ABSTRACT
Bacillus subtilis cells form multicellular biofilm communities in which spatiotemporal regulation of gene expression occurs, leading to differentiation of multiple coexisting cell types. These cell types include matrix-producing and sporulating cells. Extracellular matrix production and sporulation are linked in that a mutant unable to produce matrix is delayed for sporulation. Here, we show that the delay in sporulation is not due to a growth advantage of the matrix-deficient mutant under these conditions. Instead, we show that the link between matrix production and sporulation is through the Spo0A signaling pathway. Both processes are regulated by the phosphorylated form of the master transcriptional regulator Spo0A. When cells have low levels of phosphorylated Spo0A (Spo0A~P), matrix genes are expressed; however, at higher levels of Spo0A~P, sporulation commences. We have found that Spo0A~P levels are maintained at low levels in the matrix-deficient mutant, thereby delaying expression of sporulation-specific genes. This is due to the activity of one of the components of the Spo0A phosphotransfer network, KinD. A deletion of kinD suppresses the sporulation defect of matrix mutants, while its overproduction delays sporulation. Our data indicate that KinD displays a dual role as a phosphatase or a kinase and that its activity is linked to the presence of extracellular matrix in the biofilms. We propose a novel role for KinD in biofilms as a checkpoint protein that regulates the onset of sporulation by inhibiting the activity of Spo0A until matrix, or a component therein, is sensed.
IMPORTANCE
A question in the field of biofilm development has remained virtually unaddressed: how do the biofilm cells sense the completion of the synthesis of extracellular matrix? The presence of an extracellular matrix that holds the cells together is a defining feature of biofilms. How cells sense that matrix has been assembled and how this signal is transduced have not been investigated. Bacillus subtilis provides an excellent system in which to address this question, as the molecular pathways involved in regulation of differentiation are well characterized. We provide the first evidence for a protein that senses the presence of matrix. We identify a membrane sensor histidine kinase, KinD, that alters its activity, depending on the presence or absence of the extracellular matrix. This activity feeds back to the master regulator Spo0A to regulate expression of genes involved in producing matrix and genes necessary for the progression into sporulation.
INTRODUCTION
Bacteria often grow as elaborate surface-associated multicellular communities, commonly referred to as biofilms (1). Biofilm-associated cells are bound together by a self-generated extracellular matrix that consists of polysaccharides, proteins, and, in some cases, DNA (2). As a consequence of extracellular matrix production, bacterial colonies grown on semisolid agar surfaces develop complex architecture. Such is the case for undomesticated strains of Bacillus subtilis (3). Within these colonies, there is spatiotemporal regulation of gene expression and several different cell types coexist, including a subpopulation of extracellular-matrix-producing cells (4, 5). These extracellular-matrix-producing cells differentiate to form metabolically inactive spores, which localize preferentially to aerial projections of the biofilm (5).
The major components of the B. subtilis extracellular matrix are exopolysaccharide (EPS) and the protein TasA, encoded by the epsA-epsO and yqxM-sipW-tasA operons, respectively (6). The expression of these operons is indirectly regulated by the transcriptional regulator Spo0A (7, 8). Spo0A activity depends on its phosphorylation state. The level of phosphorylated Spo0A (Spo0A~P) is controlled by a network of kinases and phosphatases that responds to both environmental and physiological signals. The kinases function either directly on Spo0A or indirectly through a phosphorelay consisting of the response regulator Spo0F and a phosphotransfer protein, Spo0B (9). Five distinct sensor kinases input phosphate into the phosphorelay to control the level of Spo0A~P present at any moment in the cell. Two of these kinases, KinA and KinB, can have high activity and are required to achieve the high levels of phosphorylated Spo0A necessary for sporulation in response to different nutrient-limiting conditions (10–12). In the absence of KinA and KinB, KinC and KinD lead to only moderate levels of Spo0A~P. While these low levels of Spo0A~P are insufficient to trigger the sporulation pathway, they are able to induce the expression of the genes involved in biofilm formation (13–15). KinC can phosphorylate Spo0A directly in response to the action of the self-generated signaling molecule surfactin, thus triggering extracellular-matrix production (15). Exactly how KinD functions to control biofilm formation is unknown.
We have recently shown that the presence of extracellular matrix has a profound effect on sporulation in B. subtilis biofilms; mutants unable to produce matrix are defective in sporulation (5). This led us to hypothesize that cells of matrix-deficient mutants are unable to accumulate enough Spo0A~P to trigger the sporulation pathway. In the present work, we show that the extracellular matrix is indeed required to reach high levels of Spo0A~P when cells are in biofilms. We report that a kinD mutant is able to bypass the requirement for extracellular matrix prior to sporulation in B. subtilis biofilms. In addition, we report that under biofilm-inducing conditions, a kinD mutant sporulates early and that overexpressing kinD delays sporulation. These results suggest that KinD functions to maintain low levels of Spo0A~P until matrix is assembled.
