ABSTRACT
Cholera is a severe diarrheal disease, caused by Vibrio cholerae, for which there has been no reproducible, nonsurgical animal model. Here, we report that orogastric inoculation of V. cholerae into 3-day-old rabbits pretreated with cimetidine led to lethal, watery diarrhea in virtually all rabbits. The appearance and chemical composition of the rabbit diarrheal fluid were comparable to those of the “rice-water stool” produced by cholera patients. As in humans, V. cholerae mutants that do not produce cholera toxin (CT) and toxin-coregulated pilus (TCP) did not induce cholera-like disease in rabbits. CT induced extensive exocytosis of mucin from intestinal goblet cells, and wild-type V. cholerae was predominantly found in close association with mucin. Large aggregates of mucin-embedded V. cholerae were observed, both attached to the epithelium and floating within the diarrheal fluid. These findings suggest that CT-dependent mucin secretion significantly influences V. cholerae’s association with the host intestine and its exit from the intestinal tract. Our model should facilitate identification and analyses of factors that may govern V. cholerae infection, survival, and transmission, such as mucin. In addition, our results using nontoxigenic V. cholerae suggest that infant rabbits will be useful for study of the reactogenicity of live attenuated-V. cholerae vaccines.
IMPORTANCE
Cholera remains a significant threat to populations in developing nations. Currently, there is no reproducible, nonsurgical animal model of cholera, the secretory diarrheal disease caused by Vibrio cholerae. We found that oral infection of infant rabbits with V. cholerae led to lethal, watery diarrhea in most rabbits. Using this disease model, we discovered a new role for cholera toxin (CT) during infection. This toxin not only caused secretory diarrhea but also profoundly influenced how V. cholerae associates with the intestine and how the pathogen exits from the host. Rabbits inoculated with V. cholerae that does not produce CT developed mild diarrhea, suggesting that this model may prove useful for generating improved live attenuated-V. cholerae vaccine candidates. Overall, our findings suggest that the infant rabbit model will enable pursuit of several new avenues for research on cholera pathogenesis, as well as serve as a vehicle for testing new therapeutics.
INTRODUCTION
Cholera is a life-threatening diarrheal disease that is thought to have afflicted human populations for several thousand years (1). Today, cholera remains prevalent in much of the developing world, where it is a significant threat to public health (2, 3). Vibrio cholerae, a curved Gram-negative rod-shaped bacterium, is the cause of this disease. Humans become infected with V. cholerae after ingesting water or food contaminated with the microorganism. Following ingestion, bacteria that survive passage through the acidic milieu of the stomach can subsequently multiply within (colonize) the small intestine. Infection often induces the release of copious amounts of watery stool (up to 1 liter/h), which can lead to severe and rapidly progressing dehydration and shock. Without adequate rehydration therapy, severe cholera (cholera gravis) kills about half of infected individuals (4).
V. cholerae is a noninvasive enteric pathogen. The principal symptom of cholera, secretory diarrhea, is attributed to the actions of cholera toxin (CT), an A-B5 subunit-type exotoxin that is released by V. cholerae in the small intestine. Levine and colleagues reported that individuals given as little as 5 µg of CT developed secretory diarrhea, and volunteers given 25 µg of CT produced more than 25 liters of watery diarrhea (5). In the small intestine, CT induces secretory diarrhea by several mechanisms, including direct stimulation of chloride secretion by enterocytes (by elevating intracellular cyclic AMP [cAMP] levels), as well as stimulation of the enteric nervous system (reviewed in reference 6). CT also induces exocytosis of mucins from goblet cells (7–9). Mucins are a set of related glycoproteins which form the primary constituent of the mucus that covers epithelial surfaces. Thus, it is likely that the flecks of mucin in choleric stool (which account for its “rice-water” appearance) likely result from CT-induced secretion from goblet cells (7).
Human infection with V. cholerae can also induce symptoms that are not attributable to CT. Volunteers given live attenuated-V. cholerae vaccine strains that lack ctxAB, the locus encoding CT, often develop mild (noncholeric) diarrhea and abdominal cramps (10, 11). The causes of this vaccine “reactogenicity” are not known and have been difficult to study due to the lack of a suitable animal model.
Colonization of the small intestine by V. cholerae appears to be dependent upon numerous bacterial gene products (12, 13). The toxin-coregulated pilus (TCP), a type IV pilus whose expression is coregulated with that of CT, is the best characterized of these colonization factors. TCP has been shown to be essential for V. cholerae to colonize the human intestine (14). In vitro studies demonstrate that TCP mediates V. cholerae microcolony formation (15); it may also facilitate adhesion between V. cholerae and the intestinal epithelium (16). Many other factors, including the lipopolysaccharide (LPS) O antigen and various transporters, are also important for V. cholerae survival/multiplication in the intestine (reviewed in reference 17).
V. cholerae does not naturally colonize the intestines of adult mammals other than humans. Consequently, suckling mice and rabbit ligated ileal loops are the most commonly used animal models for study of V. cholerae intestinal colonization and pathogenicity (17). V. cholerae readily colonizes the suckling mouse small intestine, and studies of newborn mice have been extremely useful in identifying V. cholerae gene products that promote intestinal colonization. However, suckling mice do not develop overt diarrhea or other signs of cholera gravis; thus, they have not been as useful for studying factors underlying cholera pathology, such as the bacterial and host factors important for the secretory response. Furthermore, to date, there has been very limited use of techniques (such as confocal microscopy) to investigate the fine localization of V. cholerae in the gastrointestinal tracts of suckling mice. In contrast, the enterotoxicity of V. cholerae can be investigated using the adult rabbit ligated-ileal-loop model, which was developed more than 50 years ago (18, 19). Ligated loops have also been used in elegant scanning and transmission electron microscope studies of V. cholerae attachment to the small intestine (20) and, more recently, in studies of V. cholerae gene expression in vivo (21). However, this closed intestinal loop system requires abdominal surgery and bypasses the natural route of infection as well as several aspects of ordinary gastrointestinal tract physiology, such as peristalsis.
More than a century ago, Elie Metchnikoff observed that infant rabbits could develop profuse watery diarrhea after oral administration of V. cholerae, but the illness did not develop reliably (22). In the mid-1950s, Dutta and Habbu found that direct inoculation of V. cholerae into the small intestines of infant rabbits led to a reproducible cholera-like illness in the infected animals (23). However, during the last 40 years, this intraintestinal infant rabbit model of cholera has been used by only a few investigators (24, 25), perhaps because of the reluctance of investigators to carry out surgery and the emergence of the suckling mouse colonization model.
Here, we report the development of a nonsurgical model of cholera gravis in infant rabbits. Orogastric inoculation of V. cholerae into 3-day-old rabbits that had been pretreated with cimetidine led to lethal, watery diarrhea in virtually all animals. The observations that rabbit diarrheal fluid was chemically similar to that produced by humans and that it was not produced by rabbits infected with V. cholerae mutants that lack genes critical for inducing disease in humans suggest that this system closely models human cholera. Histological analyses of intestinal sections from infected rabbits revealed that CT induced pronounced secretion of mucin from goblet cells in the small intestine. Furthermore, confocal and scanning electron microscopic analyses suggest that CT-dependent mucin secretion has a major impact on the manner in which V. cholerae associates with the host intestine and exits from the intestinal tract. Finally, we found that a V. cholerae ctxAB mutant caused mild transient diarrhea in the infant rabbits, raising the possibility that infant rabbits will be a useful model for studying the reactogenicity of live attenuated-V. cholerae vaccines.
RESULTS
Cimetidine pretreatment improves the reliability of the infant rabbit model of cholera.
Consistent with previous reports, we found that oral infection of infant rabbits with V. cholerae could lead to a cholera-like illness, but the rabbits did not reliably develop signs of disease (22, 23). We wondered whether gastric acidity could contribute to this variability in disease and whether reducing gastric acidity would facilitate colonization. Thus, we supplemented the sodium bicarbonate customarily included in the inoculum with the histamine H2 receptor antagonist cimetidine, which inhibits the secretion of acid into the stomach and transiently increases stomach pH (26). We found that 3 h after intraperitoneal injection of cimetidine (50 mg kg of body weight−1), the stomach pH of 3-day-old rabbits was increased from pH 3-4 to pH 6-7. Furthermore, orogastric administration of ~109 CFU V. cholerae at this time point resulted in the development of cholera-like disease, with reproducible kinetics in nearly all infected rabbits. Lower doses of V. cholerae also induced disease symptoms, but the disease kinetics were less uniform. Consequently, for the experiments described below, we routinely inoculated rabbits orogastrically with ~109 CFU V. cholerae 3 h after cimetidine treatment.
Clinical and histologic signs of disease after orogastric inoculation of V. cholerae into infant rabbits.
