Abstract
Neuropathological studies suggest that the basal forebrain cholinergic system (BFCS) is affected in Alzheimer's disease (AD), but there is no in vivo evidence of early damage to this system in subjects at high risk of developing AD. Here, we found that mild cognitive impairment (MCI) patients exhibited significant volume reduction of the nucleus basalis of Meynert (NbM) using recently developed probabilistic maps of the BFCS space. In addition, volumes of different magnocellular compartments varied significantly with regional gray matter atrophy in regions known to be affected by AD and were found to correlate with cognitive decline in MCI patients. Bilateral reductions of the horizontal nucleus of the diagonal band of Broca (Ch3) and frontal lobe (medial frontal, orbital, subcallosal gyrus, anterior cingulate, and middle frontal gyrus) were significantly associated with a global decline in cognitive status, whereas volume reduction of the posterior compartment of Ch4 (NbM) and temporal lobe (including hippocampus, entorhinal cortex, and amygdala) were associated with impaired delayed recall in MCI patients. These findings establish, for the first time, a link between degeneration of specific cholinergic compartments of the BFCS and cognitive-related deficits in subjects at high risk of developing AD.
Keywords: Alzheimer's disease, basal forebrain, cerebral aging, cholinergic system, mild cognitive impairment
Introduction
A large body of evidence suggests that basal forebrain (BF) cholinergic neurons and basalo-cortical cholinergic pathways are selectively vulnerable to degeneration in Alzheimer's disease (AD). This is mainly supported by postmortem studies that found severe neurofibrillary degeneration and cell loss within the BF cholinergic system (BFCS) of AD patients, these being most pronounced in the nucleus basalis of Meynert (NbM; Whitehouse et al. 1981; McGeer et al. 1984; Arendt et al. 1985; Vogels et al. 1990), as well as by a decline of cortical choline-acetyl transferase (ChAT) activity (Nagai et al. 1983; McGeer et al. 1984). Further research has also confirmed that the extent of cholinergic loss correlates positively with dementia severity (Baskin et al. 1999; Pappas et al. 2000), suggesting that cholinergic dysfunctions could also be partially responsible for the cognitive deficits observed in AD patients (Coyle et al. 1983).
Few in vivo imaging studies have explored the integrity of BF in AD patients (Hanyu et al. 2002; Tanaka et al. 2003; Teipel et al. 2005; Whitwell, Weigand, et al. 2007). A first approach to BF morphometry showed that the thickness of the substantia innominata was significantly reduced in AD patients and that these changes correlated positively with Mini Mental State Examination (MMSE) scorings, likely revealing a cholinergic degeneration of the NbM (Hanyu et al. 2002). These findings were later corroborated (Whitwell, Weigand, et al. 2007) and demonstrated to be predictive of the magnitude of response to treatment with an acetylcholinesterase (AChE) inhibitor (Tanaka et al. 2003). By using a voxel-based morphometry (VBM) approach based on proton-diffusion-weighted images, Teipel et al. (2005) found reduced signals of BF cholinergic nuclei in AD patients by comparing the areas of reduced intensity in AD with an MNI template map of the cholinergic nuclei based on histology and magnetic resonance imaging (MRI) of one postmortem brain. They also showed a positive correlation between signal decrease in anterior–lateral NbM and reduced cortical gray matter density assessed through VBM.
Mild cognitive impairment (MCI) patients represent a high-risk population to develop AD (Petersen 2004). Supporting this assumption, MCI patients show a 4-fold increased risk for the development of AD compared with healthy elderly people (Ganguli et al. 2004). Indeed, between 19% and 50% of MCI patients progress to dementia (usually AD) over a period of 3 years (Chertkow 2002). It is still under debate whether degeneration of the BFCS appears early or late during the course of AD (Mesulam 2004) or even in populations at high risk in developing AD. Postmortem studies showed that mild AD is not associated with a loss of cortical ChAT (DeKosky et al. 2002) and counts of ChAT-positive cells revealed a similar number of cholinergic neurons in the NbM in MCI, early AD patients, and healthy elderly (Gilmor et al. 1999). But comparable amounts of BF cholinergic cells do not necessarily reflect an intact and fully functional cholinergic system because shrinkage of cholinergic neurons has also been observed in AD patients (Vogels et al. 1990), likely revealing a significant loss of synaptic contacts within cortical projection sites.
Because postmortem material is mainly obtained at advanced stages of the disease and histological approaches do not allow for longitudinal observations, in vivo imaging studies of BF integrity focused on high-risk AD groups have significant practical implications in early detection of AD. Morphometric MRI studies have started to shed light on this possibility. Thus, Teipel et al. (2007) used a deformation-based approach to compare patterns of gray matter atrophy in AD and MCI patients. They found, among other areas, a reduced volume of the BF in both MCI and AD patients when compared with elderly controls. More recently, Hall et al. (2008) searched for presymptomatic markers in asymptomatic subjects that later developed AD and observed atrophy of the BF as long as 4.5 years before symptom onset, pointing out its significance as a presymptomatic marker of AD. But there is still a lack of information on how atrophy spreads among different BF cholinergic nuclei in high-risk AD and AD patients, because mapping of cholinergic BF compartments to standard anatomical MNI space was obtained quite recently (Teipel et al. 2005; Zaborszky et al. 2008).
Here, we used a new diffeomorphic large deformation registration algorithm (Ashburner 2007) to explore global variations in the BF volume between patients diagnosed with amnestic MCI and age-matched healthy elderly subjects. Significant volumetric differences in BF were compared with the localization of cholinergic nuclei by using newly developed probabilistic, cytoarchitectonic maps of BF magnocellular compartments in MNI space (Zaborszky et al. 2008). In addition, volumes of different BF compartments were correlated with volume variations in cerebral gray matter of MCI patients, using modulated VBM with high-dimensional image registration. We further correlated volume changes of these BF magnocellular compartments with scorings obtained in neuropsychological tests to determine whether structural variations in BF cholinergic groups parallel cognitive decline in subjects at high risk of developing AD.
Material and Methods
Subjects
Thirty-three amnestic MCI patients and 28 cognitively normal volunteers were recruited from the local community and the Dementia Unit of the Neurology Service at the University Hospital Virgen del Rocio, respectively. MCI patients and healthy elderly subjects were matched for age, educational years, and handedness. Demographics and neuropsychological profile of control and MCI groups are shown in Table 1. All participants provided signed informed consent before any testing. Study protocols were previously approved by the Ethical Committee for Clinical Investigations at the University Hospital Virgen del Rocio and the Ethical Committee for Human Research at the University Pablo de Olavide.
Table 1.
Subject demographics
| Controls (n = 28) | MCI (n = 33) | P < | |
| Age, year (M ± SD) | 66.6 ± 5.1 | 69.6 ± 7.6 | 0.08 |
| Gender (F/M) | 16/12 | 13/20 | N/A |
| Education, year (M ± SD) | 11.9 ± 5.9 | 11.2 ± 6.3 | 0.3 |
| MMSE (M ± SD) | 28.4 ± 1.3 | 26.6 ± 2.5 | 0.001 |
| CDR (sum of boxes) | 0 | 0.5 | N/A |
| Immediate recall (M ± SD) | 14.3 ± 2.8 | 9.9 ± 2.2 | 10–11 |
| Delayed recall (M ± SD) | 12.9 ± 2.9 | 6.3 ± 3.1 | 10–12 |
| APOE (ϵ4/non-ϵ4) | 4/24 | 17/16 | N/A |
Note: M ± SD (mean ± standard deviation). F (females) and M (males). MMSE: Mini Mental State Exam, where the range from best to worst performance is 30–0. CDR: clinical dementia rating, where CDR = 0 no dementia, CDR = 0.5 questionable or very mild dementia. N/A (not applicable).
The diagnosis of MCI was based on consensus criteria (Petersen et al. 1999): 1) subjective memory complaints confirmed by the informant, 2) objective memory decline on neuropsychological tests evidenced by scores ≥1.5 standard deviations (SDs) below the age-appropriate mean, 3) clinical dementia rating (CDR) global score of 0.5 (questionable dementia), 4) normal independence function, judged both clinically and by means of the interview for deterioration in daily living activities validated in the Spanish population (Böhm et al. 1998), and 5) not meeting diagnostic and statistical manual of mental disorders - IV criteria for dementia. Cognitive performance was further assessed using neuropsychological tests for immediate and delayed (30 min) verbal memory (Wechsler 1987) adapted to the Spanish population. Depression was excluded by clinical interview and the Geriatric Depression Scale (GDS) of Yesavage (shorter form). The GDS cutoff to be included in the study was 0–5. The diagnosis of MCI was finally based on a clinical consensus after evaluation in the dementia unit by a senior neurologist and a clinical neuropsychologist.