RESULTS
Matrix mutants are delayed in sporulation during biofilm development.
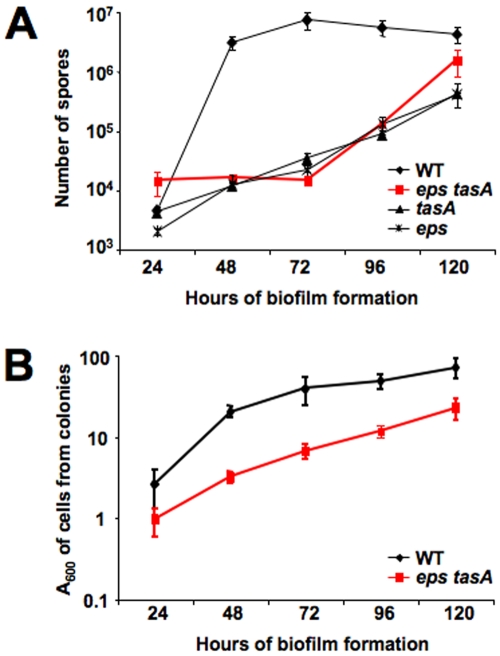
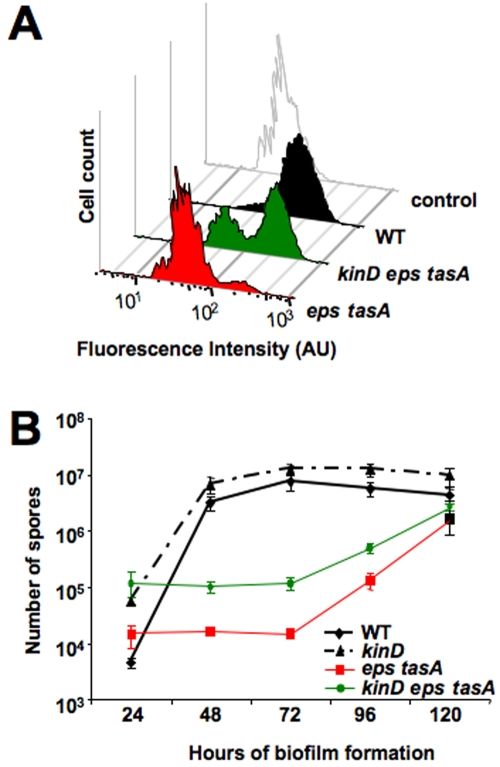
We previously reported that cells lacking either the TasA protein or the exopolysaccharide (EPS) component of the matrix were defective in sporulation when grown under biofilm-inducing conditions but not when grown in dispersed culture (5). To further understand this sporulation defect, we analyzed the kinetics of spore formation in biofilms of single mutants lacking either tasA or the eps operon as well as in a double mutant lacking both major matrix components (eps tasA) (Fig. 1A). As expected, the kinetics of spore formation in biofilms formed by the wild type showed an increasing number of spores over time, with the highest increase in the number of spores observed between 24 h and 48 h of development. In contrast, cells from biofilms of each single mutant or of the double eps tasA mutant showed a minimal increase in the number of spores up to 72 h of growth. It was only after 72 h of growth that a steady increase in the number of spores was observed. By 120 h of development, the number of spores was about 10- to 100-fold less than the wild-type level for all three mutant strains (Fig. 1A). Since the rate of spore formation of the double eps tasA mutant was similar to that of the individual tasA and eps mutants, we focused our analyses on the double mutant for the remaining experiments.
FIG 1 .
Matrix mutants are delayed in sporulation. (A) Sporulation of wild-type cells (WT) compared to the level for tasA, eps, or eps tasA matrix mutants. Cells were grown on MSgg solid medium at 30°C for the indicated time prior to harvesting, and viable spores were quantified after heat treatment for 20 min at 80°C. Error bars indicate standard errors of the means. (B) Total cells in biofilms grown on MSgg solid medium at 30°C for the indicated times. The entire colonies were harvested at the indicated times and disrupted using mild sonication conditions. Cells were resuspended in water, and absorbance at 600 nm was recorded immediately. Each strain was assayed in duplicate in at least three independent experiments. Error bars indicate standard errors of the means.
The sporulation defect is not due to a growth advantage.
The delay in sporulation of the matrix mutant might be due to a growth advantage of the eps tasA mutant over the wild type. If the energy required to synthesize the matrix components is instead funneled into other metabolic pathways, the matrix mutant cells might continue growing for longer periods of time, delaying the initiation of sporulation. To test this hypothesis, we harvested entire colonies and compared the total number of cells in the eps tasA mutant biofilm to the number of cells present in the wild-type biofilm over the course of development (Fig. 1B). Surprisingly, we found the opposite; there were significantly more cells in biofilms formed by the wild-type strain than in those formed by the eps tasA mutant, with about a 10-fold difference in cell number between the wild-type and mutant cells at all of the time points assayed. When matrix mutant cells were compared to wild-type cells visually under the microscope, there was no observable difference in cell size. We concluded that the delay observed in sporulation cannot be explained by prolonged growth of the matrix mutant cells.