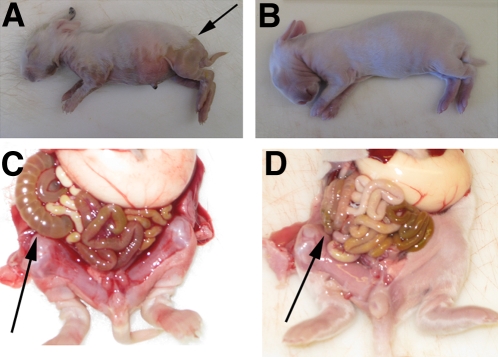
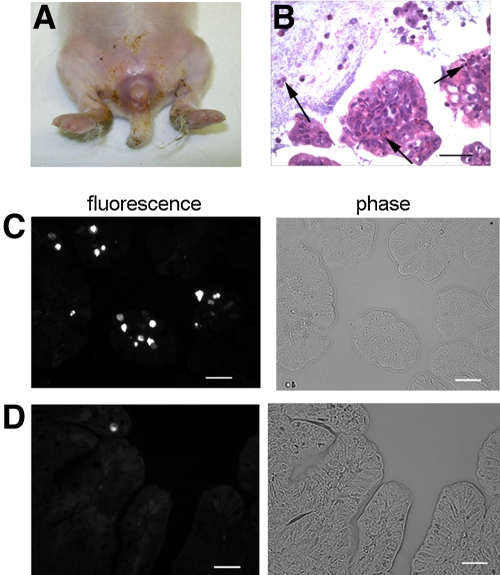
Three-day-old infant rabbits orogastrically inoculated with V. cholerae developed profuse watery diarrhea approximately 12 to 18 h postinfection. This diarrhea resulted in extensive wetting of their ventral surfaces, which was evident as a visible boundary between dry and wet fur (Fig. 1A, arrow). Subsequently, the animals became lethargic and died ~30 h postinoculation. In most of the experiments described below, we euthanized the rabbits ~22 h after inoculation, a point where severe diarrhea was present in nearly all rabbits. At this time, some infected rabbits had lost ~10% of their body weight due to the diarrhea; this also can occur during severe cholera in some patients (4). However, the extent of weight loss was not consistent between infant rabbits, perhaps because the rabbits had free access to their lactating mothers. At necropsy, the small intestines, ceca, and proximal colons of infected rabbits were markedly distended and filled with fluid (Fig. 1C). In contrast, mock-infected rabbits, which received cimetidine and an equivalent volume of sodium bicarbonate solution (the inoculum vehicle), remained dry throughout the experiment and did not exhibit signs of illness (Fig. 1B and D).
FIG 1 .
Gross findings in infant rabbits inoculated with V. cholerae (A and C) or buffer (B and D). The boundary between wet and dry fur is shown with an arrow in panel A. The arrows in panels C and D show the fluid-filled distended cecum and the normal-appearing cecum, respectively. More than 20 V. cholerae-infected and 10 mock-infected rabbits were examined.
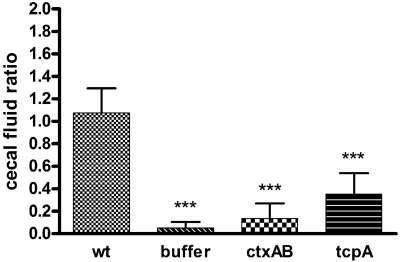
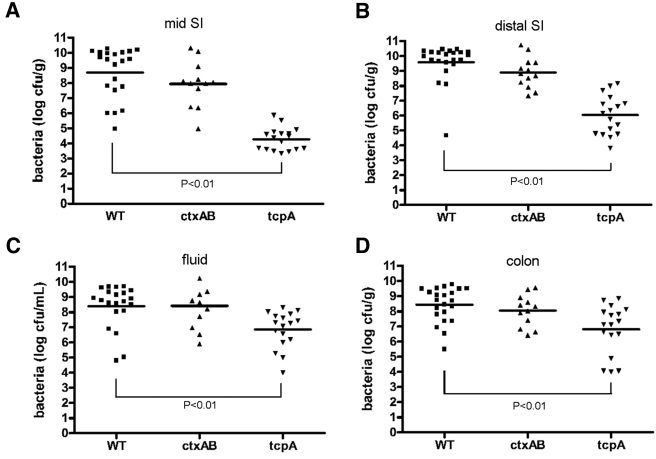
Our attempts to fashion a simple device to directly measure diarrheal fluid loss were unsuccessful. However, measuring the volume of cecal fluid was relatively simple and provided a surrogate measurement for fluid release. We determined a cecal fluid accumulation ratio, defined as the ratio of the weight of fluid drained from the cecum to the weight of cecal tissue, at necropsy for each rabbit. At 22 h postinfection, the ceca of infected rabbits were grossly distended (Fig. 1C) and their fluid accumulation ratios were more than 20 times greater than those from mock-infected rabbits (1.08 ± 0.22 versus 0.05 ± 0.05, respectively) (P < 0.001) (Fig. 2).
FIG 2 .
Cecal fluid accumulation ratios in infant rabbits inoculated with wild-type or mutant V. cholerae or buffer. Samples were collected 22 h after infection. The ratios were calculated as the weight of accumulated cecal fluid to the weight of drained cecal tissue. Error bars represent the standard deviations of the means. Values that were significantly lower (P < 0.001) than the ratio measured with wild-type (wt) V. cholerae infection are indicated by three asterisks. There were at least seven rabbits in each group.
The cecal fluid from infected rabbits appeared to be very similar to human rice-water stool in several respects. Like rice-water stool, this fluid was yellowish and alkaline (ca. pH 9), and it contained low levels of protein (<0.3 mmol/liter−1) and many mucous particles (see below). Additionally, the concentrations of certain electrolytes were elevated in rabbit cecal fluid compared with their concentrations in serum, as has been noted in comparisons of electrolytes in rice-water stool and patient serum samples. The concentrations of potassium and bicarbonate were ~3-fold (18.2 versus 6.5 mmol/liter−1) and 1.5-fold (43 versus 28 mmol/liter−1) higher, respectively, in cecal fluid than in serum in infected rabbits, changes similar in magnitude to those reported in cholera patients (27). Finally, as in rice-water stool (28), there was detectable cholera toxin in the cecal fluid. The comparable gross appearances and chemical properties of rice-water stool and cecal fluid of rabbits orally inoculated with V. cholerae suggest that similar processes, likely CT-induced secretory responses, give rise to these fluids.
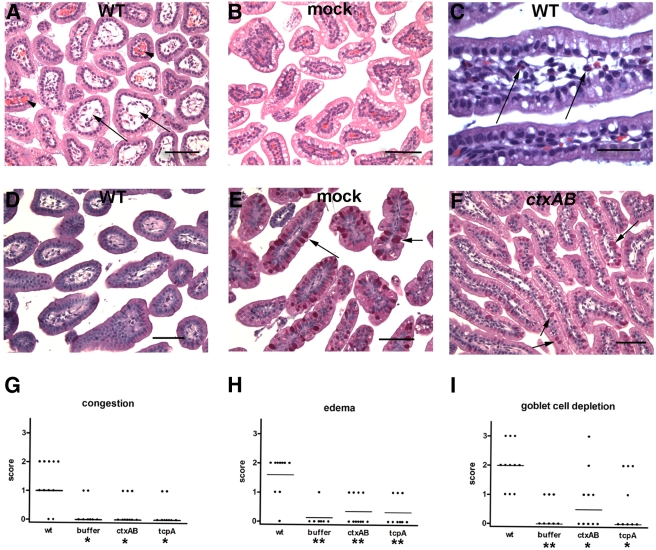
Histological analyses of sections from the intestines of infected rabbits revealed abnormalities in the distal two-thirds of the small intestine as well as in the colon. There was widespread edema and marked congestion of capillaries in the villi of the distal small intestines of infected rabbits compared to those of the mock-infected control rabbits (compare Fig. 3A and B and 3G and H). Similar findings have been observed in biopsy specimens from cholera patients (29, 30). In addition, a mild inflammatory infiltrate consisting of heterophils (the rabbit equivalent of neutrophils) was observed in the distal small intestine in about half of the infected rabbits (Fig. 3C). Histological abnormalities were less consistent in colonic sections from infected rabbits, as submucosal edema and inflammatory cells were evident in only some animals.
FIG 3 .
Histological findings in the distal small intestines of rabbits inoculated with wild-type (WT) or mutant V. cholerae. (A and B) Representative H&E-stained sections taken from rabbits at 22 h postinfection showing edema (arrows) and capillary congestion (arrowheads) in V. cholerae-infected rabbits (A), which is not evident in mock-infected rabbits (B). (C) The arrows point to heterophils present in the lamina propria of V. cholerae-infected rabbits. (D to F) PAS-stained sections show that mucin (magenta stain; arrows) is absent from goblet cells in rabbits infected with wild-type V. cholerae (D) but not in mock-infected rabbits (E) or rabbits infected with the ctxAB mutant (F). Bars, 500 µm (A, B, D, E, and F) and 100 µm (C). (G to I) Histological scores for capillary congestion (G), villous edema (H), and mucin depletion from goblet cells (I) were calculated for at least 8 rabbits. Each symbol shows the score for one rabbit. Horizontal bars represent the median values. Median values that were significantly lower than those found in wild-type-vibrio infection are indicated by asterisks below the groups on the graph (*, P < 0.05; **, P < 0.01).
