Abstract
Vascular inflammation is a common pathophysiological response to diverse cardiovascular disease processes, including atherosclerosis, myocardial infarction, congestive heart failure, and aortic aneurysms/dissection. Inflammation is an ordered process initiated by vascular injury that produces enhanced leucocyte adherence, chemotaxis, and finally activation in situ. This process is coordinated by local secretion of adhesion molecules, chemotactic factors, and cytokines whose expression is the result of vascular injury-induced signal transduction networks. A wide variety of mediators of the vascular injury response have been identified; these factors include vasoactive peptides (angiotensin II, Ang II), CD40 ligands, oxidized cholesterol, and advanced glycation end-products. Downstream, the nuclear factor-κB (NF-κB) transcription factor performs an important signal integration step, responding to mediators of vascular injury in a stimulus-dependent and cell type-specific manner. The ultimate consequence of NF-κB signalling is the activation of inflammatory genes including adhesion molecules and chemotaxins. However, clinically, the hallmark of vascular NF-κB activation is the production of interleukin-6 (IL-6), whose local role in vascular inflammation is relatively unknown. The recent elucidation for the role of the IL-6 signalling pathway in Ang II-induced vascular inflammation as one that controls monocyte activation as well as its diverse signalling mechanism will be reviewed. These new discoveries further our understanding for the important role of the NF-κB–IL-6 signalling pathway in the process of vascular inflammation.
Keywords: Nuclear factor-κB, Angiotensin II, Interleukin-6, Signal transducer and activator of transcription 3
1. Introduction
Vascular inflammation is observed to various degrees in a diverse group of vascular diseases, including large vessel atherosclerosis, myocardial infarction, congestive heart failure, and aortic aneurysm/dissections.1–3 Pathologically, the hallmark of vascular inflammation is the recruitment of circulating leucocytes, primarily monocytes, macrophages, and T lymphocytes, into the vascular wall. Mechanistically, circulating leucocytes are recruited into vascular tissues through a coordinated process of cellular adhesion, chemotaxis, and subsequent leucocyte activation,4 initiated by non-specific vascular injury.2 This recruitment process is primarily the result of coordinated expression of vascular adhesion molecules, chemotactic factors, and cytokines.
Vascular inflammation can be a protective homeostatic mechanism oriented towards lipoprotein particle opsonization and can be reversible, such as transient ‘fatty streaks’ seen in humans. However, the stable presence of activated monocyte–macrophage cells in the vessel wall is pathogenic. Here, macrophages amplify oxidized LDL or angiotensin II (Ang II)-induced reactive oxygen species (ROS) stress, to produce a self-perpetuating cycle of endothelial dysfunction, tissue remodelling, extracellular matrix degradation, enhanced reactivity to vasoactive- or inflammatory substances, and promotion of a pro-thrombotic state.
Epidemiologically, vascular inflammation is detected by enhanced circulating IL-6, a glycoprotein cytokine produced by the activated inflammatory cells within the vessel wall. IL-6 production, in turn, induces the expression of hepatic acute-phase reactants, including C-reactive protein, γ-fibrinogen, angiotensinogen, and others, some of which are measured clinically to assess atherosclerotic risk.5 Although useful clinically as a cardiovascular risk indicator, the role of IL-6 in the vasculature is only beginning to be understood.
A body of work has illuminated the central role of the nuclear factor-κB (NF-κB) transcription factor as a signal integrator controlling the process of vascular inflammation. Responsive to vasoactive peptides, oxidized LDL, activated CD40 receptor, monocyte released cytokines, or advanced glycation end-products, activated NF-κB is known to control two initial steps in the process of vascular inflammation, namely leucocyte adherence and chemotaxis. Recent work by our group has defined an additional role NF-κB plays in controlling monocyte activation via the IL-6 pathway. These studies have provided the first evidence that IL-6 via the downstream signal transducer and activator of transcription 3 (STAT3) transcription effector mediates local vascular monocyte activation and protection from ROS-induced cellular stress. In addition to its known role in mediating the systemic acute-phase response, the NF-κB–IL-6 signalling pathway plays multiple roles in initiating and sustaining vascular inflammation.
2. NF-κB regulatory pathways and modules
NF-κB is a family of structurally related inducible transcription factors known to be activated in the vessel wall in atherosclerosis, congestive heart failure, and diabetes. In vascular cells, NF-κB activation is mediated by diverse extracellular signals including Ang II, oxidized LDL, CD40 ligand, advanced glycation end-products, and inflammatory cytokines.1,6–8 This feature makes NF-κB a diverse signalling integrator of vascular injury.
The principal NF-κB proteins include the DNA-binding subunits, principally NF-κB1 and NF-κB2, that form heterodimeric partners with the transcriptional activators, RelA, C-Rel, and RelB. In most cell types, including endothelial cells, monocytes, and fibroblasts, inactivated NF-κB complexes are retained in the cellular cytoplasm by binding inhibitors of NF-κBs, IκBs, and ankryin repeat domain-containing proteins that block nuclear translocation and inhibit DNA-binding activity. These inhibitors include IκBα, -β, and -ε isoforms, as well as the 100 kDa precursor of NF-κB2 (p100). Broadly, NF-κB activation involves processes to induce nuclear translocation of distinct NF-κB heterodimeric pairs and activation of the transcriptional activator subunit initiated via post-translational modification event (phosphorylation). Mechanistic studies seeking to understand NF-κB control have now defined two bipartite regulatory pathways, known as the ‘canonical’ and ‘non-canonical’ pathways.9 The ‘canonical’ and ‘non-canonical’ pathways are distinguished by NF-κB subunits induced to translocate into the nucleus, and the distinct regulatory kinases involved.
An emerging concept is that each NF-κB pathway is composed of linked, but distinct signalling ‘modules’, one of whose action is to control NF-κB translocation and the second controls its activation. Moreover, the kinases mediating these regulatory modules are both stimulus- and cell type-specific. Predictably, inhibition of either the translocation module or the transactivation module prevents NF-κB from exerting its inflammatory or anti-apoptotic action. The canonical and non-canonical NF-κB pathways and component modules will be further discussed.
2.1. The canonical NF-κB pathway
The canonical NF-κB pathway is activated by monocyte-derived cytokines of which tumour necrosis factor (TNFα) and IL-1 are prototypes, and by Ang II stimulation. In target cells, the canonical pathway controls nuclear translocation of cytoplasmic RelA, an NF-κB family member sequestered in the cytoplasm by binding IκBα. The rate-limiting step initiating IκBα proteolysis is the multisubunit IκB kinase (IKK). Linked to RelA release is a transactivating signalling module producing RelA activation by site-specific Ser phosphorylation. By virtue of their distinct structure, the regulatory pathways activated by monokines and Ang II are described separately.