Inclusion criteria for the healthy elderly group were 1) no subjective memory complaints corroborated by neuropsychological exploration, 2) CDR global score of 0 (no dementia), and 3) normal independent function judged both clinically and by means of a standardized scale for the activities of daily living. None of them had a history of neurological, psychiatric disorders, and/or major medical illness.
The use of any pharmacological compounds (e.g., cholinesterase inhibitors) known to affect the cognitive function was considered a cause for exclusion, in both controls and MCI patients. Individuals with a history of stroke and/or significant cerebrovascular conditions, clinically significant sensory impairment, neurological conditions such as epilepsy, traumatic brain injury, and brain tumors, presence of neuropsychiatric disorders (mainly major depression), past or current alcohol abuse, or those with extremely low educational levels were also excluded from the study.
MRI Acquisition
Two high-resolution three-dimensional (3D) T1-weighted magnetization-prepared rapid gradient echo (MP-RAGE) images were acquired in the same session on a whole-body Philips Intera 1.5-T MRI scanner (Philips, The Netherlands). MP-RAGE parameters were empirically optimized for gray–white contrast (repetition time = 8.5 ms, echo time = 4 ms, flip angle = 8°, matrix dimensions 256 × 192, 184 contiguous sagittal 1.2-mm-thick slices, and time per acquisition = 5.4 min).
MRI Processing
Figure 1 shows a flowchart of the main preprocessing steps and further computational analysis used in the present study. MRI data were processed by using statistical parametric mapping (SPM5, Wellcome Trust Center for Neuroimaging). Images were visually inspected for scanner artifacts (e.g., blurring due to head motion, inadequate gray–white matter contrast, and intensity nonuniformities) and gross anatomical abnormalities unrelated to AD. Four cases were excluded from the study (2 controls and 3 MCI) based on scanner quality/low contrast criteria. These scans were excluded before any analysis was done on the images. Brain scans were manually reoriented, and the stereotaxic origin of each image was manually set to the anterior commissure at the level of the interhemispheric plane (Talairach and Tournoux 1988). More specifically, reference points were the middle of the anterior commissure in the left-right direction (x-coordinate), the middle of the anterior commissure in the anterior-posterior direction (y-coordinate), and the superior border of the anterior commissure in cranial-ventral direction (z-coordinate).
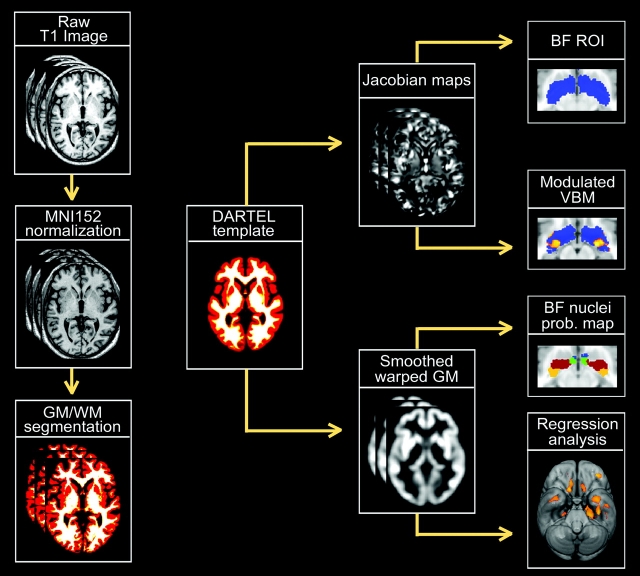
Figure 1.
Flowchart displaying a scheme of the analysis protocol. Left column (starting from top) shows the SPM5 preprocessing steps applied to each individual raw T1 MRI scan. After linearly normalizing each image to the MNI152 space, they were partitioned into different tissue classes: gray matter (GM), white matter (WM), and cerebrospinal fluid. GM and WM maps were then registered to their average by using an efficient large deformation diffeomorphic framework (DARTEL) to minimize anatomical variations between subjects (Ashburner 2007). Jacobian determinant maps were derived from the DARTEL flow fields, log transformed, and analyzed to determine volumetric changes within the BF magnocellular compartments between healthy elderly and MCI patients. The BF region of interest was based on probabilistic maps of BF compartments obtained from postmortem brains (Zaborzsky et al. 2008). In addition, the volume for each BF magnocellular group was obtained by summing up the jacobian determinant values within a mask of each corresponding compartment, which was obtained by applying a threshold above 50% to each probability map. These volumes were then regressed on the smoothed modulated warped gray matter maps.
To adjust for individual differences in global brain scale and head alignment, MR images were further normalized to the MNI152 T1-template using a 12-parameter affine transformation without nonlinear steps (Collins et al. 1994). Spatially normalized MR images were next partitioned into different tissue classes by using a unified segmentation approach (Ashburner and Friston 2005) to obtain gray and white matter maps in MNI space with an isotropic voxel size of 1.5 mm3. These gray and white matter maps were then registered to their average using the DARTEL toolbox, an efficient large deformation diffeomorphic framework implemented in SPM5 that has demonstrated to minimize anatomical variations between subjects (Ashburner 2007). Jacobian determinant maps, derived from the DARTEL flow fields, provide information about the local expansion and contraction, at the voxel level, necessary to deform one image to match the group template. These maps were resliced to a final isotropic voxel size of 1 mm3 and log transformed.
Application of Cytoarchitectonic Maps of the BF Cholinergic Nuclei to In Vivo MR Images
The primate BF is characterized by the presence of a continuous collection of aggregated and nonaggregated, large, hyperchromic neurons, the so-called magnocellular BF system. This system mainly comprises the NbM, nucleus of the diagonal band of Broca, and medial septum, with direct cholinergic projections to amygdala, hippocampus, and cortical mantle (Hedreen et al. 1984). For delineation of the magnocellular BF compartments, a modified version of the Ch1–Ch4 nomenclature of Mesulam (Mesulam et al. 1983) was used (Zaborszky et al. 2008). Areas containing such magnocellular cell groups within the septum, the horizontal and vertical limbs of the diagonal band, and in the sublenticular BF were delineated in histological sections of 10 human postmortem brains by using staining with a modified Gallyas's silver method for cell bodies (Merker 1983). This staining yielded high contrast of cell bodies for cytoarchitectonic analysis and delineation of the magnocellular cell groups (Zaborszky et al. 2008). The postmortem brains were collected and processed (Zilles et al. 2002; Amunts et al. 2007) before reliable antibodies for ChAT became available. Because cholinergic cells are typically aggregated in clusters, constitute most of the large neurons within the BF, and 90% of the magnocellular perikarya in the Ch4 compartment are cholinergic (Mesulam et al. 1983), the space identified based on the presence of large neurons in high-resolution histological sections is likely to represent the cholinergic space of the BF, that is, the volume occupied by the cortically projecting cholinergic cell bodies (Zaborszky et al. 2008).
Briefly, the Ch1–Ch2 compartment largely corresponds to the medial septal nucleus and the nucleus of the diagonal band of Broca. The more dorsal part of this area corresponds to the Ch1 group of Mesulam et al. (1983), whereas the ventral part of this compartment (Ch2 group of Mesulam) merges laterally with the Ch3 cell groups. Because the boundaries between Ch1 and Ch2 were arbitrary, these 2 regions were merged and considered to be the same compartment in all analyses performed in this study (Zaborszky et al. 2008). Our Ch3 compartment can be regarded as linking cell groups in the medial septum-vertical diagonal band nucleus (Ch1–2) with the more elaborate magnocellular cell groups in the sublenticular forebrain (Ch4) and mainly corresponds to the horizontal nucleus of the diagonal band of Broca (HDB). The Ch4 compartment largely corresponds to the NbM. We did not differentiate subcomponents of the Ch4 compartment as suggested by Mesulam et al. (1983). The large neurons in the Ch4 compartment extend as far rostrally as cell aggregates underneath the nucleus accumbens. Large aggregates of the darkly stained cell group within the ventrolateral edge of the bed nucleus of the stria terminalis at the border of the internal capsule/anterior commissure were also classified as Ch4. Ch4p included magnocellular cell groups within a well-defined area that begins caudal to the supraoptic nucleus at the level where the optic tract adjoins the internal capsule/cerebral peduncle and extends progressively laterally up to the most caudal level of the medial mammillary nucleus and the central nucleus of the amygdala. For a more detailed description of each magnocellular compartment, see Zaborszky et al. (2008).
Individual compartments were registered to the single-subject template of the MNI space, and probability maps were calculated separately for each Ch compartment. Briefly, histological sections of 10 postmortem brains were reconstructed in 3D (“histological volume”) from the digitized histological sections using the MRI scans of the fixed brain as a shape reference with an isotropic resolution of 1 mm (Amunts and Zilles 2006). Each 3D reconstructed histological volume was spatially normalized to the single-subject T1-weighted MNI reference brain (Collins et al. 1994). Based on the whole 3D brain reconstructions, volume and shape of each magnocellular cell group were computed. Probability maps were calculated separately for each Ch compartment. Based on the sample of 10 brains, they describe the relative frequency with which the same BF area (e.g., Ch1–Ch2; Ch3 = HDB, Ch4, or Ch4p) was represented in each voxel of the reference space (Amunts and Zilles 2006). A voxel was assigned to a structure that had the highest probability or that exceeded a threshold of 40%. For a full explanation of the histological processing, spatial normalization and generation of probabilistic maps, see Zaborzsky et al. (2008).