One striking histological finding in V. cholerae-infected rabbits was observed when small intestine sections were stained with periodic acid-Schiff (PAS) reagent. PAS reacts with carbohydrates and is often used to preferentially stain mucin (31). PAS staining (magenta) of the contents of goblet cells is readily apparent in sections from mock-infected rabbits (Fig. 3E); in contrast, there was a marked depletion of mucin from most of the goblet cells in infected rabbits (compare Fig. 3D and E; 3I). Such mucin depletion has also been observed in biopsy specimens from cholera patients (29, 30) and is thought to reflect CT-promoted goblet cell secretion (8). Overall, our findings indicate that orogastric infection of 3-day-old rabbits with V. cholerae yields clinical and histological signs of disease that closely resemble cholera.
V. cholerae colonization of the infant rabbit intestine.
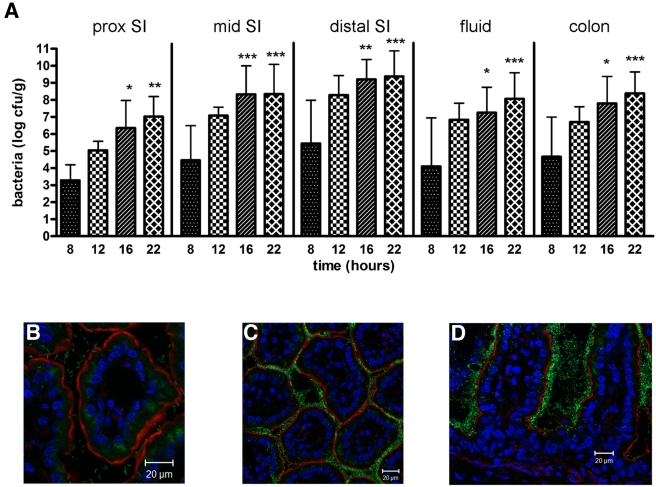
Cecal fluid and tissue homogenates from different regions of the intestine were analyzed at various times postinfection to characterize the distribution and colonization dynamics of V. cholerae in the infant rabbit intestine. Eight hours postinoculation, <10% of the initial inoculum was recovered from the intestinal tract, suggesting that there was a significant reduction in the number of V. cholerae organisms during passage through the stomach. However, by 12 and 16 h postinoculation, the number of V. cholerae CFU recovered from all regions of the intestine had increased by ~2 to 4 orders of magnitude (Fig. 4A). At all time points, the more-distal portions of the small intestine contained the highest numbers of V. cholerae, a finding consistent with analyses of cholera patients (32) (Fig. 4A). However, by the final time point, >5 × 108 CFU per gram of tissue was routinely isolated from the large intestine as well, suggesting that V. cholerae can also persist, if not multiply, in this environment. There were only small increases in the numbers of V. cholerae CFU recovered from each portion of the intestine between 16 and 22 h postinoculation, the period when secretory diarrhea begins in these animals, perhaps because diarrheal shedding limits further bacterial accumulation. By 22 h postinoculation, the cecal fluid routinely contained 108 V. cholerae CFU ml−1, similar to concentrations observed in rice-water stool (33). Collectively, these observations indicate that, following orogastric inoculation into infant rabbits, V. cholerae is able to survive and multiply within the intestinal tract.
FIG 4 .
Quantitative and microscopic assessment of wild-type V. cholerae in the intestines of infant rabbits at various times postinoculation. (A) Numbers of CFU recovered from homogenates of the indicated intestinal sections or in the cecal fluid (prox SI, proximal small intestine). Bars represent the geometric mean values. Values that were significantly greater than the values at 8 h postinfection are indicated by asterisks as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001. Each time point represented data from 5 to 15 rabbits. (B to D) Representative confocal micrographs showing the distribution of fluorescent wild-type V. cholerae at 12 h (B) and 22 h (C and D) postinoculation. Tissue sections from at least five rabbits were counterstained with phalloidin (red) to visualize filamentous actin and DAPI to stain nuclei (blue). In some sections, tissue autofluorescence (diffuse green signal within villi) is evident; this was also observed in tissues from mock-infected rabbits.
CT is required for watery diarrhea and goblet cell mucin depletion.
To date, few studies using infant rabbits have taken advantage of defined V. cholerae mutants. Here, we used defined isogenic deletion mutants to explore the importance in our infant rabbit model of two factors, CT and TCP, critical for V. cholerae virulence in humans (14). Both factors proved to be essential for pathogenesis in infant rabbits as well as humans, providing further validation of the infant rabbit host modeling the human disease.
To assess the contribution of CT to V. cholerae pathogenicity in infant rabbits, we inoculated infant rabbits with a ctxAB deletion mutant. Twenty-four hours after inoculation with this mutant, the rabbits did not exhibit diarrhea, and there was virtually no fluid accumulation in their ceca. Indeed, the cecal fluid accumulation ratios measured with the ctxAB mutant were similar to those found with mock-infected rabbits (Fig. 2). Thus, CT is essential for the accumulation of fluid in the rabbit intestine. Furthermore, these observations suggest that another V. cholerae factor(s) does not make major contributions to diarrhea at this time point; however, as discussed below, this was not the case at later time points. Edema and congestion of capillaries also proved to be CT dependent, as tissue sections from rabbits inoculated with the ctxAB mutant displayed no more congestion and edema than did those from mock-infected rabbits (Fig. 3G and H). Finally, goblet cell mucin depletion was also largely dependent on CT. There was little difference between PAS-stained sections from the rabbits inoculated with the ctxAB mutant and the control rabbits; in both cases, goblet cells were filled with mucin (compare Fig. 3E and F; 3I). In contrast, CT does not appear to be important for promoting heterophil migration into the intestinal villi; there was no discernible difference in the low numbers of heterophils observed in sections from animals infected with the ctxAB mutant versus sections from animals infected with the wild-type strain (data not shown). Thus, heterophil infiltration appears to be induced by factors other than CT.
There are conflicting reports in the literature regarding whether CT promotes V. cholerae intestinal colonization (14, 16, 34). In infant rabbits, CT does not appear to be a major V. cholerae intestinal colonization factor. At 22 h postinoculation, the numbers of CFU of the ctxAB mutant recovered from various regions of the intestine were not statistically different from those obtained after inoculation of the wild-type strain, although in general there was a trend towards fewer ctxAB mutant CFU recovered from the upper small intestine (Fig. 5). Overall, our data suggest that the failure of the ctxAB mutant to induce cholera-like illness after 22 h within the infant rabbit, as described above, is not a consequence of impaired colonization by this strain and instead reflects the absence of CT-mediated “intoxication.” Notably, since this mutant does not induce severe diarrhea and dehydration, later points in the infection cycle can be studied, as discussed below.
FIG 5 .
Recovery of wild-type or mutant V. cholerae (CFU) from homogenates of the indicated regions of the intestines or cecal fluid 22 h postinfection (SI, small intestine). Bars represent the geometric mean values from 11 to 22 rabbits. Each symbol shows the value for one rabbit.
TCP is required for V. cholerae colonization of the infant rabbit intestine.
To explore the clinical and pathological effects of TCP-deficient V. cholerae within our model system, infant rabbits were inoculated with a tcpA deletion mutant of V. cholerae. tcpA encodes the major subunit of this type IV pilus, and pili are not produced in its absence (16). The tcpA mutant exhibited a severe defect in colonization of the infant rabbit intestine. In the mid and distal small intestine, there was an ~5,000- to 30,000-fold reduction in colonization by the mutant relative to the wild type by 22 h postinoculation. The magnitude of the decline was less pronounced in the cecal fluid and colon (~30- to 100-fold reduction) (Fig. 5), suggesting that TCP may not be as important for V. cholerae colonization of the distal regions of the intestinal tract. Overall, our observations demonstrate that TCP is crucial for V. cholerae colonization of the small intestines of infant rabbits and parallel findings regarding colonization of suckling mice and human volunteers (14, 16).