2.1.1. TNFα-induced canonical pathway
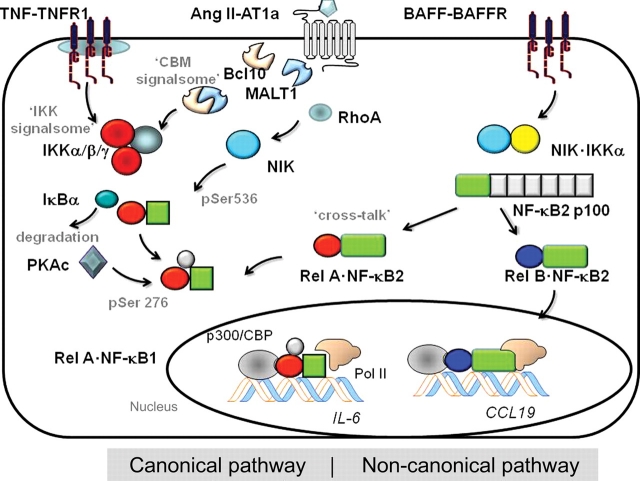
The extensively studied TNFα signalling pathway is used as an illustrative case of a monokine-induced canonical NF-κB pathway (Figure 1). Activated circulating or tissue-resident monocytes inducibly secrete TNFα, a small protein that binds ubiquitously expressed TNF receptor 1 (TNFR1), a high-affinity cell surface receptor that lacks intrinsic kinase activity. To initiate signalling, TNFα binding induces TNFR1 trimerization in specialized lipid rafts, where induced recruitment of cytoplasmic signal adapters occurs; this signalling complex includes the TNFR-associated death domain, TNF receptor-associated factors (TRAF)-2, -5, and -6, receptor-interacting protein (RIP), mitogen/ERK kinase kinase (MEKK)-3, TGFβ kinase, and others.10 Current work suggests that an important mechanism for inducible submembranous complex formation is mediated by specific Lys-63-linked ubiquitylation of TRAFs and RIP1.11,12 The major committed step in NF-κB canonical pathway activation is activation of the IKK ‘signalsome’, a multisubunit kinase complex whose core composition includes the two highly homologous serine–threonine kinases, IKKα and -β, and a regulatory subunit, IKKγ.13 Gene knockout studies have shown that IKKβ is the major effector IκBα kinase, and IKKα is largely dispensible for the canonical pathway.14 The catalytic activity of IKKβ is regulated in the signalsome by IKKγ, an essential adapter subunit that organizes the assembly of IKKs into the activated high molecular weight complex,15 binds ubiquitylated signalling adapters,16 and recruits the IκBα inhibitor into the activated IKK complex.17
Figure 1.
NF-κB activation pathways. Schematic view of the canonical (left) and non-canonical (right) NF-κB activation pathways. The canonical pathway is activated by activated TNF receptor via the IKK signalsome, composed of the catalytic kinases IKKα and -β, and the regulatory subunit IKKγ. Activated IKK is responsible for NF-κB translocation by proteolysis of the IκBα inhibitor. NF-κB activation is mediated by the PKAc phosphorylating Ser residue 276 of the RelA subunit. In contrast, Ang II activates the canonical pathway by inducing the Carma3·Bcl10·MALT1 complex, known as the ‘CBM signalsome’ upstream of IKK. Activated IKK liberates NF-κB for translocation. NF-κB activation is mediated by the RhoA-NIK signalling pathway converging on phosphorylation of RelA on Ser residue 536. The non-canonical pathway is controlled by the IKK complex composed of IKKα and NIK. Activated IKKα·NIK phosphorylates the COOH terminus of 100 kDa precursor of NF-κB2 DNA-binding subunit. Liberated NF-κB2 associates with RelA (forming the cross-talk pathway), and RelB, with the latter complex activating CCL19 and other unique genes. IKK, IκB kinase; TNF, tumour necrosis factor; NIK, NF-κB-inducing kinase; PKAc, catalytic subunit of protein kinase A; Pol II, RNA polymerase II.
In the cytosol, the active IKK signalsome phosphorylates the NH2-terminal regulatory domain of NF-κB-complexed IκBα on Ser residues 32 and 36, thereby targeting IκBα for proteolytic destruction. Consequently, IκBα degradation releases NF-κB to rapidly translocate into the nucleus; these subunits are primarily NF-κB1·RelA heterodimers. The current work has shown that the NF-κB translocation activates the synthesis of negative feedback regulators that terminate its signal; these regulators include IκB inhibitor itself, whose resynthesis recaptures NF-κB in the cytoplasm, and TNFAIP3/A20, a member of a deubiquitylation complex that removes Lys-63 from IKK-regulating signalling adapters to terminate NF-κB signalling [see Skaug et al.11 for further discussion].
Recent work by our lab and others has shown that the NF-κB ‘translocation module’ resulting in phosphorylation-induced IκBα degradation is required, but not sufficient for target gene activation.18 Additionally, a separable, NF-κB ‘activation module’ is also required for target gene activation. This latter pathway is mediated by intracellular ROS functioning as a second messenger species to induce RelA phosphorylation on Ser residue 276. This pathway is dependent on the catalytic subunit of protein kinase A (PKAc), a kinase that co-purifies with IκBα and is activated by TNFα.18,19 Because inhibition of ROS formation or short-interfering RNA (siRNA)-mediated PKAc knockdown prevents TNFα-induced RelA Ser-276 phosphorylation without affecting its nuclear translocation, the ROS-PKAc transcriptional activation module is separate from the translocation module and also necessary for NF-κB-dependent gene expression. The kinases controlling the activation module are highly stimulus-dependent—e.g. in IL-1 signalling, Ser-276 activation is initiated by a PI3K pathway, and in RNA virus infection, Ser-276 phosphorylation is mediated by the mitogen and stress-related kinase-1.20
The consequences of RelA Ser-276 phosphorylation are to reduce intermolecular NH2- and COOH-terminal interactions. This allows RelA to complex with the p300/CBP coactivator, an event that results RelA acetylation,18,19,21 stable association with endogenous chromatin targets,18 and complex formation with the cyclin-dependent kinase 9 (CDK9)–cyclin T1 transcriptional elongation complex.21 This latter association allows chromatin-associated RelA to induce phosphorylation of Ser-2 of the RNA polymerase II (Pol II) COOH-terminal domain, licensing Pol II to produce fully elongated transcripts.22
Upon activation, NF-κB induces the expression of molecules whose function broadly in the cascade of leucocyte recruitment, including vascular adhesion molecules intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) as well as monocyte chemotaxis, including monocyte chemotactic protein-1 (MCP-1) and granulocyte-macrophage colony-stimulating factor (GM-CSF).23 In this manner, the monokine-induced canonical activation pathway is composed of the two linked regulatory modules mediated by the IKK-IκBα and the ROS-PKAc-RelA Ser-276 kinase modules that impact vascular inflammation (Figure 1).