Starvation overrides the sporulation delay of matrix mutants.
Previously, we showed that the matrix mutants are not delayed in sporulation when grown in shaken culture (5). This suggests that the sporulation defect might be specific to environmental conditions under which extracellular matrix is normally produced, or it might be more generally related to growth on solid agar surfaces. To distinguish between these possibilities, we grew cells on agar surfaces using either MSgg medium, which favors extracellular-matrix production, or a nutrient-poor medium containing only 10% of the carbon source present in the regular MSgg medium (10% MSgg), in which cells proceed directly to sporulation, bypassing matrix gene expression.
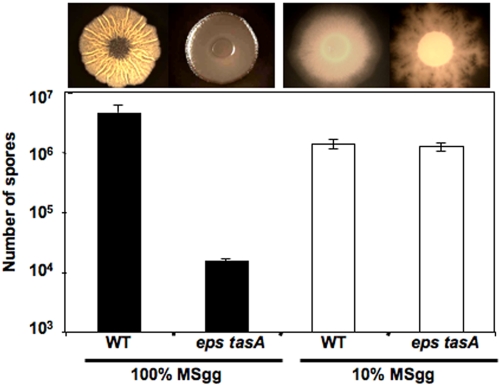
When wild-type cells were grown on agar plates containing 10% MSgg medium, extracellular matrix was not produced, as evidenced by the lack of wrinkled architecture in wild-type colonies (Fig. 2, upper panels). Under these low-nutrient-level conditions, the matrix mutant sporulated to levels similar to those observed for the wild type (Fig. 2). Furthermore, the matrix mutant did not have a growth defect compared to what was observed for the wild type under low-nutrient-level conditions; both strains grew at rates similar to those observed for the matrix mutant in 100% MSgg medium (data not shown). These findings suggest that under biofilm-forming conditions, extracellular matrix allows cells to grow to a higher density and triggers the initiation of sporulation. In the absence of matrix, sporulation is delayed. However, under low-nutrient-level conditions, which trigger sporulation regardless of the presence of matrix, the sporulation delay is not observed. It is worth noting that while the sporulation levels were similar, the colony morphology of the matrix mutant on 10% MSgg was not identical to that observed for the wild type. For reasons we do not understand, mutations in the eps genes resulted in flared colonies (Fig. 2). This was not observed in the tasA single mutant (data not shown).
FIG 2 .
The matrix mutant sporulation defect is medium dependent. Images are top views of cells grown under each condition. Spore counts of cells grown on solid 100% MSgg (black bars) or 10% MSgg (open bars) at 30°C for 72 h. Error bars indicate standard errors of the means.
Low levels of phosphorylated Spo0A are present in matrix-deficient biofilms.
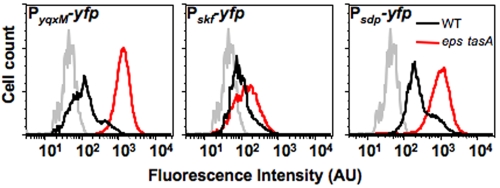
Both matrix and sporulation gene expression are controlled by the master transcriptional regulator Spo0A (16, 17). Different subsets of genes are regulated depending on the amount of phosphorylated Spo0A in the cell (7). Genes coding for matrix components are expressed at low levels of Spo0A~P, whereas sporulation gene expression is initiated only when high levels of Spo0A~P are attained. We therefore reasoned that in a matrix-deficient mutant, the cellular concentration of Spo0A~P might be maintained at low levels for longer periods during biofilm development, thereby delaying the initiation of sporulation. To test this hypothesis, we followed the expression of different genes known to be activated by low levels of Spo0A~P (yqxM, skfA, and sdpA). We generated reporter strains in which the promoters for these genes were fused to the gene encoding yellow fluorescent protein (yfp) and introduced these constructs into wild-type and matrix-deficient cells. Cells containing these promoter fusions were harvested from biofilms and were analyzed by flow cytometry after 72 h of growth (Fig. 3). Our results indicate that there is a larger subpopulation of cells expressing all three of these low-level-Spo0A~P-regulated reporters in matrix mutant biofilms than in wild-type biofilms. This supports our hypothesis that in biofilms, matrix mutant cells do not proceed to sporulation because Spo0A~P is maintained at low levels in the cell.
FIG 3 .
Matrix mutant cells retain low Spo0A~P levels. Flow cytometry of wild-type (black lines) and eps tasA (red lines) mutant cells. The grey line represents a control with cells not expressing yellow fluorescent protein (YFP). Cells were grown on MSgg solid medium at 30°C for 72 h prior to harvesting and disruption of the biofilm for flow cytometry. Cells harbored the indicated reporter fusions: PyqxM-yfp, PskfA-yfp, or PsdpA-yfp. AU, arbitrary units.