The tcpA mutant did not cause appreciable disease in the infant rabbits. None of the rabbits inoculated with this strain exhibited watery diarrhea or appeared ill, and little-to-no congestion, edema, or goblet cell mucin depletion was observed in the tissue sections (Fig. 3G to I). Furthermore, there was a statistically significant reduction in cecal fluid accumulation induced by the tcpA mutant from the level of accumulation induced by the wild-type strain (Fig. 2) (P < 0.001); however, fluid accumulation due to this mutant was greater than that observed in mock-infected rabbits (Fig. 2) (P < 0.01). Thus, robust intestinal colonization appears to be necessary for V. cholerae to elicit cholera-like diarrhea and intestinal pathology in infant rabbits. Similarly, a tcpA mutant did not elicit symptoms in studies of human volunteers (14, 35). The cause of the residual cecal fluid accumulation observed in rabbits inoculated with the tcpA mutant is unknown. It may reflect the relatively modest reduction in recovery of V. cholerae from the cecum, which may not be sufficient to prevent localized CT accumulation. Alternatively, the residual cecal fluid may result from accumulation of fluid secreted in the small intestine.
Localization of V. cholerae within the rabbit intestinal tract.
The number of V. cholerae CFU recovered from tissue homogenates reflects the organism’s capacity to survive and multiply in particular segments of the rabbit intestine. However, these experiments do not reveal the position of the bacteria within tissues or interactions that occur among bacteria and between bacteria and the intestinal epithelium. Such questions can be addressed using confocal microscopy; however, somewhat surprisingly, very few such studies of V. cholerae within an animal host have been reported. We therefore used this approach to explore the localization in orogastrically inoculated infant rabbits of wild-type V. cholerae that constitutively expressed green fluorescent protein (GFP). Preliminary experiments performed with this strain revealed that intestinal colonization by the strain expressing GFP was indistinguishable from colonization by wild-type V. cholerae, indicating that GFP production did not alter V. cholerae’s ability to survive and multiply in the rabbit intestine.
Frozen sections of intestinal tissue from numerous infected animals were stained with 4′,6′-diamidino-2-phenylindole (DAPI) and phalloidin to allow us to distinguish host cell structures as well as GFP-producing bacteria in the sections. Eight hours postinoculation, few V. cholerae were detected in any of the tissues examined. However, by 12 h after inoculation, fluorescent bacteria were detected in the lumens of the distal small intestines of infected rabbits. At this time point, most of the bacteria were present as individual cells in the intervillous spaces, rather than attached to the epithelial surface (Fig. 4B). By 16 h, abundant V. cholerae could be seen attached to this surface, both individually and within aggregates. By 22 h, much of the epithelial surface of the distal small intestine, including regions deep within crypt-like structures, was covered with V. cholerae, often several layers thick (Fig. 4C and D). However, at this time point, some clusters of V. cholerae appeared to be detaching from the villi, and many fluorescent cells were observed in the lumen, usually as part of aggregates. Collectively, these results suggest that V. cholerae within the small intestine may follow a spatially and temporally regulated program of attachment, multiplication, and release as suggested by the studies of Nelson et al. (20) and Nielsen et al. (21). However, unlike the findings reported previously for adult rabbit ileal loops, we did not observe a time point late in infection when the villi became clear of V. cholerae (20, 21). Complete detachment, which Nielsen and colleagues observed was dependent upon RpoS, may be a consequence of a “closed” system, in which fluid and bacteria continue to accumulate. In support of this idea, we found that V. cholerae in the infant rabbit intestine maintained the elongated appearance typical of log-phase cells throughout the infection cycle, rather than adopting the stubby/rounded look that characterizes stationary phase.
Although relatively large numbers of V. cholerae organisms were recovered in the tissue homogenates from the large intestine (Fig. 4A), relatively few V. cholerae cells were observed attached to the colonic epithelium by confocal microscopy. Instead, large numbers of V. cholerae cells could occasionally be seen associated with food particles, apparently descending the intestinal tract. It is possible that the apparent discordance between the abundant CFU recovered from the large intestine and the relatively few cells detected there by confocal microscopy reflects the loss of loosely attached or nonattached V. cholerae cells during preparation of tissue for microscopy.
V. cholerae is closely associated with mucin in the intestine.
Recent studies suggest that a significant proportion of the V. cholerae organisms in rice-water stool exist as aggregates, some of which are associated with mucin (33, 36). However, there is little information available regarding the association of mucin and V. cholerae within the intestine. We used confocal microscopy and scanning electron microscopy to explore whether such an association could be detected within the infant rabbit intestine and whether it might be influenced by CT-induced mucin exocytosis from goblet cells. Samples from the intestines of at least five rabbits inoculated with wild-type V. cholerae or the ctxAB mutant were examined using both techniques.
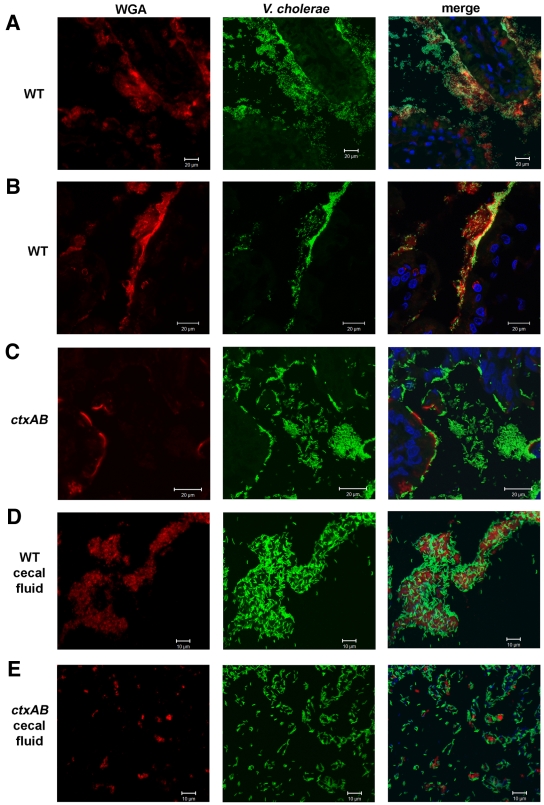
Confocal analyses of wild-type V. cholerae and mucin within the rabbit small intestine yielded striking evidence of an association between them. In tissue samples isolated from rabbits 22 h postinoculation, wheat germ agglutinin (WGA) staining revealed “clouds” of mucin-rich material tethered to the villi (Fig. 6A). Notably, the majority of V. cholerae cells appeared to be associated with intestinal mucus. Numerous fluorescent bacteria could be seen within the mucin-rich material surrounding the villi; in many cases, the layer of bacteria that coated the intestinal epithelium had boundaries that largely coincided with that of WGA staining (Fig. 6B). Large numbers of fluorescent V. cholerae bacteria were also seen surrounded by mucin-rich material in the luminal space. Mucin-associated bacteria also appeared to be detaching from the luminal surface, presumably giving rise to the large aggregates of mucin-associated bacteria detected within the lumen. Indeed, such clusters of mucin and wild-type V. cholerae could readily be visualized within the cecal fluid isolated from infected rabbits (Fig. 6D), and similar mucin-rich aggregates of V. cholerae cells have recently been reported in the rice-water stool of cholera patients (33).
FIG 6 .
Representative confocal micrographs showing fluorescent V. cholerae (green) in the rabbit distal small intestine at 22 h postinfection. Tissues were stained with wheat germ agglutinin (WGA) (red) and DAPI (blue). (A and B) Wild-type V. cholerae often colocalized with mucin-rich “clouds” of material tethered to the villi. (C) In contrast, the V. cholerae ctxAB mutant appeared less associated with mucin and was more often found in the intervillous space. The cecal fluid from rabbits infected with wild-type V. cholerae contained many large mucin-rich aggregates of V. cholerae cells compared with the fluid from rabbits infected with the ctxAB mutant (compare panels D and E). Tissues from at least 10 rabbits were examined.
A significantly different distribution of bacteria and mucin was detected in intestinal sections from infant rabbits infected with the V. cholerae ctxAB mutant. Consistent with the PAS staining results presented in Fig. 3F, mucin in these animals appeared to be largely tissue-associated and presumably contained within goblet cells at the luminal surface. There appeared to be minimal colocalization of bacteria and mucin-rich material (Fig. 6C); the mutant bacteria were more dispersed in the intervillous spaces and less closely associated with the epithelial surface than were wild-type V. cholerae. Some mucin-rich structures were detected within the lumen and also found in the cecal fluid from these animals, but they were considerably smaller than the clumps observed in animals infected with wild-type V. cholerae (compare Fig. 6D and E). Together, these observations suggest that CT-induced mucin secretion can promote a close association between V. cholerae (embedded in mucin) and the epithelium, as well as potentially providing a vehicle in which bacteria can be shed.