2.1.2. The Ang II-induced canonical pathway
The potent vasopressor peptide, Ang II, activates NF-κB via the high-affinity type 1 angiotensin receptor (AT1A) in vascular tissues and hepatocytes in a heterotrimeric G protein-dependent manner.24,25 The G protein-dependent signals activate phospholipase Cβ to generate rises in inositol trisphosphate and diacylglycerol, which increase intracellular calcium and activate typical protein kinase C (PKC) isoforms. In addition, the RhoA GTPase is activated by GDP–GTP exchange factors, isoprenylation by geranylgeranyl pyrophosphate and membrane translocation to affect cytoskeletal rearrangement, ROS production, and intracellular signalling.26 Although initially the Ang II signalling pathway was thought to induce the canonical pathway, detailed studies have shown that the Ang II-induced pathway is quite distinct from that induced by TNFα (Figure 1).
Work in hepatocytes has shown that activated PKC phosphorylates a tissue-specific member of the membrane associated guanylate-kinase superfamily of scaffolding proteins, known as CARMA3.27 Phospho-CARMA3 forms a complex with two other proteins: Bcl10, an intermediate bridging factor, and mucosa-associated lymphoid tissue lymphoma translocation 1 (MALT1). The oligomeric CARMA3·Bcl10·MALT1 complex is referred to as the ‘CBM signalsome’, an acronym for its major protein components.28 In its activated form, MALT1 of the CBM complex induces Lys-63-linked ubiquitylation of IKK and cleaves the IKK inhibitory TNFAIP3/A20 molecule, a protein complex involved in deubiquitylation of Lys-63-linked TRAF6, RIP1, and IKKγ.29 Together, these two actions result in accumulation of the activated Lys-63-linked TRAF6, RIP1, and IKKγ proteins, producing activation of NF-κB translocation by degradation of IκBα through the mechanisms described for TNFα above.
Although the CBM signalsome plays a major role in the NF-κB translocation module in hepatocytes, the definition of a second Ang II-induced signalling module was discovered in experiments from our lab on vascular smooth muscle cells (VSMCs), where inactive NF-κB isoforms could be identified in unstimulated cells, and no significant changes in NF-κB abundance was observed in response to Ang II stimulation.30 Instead, we found that Ang II stimulation rapidly induces Ser phosphorylation of RelA at residue 536, a site located in its COOH-terminal transactivating domain, without significantly affecting Ser-276 phosphorylation.30 We further found that Ser-536 phosphorylation was mediated upstream by RhoA, since Ser-536 Rel formation was blocked by either adenoviral-mediated expression of dominant-negative RhoA (RhoA-Thr17Asn) or treatment with the specific irreversible RhoA inhibitor, the exoenzyme C3 from Clostridium botulinum. Ang II-inducible phospho-Ser-536 RelA was required for IL-6 activation because C3 toxin treatment or expression of RhoA-Thr17Asn also blocked Ang II-induced IL-6 expression.
Interestingly, phospho-Ser-536 RelA is a form of RelA that is free from IκBα inhibition, and this pool of phospho-Ser-536 RelA independently recycles through the nucleus,31 where it is in hyperdynamic exchange with chromatin-associated NF-κB.32 The effect of Ang II, then, is to increase the relative abundance of phospho-Ser-536 RelA in the nucleoplasmic pool. To confirm this phenomenon, application of chromatin immunoprecipitation assays in Ang II-stimulated VSMCs showed that no significant changes in total RelA binding could be observed on the native IL-6 promoter, but Ang II induced an increase in fractional binding of phospho-Ser-536 RelA to the IL-6 promoter at times coincident with IL-6 expression. In this way, Ang II induces NF-κB/RelA activation in VSMCs without significantly changing its total nuclear abundance. We also observed enhanced phospho-Ser-536 RelA formation in the aortae of rats chronically infused with Ang II, establishing relevance to vascular signalling.33 Interestingly, this RelA activation pathway was also found to be operative in hepatocytes, indicating its broad involvement in signalling.33
Our work has further defined the identity of the RelA Ser-536 kinase. Currently, it is thought that the primary Ser-536 kinases are IKKβ and the NF-κB-inducing kinase (NIK)/MEKK14. Because Ang II only weakly induces IKKβ, we investigated the role of NIK in Ang II-induced RelA transactivation. Either expressing a dominant-negative NIK or inhibiting NIK expression using siRNA-mediated knockdown inhibited Ang II-induced Ser-536 phosphorylation and NF-κB-dependent transcription.33 To further show that its actions are at least partly independent of the NF-κB translocation module, we found that NIK induces the activity of the RelA transactivation domain-1 and -2 in constitutively nuclear RelA proteins.
Work using non-denaturing coimmunoprecipitation assays has yielded the intriguing finding that RelA forms an inducible nuclear complex with NIK in response to Ang II stimulation.33 The function and mechanism of the NIK·RelA complex in Ang II-induced vascular inflammation will require further exploration.
Taken together, the relative contributions of the Ang II-induced ‘transactivation modules’ and the ‘translocation modules’ are cell type-dependent. In VSMCs, where inactive NF-κB is constitutively nuclear, the predominant signalling event is the activation of the NF-κB transactivation module mediated by RhoA-NIK; conversely, in hepatocytes where NF-κB is primarily sequestered in the cytoplasm, both the translocation (CBM signalsome) and transactivation modules (RhoA-NIK) are required for Ang II induction.
2.2. The non-canonical (and cross-talk) NF-κB activation pathways
The non-canonical pathway is activated by diverse signals, B cell-activating factor (BAFF), lymphotoxin β (LTβ) and RNA viral infection.34 Although genetic knockouts of this pathway indicate that the non-canonical pathway plays a major role in development of the immune system, recent work has also shown that vascular pro-inflammatory factors, such as platelet-derived CD154 or LTβ activates the non-canonical pathway.35–37 The hallmark of non-canonical pathway activation is the inducible processing of the NF-κB2 p100 precursor into its mature 52 kDa DNA-binding form (NF-κB2) and liberation of sequestered NF-κB complexes.9 Here, p100 processing is mediated by IKKα-mediated phosphorylation at two Ser residues in the p100 COOH terminus, targeting the complex for limited proteasomal-mediated degradation.38
Neither IKKβ nor IKKγ, key regulators of the canonical pathway, is required for signalling in the non-canonical pathway. Instead, IKKα activation is required, and its activation is absolutely NIK-dependent (Figure 1). Here, NIK serves both as a rate-limiting upstream activator that phosphorylates IKKα and serves a docking site to recruit both p100 NF-κB2 and IKKα into a complex.38 In unstimulated cells, intracellular NIK abundance is very low, and hormone induction of NIK is mediated by post-translational stabilization. This constitutively active degradation process is the result of NIK binding a complex of TRAF isoforms, including TRAF- 2, -3, and associated cIAP ubiquitin ligases.39 Activation of the BAFF receptor induces TRAF3 degradation, resulting in stabilization of NIK, its oligomerization, and autoactivation by threonine phosphorylation.40 The effect of Ang II on TRAF isoforms has not yet been investigated.