A kinD mutant sporulates early in biofilms.
The finding that the presence of extracellular matrix alters the concentration of Spo0A~P within cells compelled us to explore the mechanism behind this regulation. Because two-component-system histidine kinases can have either kinase or phosphatase activity (18–20), we envisioned two possibilities for how concentrations of Spo0A~P could increase: (i) a kinase senses a signal and phosphorylates Spo0A~P, or (ii) a phosphatase senses a signal and stops dephosphorylating Spo0A. In the first scenario, one of the kinases would sense the presence of matrix and respond by phosphorylating Spo0A to levels that allow sporulation. In matrix mutants, the kinase would not detect the presence of matrix and the concentration of Spo0A~P would not increase, and thus, sporulation would be delayed compared to the wild type until another sporulation signal (probably nutrient depletion) is sensed. If this is the case, then mutating the matrix-sensing kinase should cause a sporulation delay in otherwise wild-type cells, mimicking the matrix mutant phenotype. In the second scenario, a phosphatase would be active until matrix was produced. Once matrix was produced, phosphatase activity would shut off and the level of Spo0A~P would increase to allow sporulation. If this is the case, then mutating the matrix-sensing phosphatase should result in increased Spo0A~P levels and early sporulation, regardless of the presence or absence of matrix.
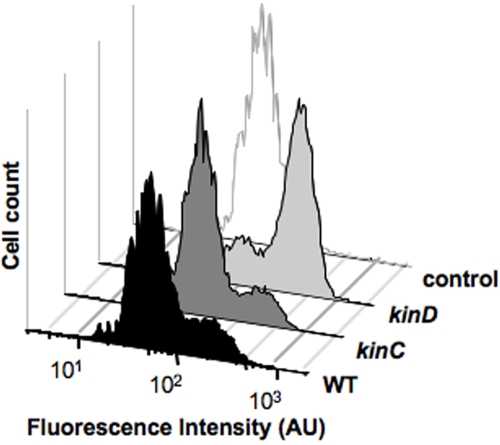
Previous work has shown that the two major kinases involved in regulating Spo0A~P levels in colony biofilm formation are KinC and KinD (15). Therefore, we used flow cytometry to analyze the expression of a sporulation-specific reporter (PsspB-yfp) in either a kinC or a kinD mutant compared to the wild type. Neither kinase mutant showed a delay in sporulation similar to that observed for the matrix mutant at 72 h of development (data not shown). Instead, and consistent with the second scenario described above, at 24 h of biofilm formation the kinD mutant displayed an increased level of expression of the sporulation reporter relative to what was observed for the wild type and the kinC mutant (Fig. 4). This suggests that kinD mutant cells prematurely have sufficiently high levels of Spo0A~P to trigger sporulation, indicating that in early stages of biofilm development KinD has an active role, either direct or indirect, in decreasing the levels of Spo0A~P.
FIG 4 .
A kinD mutant shows early sporulation. Flow cytometry of wild-type, kinD, or kinC mutant cells harboring the PsspB-yfp promoter fusion. Cells were grown on MSgg solid medium at 30°C for 24 h prior to harvesting and disruption of the biofilm for flow cytometry. The control is wild-type cells with no fluorescent protein. AU, arbitrary units.
A kinD mutant suppresses the sporulation defect in matrix-defective biofilms.
To determine if KinD’s ability to decrease the levels of Spo0A~P is necessary for the sporulation delay in the mutant lacking matrix, we introduced the kinD deletion into the eps tasA matrix-deficient strain and analyzed the expression of the PsspB-yfp sporulation reporter in the kinD eps tasA triple mutant. Our results indicate that while expression of the sporulation reporter was severely diminished in the double eps tasA mutant at 72 h of biofilm development, the level of PsspB-yfp reporter expression in the kinD eps tasA triple mutant was closer to wild-type levels (Fig. 5A). This increase in sporulation gene expression is independent of biofilm architecture, as the triple mutant colonies phenocopy the eps tasA mutant (see Fig. S1 in the supplemental material).
FIG 5 .
A kinD deletion restores sporulation to the eps tasA matrix mutant under biofilm-inducing conditions. (A) Flow cytometry of cells harboring the PsspB-yfp promoter fusion. Cells were grown on MSgg solid medium at 30°C for 72 h prior to harvesting and disruption of the biofilm for flow cytometry. The control is wild-type cells with no fluorescent protein. AU, arbitrary units. (B) Sporulation of wild type, eps tasA, kinD, and kinD eps tasA cells. Cells were grown on MSgg solid medium at 30°C for the indicated time prior to harvesting and counting of viable spores after heat treatment. Error bars represent standard errors of the means.