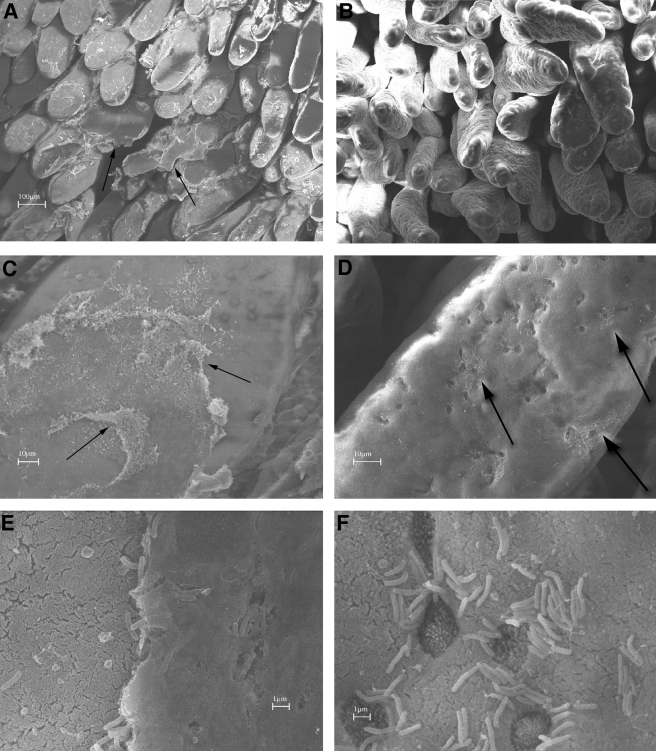
Scanning electron microscopy (SEM) images of tissue from rabbits infected with wild-type V. cholerae or the ctxAB mutant support this model. Low-magnification SEM images revealed striking differences between rabbits infected with wild-type V. cholerae and those infected with the ctxAB mutant. In samples from rabbits inoculated with wild-type V. cholerae, significant quantities of macroscopic material partially covered the villi (Fig. 7A and C, arrows). This material was not observed in samples from rabbits infected with the ctxAB mutant (compare Fig. 7A and B). Higher-magnification analyses revealed that this material corresponds to a dense matrix, presumably containing mucin as well as bacteria (Fig. 7E). These images also suggest that this matrix is only loosely adherent to the underlying tissue; numerous sites where it appeared to be detaching from the epithelium were detected. In contrast, high-magnification SEM images of intestines containing the V. cholerae ctxAB mutant reveal that these bacteria were not surrounded by an adherent matrix but instead are in direct contact with the brush border microvilli (Fig. 7D and F). Thus, these studies reveal a profound difference between the means by which wild-type V. cholerae and the ctxAB mutant adhere to epithelial tissue, which presumably reflects their differential effects on the exocytosis of mucin from goblet cells.
FIG 7 .
Scanning electron micrographs of the distal small intestines of rabbits inoculated with wild-type V. cholerae (A, C, and E) or the ctxAB mutant (B, D, and F). (A and B) Low-magnification images showing macroscopic material (arrow) on the villi in rabbits inoculated with the wild type (A) but not the ctxAB mutant (B). (C to F) Higher magnification reveals that this material contains numerous V. cholerae organisms (C and E), whereas the ctxAB mutant has a patchy distribution and is in direct apposition to the brush border microvilli (D [arrows] and F). Goblet cells, identified by their shorter, darker microvilli, are seen in panel F. Tissue samples from at least five rabbits were examined.
Taken together, our microscopic observations suggest that V. cholerae, via CT-induced mucin secretion, creates a mucin-rich niche for itself near the intestinal epithelium. Later in infection, mucin-rich V. cholerae aggregates detach from the epithelium and become a vehicle for V. cholerae passage through the intestine and back into the environment.
The V. cholerae ctxAB mutant causes mild diarrhea in infant rabbits.
Although the V. cholerae ctxAB mutant does not induce secretory diarrhea in humans, it has frequently been observed to induce a mild (noncholeric) transient diarrhea in trials of live attenuated-V. cholerae vaccine candidates (10, 11, 37). The causes of this vaccine “reactogenicity” are not clear. Similarly, while the V. cholerae ctxAB mutant did not cause a cholera-like disease or death in infant rabbits by 30 h, these animals did not remain free of signs of disease. Instead, most rabbits developed mild transient “fecal” diarrhea at a later time point. By 3 days postinoculation, 13 of 16 infant rabbits inoculated with the V. cholerae ctxAB mutant had developed nonwatery diarrhea, as evident by the accumulation of fecal material on their perianal areas, hind limbs, and tails (Fig. 8A). The diarrhea resolved over ~24 h; by day 5 postinoculation, all the rabbits were free of diarrhea and appeared healthy. We did not observe diarrhea in mock-infected (control) rabbits at any time. Thus, infant rabbits may prove useful in deciphering the causes of “reactogenic” diarrhea induced by V. cholerae ctxAB mutants.
FIG 8 .
V. cholerae ctxAB mutant caused mild diarrhea in infant rabbits 3 days postinoculation. (A) Diarrhea was manifest as fecal contamination of the hind legs and tail. (B) A moderate inflammatory infiltrate was observed in the colonic tissue of all infected rabbits (arrows indicate heterophils). (C and D) Representative images showing the increased numbers of TUNEL-positive cells in the colons of the three infected rabbits examined (C) compared to those in the three mock-infected control animals examined (D). Bars, 50 µm.
Intestinal sections from the rabbits inoculated with the ctxAB mutant were examined histologically for possible clues regarding the cause of the diarrhea. Interestingly, most of the pathology observed at 3 days postinoculation of the ctxAB mutant was found in the colon. Although this organ exhibited minimal histological abnormalities at 22 h, by day 3 postinoculation, the rabbit colons contained moderate numbers of heterophils in the lamina propria, some of which appeared to be transversing the epithelium, and amid the digesta descending the gastrointestinal tract (Fig. 8B). This finding is consistent with the increased levels of lactoferrin, an inflammatory mediator, present in the stools of individuals given live attenuated-V. cholerae vaccines (38).
In addition, terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining of sections from the colon showed elevated numbers of apoptotic cells compared to numbers in the mock-infected age-matched rabbits (Fig. 8C and D). There was ~3-fold more apoptotic cells in the colons of rabbits infected with the ctxAB mutant than in mock-infected rabbits (14.4 ± 1.8 [mean ± standard deviation {SD}] compared to 4.6 ± 0.2, respectively) (P < 0.05). These observations raise the possibility that reactogenicity may be linked to proinflammatory and/or proapoptotic effects of the V. cholerae ctxAB mutant in the colon.
DISCUSSION
Here, we developed a simple nonsurgical animal model of cholera gravis. Orogastric inoculation of cimetidine-treated 3-day-old infant rabbits with V. cholerae routinely resulted in a disease that closely resembles severe human cholera. The appearance and chemical composition of the diarrheal fluid produced by rabbits were reminiscent of human rice-water stool; furthermore, CT and TCP, the two validated V. cholerae virulence factors, were essential for induction of cholera-like illness in rabbits. In addition to enabling studies of V. cholerae factors required for severe diarrhea, this model provides opportunities to study the histopathologic changes induced by the interaction between V. cholerae and an orogastrically infected host. Using this model, we observed striking CT-dependent mucin exocytosis from goblet cells in the small intestine, which seemed to have a major impact on the manner in which V. cholerae associated with and exited from the host intestinal tract. Confocal microscopy studies of fluorescently labeled V. cholerae within the intestine revealed that the majority of wild-type V. cholerae organisms become enmeshed within a mucin-rich matrix. The results of SEM analyses corroborated this idea and suggested that CT-induced mucin secretion leads to accumulation of a thick layer of loosely adherent material laden with V. cholerae cells on the surfaces of most villi. Cecal fluid appears to contain large detached fragments of this layer, and these are likely released in the secretory diarrhea. Comparable coating and shedding were not detected in rabbits infected with the V. cholerae ctxAB mutant, which does not induce goblet cell exocytosis. This mutant instead appears to interact directly with the surfaces of the microvilli and is less frequently observed within large aggregates in cecal fluid. Together, the confocal microscopy and SEM experimental results suggest that CT-induced mucin secretion influences both how V. cholerae associates with the intestinal epithelium and how the organism exits from the intestinal tract. These results raise many interesting questions regarding the role of mucin in disease pathogenesis and bacterial dissemination, which can be addressed using this model in future studies.
Cimetidine-treated infant rabbits provide a new opportunity for exploring the stages of the V. cholerae infection cycle using intact orally inoculated animals. Most of the previous studies of the phases of the V. cholerae infection cycle have been carried out using ligated rabbit ileal loops. SEM studies using this model carried out more than 30 years ago revealed that after an initial lag period, V. cholerae attached to and multiplied along much of the villous surface to form a thick multilayered mat of bacteria. However, by 12 to 16 h after loop inoculation, the bacteria disappeared from the surfaces of the villi (20). Similar observations were made by Nielsen et al. using confocal microscopy (21). They reported that 12 h after injection into ileal loops, V. cholerae undergoes an RpoS-dependent “mucosal escape response” from the villous surface (21). Our work generally corroborates the kinetics defined using ligated loops. We also detected an apparent lag period prior to V. cholerae attachment to the villous surface (Fig. 4B), followed by large increases in the numbers of adherent organisms (Fig. 4C). In addition, we also observed detachment of large clumps of mucin-embedded V. cholerae cells by 22 h. However, we did not see as uniform a clearance of the villous surface as observed in the ligated-loop studies. Instead, at 22 h, there were large numbers of adherent V. cholerae organisms remaining on the villous surfaces (Fig. 4C, 6, and 7). It is possible that this difference arises from the distinct conditions produced in the closed versus open intestinal systems in these two models.