Originally, it was thought that non-canonical pathway only releases RelB·NF-κB2, a complex known to have distinct DNA-binding specificity from the canonical RelA·NF-κB1 complex. In particular, RelB·NF-κB2 selectively controls EBV-induced molecule 1 chemokine ligand 1 (ELC/CCL19), stromal cell-derived factor, and BAFF, genes important in lymph node organogenesis and the immune response.41 Recent work has indicated that non-canonical pathway also releases p100-sequestered RelA·NF-κB1 complexes.36 This is termed the ‘cross-talk’ pathway because a canonical RelA·NF-κB1 DNA-binding complex is released as a consequence of activating the non-canonical NIK-IKKα kinases. The discovery that the RelA.NF-kB1 complex is downstream of the noncanonical pathway is important because it makes the cross-talk pathway a potential mediator of vascular inflammation by its ability to activate cytokines and adhesion molecules.
The significance of the non-canonical pathway and its role in chronic vascular inflammation are relatively unknown, although unbiased proteomics studies identified NIK up-regulation in diabetic nephropathy and may indicate that non-canonical pathway activation may play a significant role in diabetic nephropathy.42 Apart from the distinct regulatory kinases, we know that the relative activation kinetics of the canonical and non-canonical NF-κB activation pathways are quite distinct; e.g. the non-canonical pathway is activated much more slowly than the canonical pathway in response to LTβ.36 The significance of the interplay between these two pathways and their relative contributions in vascular inflammation will need to be further explored.
3. The NF-κB–IL-6 signalling pathway
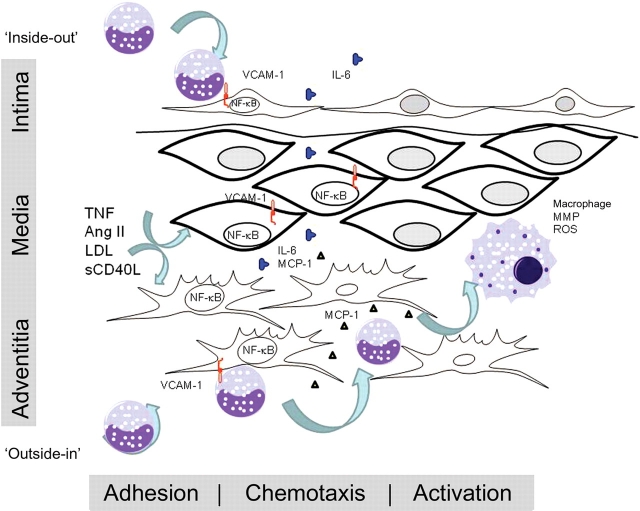
The ‘outside-in’ model for vascular injury2 states that the recruitment of circulating leucocytes is a coordinated process initially occurring in the vascular adventitia consisting of discrete steps of adhesion, chemotaxis, and cellular activation (Figure 2). Adhesion is a process mediated by cell surface presentation of VCAM-1/CD106, E-selectin, and ICAM, whereas chemotaxis is mediated by secretion of chemotactic cytokines (MCP-1 and GM-CSF) or elastin degradation products. Mentioned earlier, VCAM-1, ICAM, MCP-1, and GM-CSF are all downstream of the NF-κB signalling pathway; through expression of these adhesion molecules and chemotaxins, NF-κB signalling enhances cellular infiltration of the vessel wall.
Figure 2.
Overview of NF-κB–IL-6 in the major regulated steps in vascular inflammation. A variety of pro-inflammatory agonists activate resident VSMCs, fibroblasts, and endothelial cells via the NF-κB transcription factor, including TNF, local and systemic Ang II production, and CD40 ligands, sCD40 and CD145, advanced glycation end-products, and oxidized LDL. Downstream, activated NF-κB coordinates the expression of monocyte adhesion proteins, including VCAM-1 and ICAM-1. Circulating monocytes enter the vessel wall either from ‘inside-out’ via endothelial cell interaction, or from ‘outside-in’ via the adventitial vaso vasorum.2 Monocyte chemotaxis is controlled by NF-κB-inducing expression of MCP-1, GM-CSF, and other chemokines. Monocyte activation is controlled by local IL-6 production. IL-6 acts through trans-signalling and trans-membrane signalling result in monocyte activation (MMP expression) and ROS resistance. Enhanced monocytic interaction with adventitial fibroblasts produce an amplification loop, enhancing MCP-1 and IL-6 production. Activated monocyte/macrophages oxidize cholesterol, release ROS, and matrix metalloproteinases to produce ECM remodelling that contribute to vascular pathology.
One of the most highly induced NF-κB-dependent cytokines is IL-6, a pleiotropic glycoprotein secreted by multiple vascular cell types, including macrophages, lymphocytes, fibroblasts, endothelial cells, and smooth muscle cells. Quantitative analysis of inducible IL-6 production in a rodent model of Ang II infusion has yielded the interesting finding that the aortic adventitia is the greatest source of IL-6 secretion,43 a site where the majority of monocytes are found. These data and others are consistent with the ‘outside-in’ hypothesis of vascular inflammation.2,44
4. Local vascular effects of inducible IL-6 signalling
Because of the stress responsiveness of the NF-κB signal integrator, IL-6 secretion is up-regulated in response to Ang II, cytokines, ROS stress, and vascular injury.6,43 Downstream, IL-6 signals responsive cells via the Jak-STAT3 pathway. The role of the IL-6–STAT3 signalling in inducing the hepatic acute-phase response is well established;45 however, the local role of IL-6 signalling in vascular inflammation has not been. In recently published work, we have made progress in understanding its local vascular role in a mouse model of Ang II-induced aortic aneurysms.
4.1. Monocyte/macrophage activation
In a rodent module of subcutaneous Ang II infusion, Ang II induces ‘outside-in’ aortic inflammation, with marked adventitial expansion and IL-6 secretion, followed by aortic remodelling, aneurysms, and dissections.3 Using a method for flow cytometric quantitation of aortic cellular constituents, we found that Ang II induces recruitment of CCR2+ CD14hiCD11bhiF4/80− macrophages into ‘hot spots’ in the ascending and supra-renal sections of the aorta. These areas of enhanced adventitial monocyte recruitment coincide with regions of enhanced ROS formation and represented the primary sites for subsequent development of aortic aneurysms and dissections in this model.