In order to search volume changes within the whole BFCS between healthy controls and MCI patients, we created a binary mask of the combined probability maps (Ch1–Ch2, Ch3 = HDB, Ch4, and Ch4p) including all voxels showing any probability of belonging to a cholinergic nucleus (BF region of interest [ROI]). For further correlation analysis, a mask of each magnocellular compartment (Ch1–Ch2, Ch3 = HDB, Ch4, and Ch4p) was created by applying a threshold of 50% to the corresponding probability map. Thus, for these masks, only those voxels were considered that were present in more than 5 postmortem brains. To obtain a more accurate matching of these masks with the average anatomy of our population, we estimated a DARTEL warping (Ashburner 2007) from the MNI single-subject template to our group average template, and the resulting warps were applied to the masks (see Fig. 1 for a schematic overview of the different masks).
Determining Changes in Gray Matter Volume: DARTEL-Modulated Voxel-Based Morphometry
We used modulated VBM in combination with the DARTEL framework to evaluate whether or not changes in cerebral gray matter volume accompany volume variations of different BF magnocellular compartments in MCI patients.
Individual gray matter maps were warped to the group average template and rescaled by the Jacobian determinants on a voxel-by-voxel basis. This “modulation” adjusts for alterations of voxel volumes due to shrinkage or expansion during nonlinear registration and tends to preserve the absolute amount (i.e., volume) of gray matter before image warping (Good et al. 2001). For statistical purposes, modulated warped gray matter maps were smoothed with a Gaussian kernel of full width at half maximum of 8 mm.
Statistical Analysis
Testing for Volumetric Changes in BF Magnocellular Compartments
To test the null hypothesis of no volumetric differences in the BFCS between the 2 groups (healthy elderly subjects and MCI patients), voxelwise statistical tests (2-sample t-test) were applied to the logged Jacobian determinant maps. Analyses were restricted to voxels within the BF ROI, and the contrast matrix was set to show only effects in the direction of reduced volume in MCI group compared with controls. Due to our previous hypothesis and the relatively small search volume restricted to the BF ROI, effects were considered significant if they reached a statistical threshold of P < 0.01, uncorrected for multiple comparisons (Teipel et al. 2005). The minimal cluster size was set at 5 contiguous voxels (5 mm3). Because age was slightly different (but statistically similar) in the 2 groups, differences were additionally corrected for age in an independent regression model where this variable was introduced as a covariate of no interest. To further test for a possible influence of APOE epsilon4 status on the BF structural integrity, we performed additional analyses between MCI APOE4 carriers (N = 17) and noncarriers (N = 16). For interpretation purposes, the t-maps of effects within the BF ROI were compared with the probabilistic maps of BF magnocellular groups, and peak coordinates of effects were transformed into the Talairach space (Lancaster et al. 2007).
Correlations between Volumes of BF Magnocellular Compartments and Volumes of Cerebral Gray Matter
We explored if the volume of each BF magnocellular group correlated with significant regional volume loss of cerebral gray matter in MCI patients. To this end, the sum of Jacobian determinant values within the more stringent mask of each magnocellular compartment (above 50% threshold) was calculated for each MCI patient. This measure of relative volume was then regressed on the smoothed modulated gray matter maps, which were proportionally scaled to the global mean, and a relative threshold of 0.4 was applied. Due to the a priori hypothesis of effects within cortical projection sites of cholinergic nuclei known to be affected early in AD pathology, results were assessed at a statistical threshold of P < 0.005 uncorrected for multiple comparisons (Teipel et al. 2005), and the cluster extend threshold was set at 50 contiguous voxels (168.75 mm3).
Correlations between Volumes of BF Magnocellular Compartments and Cognitive Decline
Different measures of cognitive decline were correlated with volumes of specific BF magnocellular compartments (see Fig. 5), and the output of the abovementioned regression on modulated gray matter maps, respectively. To this end, BF probabilistic masks (above 50% threshold) were used to determine the volume of each BF compartment that would be correlated with scores of cognitive tests. Scorings of MMSE, immediate recall and delayed recall tests were separately considered as dependent variables in the Pearson product–moment correlation test. As for the regression on gray matter maps, we used the sum of the Jacobian determinant values within the more stringent mask for each BF magnocellular compartment as a measure of volume. Because volumes of the different BF compartments were associated with volumes of partly distinct regions of cerebral gray matter, we also tested for correlations between gray matter volumes of these regions and the measures of cognitive function. For this, we calculated individual gray matter volumes from the modulated gray matter maps within the clusters that resulted from the regression analyses.
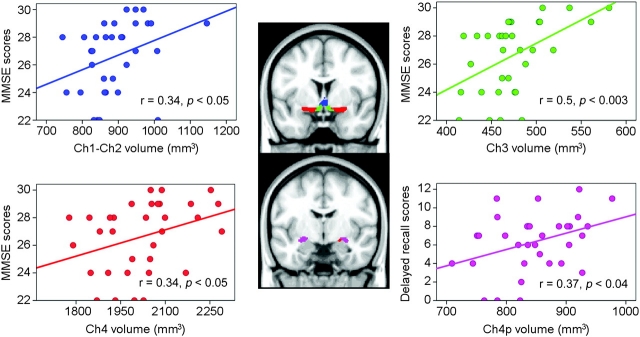
Figure 5.
Scatter plots showing significant relationships between cognitive impairment and volume loss of each BF cholinergic compartment in MCI patients. Note that cognitive function is differently associated with volume reductions of BF nuclei in MCI patients: Global cognitive decline (MMSE scores) are related to volumetric changes in Ch1−Ch2, Ch3 = HDB, and Ch4, whereas impaired delayed recall is associated with volume loss in Ch4p. R (Pearson correlation coefficient) and P (level of significance) values were added to each subplot. Cholinergic nucleus masks are projected onto the MNI152 anatomical space, and color-coded: Ch1–2 (blue), Ch3 = HDB (green), Ch4 (red), and Ch4p (purple). Identical color identification is maintained in scatter plots for each BF nucleus.
Results
Cognitive Performance and APOE4 Genotype
Table 1 shows the demographics, neuropsychological profile and proportion of subjects in each group who presented the allele epsilon4 in the apolipoprotein E (APOE4). MCI patients showed significantly lower scorings when compared with controls in MMSE, immediate, and delayed verbal memory tests. The apolipoprotein E4 seems to be linked to a major risk of early beginning of AD (Herukka et al. 2007; Artero et al. 2008). Our study confirmed this hypothesis; the presence of APOE4 was 3.6 times higher in MCI patients than in healthy controls.
BF Changes in MCI Patients
Talairach coordinates showing maximum reduction of BF regions in MCI patients compared with elderly controls are listed in Table 2. Figure 2 illustrates the location of significant volume reductions in the BF (t-map results), superimposed on the BF ROI (binary mask of the combined probability maps without applying probability thresholds). All significant volume loss in MCI patients (P < 0.01, uncorrected) was restricted to the (NbM) (Ch4 and Ch4p magnocellular groups according to Zaborszky et al. 2008). The most pronounced changes were evident in bilateral posterior and intermediate lateral regions of the nucleus basalis ROI (Ch4 and Ch4p, no thresholded mask). Bilateral volume reductions were also observed in anterior to intermediate medial regions of the nucleus basalis ROI, whereas cluster size was larger on the left hemisphere. Further areas of volume loss included bilateral anterior lateral regions and most posterior lateral regions on the right hemisphere.
Table 2.