To test if the increased level of PsspB-yfp expression correlates with an increased number of spores, we quantified the viable spores present in the triple kinD eps tasA and the single kinD mutant biofilms in comparison to the levels for the wild type and the eps tasA mutant strain. Indeed, the kinD eps tasA mutant had increased levels of viable spores at all of the time points that we assayed compared to the level for the eps tasA mutant (Fig. 5B). We note that the kinD mutation does not fully restore wild-type levels of sporulation to the eps tasA mutant strain, as the overall numbers of spores in the triple mutant are still lower than those in the wild type. Additionally, we observed that the kinD strain and the triple mutant both had about 10-fold more spores than the wild type at 24 h, consistent with the early PsspB-yfp expression we observed in the kinD mutant (Fig. 4 and 5B). These results are consistent with the idea that KinD functions as a phosphatase to normally keep levels of Spo0A~P low in the cell until matrix is assembled.
KinD inhibits Spo0A activity.
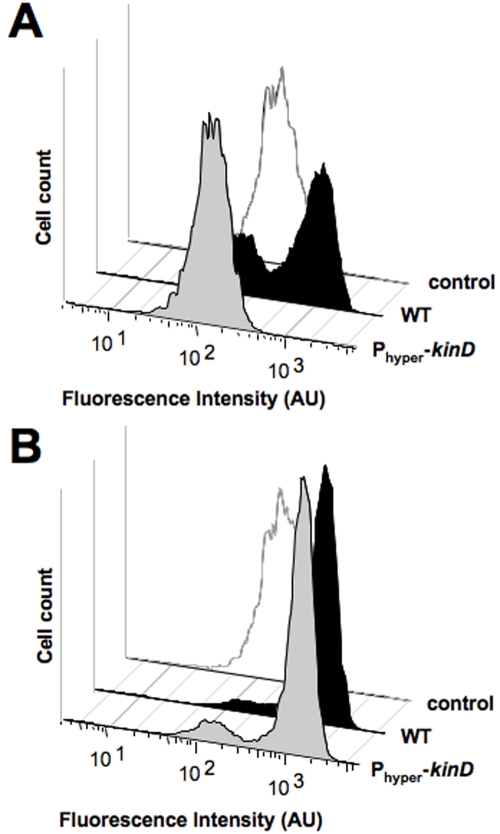
If KinD is indeed acting as a phosphatase to decrease levels of Spo0A~P, then overexpressing the kinD gene in the wild-type background should hinder sporulation in biofilms. This was in fact the case. When we assayed a strain in which kinD expression was under the control of an IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible promoter, we found that in MSgg medium, expression of PsspB-yfp was decreased relative to the level for the wild type (Fig. 6A). This decrease was not observed at the same time under lower-nutrient-level conditions, such as those obtained with 10% MSgg medium (Fig. 6B). It is likely that under low-nutrient-level conditions, the kinases KinA and KinB counteract the phosphatase activity of KinD, leading to higher levels of Spo0A~P that initiate sporulation. We noticed that even under low-nutrient-level conditions, there was a brief interval at early time points in which the strain overexpressing kinD displayed fewer cells expressing the sporulation reporter than the wild type (data not shown).
FIG 6 .
Sporulation is delayed in a strain overexpressing kinD. Flow cytometry of wild-type and Phyperspank-kinD cells harboring the PsspB-yfp promoter fusion. Cells were grown on solid medium with IPTG at 30°C for 48 h prior to harvesting and disrupting of the biofilm for flow cytometry. The control is cells with no fluorescent protein. (A) One hundred percent MSgg medium. (B) Ten percent MSgg medium. AU, arbitrary units.
DISCUSSION
B. subtilis uses a complex phosphotransfer network to control the activity of Spo0A, a transcriptional regulator of numerous cellular processes, including extracellular-matrix production and sporulation (7, 9). We found that mutants defective in extracellular-matrix production are delayed in accumulation of high levels of Spo0A~P under biofilm-inducing conditions, resulting in a sporulation delay when cells are in biofilms. A deletion of the gene for the KinD sensor histidine kinase at least partially restored sporulation in matrix mutants. Furthermore, the absence of KinD in the wild type resulted in early sporulation in a biofilm. These results strongly suggest that KinD functions to maintain Spo0A~P at low levels in cells during early stages of biofilm formation, possibly by acting as a phosphatase.