To determine the role of IL-6, similar studies were performed in IL-6−/− mice, where a significantly reduced rate of aortic dissections was found. Analysis of the monocyte activation markers showed that the monocytes were CD14loCD11blo F4/80+. These data indicate that the loss of the F4/80 marker was Ang II- and IL-6-dependent and a hallmark of monocyte activation. Importantly, aortic monocytes from Ang II-stimulated wild-type mice were positive for phospho-Tyr STAT3, but not those isolated from an IL-6−/− background, indicating that IL-6 signalling was required for monocyte activation in the vascular wall. These data indicate that the Jak-STAT3 pathway induced by Ang II and mediated by IL-6 is involved in the monocyte activation step of vascular inflammation.3
Interestingly, we further found that MCP-1 expression was reduced in IL-6 knockout mice and that IL-6 expression was reduced in MCP-1 knockout mice, suggesting an important cross-amplication pathway. In vitro, we have found that leucocyte–fibroblast interaction in the aortic adventitia potentiates IL-6 production, inducing local monocyte recruitment and activation, thereby promoting MCP-1 secretion, vascular inflammation, ECM remodelling, and aortic destabilization.3 These findings suggest that a local IL-6-MCP-1 amplification loop underlies Ang II-induced vascular inflammation, where co-regulation of MCP-1 enhances monocyte chemotaxis, and IL-6 mediates their direct activation. Further work will be required to understand the role of IL-6 in macrophage recruitment and activation in other types of vascular diseases.
4.2. Protection from ROS-induced cell injury
Enhanced vascular ROS production is a hallmark of Ang II stimulation, thrombin formation, and LDL oxidation; ROS may contribute to endothelial dysfunction seen in vascular inflammation46–48 or may functioning as a second messenger. For example, ROS activates Ras by cysteine oxidation,49 potentiates cytokine-induced RelA Ser-276 phosphorylation via a PKAc pathway,18 or induce cellular apoptosis.
In addition to its role in potentiating an activated monocyte phenotype, IL-6 signalling via STAT3 may also play an important role in protection from ROS-induced apoptosis. This phenomenon was first suggested by experiments where expression of activated STAT3, STAT3-C, protects against Fas-mediated liver injury in the mouse.50 This group showed that STAT3-C up-regulated expression of FLICE inhibitor protein, Bcl-xL, Bcl-2, and redox-associated protein redox factor-1 (Ref-1), and down-regulated expression of pro-apoptotic FLICE and caspase-3. These data strongly indicate that IL-6 acts at several points in the apoptotic pathway to protect against oxidant injury-induced cell death. More work will be required to determine the role of IL-6–STAT3 signalling in local protection against ROS-induced vascular injury.
5. IL-6 signalling mechanisms
IL-6 is the prototype for one of the most pleiotropic of mammalian cytokine families, a family that includes IL-11, oncostatin M, cardiotrophin-1, ciliary neurotrophic factor, cardiotrophin-like cytokine, leukaemia inhibitory factor, and IL-27p28.51 These proteins signal by binding a unique α-receptor subunit on the cell surface responsible for ligand binding that associates with a shared 130 kDa glycoprotein (gp130) β-subunit responsible for signal transduction. Two distinct pathways for vascular IL-6 signal transduction have been defined, discussed below.
5.1. Membrane/‘classic’ IL-6 signalling
The classic mechanism for IL-6 signalling involves its binding to cell surface-attached IL-6 receptor α-subunits (IL-6Rα), an event that triggers their association with the gp130 β-subunit, inducing gp130 homodimerization, and subsequent formation of a hexameric IL-6·IL-6Rα·gp130 high-affinity complex.52 Receptor ligation induces conformational changes in the gp130 cytoplasmic domain, an event that brings the Jak tyrosine kinases into close proximity. This molecular interaction results in trans-autophosphorylation of Jak153 and gp130 phosphorylation on the docking sites for the STAT. Although IL-6·IL-6Rα·gp130 signals through several pathways including the Ras-ERK-MAPK cascade,53,54 the STAT pathway is the one that appears to play a major role in vascular inflammation. Jak1 induces Tyr phosphorylation of STAT isoforms-1 and -3, inducing homo- and heterodimerization, and nuclear translocation to enhance target gene transcription.55
5.2. IL-6 trans-signalling
Interestingly, endothelial cells and VSMCs do not normally express high levels of IL-6R, making it difficult to understand how these cells could be responsive to IL-6. Recently, it has been appreciated that the IL-6 response can be mediated by a phenomenon termed ‘trans-signalling’.56 This mechanism is mediated by the presence of a soluble IL-6Rα that binds free IL-6. Upon IL-6 binding, the soluble IL-6–IL-6Rα complex can associate with ubiquitous gp130 on a cell type, expanding the repertoire of IL-6 responsive cells to include endothelial cells and VSMCs.56 We know that sIL-6Rα is produced via ectodomain shedding by a disintegrin and metalloproteinases (ADAMs)-17 and -10, proteases activated by the presence of inflammation.
Determining the relative contributions of IL-6 membrane vs. trans-signalling has been made possible by the application of a soluble gp130 that competitively inhibits IL-6 trans-signalling without affecting membrane signalling. In one study, soluble recombinant gp130 inhibited Ang II-dependent hypertension in a rodent model, but not vascular hypertrophy and STAT3 activation,57 indicating these latter responses mediated through IL-6 membrane signalling. Transgenic overexpression of soluble gp130 inhibited mononuclear inflammation, indicating that this process was mediated by the IL-6 trans-signalling mechanism.58 Together, these data indicate that IL-6 trans-signalling is an important mechanism mediating IL-6's effects on vascular inflammation.
5.3. Mechanisms of IL-6–STAT3 transactivation
Activated nuclear STAT3 controls the expression of genes important in tissue remodelling (ColA1, fibronectin), cell growth (cyclin D1, and c-myc), and the hepatic acute-phase response (angiotensinogen, C-reactive protein, and γ-fibrinogen).59 Although it is well-established that STAT3 activation requires specific tyrosine phosphorylation, mediated by Jaks, more recent studies indicate the requirement of additional protein interactions mediated by protein acetylation.