Volume loss of BF compartments in MCI patients compared with healthy elderly subjects
| BF compartments | Side | CS | Coordinates |
P | T59 | ||
| x | y | Z | |||||
| Ch4 | L | 465 (312) | −19 | −4 | −11 | 0.001 | 4.63 |
| Ch4p | L | −29 | 1 | −11 | 0.001 | 4.21 | |
| Ch4 | L | −21 | 5 | −11 | 0.01 | 2.58 | |
| Ch4 | L | 378 (49) | −15 | −4 | 4 | 0.001 | 3.72 |
| Ch4 | L | (15) | −9 | −4 | −2 | 0.001 | 3.29 |
| Ch4p | L | −19 | −11 | −5 | 0.001 | 3.14 | |
| Ch4/Ch4p | R | 523 (365) | 25 | −4 | −9 | 0.001 | 4.81 |
| Ch4p | R | 32 | −14 | −9 | 0.005 | 2.86 | |
| Ch4 | R | 21(5) | 21 | 8 | −10 | 0.001 | 3.25 |
| Ch4p | R | 70 (5) | 31 | −19 | −2 | 0.001 | 3.22 |
| Ch4 | R | 41 | 7 | −2 | 0 | 0.005 | 3.08 |
| Ch4 | R | 8 | 15 | 1 | 5 | 0.005 | 2.91 |
| Ch4p | R | 6 | 26 | 4 | −8 | 0.005 | 2.86 |
| Ch4p | R | 6 | 31 | −19 | −8 | 0.005 | 2.84 |
Note: L/R (left and right cerebral hemispheres, respectively). CS (cluster size) was set at 5 contiguous voxels, and values were presented uncorrected and corrected by age (in parentheses). Coordinates in bold delineate a cluster and the peak-effect coordinate (in the Talairach reference space) within the cluster. Subsequent nonbold coordinates identify further peaks within the same cluster. Statistical values are uncorrected voxel-level P values and their corresponding T-values (59 degrees of freedom).
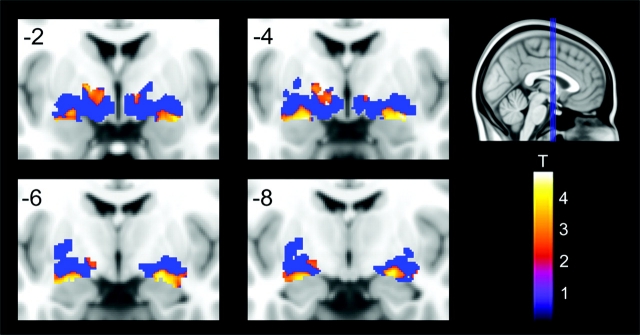
Figure 2.
Bilateral volume reductions of BF in MCI patients compared with healthy elderly subjects, no age corrections (P < 0.01, uncorrected). Coronal slices are from anterior to posterior (y = −2 to y = −8) in intervals of 2 mm. Blue area represents the combined probability maps (Ch1−Ch2, Ch3 = HDB, Ch4, and Ch4p) in MNI space including all voxels showing any probability of belonging to a cholinergic nucleus (BF ROI). Significant volume reductions are coded by the color scale (significance levels represented by T-values).
Most of these clusters remained significant (although reduced in size) after correction for age (Table 2). When compared with the Ch4 and Ch4p probability maps (Zaborszky et al. 2008), significant voxels within bilateral posterior and intermediate lateral regions of the nucleus basalis ROI showed high probability (up to 100%) of corresponding to the Ch4 and Ch4p magnocellular compartments. On the other hand, significant voxels/clusters in anterior lateral and medial regions of the nucleus basalis ROI exhibited less than 40% probability of belonging to the Ch4 + Ch4p compartments. No significant differences in BF volume were found between MCI APOE4 carriers and MCI APOE4 noncarriers, which might be accounted for by the small MCI sample used in the present study. Also, the fact that not all the amnestic MCI patients will evolve to AD, independently of genotype, might critically affect the integrity of BF.
Changes in BF Magnocellular Compartments Associated with Reductions in Regional Cerebral Gray Matter Volume
Results of the correlations between volumes of the BF magnocellular compartments and cerebral gray matter volumes are summarized in Table 3–6 and illustrated in Figures 3 and 4. Volumes of all magnocellular compartments (Ch1–Ch2, Ch3 = HDB, Ch4, and Ch4p) correlated significantly with gray matter volumes of the right medial frontal gyrus, right orbital gyrus, and bilateral precuneus. Volumes of Ch1–Ch2 additionally correlated with gray matter volumes of the bilateral subcallosal gyrus, right anterior cingulate, left middle frontal gyrus, and left temporal pole (Table 3). Volume of Ch3 = HDB compartment further correlated with bilateral hypothalamus (Table 4), and the volume of the NbM (Ch4) varied significantly with the gray matter volume of the right anterior thalamus and bilateral basal ganglia, including the caudate head, putamen, and globus pallidus.
Table 3.
Correlation between Ch1–Ch2 volume and gray matter volume in MCI
| Brain region | CS | Coordinates |
P | T31 | |||
| x | y | z | |||||
| Medial frontal gyrus (BA 9) | R | 1124 | 5 | 47 | 28 | 0.001 | 4.79 |
| Subcallosal gyrus (BA 25) | L | 1123 | −9 | 21 | −7 | 0.001 | 3.77 |
| Subcallosal gyrus (BA 25) | R | 8 | 13 | −5 | 0.001 | 3.64 | |
| Septum | L | −3 | 7 | −1 | 0.001 | 3.49 | |
| Middle frontal gyrus (BA 47) | L | 247 | −42 | 37 | 0 | 0.001 | 3.69 |
| Middle frontal gyrus (BA 10) | L | −36 | 40 | 8 | 0.001 | 3.45 | |
| Precuneus (BA 7) | R | 140 | 1 | −66 | 29 | 0.001 | 3.64 |
| Anterior cingulate (BA 32) | R | 161 | 8 | 40 | 8 | 0.001 | 3.51 |
| Temporal pole (BA 38) | L | 143 | −36 | 14 | −36 | 0.001 | 3.36 |
| Middle frontal gyrus (BA 10) | R | 52 | 32 | 37 | 11 | 0.005 | 3.12 |
| Fusiform gyrus (BA 37) | L | 61 | −36 | −22 | −19 | 0.005 | 3.07 |
| Fusiform gyrus (BA 37) | L | −34 | −33 | −21 | 0.005 | 2.99 | |
Note: L/R (left and right cerebral hemisphere, respectively). CS (cluster size) was set at 50 contiguous voxels. Coordinates in bold delineate a cluster and the peak-effect coordinate (in the Talairach reference space) within the cluster. Subsequent nonbold coordinates identify further peaks within the same cluster. Statistical values are uncorrected voxel-level P values and their corresponding T-values (31 degrees of freedom). BA (Brodmann area).
Table 4.
Correlation between Ch3 (HDB) volume and gray matter volume in MCI
| Brain region | CS | Coordinates |
P | T31 | |||
| x | y | z | |||||
| Precuneus (BA 7) | L | 148 | −1 | −65 | 30 | 0.001 | 3.9 |
| Orbital gyrus (BA 11) | R | 142 | 10 | 41 | −12 | 0.001 | 3.8 |
| Medial frontal gyrus (BA 9) | R | 408 | 7 | 53 | 14 | 0.001 | 3.47 |
| Medial frontal gyrus (BA 9) | R | 4 | 49 | 29 | 0.001 | 3.45 | |
| Hypothalamus | L | 241 | −4 | −7 | −7 | 0.001 | 3.39 |
| Anterior prefrontal cortex (BA 10) | L | 50 | −16 | 52 | 1 | 0.005 | 3.17 |
Note: L/R (left and right cerebral hemispheres, respectively). CS (cluster size) was set at 50 contiguous voxels. Coordinates in bold delineate a cluster and the peak-effect coordinate (in the Talairach reference space) within the cluster. Subsequent nonbold coordinates identify further peaks within the same cluster. Statistical values are uncorrected voxel-level P values and their corresponding T-values (31 degrees of freedom). BA (Brodmann area).
Table 5.
Correlation between Ch4 volume and gray matter volume in MCI
| Brain region | Side | CS | Coordinates |
P | T31 | ||
| x | y | z | |||||
| Putamen | R | 1810 | 20 | 11 | −8 | 0.001 | 4.82 |
| Orbital gyrus (BA 11) | R | 9 | 44 | −13 | 0.001 | 3.3 | |
| Caudate head | R | 11 | 15 | 3 | 0.005 | 3.14 | |
| Putamen | L | 1614 | −25 | 10 | −8 | 0.001 | 5.11 |
| Ventral striatum | L | −15 | 13 | −7 | 0.001 | 4.69 | |
| Caudate head | L | −14 | 12 | 3 | 0.001 | 3.66 | |
| Superior frontal gyrus (BA 9) | R | 395 | 11 | 57 | 30 | 0.001 | 4.33 |
| Middle frontal gyrus (BA 10) | L | 352 | −43 | 41 | 0 | 0.001 | 4.38 |
| Middle frontal gyrus (BA 10) | L | −31 | 41 | 6 | 0.005 | 3.17 | |
| Precuneus (BA 7) | L | 267 | −2 | −65 | 31 | 0.001 | 4.17 |
| Thalamus (anterior nucleus) | R | 59 | 5 | −5 | 7 | 0.001 | 3.40 |
Note: L/R (left and right cerebral hemispheres, respectively). CS (cluster size) was set at 50 contiguous voxels. Coordinates in bold delineate a cluster and the peak-effect coordinate (in the Talairach reference space) within the cluster. Subsequent nonbold coordinates identify further peaks within the same cluster. Statistical values are uncorrected voxel-level P values and their corresponding T-values (31 degrees of freedom). BA (Brodmann area).