Initially, we hypothesized that the sporulation defect observed in the biofilms of a matrix-deficient mutant could simply be attributed to increased nutrient availability; a mutant unable to synthesize extracellular matrix would expend less energy and thus have more nutrients available, allowing cells to grow for longer periods than wild-type cells. In contrast to our expectations, there were significantly fewer cells present in matrix mutant biofilms than in wild-type biofilms, indicating that the production of extracellular matrix somehow allows cells to grow vegetatively for longer periods of time. It is possible that matrix itself can serve as a carbon source for cells once the nutrients from the medium are depleted. An alternative hypothesis is that the matrix could function as a signal to trigger sporulation. This is supported by the fact that coculturing matrix mutant cells with sporulation mutants (that produce wild-type matrix) restored sporulation of matrix mutant cells in biofilms (5). Thus, we favor the second hypothesis and propose that the presence of extracellular matrix (or some component therein) is sensed by KinD in mature biofilms. When the signal is sensed, KinD’s activity is altered and Spo0A~P accumulates to high levels that trigger the sporulation pathway, thereby shutting down vegetative growth. While our data support this model, we cannot rule out the possibility that the regulation of sporulation in biofilms may also occur via a KinD-independent pathway and that deleting KinD simply increases the total concentration of Spo0A~P to allow sporulation to progress.
We observed similar sporulation delays in mutants lacking either the major protein component (TasA) or the exopolysaccharide component (EPS) of the matrix. Both TasA and exopolysaccharides are required to assemble wild-type biofilms (6). It is likely that TasA and EPS interact and that in a strain lacking one, the other is altered in structure or function. Current data are not sufficient to distinguish if the defect observed in the matrix mutants is due to a lack of signaling from specific components of the matrix or if there is another extracellular signal that is normally either excluded from or maintained close to the cells when wild-type matrix is present. This concept is not unprecedented in B. subtilis; we have previously shown that the matrix of B. subtilis does alter signaling and that once cells have produced extracellular matrix they become immune to certain signaling molecules, e.g., ComX, which is necessary for differentiation of certain cell types (21).
The simplest interpretation of our results is that KinD displays phosphatase activity towards either Spo0F~P or Spo0A~P, thus delaying sporulation. In fact, in vitro data suggest that KinD (as well as KinA, KinC, and KinE) is capable of both phosphorylating Spo0F and dephosphorylating Spo0F~P (14). While in vivo and in vitro data show that KinC (and to a lesser extent KinD) was able to phosphorylate Spo0A directly, the reverse reaction was not tested with Spo0A~P (14). An alternative possibility is that KinD acts indirectly on the phosphorelay by activating one of the many proteins that have phosphatase activity on Spo0A or Spo0F, such as the various Phr-Rap systems or the Spo0A phosphatase Spo0E (22, 23). Our data show that overproduction of KinD results in a delay of sporulation. This fact, together with the precedent of bifunctional kinases in other microorganisms (18, 24, 25), makes us favor the hypothesis that KinD is bifunctional, having either kinase or phosphatase activity, depending on the growth conditions.
There are numerous examples of two-component histidine kinases that function as phosphatases (19, 20). One well-studied example of these is the Escherichia coli CpxA protein (26). CpxA is a bifunctional sensor kinase that regulates the activity of its cognate response regulator CpxR. Depending on the environmental conditions, such as the presence of envelope stress, CpxA displays either kinase or phosphatase activity (27, 28). Activity of CpxA is also regulated by a small periplasmic protein, CpxP, which binds to the sensing domain of CpxA and decreases autokinase activity (29). It has been proposed that the phosphatase activity of bifunctional kinases helps to prevent cross talk between two-component systems within the cell (18, 30).
In uropathogenic E. coli, QseC is a bifunctional sensor kinase that acts on the response regulator QseB to control factors involved in biofilm formation (type I pili, curli, and flagella) (25). The QseC kinase and phosphatase activities are proposed to depend on environmental conditions, analogous to what we observe in B. subtilis, where the growth conditions (lack of an extracellular matrix or low nutrient levels in the medium) affect Spo0A~P levels.
There are also examples of systems similar to B. subtilis in which multiple histidine kinases converge on the same phosphotransfer pathway. For example, Vibrio harveyi exhibits this “many-to-one” circuitry to control the levels of the phosphorylated response regulator in the cell (LuxO). LuxO~P accumulates when the kinases are active, but inactive kinases function as phosphatases on LuxO~P, preventing LuxO~P accumulation unless all three kinases are active (18). Despite the fact that many kinases feed into one output protein in both V. harveyi and B. subtilis, in both cases the respective sensor kinases are thought to regulate different sets of genes, presumably due to variations in the concentrations of the phosphorylated response regulators (18).
The results presented in this paper provide evidence for a novel role for KinD in B. subtilis. We propose that, analogous to other two-component systems, KinD displays phosphatase activity over the cognate response regulator that results in a repression of the accumulation of Spo0A~P. The phosphatase activity switches to kinase activity once a yet unknown signal or signals in mature biofilms (possibly extracellular matrix) are present, allowing the cells to continue with the development pathway leading to the formation of spores. While we do not know the exact nature of the signal that KinD senses, the fact that its activity is altered in response to the presence of extracellular matrix is reminiscent of integrin signaling in eukaryotic wound healing (31). Integrins are cell surface receptors that bind to various components of the extracellular matrix (such as collagen) and control differentiation signaling pathways by regulating growth factor production. Furthermore, much like what we observe in B. subtilis, where the presence of extracellular matrix regulates both extracellular-matrix production and differentiation into spores, in eukaryotes, interaction of extracellular matrix with growth factors is bidirectional. Not only can the presence of matrix induce growth factor expression, but growth factors also regulate production of the extracellular matrix (31). It is interesting to note that this type of regulatory circuitry represents another example of similar mechanistic strategies that have evolved to regulate differentiation in multicellular development of eukarya and bacteria (32).