Recent studies have shown that STAT activity is modulated by co-factor interaction. Upon nuclear entry, STAT3 associates with the p300/CBP coactivator, an enhancer protein with intrinsic histone acetyltranferase activity which is able to open chromatin structure, allowing other chromatin-modifying proteins to bind to DNA and activate transcription.55 Stable p300/CBP association requires both the NH2-terminal modulatory domain and the COOH-terminal transactivation domain of STAT3. Studies from our laboratory first described two novel inducible acetylation sites on the STAT3 NH2 terminus at Lys-49 and -87 that are required to stabilize interaction with the p300/CBP complex. This activity is produced by the acetylated (Ac) STAT3 NH2 terminus binding to the p300 bromodomain.60 As a result, the AcSTAT3·p300 complex stably associates with target chromatin and modifies nucleosomal histones, allowing for enhanced target gene expression. Others have found that STAT3 acetylation on its COOH-terminal region at Lys-685 is critical for stable dimer formation and DNA-binding activity, making acetylation a critical step in STAT3 signalling.61
We have recently discovered that STAT3 also regulates downstream gene expression by also promoting transcription elongation.59 This function is realized by an additional inducible interaction between STAT3 and the CDK9·CcnT1 complex. STAT association with CDK9·CcnT1and recruitment to target genes result in COOH-terminal phosphorylation of RNA Pol II. Phosphorylated Pol II then produces full-length mRNA transcripts. Importantly, we have found that it is possible to modulate the systemic inflammatory response mediated by the NF-κB–IL-6–STAT3 pathway by inhibiting CDK9.59 These findings may suggest additional mechanisms to modulate vascular inflammation clinically, with some of the cyclin-dependent kinase inhibitors being in late-stage clinical trials.62
6. Summary and perspectives
In this review, I have illustrated the multiple pathways of inflammatory responses mediated by the NF-κB transcription factor (summarized in Figure 2). Dissection of these pathways is more than an academic exercise, because this knowledge allows for interruption of this important stress responsive signalling integrator having identified key regulators whose inhibition would not interfere with its normal roles in adaptive immunity and anti-apoptosis. One of its important targets in vascular inflammation is IL-6 expression. Despite IL-6's properties as being one of the most highly induced adventitial cytokines and used clinically as an indicator of significant vascular inflammation, its contribution to vascular inflammation has been essentially unknown. Using a mouse model of Ang II infusion, some of the local roles of IL-6 are beginning to be understood; these studies suggest a role in monocyte activation and potentially a role in protection from ROS-induced cell stress. We now know that IL-6 signals by classic membrane receptor or trans-signalling mechanisms that converge on the STAT3 transcription factor. Investigation into the biochemistry of STAT3 activation has led to the discovery of novel post-translational modifications and association with cyclin kinase-containing coactivators required for activity; these discoveries may also be targets for anti-vascular inflammatory therapies. These studies underscore the central relevance and mechanisms for the NF-κB–IL-6–STAT3 signalling pathway in mediating vascular inflammation.
Conflict of interest: none declared.
Funding
Supported by National Heart Lung and Blood Institute grants P50 HL083794 and HL70925 (A.R.B.). Core Laboratory support was from National Institute of Environmental Health grant P30 ES06676 and National Heart Lung and Blood Institute contract BAA-HL-02-04 (A. Kurosky, UTMB).
References
- 1.Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2006;26:987–994. doi: 10.1161/01.ATV.0000214999.12921.4f. doi:10.1161/01.ATV.0000214999.12921.4f. [DOI] [PubMed] [Google Scholar]
- 2.Maiellaro K, Taylor WR. The role of the adventitia in vascular inflammation. Cardiovasc Res. 2007;75:640–648. doi: 10.1016/j.cardiores.2007.06.023. doi:10.1016/j.cardiores.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tieu B, Lee C, Sun H, LeJeune W, Ju X, Spratt H, et al. Adventitial IL-6-MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection. J Clin Invest. 2009;119:3637–3651. doi: 10.1172/JCI38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Springer T. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multiple paradigm. Cell. 1994;76:301. doi: 10.1016/0092-8674(94)90337-9. doi:10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. doi:10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 6.Han Y, Runge MS, Brasier AR. Angiotensin II induces IL-6 transcription in vascular smooth muscle cells through pleiotropic activation of NF-kB transcription factors. Circ Res. 1999;84:695–703. doi: 10.1161/01.res.84.6.695. [DOI] [PubMed] [Google Scholar]
- 7.Chakrabarti S, Blair P, Freedman JE. CD40-40L signaling in vascular inflammation. J Biol Chem. 2007;282:18307–18317. doi: 10.1074/jbc.M700211200. doi:10.1074/jbc.M700211200. [DOI] [PubMed] [Google Scholar]
- 8.Basta G, Schmidt AM, De Caterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004;63:582–592. doi: 10.1016/j.cardiores.2004.05.001. doi:10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, et al. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. doi:10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- 10.Hsu H, Huang J, Shu HB, Baichwal V, Goeddel DV. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996;4:387–396. doi: 10.1016/s1074-7613(00)80252-6. doi:10.1016/S1074-7613(00)80252-6. [DOI] [PubMed] [Google Scholar]
- 11.Skaug B, Jiang X, Chen ZJ. The role of ubiquitin in the NF-kB regulatory pathways. Annu Rev Biochem. 2009;78:769–796. doi: 10.1146/annurev.biochem.78.070907.102750. doi:10.1146/annurev.biochem.78.070907.102750. [DOI] [PubMed] [Google Scholar]
- 12.Lamothe B, Besse A, Campos AD, Webster WK, Wu H, Darnay BG. Site-specific Lys-63-linked tumor necrosis factor receptor-associated factor 6 auto-ubiquitination is a critical determinant of I{kappa}B kinase activation. J Biol Chem. 2007;282:4102–4112. doi: 10.1074/jbc.M609503200. doi:10.1074/jbc.M609503200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karin M, Ben Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. doi:10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 14.Li Q, Van Antwerp D, Mercurio F, Lee K-F, Verma IM. Severe liver degeneration in mice lacking the IkB kinase 2 gene. Science. 1999;284:321–325. doi: 10.1126/science.284.5412.321. doi:10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- 15.Poyet J-L, Srinivasula SM, Lin J-H, Fernandes-Alnemri T, Yamaoka S, Tsichlis PN, et al. Activation of the IkB kinases by RIP via IKKg/NEMO-mediated oligomerization. J Biol Chem. 2000;275:37966–37977. doi: 10.1074/jbc.M006643200. doi:10.1074/jbc.M006643200. [DOI] [PubMed] [Google Scholar]