Table 6.
Correlation between Ch4p volume and gray matter volume in MCI
| Brain region | Side | CS | Coordinates |
P | T31 | ||
| x | y | z | |||||
| Medial frontal gyrus (BA 9) | R | 689 | 8 | 46 | 28 | 0.001 | 4.58 |
| Posterior hippocampus | L | 355 | −33 | −30 | −5 | 0.001 | 4.46 |
| Middle frontal gyrus (BA 10) | L | 285 | −31 | 41 | 6 | 0.001 | 4.39 |
| Uncus (BA 20) | L | 755 | −36 | −14 | −27 | 0.001 | 4.11 |
| Temporal pole (BA 38) | L | −33 | 7 | −28 | 0.001 | 3.62 | |
| Ch4p/extended amygdala | L | 1096 | −19 | −10 | −10 | 0.001 | 4.08 |
| Parahippocampal gyrus (BA 35) | L | −23 | −22 | −19 | 0.001 | 3.76 | |
| Parahippocampal gyrus (BA 35) | L | −17 | −16 | −27 | 0.001 | 3.41 | |
| Subcallosal gyrus (BA 25) | R | 267 | 11 | 21 | −4 | 0.001 | 4.01 |
| Subcallosal gyrus (BA 25) | R | 10 | 32 | −9 | 0.001 | 3.58 | |
| Posterior cingulate/precuneus (BA 31) | L | 365 | −13 | −55 | 26 | 0.001 | 4.01 |
| Superior temporal gyrus (BA 22) | L | 295 | −50 | −36 | 3 | 0.001 | 3.71 |
| Anterior cingulate (BA 32) | R | 197 | 11 | 42 | 7 | 0.001 | 3.60 |
| Posterior hippocampus | R | 124 | 32 | −30 | −1 | 0.001 | 3.54 |
| Middle temporal gyrus (BA 21) | R | 299 | 42 | −3 | −21 | 0.001 | 3.50 |
| Temporal pole (BA 38) | R | 36 | 8 | −24 | 0.001 | 3.30 | |
| Subcallosal gyrus (BA 25) | L | 109 | −12 | 19 | −5 | 0.001 | 3.34 |
| Middle temporal gyrus (BA 21) | L | 194 | −47 | −14 | −19 | 0.001 | 3.31 |
| Middle temporal gyrus (BA 21) | L | −48 | −3 | −23 | 0.005 | 3.20 | |
| Fusiform gyrus (BA 37) | R | 186 | 38 | −55 | −10 | 0.001 | 3.25 |
| Middle temporal gyrus (BA 37) | R | 41 | −52 | −3 | 0.005 | 3.08 | |
| Angular gyrus (BA 39) | L | 81 | −43 | −63 | 17 | 0.005 | 3.22 |
| Parahippocampal gyrus (BA 28) | R | 59 | 22 | −20 | −19 | 0.005 | 3.19 |
| Parahippocampal gyrus (BA 28) | R | 18 | −17 | −27 | 0.005 | 2.97 | |
| Superior temporal gyrus (BA 41) | R | 96 | 40 | −38 | 12 | 0.005 | 3.14 |
| Middle temporal gyrus (BA 21) | L | 74 | −50 | −26 | −12 | 0.005 | 2.97 |
| Superior temporal gyrus (BA 21) | L | −51 | −22 | −4 | 0.005 | 2.89 | |
Note: L/R (left and right cerebral hemispheres, respectively). CS (cluster size) was set at 50 contiguous voxels. Coordinates in bold delineate a cluster and the peak-effect coordinate (in the Talairach reference space) within the cluster. Subsequent nonbold coordinates identify further peaks within the same cluster. Statistical values are uncorrected voxel-level P values and their corresponding T-values (31 degrees of freedom). BA (Brodmann area).
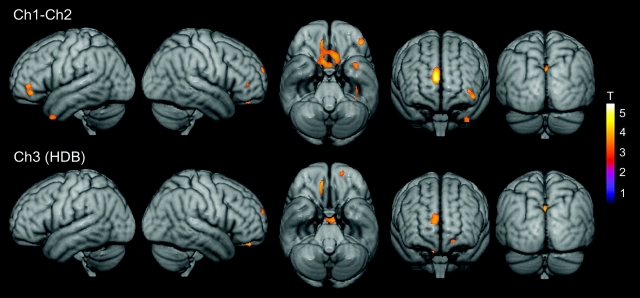
Figure 3.
Changes in the volume of Ch1−Ch2 (top panel) and Ch3 = HDB (bottom panel) associated with reductions in regional gray matter concentration in MCI patients. Statistical threshold was set at P < 0.005, uncorrected, and cluster extension was set at 50 contiguous voxels as minimum. Significant correlations are coded by the color scale (significance levels represented by T-values).
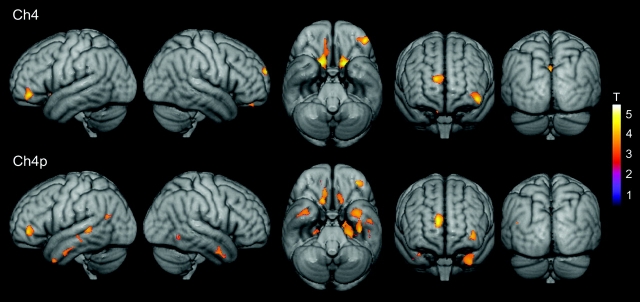
Figure 4.
Changes in the volume of Ch4 (top panel) and Ch4p (bottom panel) associated with reductions in regional gray matter concentration in MCI patients. Statistical threshold was set at P < 0.005, uncorrected, and cluster extension was set at 50 contiguous voxel as minimum. Significant correlations are coded by the color scale (significance levels represented by T-values).
Volume of Ch4p magnocellular groups significantly correlated with gray matter volume loss of the temporal lobes including its medial, polar, and lateral regions, differing from the correlation pattern obtained for the Ch4 compartment (Tables 5 and 6).
BF Changes and Cognitive Decline in MCI Patients
Correlation analyses revealed that the volume of different BF magnocellular compartments varied significantly with cognitive function in MCI patients (Fig. 5) but not in healthy elderly subjects. Among the BF Ch groups, the volume of Ch3 = HDB correlated most significantly and positively with MMSE scores (r = 0.5, P < 0.005), followed by Ch1–Ch2 (r = 0.34, P < 0.05) and Ch4 (r = 0.34, P < 0.05). The volume of Ch4p did not correlate with MMSE scores, but it was only the Ch compartment that correlated significantly and positively with scoring of the delayed recall test (r = 0.37, P < 0.05). Scoring obtained in the immediate recall test showed no significant correlations with volume reduction in any of the BF magnocellular compartments.
Combined gray matter volumes of frontal lobe regions that correlated with BF volumes (i.e., medial frontal, orbital and subcallosal gyrus as well as parts of the anterior cingulate and middle frontal gyrus) exhibited high correlation coefficients for scorings of MMSE (r = 0.48, P < 0.005) and delayed recall test (r = 0.47, P < 0.005). Both the cluster in right medial frontal cortex (r[MMSE] = 0.43, P < 0.01; r[delayed recall] = 0.38, P < 0.05) and the one in the right orbital cortex (r[MMSE] = 0.49, P < 0.005; r[delayed recall] = 0.41, P < 0.01) showed higher correlation with MMSE than delayed recall scores. Combined gray matter volumes of temporal lobe regions that correlated with BF volumes were also positively correlated with MMSE scores (r = 0.39, P < 0.05) but especially with scoring in the delayed recall test (r = 0.59, P < 0.001). Interestingly, the smaller the scores in the delayed recall test, the smaller the gray matter volumes of the temporal poles (left: r = 0.66, P < 0.0001; right: r = 0.63, P < 0.0001) and medial temporal lobe (MTL), including hippocampus, entorhinal cortex, and amygdala (left: r = 0.61, P < 0.0001; right: r = 0.45, P < 0.005). The precuneus, as the only region in the parietal lobe that correlated with BF volumes, showed significant correlation coefficients for both MMSE scoring (r = 0.38, P < 0.05) and delayed recall test (r = 0.40, P < 0.05). The lateral temporal lobe (including superior and middle temporal gyrus) also correlated with MMSE (left: r = 0.42, P = 0.01; right: r = 0.34, P = 0.05) and delayed recall scores (left: r = 0.52, P = 0.001; right: r = 0.34, P = 0.05). The left lateral temporal lobe was further the only region that correlated significantly with immediate recall scores (r = 0.32, P < 0.05).