While the production of CT within the intestine appears to have a major impact on the way in which V. cholerae interacts with the intestinal epithelium, this toxin did not appear to promote V. cholerae intestinal colonization. There was not a significant difference in the numbers of CFU of wild-type V. cholerae and the ctxAB mutant recovered from intestinal homogenates of infected rabbits, though in general slightly more wild-type vibrios than ctxAB mutant vibrios were recovered, particularly in the more proximal regions of the small intestine (Fig. 5). This observation is consistent with prior studies of colonization of infant mice and humans by ctx mutants (14, 16). It contrasts with results obtained using an adult rabbit model in which fewer numbers of the ctx mutant were recovered (34). Still, it should be noted that the dramatic difference in symptoms induced by infection with wild-type V. cholerae and the ctxAB mutant likely distorts comparisons of their abilities to colonize. For example, it is not possible to enumerate wild-type V. cholerae bacteria shed within diarrhea, and an increase in CFU within tissues may be masked by tissue edema, as colonization is normalized per gram of tissue. Consequently, although CT clearly is not required for colonization by V. cholerae, the possibility that it enhances colonization is not ruled out by our findings.
Why does V. cholerae produce CT if the toxin is not required for V. cholerae multiplication in the host? The profuse watery diarrhea elicited by CT has long been thought to promote V. cholerae dissemination in the environment and to new hosts. Our work suggests that CT also augments the formation of V. cholerae-laden mucin particles, which have been observed in rice-water stool (33). We speculate that these particles may benefit the bacteria by promoting their environmental persistence. Mucin is potentially a rich source of nutrients for V. cholerae to utilize once it has been expelled from the host intestinal tract. Mucin might also protect V. cholerae from infectious phages, which are prevalent both within the human intestine and in the environment (39) or from other harmful/antimicrobial factors (40). Finally, interaction with mucin has been shown to alter V. cholerae’s gene expression profile (41); such changes might also promote the survival of the bacterium as it is shed. The formation of mucin-rich aggregates might thus provide a signal to the bacterium to prepare itself for a new environment. Previous studies have suggested that changes in V. cholerae’s gene expression profile late in infection may promote environmental survival (42). These studies were performed with mice, which do not exhibit as dramatic a secretory response to CT as rabbits; analogous studies of gene expression in rabbits might thus yield a distinct set of changes.
It also seems reasonable that mucin embedding of V. cholerae might enhance the infectivity of the pathogen, potentially by protecting the organism from host factors that limit infection. Previous studies suggested that encapsulation of V. cholerae aggregates within biofilms enhanced the organism’s infectivity (36, 43). Infant rabbits may prove to be an extremely useful model for investigating the determinants of V. cholerae transmissibility, since we found that infant rabbit littermates in the same cages as rabbits inoculated with V. cholerae often developed cholera-like illness.
It is important to recognize that mucin secretion may also be beneficial for V. cholerae’s host. Mucosal pathogens routinely confront mucus-lined epithelial surfaces, and host mucin secretion is generally thought to function as an important physical barrier against enteric pathogens, including enteropathogenic Escherichia coli, Yersinia enterocolitica, Shigella flexneri, and rhesus rotavirus (44–47). Additionally, infection by several other pathogens has been observed to modulate mucin synthesis and secretion. In contrast to V. cholerae, Helicobacter pylori is thought to reduce mucin exocytosis and synthesis in the stomach, thereby promoting the association of bacteria with the gastric epithelium (48). The effect of Listeria monocytogenes is perhaps more analogous to that of V. cholerae. L. monocytogenes promotes mucin exocytosis through the action of an exotoxin, listeriolysin O; this response is thought to benefit the host by inhibiting L. monocytogenes from entering host cells (49). It is possible that CT-induced mucin secretion is also protective for the rabbit, perhaps exposing the bacteria to host antimicrobial agents, limiting diffusion of secreted bacterial factors to the epithelial layer, or facilitating bacterial shedding from the small intestine.
Orogastric infection of infant rabbits should facilitate the investigation of several additional important questions in cholera research that have been difficult to approach due to the lack of a suitable animal model. First, this model should facilitate a variety of new studies on the in vivo physiology of V. cholerae. Such work could include multiple “-omics” studies on the high numbers of V. cholerae bacteria easily accessible in the rabbit cecum. Second, this model can be used to investigate whether the fine localization of individual V. cholerae cells in the intestine (e.g., luminal versus epithelial) influences their gene expression patterns (using fluorescent reporters) at different times after inoculation. Third, since infant rabbits die from severe cholera, this model should also be useful for testing new cholera therapeutics, including novel antimicrobial agents.
Finally, our work suggests that the infant rabbit model of cholera will be useful for dissecting the molecular bases of diarrhea caused by nontoxigenic, live attenuated-V. cholerae vaccine candidates. We found that rabbits inoculated with a ctxAB mutant routinely developed mild fecal diarrhea reminiscent of the diarrhea often observed in human trials of live attenuated-V. cholerae vaccine candidates. These rabbits also routinely developed an inflammatory response in the colon, suggesting that human reactogenic diarrhea may likewise originate from a colon-derived inflammatory response. Currently, we are taking advantage of the simplicity of the infant rabbit model described here to identify the reactogenic component of live attenuated-V. cholerae vaccines.
MATERIALS AND METHODS
Bacterial strains and media.
A streptomycin-resistant mutant of V. cholerae El Tor O1 strain C6706, a 1991 Peruvian clinical isolate, was used as the wild-type strain in this study (50). Derivatives of strain C6706 harboring deletions of tcpA or ctxAB were constructed by allelic exchange (51). Bacteria were routinely grown in LB broth containing streptomycin (200 µg ml−1) at 37°C unless otherwise noted. V. cholerae C6706 lacZ::gfp, which constitutively expresses a chromosomal copy of the gene encoding green fluorescent protein (GFP) from the lac promoter, was made by introducing a suicide plasmid, pJZ111 (a kind gift from Jun Zhu), into C6706 or one of its derivatives. In LB broth, there were no discernible differences in growth between C6706 and C6706 lacZ::gfp.
Infant rabbit model.
All animal protocols were reviewed and approved by the Harvard Medical Area Standing Committee on Animals. Litters of 2-day-old New Zealand White infant rabbits were obtained from a commercial breeder (Pine Acre Rabbitry, Norton, MA) and housed together with the adult female for the duration of the experiments. Three-day-old infant rabbits were treated with cimetidine (50-mg/kg intraperitoneal injection) 3 h prior to orogastric inoculation with either V. cholerae or a buffer control with a size 5 French catheter (Arrow International, Reading, PA). In most experiments, rabbits were inoculated with ~1 × 109 CFU of V. cholerae. To prepare the inocula, overnight cultures of V. cholerae grown at 30°C were harvested by centrifugation, and the cell pellets were resuspended at a final concentration of ~2 × 109 CFU/ml in a sodium bicarbonate solution (2.5 g in 100 ml; pH 9). After the rabbits were inoculated, they were monitored for clinical signs of illness. Diarrhea was assessed by the presence of watery or fecal material on the hind legs, tails, or ventral surfaces of individual rabbits. Rabbits receiving wild-type V. cholerae were typically euthanized at 22 h postinfection. At necropsy, the entire intestinal tract from the duodenum to the rectum was removed. The amount of cecal fluid was measured by first isolating the cecum from the rest of the intestine with silk ligatures; then, the cecal contents were collected by snipping the end of the cecum and allowing the contents to drain under gravity into a preweighed collection tube. A cecal fluid accumulation ratio was calculated as the ratio of the cecal fluid weight to the remaining cecal tissue weight. To limit any litter-specific effects, at least two independent litters were used to test each condition; each litter on average contained eight kits.
The number of V. cholerae CFU in tissue samples was determined by plating on selective media. Tissue samples were homogenized in 2 ml sterile phosphate-buffered saline (PBS), serially diluted, and plated on LB agar containing streptomycin (200 µg ml−1) for enumeration of CFU per gram of tissue. The number of CFU in cecal fluid samples was determined following serial dilution and plating, and the values were expressed per milliliter of fluid. The detection limit of these assays was ~100 CFU/g or 10 CFU/ml. In samples where no bacterial colonies were detected at the lowest dilution, the mean values present in Fig. 4 and 5 were calculated using the lower limit of detection as a value.
