeffer MA, Yusuf S, Connolly SJ. Incidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy: an ACTIVE W Substudy. J Am Coll Cardiol. 2007;50:2156–2161. doi: 10.1016/j.jacc.2007.07.076. [DOI] [PubMed] [Google Scholar]