Discussion
The present study examined the anatomical integrity of the BF magnocellular system in patients at high risk of developing AD as well as its potential relationship with cognitive decline in healthy elderly and MCI patients. To this end, we used a relatively new deformation-based approach (Ashburner 2007) in combination with recently developed probabilistic maps of different BF magnocellular compartments in MNI space (Zaborszky et al. 2008). We found a significant reduction of the NbM (Ch4) and its posterior regions (Ch4p) in MCI patients when compared with elderly controls. Furthermore, volumes of different BF cholinergic compartments not only correlated with cognitive impairment but also with a significant loss of gray matter volumes in brain regions known to be severely affected in AD. Taken together, these results support the hypothesis that dysfunctions (synaptic and/or cell death) of the BF magnocellular system may be partially responsible for cognitive decline in AD (Coyle et al. 1983), even years before the diagnosis is made (Sarter and Bruno 2002, 2004).
Degeneration of BF Cholinergic Cells in MCI
Manual segmentation of NbM in MRI studies poses important constraints, one of the most important obstacles being the lack of internal landmarks. The BF areas consist of scattered small cell groups, which are loosely distributed (see, e.g., Figs. 1 and 16 in Zaborszky et al. 2008). The precise delineation of these structures in MR images is impossible due to the limited spatial resolution and contrast of MRI. Therefore, manual segmentation of this complex structure can only estimate selected features, for example, the thickness of the substantia innominata at a certain position (Hanyu et al. 2002; Whitwell, Weigand, et al. 2007) but not the whole extent of the nuclei. Automated methods based on deformation-based morphometry techniques using a diffeomorphic approach (DARTEL) have demonstrated high accuracy at spatial registration of cerebral images, even in those with small sizes (Ashburner 2007). The combination of state-of-the-art deformation-based morphometry with probabilistic maps from postmortem brains is recently the most accurate method to measure volume changes in this region. Although neurofibrillary degeneration and damage/loss of cholinergic cells within the BF is a consistent finding of advanced AD (Whitehouse et al. 1981; McGeer et al. 1984; Arendt et al. 1985; Vogels et al. 1990), there is still controversy about the integrity of the basocortical cholinergic system in preclinical and/or early stages of AD. For instance, Gilmor et al. (1999) determined that the number of cholinergic cells contained within the NbM did not differ significantly between healthy elderly subjects, MCI, and mild AD, suggesting that cholinergic neurons are quite well preserved in early stages of AD. In support of this hypothesis, others found that cortical ChAT levels were preserved or even increased at early stages of the disease (DeKosky et al. 2002). In contrast, the formation of neurofibrillary tangles in cholinergic cells of the nucleus basalis as well as an impairment of trophic support by nerve growth factor was found early in the neurodegenerative process (Sassin et al 2000; Mufson et al. 2003; Mesulam et al. 2004). Given the negative effects of neurofibrillary tangle formation on cell metabolism and the nerve growth factor dependency of BF cholinergic cells, it is more likely that our findings reflect shrinkage of cholinergic cells than gross cell loss. A few in vivo imaging studies aimed at determining patterns of atrophy throughout the whole brain found a significant reduction of BF volume in MCI patients (Teipel et al. 2007; Whitwell, Petersen, et al. 2007; Hall et al. 2008), whereas most studies did not report changes in this region (Chetelat et al. 2002; Karas et al. 2004; Pennanen et al. 2005; Hämäläinen et al. 2007). Contradictory results may not only arise from the intrinsic heterogeneity of symptoms associated with MCI and subtle differences with diagnostic criteria (Winblad et al. 2004) but also from different technical approaches and statistical thresholds used in each study. Because neurofibrillary formation and decrease of cell numbers in the nucleus basalis have also been observed in the healthy elderly (Hanyu et al. 2002; Mesulam et al. 2004), one should expect no more than subtle volume variations between MCI patients and age-matched healthy elderly (Mesulam 2004).
VBM studies performed on AD patients do not usually report volume loss of the BF (reviewed in Busatto et al. 2008) despite the consistent finding of nucleus basalis atrophy in postmortem AD studies. The absence of changes might be due to heavy spatial smoothing (typically about 12 mm of Gaussian kernel in VBM studies) to compensate for imprecise image registration (Bookstein 2001). Given the small size of the BF cholinergic compartments, this smoothing step likely blurs small anatomical changes in this region. A further problem of classical VBM approaches on BF integrity could be deficient tissue class segmentation within the BF region. This potential confusion is only partly circumvented with the registration algorithm we used here. Because DARTEL aligns tissue class maps (Ashburner 2007), registration accuracy will still depend on the initial segmentation. However, DARTEL flow fields, and thus the jacobian determinants, were calculated by aligning gray matter and white matter simultaneously, which might partially compensate for a potentially incorrect initial segmentation.
In the present study, significant volume reductions in the BF of MCI patients were restricted to the NbM (Ch4) and most pronounced in its posterior aspects (Ch4p). Most of the effects survived after correction for age, which makes it unlikely that these differences can be accounted for by the slight, although not significant, variation in age between the 2 groups. The Ch4 magnocellular compartment together with the amygdala, hippocampus, and entorhinal cortex comprises a set of limbic regions known to be affected by early AD pathology (Braak and Braak 1996). As suggested by Mesulam and colleagues (Mesulam 2004), it might be its anatomical position and connectivity among these brain regions that contributes to the vulnerability of the cholinergic cells within the BF. Although plausible, further histological and neuroimaging research is needed to verify this hypothesis.
Effects were most pronounced in posterior and intermediate lateral regions of the nucleus basalis ROI, and probabilistic maps of BF magnocellular compartments revealed probabilities of up to 100% for these voxels to correspond to Ch4 and Ch4p, respectively. Both BF compartments constitute the cholinergic NbM and are known to be significantly affected in AD patients (Whitehouse et al. 1981; McGeer et al. 1984; Arendt et al. 1985; Vogels et al. 1990). Given that posterior regions of the Ch4 compartment are in close proximity to the amygdala, it could be that some of the voxels within our ROI cluster that show less probability of corresponding to magnocellular groups, instead belong to superior parts of the amygdaloid complex (Zaborszky et al. 2008). Our findings of most pronounced degeneration within posterior parts of the cholinergic nucleus basalis at preclinical stages of AD fit well with previous studies, which found cholinergic cell loss in AD to be most severe over posterior parts of Ch4 (Mesulam et al. 2004).
Interestingly, no volume differences in Ch1–Ch2 magnocellular compartments were found between MCI patients and healthy elderly subjects. Given that the hippocampus receives most of its cholinergic input from these nuclei (Mesulam and Geula 1988), it seems unlikely that cholinergic denervation is the major cause for the reduced hippocampus volume typically reported in MCI patients when compared with the healthy elderly (Whitwell, Petersen, et al. 2007; Ries et al. 2008). However, it is hard to say to which spatial scale our analytic approach is sensitive to detecting volumetric variations in these BF magnocellular compartments. Our findings of BF atrophy were obtained on a voxel level, that is, voxelwise statistical tests within the BF ROI. Given that this BF ROI consisted of an overestimated mask of the whole BFCS, it seems unlikely that the small size of the rostral nuclei (Ch1–Ch2 and Ch3) accounts for missing effects on a voxel level within this region. A more probable confusion for these rostral elements is the direct vicinity to the ventricular space. The ventricular space appears typically enlarged in MCI patients, which means that voxels in the ventricular space have to be substantially shrunk to match the template. Brain-tissue voxels that directly neighbor ventricular voxels are therefore prone to partial volume effects, which may compensate a possibly smaller volume of these voxels, resulting in Jacobian determinant values equal to or higher than 1, indicative of equal or higher volumes of the ventricle neighboring gray/white matter compared with the template. The deformation-based approach is therefore less exact for regions that directly neighbor the ventricular space, and therefore these results should be taken with caution.
BF Changes Associated with Regional Volume Loss of Cerebral Gray Matter
Previous postmortem (Arendt et al. 1985; Cullen et al. 1997) and in vivo MRI studies (Teipel et al. 2005) conducted in AD patients found high positive correlations between degeneration of the NbM and volume loss in its cortical projection sites. Consistent with these findings, our MCI group showed significant correlations between the volume of the Ch4 magnocellular compartment and the volume of regions of the frontal (medial, orbital, and middle frontal gyri) and parietal (precuneus) cortices. Further brain areas that showed correlations with volumetric changes in specific magnocellular compartments included the anterior cingulate and subcallosal gyrus (both Ch1–Ch2 and Ch4p), basal ganglia, and right anterior thalamus (Ch4), as well as medial, polar, and lateral regions of the temporal lobes (Ch4p). Most of these areas are known to be severely affected in AD (Busatto et al. 2008). In particular, the frontal and temporal lobe showed an early involvement at preclinical stages of this neurodegenerative disease (Whitwell, Petersen, et al. 2007; Ries et al. 2008). Our results neither resemble the projection patterns of the corresponding cholinergic nuclei (Selden et al. 1998) nor allow further inference on causal effects, but they provide novel insights on how early damage of BF magnocellular compartments parallels regional volume loss of cortical gray matter in high-risk subjects who will eventually progress to AD.