- 16.Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ. Activation of IKK by TNF[alpha] requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell. 2006;22:245–257. doi: 10.1016/j.molcel.2006.03.026. doi:10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto Y, Kim DW, Kwak YT, Prajapati S, Verma U, Gaynor RB. IKKgamma/NEMO facilitates the recruitment of the IkappaB proteins into the IkappaB kinase complex. J Biol Chem. 2001;276:36327–36336. doi: 10.1074/jbc.M104090200. doi:10.1074/jbc.M104090200. [DOI] [PubMed] [Google Scholar]
- 18.Jamaluddin M, Wang S, Boldogh I, Tian B, Brasier AR. TNF-α-Induced NF-κB/Rel A Ser 276 phosphorylation and enhanceosome formation on the IL-8 promoter is mediated by a reactive oxygen species (ROS)-dependent pathway. Cell Signal. 2007;9:1419–1433. doi: 10.1016/j.cellsig.2007.01.020. doi:10.1016/j.cellsig.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Zhong H, Voll RE, Ghosh S. Phosphorylation of NF-kB p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell. 1998;1:661–671. doi: 10.1016/s1097-2765(00)80066-0. doi:10.1016/S1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]
- 20.Jamaluddin M, Tian B, Boldogh I, Garofalo R, Brasier AR. Respiratory syncyctial virus infection induces a ROS-MSK1-Phospho-Ser-276 RelA pathway required for cytokine expression. J Virol. 2009;83:10605–10615. doi: 10.1128/JVI.01090-09. doi:10.1128/JVI.01090-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nowak DE, Tian B, Jamaluddin M, Boldogh I, Vergara LA, Choudhary S, et al. RelA Ser276 phosphorylation is required for activation of a subset of NF-{kappa}B-dependent genes by recruiting cyclin-dependent kinase 9/Cyclin T1 complexes. Mol Cell Biol. 2008;28:3623–3638. doi: 10.1128/MCB.01152-07. doi:10.1128/MCB.01152-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Price DH. P-TEFb, a cyclin-dependent kinase controlling elongation by RNA polymerase II. Mol Cell Biol. 2000;20:2629–2634. doi: 10.1128/mcb.20.8.2629-2634.2000. doi:10.1128/MCB.20.8.2629-2634.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pahl H. Activators and target genes of Rel/NF-kB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. doi:10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 24.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. doi:10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 25.Zhai P, Yamamoto M, Galeotti J, Liu J, Masurekar M, Thaisz J, et al. Cardiac-specific overexpression of AT1 receptor mutant lacking G{alpha}q/G{alpha}i coupling causes hypertrophy and bradycardia in transgenic mice. J Clin Invest. 2005;115:3045–3056. doi: 10.1172/JCI25330. doi:10.1172/JCI25330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bishop A, Hall A. Rho GTPases and their effector function. Biochem J. 2000;348:241–255. doi:10.1042/0264-6021:3480241. [PMC free article] [PubMed] [Google Scholar]
- 27.McAllister-Lucas LM, Ruland J, Siu K, Jin X, Gu S, Kim DSL, et al. CARMA3/Bcl10/MALT1-dependent NF-{kappa}B activation mediates angiotensin II-responsive inflammatory signaling in nonimmune cells. Proc Natl Acad Sci USA. 2007;104:139–144. doi: 10.1073/pnas.0601947103. doi:10.1073/pnas.0601947103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McAllister-Lucas LM, Lucas PC. Finally, MALT1 is a protease! Nat Immunol. 2008;9:231–233. doi: 10.1038/ni0308-231. doi:10.1038/ni0308-231. [DOI] [PubMed] [Google Scholar]
- 29.Zhou H, Du MQ, Dixit VM. Constitutive NF-[kappa]B activation by the t(11;18)(q21;q21) product in MALT lymphoma is linked to deregulated ubiquitin ligase activity. Cancer Cell. 2005;7:425–431. doi: 10.1016/j.ccr.2005.04.012. doi:10.1016/j.ccr.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 30.Cui R, Tieu B, Recinos AI, Tilton RG, Brasier AR. Rho A mediates angiotensin II-induced phospho-Ser536 NF-kB/RelA subunit exchange on the IL-6 promoter in VSMCs. Circ Res. 2006;99:723–730. doi: 10.1161/01.RES.0000244015.10655.3f. doi:10.1161/01.RES.0000244015.10655.3f. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki CY, Barberi TJ, Ghosh P, Longo DL. Phosphorylation of RelA/p65 on serine 536 defines an I{kappa}B{alpha}-independent NF-{kappa}B Pathway. J Biol Chem. 2005;280:34538–34547. doi: 10.1074/jbc.M504943200. doi:10.1074/jbc.M504943200. [DOI] [PubMed] [Google Scholar]
- 32.Bosisio D, Marazzi I, Agresti A, Shimizu N, Bianchi ME, Natoli G. A hyper-dynamic equilibrium between promoter-bound and nucleoplasmic dimers controls NF-kappaB-dependent gene activity. EMBO J. 2006;25:798–810. doi: 10.1038/sj.emboj.7600977. doi:10.1038/sj.emboj.7600977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choudhary S, Lu M, Cui R, Brasier AR. Involvement of a novel Rac/RhoA GTPase-NF-κB inducing kinase (NIK) signaling pathway mediating angiotensin II-induced RelA transactivation. Mol Endocrinol. 2007;21:2203–2217. doi: 10.1210/me.2006-0465. doi:10.1210/me.2006-0465. [DOI] [PubMed] [Google Scholar]
- 34.Morrison MD, Reiley W, Zhang M, Sun SC. An atypical tumor necrosis factor (TNF) receptor-associated factor-binding motif of B cell-activating factor belonging to the TNF family (BAFF) receptor mediates induction of the noncanonical NF-{kappa}B signaling pathway. J Biol Chem. 2005;280:10018–10024. doi: 10.1074/jbc.M413634200. doi:10.1074/jbc.M413634200. [DOI] [PubMed] [Google Scholar]
- 35.Cheng G, Baltimore D. TANK, a co-inducer with TRAF2 of TNF and CD40L-mediated NF-kB activation. Genes Dev. 1996;10:963–973. doi: 10.1101/gad.10.8.963. doi:10.1101/gad.10.8.963. [DOI] [PubMed] [Google Scholar]
- 36.Basak S, Kim H, Kearns JD, Tergaonkar V, O'Dea E, Werner SL, et al. A Fourth I[kappa]B protein within the NF-[kappa]B signaling module. Cell. 2007;128:369–381. doi: 10.1016/j.cell.2006.12.033. doi:10.1016/j.cell.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madge LA, Kluger MS, Orange JS, May MJ. Lymphotoxin-{alpha}1{beta}2 and LIGHT induce classical and noncanonical NF-{kappa}B-dependent proinflammatory gene expression in vascular endothelial cells. J Immunol. 2008;180:3467–3477. doi: 10.4049/jimmunol.180.5.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao G, Fong A, Sun SC. Induction of p100 processing by NF-{kappa}B-inducing kinase involves docking I{kappa}B Kinase {alpha} (IKK{alpha}) to p100 and IKK{alpha}-mediated phosphorylation. J Biol Chem. 2004;279:30099–30105. doi: 10.1074/jbc.M401428200. doi:10.1074/jbc.M401428200. [DOI] [PubMed] [Google Scholar]