MATERIALS AND METHODS
Strains, media, and culture conditions.
The strains used in the study are listed in Table S1 in the supplemental material. B. subtilis strain NCIB3610 was used as the wild type. For routine growth and spore quantification, cells were propagated on Luria-Bertani (LB) medium. For biofilm assays, cells were scraped from overnight growth on LB plates and resuspended in LB liquid medium to an optical density at 600 nm (OD600) of 1, and 2 µl was spotted on the indicated medium solidified with 1.5% agar (3). MSgg medium (100% MSgg) consists of 5 mM potassium phosphate (pH 7), 100 mM MOPS (morpholinepropanesulfonic acid; pH 7), 2 mM MgCl2, 700 µM CaCl2, 50 µM MnCl2, 50 µM FeCl3, 1 µM ZnCl2, 2 µM thiamine, 0.5% glycerol, 0.5% glutamate, 50 µg/ml tryptophan, 50 µg/ml phenylalanine, and 50 µg/ml threonine (3). Low-nutrient-level MSgg (10% MSgg) was the same as 100% MSgg, but with 0.05% glycerol and 0.05% glutamate. Biofilms were incubated at 30°C for the indicated times. The antibiotic concentrations (final) were as follows: for macrolides-lincosamides-streptogramin B (MLS), 1 µg ml−1 erythromycin, 25 µg ml−1 lincomycin; for spectinomycin, 100 µg ml−1; for tetracycline, 10 µg ml−1; for kanamycin, 10 µg ml−1; and for chloramphenicol, 5 µg ml−1. When needed, IPTG was added to the medium to give a final concentration of 20 µM.
Strain and plasmid construction.
Mutant strains were generated by SPP1-mediated generalized transduction to transfer the mutant alleles into the B. subtilis strain NCIB3610 (or a derivative) (33). To generate strain HV1235 (3610 epsA-epsO::tet tasA::kan), strain SSB488 (3610 epsA-epsO::tet) (6) was transduced with SPP1 lysate from CA017 (3610 tasA::kan) (5) and kanamycin-resistant colonies were selected. The donor strain for all kinC::cm and kinD::mls mutations was FC405 (3610 kinC::cm kinD::mls), kindly donated by Frances Chu. The donor strain for kinDΩPhyperspank-kinD was MF2147 (34).
Transcriptional fusions were introduced by natural competence into the amyE locus of B. subtilis strain 168 prior to transfer to the recipient by use of SPP1-mediated generalized transduction. Plasmids were constructed using standard methods and amplified in Escherichia coli DH5α. PCR fragments were amplified from NCIB3610 chromosomal DNA, using Pfu DNA polymerase. Oligonucleotide primers were purchased from IDT (Coralville). To construct PskfA-yfp, the primers PskfA-for (5′-CCGGAATTCAAGCAGCGTAATGAAGAGTG-3′) and PskfA-rev (5′-GGCAAGCTTTTTTTGCATAGAGTCTATTGAC-3′) were used to amplify a 486-bp region containing the promoter of the skf operon. The PCR fragment was digested with EcoRI and HindIII and cloned into an amyE integration vector harboring yfp (pKM003), which was cut with the same enzymes. The PsdpA-yfp fusion was constructed in the same way, using primers PsdpA-for (5′-CCGGAATTCGAGAGCGAAGACATTTTTAA-3′) and PsdpA-rev (5′-GGCAAGCTTCTAATATAATCATTTCAAAAAGA-3′) to amplify a 216-bp region upstream of sdpA.
Spore and total cell quantification.
Cells were grown as biofilms on MSgg plates and incubated at 30°C for the indicated times prior to harvesting for quantification. Samples were kept on ice during the following procedure to minimize growth during processing. Entire colonies were harvested, resuspended in 500 µl H2O, and subjected to mild sonication to obtain single cells as described for flow cytometry preparation. The sonicated samples were centrifuged, and matrix was removed prior to resuspension in 500 µl of sterile H2O. The optical density (OD600) of each sample was recorded, and this value represents the total cell density and was used for Fig. 1B. For quantification of spores, each preparation was normalized to an OD600 of 1 and then incubated at 80°C for 20 min to kill vegetative cells. To determine viable cell counts, serial dilutions were plated from the normalized preparation before and after the 80°C incubation.
Image analysis.
Whole colonies were photographed at low magnification (×0.8) using a Stemi SV6 stereomicroscope (Zeiss) equipped with a 1.0× Achromat S objective lens, an AxioCam charge-coupled-device (CCD) video camera system (Zeiss), and a computer interface. Data were captured using AxioVision suite software (Zeiss).