Linking BF Cholinergic Integrity with Cognitive Deterioration in MCI
Although growing evidence supports the hypothesis that dysfunction of the cholinergic system is, at least partially, responsible for cognitive symptoms in AD patients (Coyle et al. 1983; Baskin et al. 1999; Pappas et al. 2000; Hanyu et al. 2002), its role in the cognitive impairment at early AD and the preclinical stages of AD is still under debate. For instance, AChE inhibitors failed to delay conversion from MCI to AD (Jack et al. 2008) but improved late episodic learning, delayed recall, and recruitment of the hippocampus while MCI patients performed a spatial navigation task (Grön et al. 2006). Prolonged administration of AChE inhibitors to MCI patients further enhanced brain activation and behavioral gains during episodic and working memory tasks (Goekoop et al. 2004).
By using an in vivo morphometric approach, we showed here for the first time that volume reductions of BF magnocellular groups correlated significantly with decline in global cognitive status and impaired delayed recall in MCI patients. In particular, volume reduction of Ch3 = HDB compartment mainly predicted MMSE scores, whereas the posterior part of the NbM (Ch4p) was the only magnocellular compartment whose changes in volume were correlated with delayed recall scores of MCI patients. Because reductions of all cholinergic nuclei correlated with volume loss in partially distinct brain areas, we further tested for associations between gray matter volumes of these cortical regions and cognitive performance. Gray matter volume loss of the frontal lobe was associated with impairment in global cognition and specific memory functions. Among the examined regions, gray matter loss in the temporal poles and the left MTL exhibited the highest correlation with delayed recall scores. Consistent with these results, atrophy in regions of the left MTL, which are involved in encoding and retrieval of episodic memory traces (e.g., Pelage et al. 1998; Schacter and Wagner 1999), was found to correlate with episodic memory performance in MCI and AD patients (Leube et al. 2008). We further found that volume changes in Ch4p correlated with scoring in the delayed recall test. Taken together, our results suggest that dysfunctions in the left MTL and the posterior NbM may both affect encoding and retrieval of episodic memories in subjects at high risk of developing AD. Nevertheless, further investigation is required to elucidate the specific role of each BF magnocellular group in memory formation in general and in memory deterioration in particular.
In summary, by combining a deformation-based morphometry approach and stereotaxic probabilistic maps of different compartments of the BF magnocellular system, we found significant volume reductions of the NbM (Ch4) and its posterior compartments (Ch4p) in MCI patients as compared with healthy elderly controls. Reduction of specific BF magnocellular compartments was associated with cognitive decline (global and memory specific) in MCI patients but not in the healthy elderly. Furthermore, the volume loss of these BF magnocellular compartments correlated significantly with volume loss of gray matter in distinct brain regions that, in turn, showed varying association with cognitive function. We hope that these results provide further insights for in vivo assessment of the structural and functional integrity of the basocortical cholinergic system in early neurodegenerative processes.
Funding
European Union (GABA project, contract 043309 to J.L.C.); Spanish Ministry of Science and Innovation (grant SAF2008-3300 to J.L.C.); Regional Ministry of Innovation, Science and Enterprise, Junta de Andalucia (grant CTS-4604 to J.L.C.); and National Institute of Health (grant NS023945 to L.Z.).
Acknowledgments
Conflict of Interest: None declared.
References
- Amunts K, Schleicher A, Zilles K. Cytoarchitecture of the cerebral cortex– more than localization. Neuroimage. 2007;37:1061–1065. doi: 10.1016/j.neuroimage.2007.02.037. [DOI] [PubMed] [Google Scholar]
- Amunts K, Zilles K. Atlases of the human brain: tools for functional neuroimaging. In: Zaborsky L, Wouterlood FG, Lanciego JL, editors. Neuroanatomical tract-tracing 3: molecules, neurons, and systems. 3rd ed. New York: Springer; 2006. pp. 566–603. [Google Scholar]
- Arendt T, Bigl V, Tennstedt A, Arendt A. Neuronal loss in different parts of the nucleus basalis is related to neuritic plaque formation in cortical target areas in Alzheimer's disease. Neuroscience. 1985;14:1–14. doi: 10.1016/0306-4522(85)90160-5. [DOI] [PubMed] [Google Scholar]
- Artero S, Ancelin ML, Portet F, Dupuy A, Berr C, Dartigues JF, Tzourio C, Rouaud O, Poncet M, Pasquier F, et al. Risk profiles for mild cognitive impairment and progression to dementia are gender specific. J Neurol Neurosurg Psychiatry. 2008;79:979–984. doi: 10.1136/jnnp.2007.136903. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baskin DS, Browning JL, Pirozzolo FJ, Korporaal S, Baskin JA, Appel SA. Brain choline acetyltransferase and mental function in Alzheimer disease. Arch Neurol. 1999;56:1121–1123. doi: 10.1001/archneur.56.9.1121. [DOI] [PubMed] [Google Scholar]
- Böhm P, Peña-Casanova J, Aguilar M, Hernandez G, Sol JM, Blesa R NORMACODEN Group. Clinical validity and utility of the interview for deterioration of daily living in dementia for Spanish-speaking communities. Int Psychogeriatr. 1998;10:261–270. doi: 10.1017/s1041610298005377. [DOI] [PubMed] [Google Scholar]
- Bookstein FL. “Voxel-based morphometry” should not be used with imperfectly registered images. Neuroimage. 2001;14:1454–1462. doi: 10.1006/nimg.2001.0770. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Evolution of the neuropathology of Alzheimer's disease. Acta Neurol Scand. 1996;(Suppl. 165):3–12. doi: 10.1111/j.1600-0404.1996.tb05866.x. [DOI] [PubMed] [Google Scholar]
- Busatto GF, Diniz BS, Zanetti MV. Voxel-based morphometry in Alzheimer's disease. Expert Rev Neurother. 2008;8:1691–1702. doi: 10.1586/14737175.8.11.1691. [DOI] [PubMed] [Google Scholar]
- Chertkow H. Mild cognitive impairment. Curr Opin Neurol. 2002;15:401–407. doi: 10.1097/00019052-200208000-00001. [DOI] [PubMed] [Google Scholar]
- Chetelat G, Desgranges B, De La Sayette V, Viader F, Eustache F, Baron JC. Mapping gray matter loss with voxel-based morphometry in mild cognitive impairment. Neuroreport. 2002;13:1939–1943. doi: 10.1097/00001756-200210280-00022. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Coyle JT, Price DL, DeLong MR. Alzheimer's disease: a disorder of cortical cholinergic innervation. Science. 1983;219:1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- Cullen KM, Halliday GM, Double KL, Brooks WS, Creasey H, Broe GA. Cell loss in the nucleus basalis is related to regional cortical atrophy in Alzheimer's disease. Neuroscience. 1997;78:641–652. doi: 10.1016/s0306-4522(96)00569-6. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Ikonomovic MD, Styren SD, Beckett L, Wisniewski S, Bennett DA, Cochran EJ, Kordower JH, Mufson EJ. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol. 2002;51:145–155. doi: 10.1002/ana.10069. [DOI] [PubMed] [Google Scholar]
- Ganguli M, Dodge HH, Shen C, DeKosky ST. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology. 2004;63:115–121. doi: 10.1212/01.wnl.0000132523.27540.81. [DOI] [PubMed] [Google Scholar]
- Gilmor ML, Erickson JD, Varoqui H, Hersh LB, Bennett DA, Cochran EJ, Mufson EJ, Levey AI. Preservation of nucleus basalis neurons containing choline acetyltransferase and the vesicular acetylcholine transporter in the elderly with mild cognitive impairment and early Alzheimer's disease. J Comp Neurol. 1999;411:693–704. [PubMed] [Google Scholar]
- Goekoop R, Rombouts SA, Jonker C, Hibbel A, Knol DL, Truyen L, Barkhof F, Scheltens P. Challenging the cholinergic system in mild cognitive impairment: a pharmacological fMRI study. Neuroimage. 2004;23:1450–1459. doi: 10.1016/j.neuroimage.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grön G, Brandenburg I, Wunderlich AP, Riepe MW. Inhibition of hippocampal function in mild cognitive impairment: targeting the cholinergic hypothesis. Neurobiol Aging. 2006;27:78–87. doi: 10.1016/j.neurobiolaging.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Hall AM, Moore RY, Lopez OL, Kuller L, Becker JT. Basal forebrain atrophy is a presymptomatic marker for Alzheimer's disease. Alzheimers Dement. 2008;4:271–279. doi: 10.1016/j.jalz.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen A, Tervo S, Grau-Olivares M, Niskanen E, Pennanen C, Huuskonen J, Kivipelto M, Hänninen T, Tapiola M, Vanhanen M, et al. Voxel-based morphometry to detect brain atrophy in progressive mild cognitive impairment. Neuroimage. 2007;37:1122–1131. doi: 10.1016/j.neuroimage.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Hanyu H, Asano T, Sakurai H, Tanaka Y, Takasaki M, Abe K. MR analysis of the substantia innominata in normal aging, Alzheimer disease, and other types of dementia. Am J Neuroradiol. 2002;23:27–32. [PMC free article] [PubMed] [Google Scholar]