For routine histological analyses, tissue segments were fixed in 10% neutral-buffered formalin, processed for paraffin embedding, and stained with hematoxylin and eosin (H&E) or periodic-acid Schiff (PAS) reagent. The slides were semiquantitatively assessed for capillary congestion, inflammatory infiltrates, edema, and mucin loss from goblet cells by a pathologist with no knowledge of the sample source. Each histological parameter mentioned above was evaluated and given a score as follows: 0 for normal, 1 for mild, 2 for moderate, and 3 for severe. TUNEL staining was performed on colonic tissues from mock-infected rabbits and rabbits infected with the ctxAB mutant to evaluate apoptosis (in situ cell death detection kit; Roche, IN). Cecal fluid electrolytes were measured on an Olympus Analyzer (AU-2700) at the Brigham and Women’s Hospital clinical laboratory.
Confocal microscopy.
For confocal microscopy, tissue segments from rabbits infected with GFP-producing V. cholerae were fixed in 4% paraformaldehyde (in PBS) on ice for 2 h before being placed in 30% sucrose (in PBS) at 4°C overnight. The next day, the tissue segments were briefly washed in PBS, the outer surface was dried on filter paper, and trimmed pieces were placed in optimal cutting temperature (OCT) compound (Electron Microscopy Sciences, PA). Each tissue block was quick-frozen over a mixture of dry ice and ethanol and stored at −80°C prior to sectioning. Sections that were approximately 5 μm thick were cut, placed on glass slides, and processed for immunofluorescence. Initially, OCT compound was removed from the tissue section by washing the slides three times in PBS (5 min each). The slides were stained for 1 h with Alexa Fluor 568 phalloidin (1/50) (catalog no. A12380; Invitrogen, OR) and/or Alexa Fluor 633 wheat germ agglutinin (1/200) (catalog no. W21404; Invitrogen, OR) at room temperature in the dark. After being washed further in PBS (twice for 5 min each time), the slides were counterstained with DAPI for 5 min (1 µg/ml), washed in PBS (twice for 5 min each time), covered in mounting medium (Vector Laboratories, CA), covered with the coverslips, sealed with VALAP (equal mixture of Vaseline, lanolin, and paraffin), and stored at −20°C. Slides were examined for fluorescence using a Zeiss LSM510 Meta upright confocal microscope, and images were taken with LSM510 software.
Diarrheal fluid was collected from the ceca of infected rabbits at necropsy and immediately fixed in 2% paraformaldehyde in PBS for 30 min. Triplicate 10-μl aliquots of the fluid were spotted on glass slides coated with 1% agarose (in PBS) and allowed to absorb for 10 min. The slides were then counterstained with wheat germ agglutinin and DAPI as described above and examined using the upright confocal microscope.
Electron microscopy.
For scanning electron microscopy, segments of intestinal tissue were tied with silk ligatures and immediately fixed in 0.02% picric acid, 5% gluteraldehyde, and 1% paraformaldehyde buffered in 0.1 M sodium cacodylate buffer (pH 7.4). The segments were opened longitudinally, and tissue sections were washed once in fresh 0.1 M sodium cacodylate buffer (pH 7.4) for 5 min. The tissue pieces were then incubated in 1% osmium tetraoxide in 0.1 M sodium cacodylate buffer (pH 7.4) for 30 min at room temperature. After removal of the osmium tetraoxide solutions, the tissue pieces were washed two times in 0.1 M sodium cacodylate buffer (pH 7.4) and dehydrated in a series of ethanol solutions with increasing percentages of ethanol. The tissue pieces were dried in the critical point dryer (Samdri PVT-3B), oriented on stubs, and sputter-coated using a gold-palladium target (Hummer V sputter coater). Samples were examined using an LEO 1450VP scanning electron microscope and images stored using the LEO-32 V04.00.10 operating program.
Statistical analysis.
Cecal fluid accumulation ratios and bacterial counts (after log transformation) were statistically analyzed using one-way analysis of variance (ANOVA) and Bonferroni’s test for multiple comparisons (GraphPad Prism, San Diego, CA). Histopathogic scores for edema, congestion, and goblet cell content were statistically analyzed using the Kruskal-Wallis statistic with Dunn’s posttest for multiple comparisons (GraphPad Prism, San Diego, CA). TUNEL-stained slides were evaluated for the number of apoptotic cells per field of view using the Student t test.
ACKNOWLEDGMENTS
We thank Rebecca Stearns for expert help with the scanning electron microscope, Lay Hong Ang for assistance with the confocal microscope in the Imaging Core of the Harvard Digestive Disease Center, Liza Shakhnovich for the ctxAB and tcpA mutants, Leyla Slamti for translation of Metchnikoff’s original paper, and Brigid Davis for critical review of the manuscript.
This study was funded by grants from NIH (R37 AI-42347), HHMI, the Institute for One World Health, and the Harvard Catalyst (NIH grant 1 UL1 RR 025758-02).
We declare no conflicts of interest.
Footnotes
Citation Ritchie, J. M., H. Rui, R. T. Bronson, and M. K. Waldor. 2010. Back to the future: studying cholera pathogenesis using infant rabbits. mBio 1(1):e00047-10. doi:10.1128/mBio.00047-10.
REFERENCES
- 1. Lacey S. W. 1995. Cholera: calamitous past, ominous future. Clin. Infect. Dis. 20:1409–1419 [DOI] [PubMed] [Google Scholar]
- 2. Sack D. A., Sack R. B., Chaignat C. L. 2006. Getting serious about cholera. N. Engl. J. Med. 355:649–651 [DOI] [PubMed] [Google Scholar]
- 3. WHO 2009. Cholera: global surveillance summary, 2008. Wkly. Epidemiol. Rec. 84:309–324 [PubMed] [Google Scholar]
- 4. Sack D. A., Sack R. B., Nair G. B., Siddique A. K. 2004. Cholera. Lancet 363:223–233 [DOI] [PubMed] [Google Scholar]
- 5. Levine M. M., Kaper J. B., Black R. E., Clements M. L. 1983. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol. Rev. 47:510–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sears C. L., Kaper J. B. 1996. Enteric bacterial toxins: mechanisms of action and linkage to intestinal secretion. Microbiol. Rev. 60:167–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sherr H. P., Mertens R. B. 1979. Cholera toxin-induced glycoprotein secretion in rabbit small intestine. Gastroenterology 77:18–25 [PubMed] [Google Scholar]
- 8. Forstner J. F., Roomi N. W., Fahim R. E., Forstner G. G. 1981. Cholera toxin stimulates secretion of immunoreactive intestinal mucin. Am. J. Physiol. 240:G10–G16 [DOI] [PubMed] [Google Scholar]
- 9. Leitch G. J. 1988. Cholera enterotoxin-induced mucus secretion and increase in the mucus blanket of the rabbit ileum in vivo. Infect. Immun. 56:2871–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tacket C. O., Losonsky G., Nataro J. P., Cryz S. J., Edelman R., Fasano A., Michalski J., Kaper J. B., Levine M. M. 1993. Safety and immunogenicity of live oral cholera vaccine candidate CVD one hundred and ten, a delta ctxA delta zot delta ace derivative of El Tor Ogawa Vibrio cholerae. J. Infect. Dis. 168:1536–1540 [DOI] [PubMed] [Google Scholar]
- 11. Taylor D. N., Killeen K. P., Hack D. C., Kenner J. R., Coster T. S., Beattie D. T., Ezzell J., Hyman T., Trofa A., Sjogren M. H. 1994. Development of a live, oral, attenuated vaccine against El Tor cholera. J. Infect. Dis. 170:1518–1523 [DOI] [PubMed] [Google Scholar]
- 12. Merrell D. S., Hava D. L., Camilli A. 2002. Identification of novel factors involved in colonization and acid tolerance of Vibrio cholerae. Mol. Microbiol. 43:1471–1491 [DOI] [PubMed] [Google Scholar]
- 13. Chiang S. L., Mekalanos J. J. 1998. Use of signature-tagged transposon mutagenesis to identify Vibrio cholerae genes critical for colonization. Mol. Microbiol. 27:797–805 [DOI] [PubMed] [Google Scholar]
- 14. Herrington D. A., Hall R. H., Losonsky G., Mekalanos J. J., Taylor R. K., Levine M. M. 1988. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J. Exp. Med. 168:1487–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kirn T. J., Lafferty M. J., Sandoe C. M., Taylor R. K. 2000. Delineation of pilin domains required for bacterial association into microcolonies and intestinal colonization by Vibrio cholerae. Mol. Microbiol. 35:896–910 [DOI] [PubMed] [Google Scholar]
- 16. Taylor R. K., Miller V. L., Furlong D. B., Mekalanos J. J. 1987. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc. Natl. Acad. Sci. U. S. A. 84:2833–2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ritchie J. M., Waldor M. K. 2009. Vibrio cholerae interactions with the gastrointestinal tract: lessons from animal studies. Curr. Top. Microbiol. Immunol. 337:37–59 [DOI] [PubMed] [Google Scholar]