- 39.Liao GX, Zhang MY, Harhaj EW, Sun SC. Regulation of the NF-kappa B-inducing kinase by tumor necrosis factor receptor-associated factor 3-induced degradation. J Biol Chem. 2004;279:26243–26250. doi: 10.1074/jbc.M403286200. doi:10.1074/jbc.M403286200. [DOI] [PubMed] [Google Scholar]
- 40.Lin X, Mu Y, cunningham ET, Marcu KB, Geleziunas R, Greene WG. Molecular determinants of NF-kB inducing kinase action. Mol Cell Biol. 1998;18:5899–5907. doi: 10.1128/mcb.18.10.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonizzi G, Bebien M, Otero DC, Johnson-Vroom K, Cao Y, Vu D, et al. Activation of IKKalpha target genes depends on recognition of specific kappaB binding sites by RelB:p52 dimers. EMBO J. 2004;23:4202–4210. doi: 10.1038/sj.emboj.7600391. doi:10.1038/sj.emboj.7600391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Starkey JM, Haidacher SJ, LeJeune WS, Zhang X, Tieu B, Choudhary S, et al. Diabetes-induced activation of canonical and noncanonical nuclear factor-kB pathways in renal cortex. Diabetes. 2006;55:1252–1259. doi: 10.2337/db05-1554. doi:10.2337/db05-1554. [DOI] [PubMed] [Google Scholar]
- 43.Recinos AI, LeJeune W, Sun H, Lee C, Tieu B, Lu M, et al. Angiotensin II Induces IL-6 expression and the Jak-STAT3 pathway in aortic adventitia of LDL receptor-deficient mice. Atherosclerosis. 2005;194:125–133. doi: 10.1016/j.atherosclerosis.2006.10.013. doi:10.1016/j.atherosclerosis.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michel JB, Thaunat O, Houard X, Meilhac O, Caligiuri G, Nicoletti A. Topological determinants and consequences of adventitial responses to arterial wall injury. Arterioscler Thromb Vasc Biol. 2007;27:1259–1268. doi: 10.1161/ATVBAHA.106.137851. doi:10.1161/ATVBAHA.106.137851. [DOI] [PubMed] [Google Scholar]
- 45.Alonzi T, Maritano D, Gorgoni B, Rizzuto G, Libert C, Poli V. Essential role of STAT3 in the control of the acute-phase response as revealed by inducible gene inactivation [correction of activation] in the liver. Mol Cell Biol. 2001;21:1621–1632. doi: 10.1128/MCB.21.5.1621-1632.2001. (erratum appears in Mol Cell Biol 2001;21:2967) doi:10.1128/MCB.21.5.1621-1632.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Griendling KK, Sorescu D, Ushio-Fukai M. NADPH oxidase role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 47.Patterson C, Ruef J, Madamanchi NR, Barry-Lane P, Hu Z, Horaist C, et al. Stimulation of a vascular smooth muscle cell NAD(P)H oxidase by thrombin. Evidence that p47(phox) may participate in forming this oxidase in vitro and in vivo. J Biol Chem. 1999;274:19814–19822. doi: 10.1074/jbc.274.28.19814. doi:10.1074/jbc.274.28.19814. [DOI] [PubMed] [Google Scholar]
- 48.Gimbrone MA, Jr, Topper JN, Nagel T, Anderson KR, Garcia-Cardena G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann NY Acad Sci. 2000;902:230–239. doi: 10.1111/j.1749-6632.2000.tb06318.x. [DOI] [PubMed] [Google Scholar]
- 49.Adachi T, Pimentel DR, Heibeck T, Hou X, Lee YJ, Jiang B, et al. S-Glutathiolation of Ras mediates redox-sensitive signaling by angiotensin II in vascular smooth muscle cells. J Biol Chem. 2004;279:29857–29862. doi: 10.1074/jbc.M313320200. [DOI] [PubMed] [Google Scholar]
- 50.Haga S, Terui K, Zhang HQ, Enosawa S, Ogawa W, Inoue H, et al. Stat3 protects against Fas-induced liver injury by redox-dependent and -independent mechanisms. J Clin Invest. 2003;112:989–998. doi: 10.1172/JCI17970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.New IL-6 (gp130) Family cytokine members, CLC/NNT1/BSF3 and IL-27. Growth Factors. 2004;22:75–77. doi: 10.1080/08977190410001715181. doi:10.1080/08977190410001715181. [DOI] [PubMed] [Google Scholar]
- 52.Boulanger MJ, Chow Dc, Brevnova EE, Garcia KC. Hexameric structure and assembly of the interleukin-6/IL-6 {alpha}-receptor/gp130 complex. Science. 2003;300:2101–2104. doi: 10.1126/science.1083901. doi:10.1126/science.1083901. [DOI] [PubMed] [Google Scholar]
- 53.Heinrich PC, Behrmann I, Haan S, Hermanns HM, M++ller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. doi:10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakaoka Y, Nishida K, Fujio Y, Izumi M, Terai K, Oshima Y, et al. Activation of gp130 transduces hypertrophic signal through interaction of scaffolding/docking protein Gab1 with tyrosine phosphatase SHP2 in cardiomyocytes. Circ Res. 2003;93:221–229. doi: 10.1161/01.RES.0000085562.48906.4A. doi:10.1161/01.RES.0000085562.48906.4A. [DOI] [PubMed] [Google Scholar]
- 55.Ray S, Boldogh S, Brasier AR. STAT3 NH2-terminal acetylation is activated by the hepatic acute-phase response and required for IL-6 induction of angiotensinogen. Gastroenterology. 2005;129:1616–1632. doi: 10.1053/j.gastro.2005.07.055. doi:10.1053/j.gastro.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 56.Jones SA, Richards PJ, Scheller J, Rose-John S. IL-6 transsignaling: the in vivo consequences. J Interferon Cytokine Res. 2005;25:241–253. doi: 10.1089/jir.2005.25.241. doi:10.1089/jir.2005.25.241. [DOI] [PubMed] [Google Scholar]
- 57.Coles B, Fielding CA, Rose-John S, Scheller J, Jones SA, O'Donnell VB. Classic interleukin-6 receptor signaling and interleukin-6 trans-signaling differentially control angiotensin II-dependent hypertension, cardiac signal transducer and activator of transcription-3 activation, and vascular hypertrophy in vivo. Am J Pathol. 2007;171:315–325. doi: 10.2353/ajpath.2007.061078. doi:10.2353/ajpath.2007.061078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rabe B, Chalaris A, May U, Waetzig GH, Seegert D, Williams AS, et al. Transgenic blockade of interleukin 6 transsignaling abrogates inflammation. Blood. 2008;111:1021–1028. doi: 10.1182/blood-2007-07-102137. doi:10.1182/blood-2007-07-102137. [DOI] [PubMed] [Google Scholar]
- 59.Hou T, Ray S, Brasier AR. The functional role of an IL-6 inducible CDK9-STAT3 complex in human ã-fibrinogen gene expression. J Biol Chem. 2007;282:37091–37102. doi: 10.1074/jbc.M706458200. doi:10.1074/jbc.M706458200. [DOI] [PubMed] [Google Scholar]
- 60.Hou T, Ray S, Lee C, Brasier AR. The STAT3 NH2-terminal domain stabilizes enhanceosome assembly by interacting with the p300 bromodomain. J Biol Chem. 2008;283:30725–30734. doi: 10.1074/jbc.M805941200. doi:10.1074/jbc.M805941200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yuan Zl, Guan YJ, Chatterjee D, Chin YE. Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science. 2005;307:269–273. doi: 10.1126/science.1105166. doi:10.1126/science.1105166. [DOI] [PubMed] [Google Scholar]
- 62.Shapiro GI. Preclinical and clinical development of the cyclin-dependent kinase inhibitor flavopiridol. Clin Cancer Res. 2004;10:4270s–4275s. doi: 10.1158/1078-0432.CCR-040020. doi:10.1158/1078-0432.CCR-040020. [DOI] [PubMed] [Google Scholar]