- Hedreen JC, Struble RG, Whitehouse PJ, Price DL. Topography of the magnocellular basal forebrain system in human brain. J Neuropath Exp Neurol. 1984;43:1–21. doi: 10.1097/00005072-198401000-00001. [DOI] [PubMed] [Google Scholar]
- Herukka SK, Helisalmi S, Hallikainen M, Tervo S, Soininen H, Pirttilä T. CSF Abeta42, Tau and phosphorylated Tau, APOE epsilon4 allele and MCI type in progressive MCI. Neurobiol Aging. 2007;28:507–514. doi: 10.1016/j.neurobiolaging.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, Grundman M, Jin S, Gamst A, Ward CP, encakova D, Doody RS, Thal LJ Members of the Alzheimer's Disease Cooperative Study (ADCS) Longitudinal MRI findings from the vitamin E and donepezil treatment study for MCI. Neurobiol Aging. 2008;2008(29):1285–1295. doi: 10.1016/j.neurobiolaging.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karas GB, Scheltens P, Rombouts SA, Visser PJ, van Schijndel RA, Fox NC, Barkhof F. Global and local gray matter loss in mild cognitive impairment and Alzheimer's disease. Neuroimage. 2004;23:708–716. doi: 10.1016/j.neuroimage.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage M, Habib R, Tulving E. Hippocampal PET activations of memory encoding and retrieval: the HIPER model. Hippocampus. 1998;8:313–322. doi: 10.1002/(SICI)1098-1063(1998)8:4<313::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Leube DT, Weis S, Freymann K, Erb M, Jessen F, Heun R, Grodd W, Kircher TT. Neural correlates of verbal episodic memory in patients with MCI and Alzheimer's disease–a VBM study. Int J Geriatr Psychiatry. 2008;23:1114–1118. doi: 10.1002/gps.2036. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG, Suzuki J, Dolman CE, Nagai T. Aging, Alzheimer's disease, and the cholinergic system of the basal forebrain. Neurology. 1984;34:741–745. doi: 10.1212/wnl.34.6.741. [DOI] [PubMed] [Google Scholar]
- Merker B. Silver staining of cell bodies by means of physical development. J Neurosci Meth. 1983;9:235–241. doi: 10.1016/0165-0270(83)90086-9. [DOI] [PubMed] [Google Scholar]
- Mesulam M. The cholinergic lesion of Alzheimer's disease: pivotal factor or side show? Learn Mem. 2004;11:43–49. doi: 10.1101/lm.69204. [DOI] [PubMed] [Google Scholar]
- Mesulam M, Shaw P, Mash D, Weintraub S. Cholinergic nucleus basalis tauopathy emerges early in the aging-MCI-AD continuum. Ann Neurol. 2004;55:815–828. doi: 10.1002/ana.20100. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Geula C. Nucleus basalis (Ch4) and cortical cholinergic innervation in the human brain: observations based on the distribution of acetylcholinesterase and choline acetyltransferase. J Comp Neurol. 1988;275:216–240. doi: 10.1002/cne.902750205. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1–Ch6) Neuroscience. 1983;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Ginsberg SD, Ikonomovic MD, DeKosky ST. Human cholinergic basal forebrain: chemoanatomy and neurologic dysfunction. J Chem Neuroanat. 2003;26:233–242. doi: 10.1016/s0891-0618(03)00068-1. [DOI] [PubMed] [Google Scholar]
- Nagai T, McGeer PL, Peng JH, McGeer EG, Dolman CE. Choline acetyltransferase immunohistochemistry in brains of Alzheimer's disease patients and controls. Neurosci Lett. 1983;36:195–199. doi: 10.1016/0304-3940(83)90264-1. [DOI] [PubMed] [Google Scholar]
- Pappas BA, Bayley PJ, Bui BK, Hansen LA, Thal L. Choline acetyltransferase activity and cognitive domain scores of Alzheimer's patients. Neurobiol Aging. 2000;21:11–17. doi: 10.1016/s0197-4580(00)00090-7. [DOI] [PubMed] [Google Scholar]
- Pennanen C, Testa C, Laakso MP, Hallikainen M, Helkala EL, Hänninen T, Kivipelto M, Könönen M, Nissinen A, Tervo S, et al. A voxel based morphometry study on mild cognitive impairment. J Neurol Neurosurg Psychiatry. 2005;76:11–14. doi: 10.1136/jnnp.2004.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment. Clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Ries ML, Carlsson CM, Rowley HA, Sager MA, Gleason CE, Asthana S, Johnson SC. Magnetic resonance imaging characterization of brain structure and function in mild cognitive impairment: a review. J Am Geriatr Soc. 2008;56:920–934. doi: 10.1111/j.1532-5415.2008.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Bruno JP. Mild cognitive impairment and the cholinergic hypothesis: a very different take on recent data. Ann Neurol. 2002;52:384–385. doi: 10.1002/ana.10308. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP. Developmental origins of the age-related decline in cortical cholinergic function and associated cognitive abilities. Neurobiol Aging. 2004;25:1127–1139. doi: 10.1016/j.neurobiolaging.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Sassin I, Schultz C, Thal DR, Rüb U, Arai K, Braak E, Braak H. Evolution of Alzheimer's disease-related cytoskeletal changes in the basal nucleus of Meynert. Acta Neuropathol. 2000;100:259–269. doi: 10.1007/s004019900178. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Selden NR, Gitelman DR, Salamon-Murayama N, Parrish TB, Mesulam MM. Trajectories of cholinergic pathways within the cerebral hemispheres of the human brain. Brain. 1998;121:2249–2257. doi: 10.1093/brain/121.12.2249. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Coplanar stereotaxic atlas of the human brain. New York: Thieme Medical; 1988. 122 p. [Google Scholar]
- Tanaka Y, Hanyu H, Sakurai H, Takasaki M, Abe K. Atrophy of the substantia innominata on magnetic resonance imaging predicts response to donepezil treatment in Alzheimer's disease patients. Dement Geriatr Cogn Disord. 2003;16:119–125. doi: 10.1159/000070998. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Born C, Ewers M, Bokde AL, Reiser MF, Moller HJ, Hampel H. Multivariate deformation-based analysis of brain atrophy to predict Alzheimer's disease in mild cognitive impairment. Neuroimage. 2007;38:13–24. doi: 10.1016/j.neuroimage.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Flatz WH, Heinsen H, Bokde AL, Schoenberg SO, Stockel S, Dietrich O, Reiser MF, Moller HJ, Hampel H. Measurement of basal forebrain atrophy in Alzheimer's disease using MRI. Brain. 2005;128:2626–2644. doi: 10.1093/brain/awh589. [DOI] [PubMed] [Google Scholar]
- Vogels OJ, Broere CA, ter Laak HJ, ten Donkelaar HJ, Nieuwenhuys R, Schulte BP. Cell loss and shrinkage in the nucleus basalis Meynert complex in Alzheimer's disease. Neurobiol Aging. 1990;11:3–13. doi: 10.1016/0197-4580(90)90056-6. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Memory Scale-Revised. San Antonio (TX): The Psychological Corporation; 1987. [Google Scholar]
- Whitehouse PJ, Price DL, Clark AW, Coyle JT, DeLong MR. Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol. 1981;10:122–126. doi: 10.1002/ana.410100203. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Petersen RC, Negash S, Weigand SD, Kantarci K, Ivnik RJ, Knopman DS, Boeve BF, Smith GE, Jack CR., Jr Patterns of atrophy differ among specific subtypes of mild cognitive impairment. Arch Neurol. 2007;64:1130–1138. doi: 10.1001/archneur.64.8.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Weigand SD, Shiung MM, Boeve BF, Ferman TJ, Smith GE, Knopman DS, Petersen RC, Benarroch EE, Josephs KA, et al. Focal atrophy in dementia with Lewy bodies on MRI: a distinct pattern from Alzheimer's disease. Brain. 2007;130:708–719. doi: 10.1093/brain/awl388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Bäckman L, Albert M, Almkvist O, et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Zaborzsky L, Hoemke L, Mohlberg H, Schleicher A, Amunts K, Zilles K. Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. Neuroimage. 2008;42:1127–1141. doi: 10.1016/j.neuroimage.2008.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Schleicher A, Palomero-Gallagher N, Amunts K. Quantitative analysis of cyto- and receptor architecture of the human brain. In: Mazziotta JC, Toga A, editors. Brain mapping: the methods. 2nd ed. San Diego (CA): Elsevier; 2002. pp. 573–602. [Google Scholar]