- 18. De S. N. 1959. Enterotoxicity of bacteria-free culture-filtrate of Vibrio cholerae. Nature 183:1533–1534 [DOI] [PubMed] [Google Scholar]
- 19. De S. N., Chatterje D. N. 1953. An experimental study of the mechanism of action of Vibrio cholerae on the intestinal mucous membrane. J. Pathol. Bacteriol. 66:559–562 [DOI] [PubMed] [Google Scholar]
- 20. Nelson E. T., Clements J. D., Finkelstein R. A. 1976. Vibrio cholerae adherence and colonization in experimental cholera: electron microscopic studies. Infect. Immun. 14:527–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nielsen A. T., Dolganov N. A., Otto G., Miller M. C., Wu C. Y., Schoolnik G. K. 2006. RpoS controls the Vibrio cholerae mucosal escape response. PLoS Pathog. 2:e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Metchnikoff E. 1894. Recherches sur le cholera et les vibrions. Receptivite des jeunes lapins pour le cholera intestinal. Ann. Inst. Pasteur (Paris) 8:557 [Google Scholar]
- 23. Dutta N. K., Habbu M. K. 1955. Experimental cholera in infant rabbits: a method for chemotherapeutic investigation. Br. J. Pharmacol. Chemother. 10:153–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Finkelstein R. A., Boesman-Finkelstein M., Chang Y., Hase C. C. 1992. Vibrio cholerae hemagglutinin/protease, colonial variation, virulence, and detachment. Infect. Immun. 60:472–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Madden J. M., Nematollahi W. P., Hill W. E., McCardell B. A., Twedt R. M. 1981. Virulence of three clinical isolates of Vibrio cholerae non-O-one serogroup in experimental enteric infections in rabbits. Infect. Immun. 33:616–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schlech W. F., III, Chase D. P., Badley A. 1993. A model of food-borne Listeria monocytogenes infection in the Sprague-Dawley rat using gastric inoculation: development and effect of gastric acidity on infective dose. Int. J. Food Microbiol. 18:15–24 [DOI] [PubMed] [Google Scholar]
- 27. Bennish M. L. 1994. Cholera: pathophysiology, clinical features and treatment, p. 229–255 In Wachsmuth I. K., Blake P. A., Olsvik O., Vibrio cholerae and cholera: molecular to global perspectives. American Society for Microbiology, Washington, DC [Google Scholar]
- 28. Aziz K. M., Mosley W. H. 1972. Quantitative studies of toxin in the stools and jejunal aspirates of patients with cholera. J. Infect. Dis. 125:36–44 [DOI] [PubMed] [Google Scholar]
- 29. Gangarosa E. F., Beisel W. R., Benyajati C., Sprinz H., Piyaratn P. 1960. The nature of the gastrointestinal lesion in Asiatic cholera and its relation to pathogenesis: a biopsy study. Am. J. Trop. Med. Hyg. 9:125–135 [DOI] [PubMed] [Google Scholar]
- 30. Chen H. C., Reyes V., Fresh J. W. 1971. An electron microscopic study of the small intestine in human cholera. Virchows Arch. B Cell Pathol. 7:236–259 [DOI] [PubMed] [Google Scholar]
- 31. Thornton D. J., Holmes D. F., Sheehan J. K., Carlstedt I. 1989. Quantitation of mucus glycoproteins blotted onto nitrocellulose membranes. Anal. Biochem. 182:160–164 [DOI] [PubMed] [Google Scholar]
- 32. Gorbach S. L., Banwell J. G., Jacobs B., Chatterjee B. D., Mitra R., Brigham K. L., Neogy K. N. 1970. Intestinal microflora in Asiatic cholera. II. The small bowel. J. Infect. Dis. 121:38–45 [DOI] [PubMed] [Google Scholar]
- 33. Nelson E. J., Chowdhury A., Harris J. B., Begum Y. A., Chowdhury F., Khan A. I., Larocque R. C., Bishop A. L., Ryan E. T., Camilli A., Qadri F., Calderwood S. B. 2007. Complexity of rice-water stool from patients with Vibrio cholerae plays a role in the transmission of infectious diarrhea. Proc. Natl. Acad. Sci. U. S. A. 104:19091–19096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pierce N. F., Kaper J. B., Mekalanos J. J., Cray W. C., Jr. 1985. Role of cholera toxin in enteric colonization by Vibrio cholerae O1 in rabbits. Infect. Immun. 50:813–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tacket C. O., Taylor R. K., Losonsky G., Lim Y., Nataro J. P., Kaper J. B., Levine M. M. 1998. Investigation of the roles of toxin-coregulated pili and mannose-sensitive hemagglutinin pili in the pathogenesis of Vibrio cholerae O139 infection. Infect. Immun. 66:692–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Faruque S. M., Biswas K., Udden S. M., Ahmad Q. S., Sack D. A., Nair G. B., Mekalanos J. J. 2006. Transmissibility of cholera: in vivo-formed biofilms and their relationship to infectivity and persistence in the environment. Proc. Natl. Acad. Sci. U. S. A. 103:6350–6355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tacket C. O., Kotloff K. L., Losonsky G., Nataro J. P., Michalski J., Kaper J. B., Edelman R., Levine M. M. 1997. Volunteer studies investigating the safety and efficacy of live oral El Tor Vibrio cholerae O1 vaccine strain CVD 111. Am. J. Trop. Med. Hyg. 56:533–537 [DOI] [PubMed] [Google Scholar]
- 38. Silva T. M., Schleupner M. A., Tacket C. O., Steiner T. S., Kaper J. B., Edelman R., Guerrant R. 1996. New evidence for an inflammatory component in diarrhea caused by selected new, live attenuated cholera vaccines and by El Tor and O139 Vibrio cholerae. Infect. Immun. 64:2362–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Faruque S. M., Islam M. J., Ahmad Q. S., Faruque A. S., Sack D. A., Nair G. B., Mekalanos J. J. 2005. Self-limiting nature of seasonal cholera epidemics: role of host-mediated amplification of phage. Proc. Natl. Acad. Sci. U. S. A. 102:6119–6124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lievin-Le Moal V., Servin A. L. 2006. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: mucins, antimicrobial peptides, and microbiota. Clin. Microbiol. Rev. 19:315–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu Z., Miyashiro T., Tsou A., Hsiao A., Goulian M., Zhu J. 2008. Mucosal penetration primes Vibrio cholerae for host colonization by repressing quorum sensing. Proc. Natl. Acad. Sci. U. S. A. 105:9769–9774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schild S., Tamayo R., Nelson E. J., Qadri F., Calderwood S. B., Camilli A. 2007. Genes induced late in infection increase fitness of Vibrio cholerae after release into the environment. Cell Host Microbe 2:264–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhu J., Mekalanos J. J. 2003. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Dev. Cell 5:647–656 [DOI] [PubMed] [Google Scholar]
- 44. Mack D. R., Michail S., Wei S., McDougall L., Hollingsworth M. A. 1999. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. 276:G941–G950 [DOI] [PubMed] [Google Scholar]
- 45. Mantle M., Basaraba L., Peacock S. C., Gall D. G. 1989. Binding of Yersinia enterocolitica to rabbit intestinal brush border membranes, mucus, and mucin. Infect. Immun. 57:3292–3299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nutten S., Sansonetti P., Huet G., Bourdon-Bisiaux C., Meresse B., Colombel J. F., Desreumaux P. 2002. Epithelial inflammation response induced by Shigella flexneri depends on mucin gene expression. Microbes Infect. 4:1121–1124 [DOI] [PubMed] [Google Scholar]
- 47. Chen C. C., Baylor M., Bass D. M. 1993. Murine intestinal mucins inhibit rotavirus infection. Gastroenterology 105:84–92 [DOI] [PubMed] [Google Scholar]
- 48. Micots I., Augeron C., Laboisse C. L., Muzeau F., Megraud F. 1993. Mucin exocytosis: a major target for Helicobacter pylori. J. Clin. Pathol. 46:241–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lievin-Le Moal V., Servin A. L., Coconnier-Polter M. H. 2005. The increase in mucin exocytosis and the upregulation of MUC genes encoding for membrane-bound mucins induced by the thiol-activated exotoxin listeriolysin O is a host cell defence response that inhibits the cell-entry of Listeria monocytogenes. Cell. Microbiol. 7:1035–1048 [DOI] [PubMed] [Google Scholar]
- 50. Dziejman M., Balon E., Boyd D., Fraser C. M., Heidelberg J. F., Mekalanos J. J. 2002. Comparative genomic analysis of Vibrio cholerae: genes that correlate with cholera endemic and pandemic disease. Proc. Natl. Acad. Sci. U. S. A. 99:1556–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mekalanos J. J., Swartz D. J., Pearson G. D., Harford N., Groyne F., de Wilde M. 1983. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature 306:551–557 [DOI] [PubMed] [Google Scholar]