Flow cytometry.
Biofilms were scraped from the surface of the agar plate and resuspended in 500 µl of phosphate-buffered saline (PBS). Biofilms were then immediately disrupted by repetitive passes through a needle (size, 23g1) and fixed in a 4% paraformaldehyde solution for 7 min. After fixation, cells were washed with PBS, resuspended in GTE buffer (50 mM glucose, 10 mM EDTA, pH 8, 20 mM Tris-HCl, pH 8), and stored at 4°C. Prior to flow cytometry analysis, cells were subjected to mild sonication under conditions that disrupt the cells from the extracellular matrix but that do not lyse cells at detectable levels (6).
For flow cytometry analysis, cells were diluted in PBS and directly measured with an LSR II flow cytometer (BD Biosciences, United States) operating a solid-state laser at 488 nm. Data containing the fluorescent signals were collected by a 505LP 530/30-bp filter, and the photomultiplier voltage was set between 300 and 500 V. For each sample, at least 30,000 events were analyzed. Data were captured using FACSDiva software (BD Biosciences) and further analyzed using FlowJo 8.6.3 software (Tree Star, Inc.). Figures were prepared for publication using FlowJo 8.6.3 and Microsoft PowerPoint.
Supplemental Material
ACKNOWLEDGMENTS
We thank past and present members of the Kolter and Losick laboratories for helpful discussions. We thank D. Lopez, D. Romero, K. Lemon, and E. Shank for critical reading of the manuscript.
This work was supported by NIH grants GM58213 (to R.K.) and GM18568 (to R.L.) and by grants from BASF to both R.K. and R.L.
Footnotes
Citation Aguilar, C., H. Vlamakis, A. Guzman, R. Losick, and R. Kolter. 2010. KinD is a checkpoint protein linking spore formation to extracellular-matrix production in Bacillus subtilis biofilms. mBio 1(1):e00035-10. doi:10.1128/mBio.00035-10.
REFERENCES
- 1. Kolter R., Greenberg E. 2006. Microbial sciences: the superficial life of microbes. Nature 441:300–302 [DOI] [PubMed] [Google Scholar]
- 2. Stewart P., Franklin M. 2008. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 6:199–210 [DOI] [PubMed] [Google Scholar]
- 3. Branda S., González-Pastor J., Ben-Yehuda S., Losick R., Kolter R. 2001. Fruiting body formation by Bacillus subtilis. Proc. Natl. Acad. Sci. U. S. A. 98:11621–11626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chai Y., Chu F., Kolter R., Losick R. 2008. Bistability and biofilm formation in Bacillus subtilis. Mol. Microbiol. 67:254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vlamakis H., Aguilar C., Losick R., Kolter R. 2008. Control of cell fate by the formation of an architecturally complex bacterial community. Genes Dev. 22:945–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Branda S. S., Chu F., Kearns D. B., Losick R., Kolter R. 2006. A major protein component of the Bacillus subtilis biofilm matrix. Mol. Microbiol. 59:1229–1238 [DOI] [PubMed] [Google Scholar]
- 7. Fujita M., González-Pastor J., Losick R. 2005. High- and low-threshold genes in the Spo0A regulon of Bacillus subtilis. J. Bacteriol. 187:1357–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Molle V., Fujita M., Jensen S., Eichenberger P., González-Pastor J., Liu J., Losick R. 2003. The Spo0A regulon of Bacillus subtilis. Mol. Microbiol. 50:1683–1701 [DOI] [PubMed] [Google Scholar]
- 9. Burbulys D., Trach K., Hoch J. 1991. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell 64:545–552 [DOI] [PubMed] [Google Scholar]
- 10. Grossman A. 1995. Genetic networks controlling the initiation of sporulation and the development of genetic competence in Bacillus subtilis. Annu. Rev. Genet. 29:477–508 [DOI] [PubMed] [Google Scholar]
- 11. LeDeaux J., Yu N., Grossman A. 1995. Different roles for KinA, KinB, and KinC in the initiation of sporulation in Bacillus subtilis. J. Bacteriol. 177:861–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Piggot P., Hilbert D. 2004. Sporulation of Bacillus subtilis. Curr. Opin. Microbiol. 7:579–586 [DOI] [PubMed] [Google Scholar]
- 13. Hamon M., Lazazzera B. 2001. The sporulation transcription factor Spo0A is required for biofilm development in Bacillus subtilis. Mol. Microbiol. 42:1199–1209 [DOI] [PubMed] [Google Scholar]
- 14. Jiang M., Shao W., Perego M., Hoch J. 2000. Multiple histidine kinases regulate entry into stationary phase and sporulation in Bacillus subtilis. Mol. Microbiol. 38:535–542 [DOI] [PubMed] [Google Scholar]
- 15. López D., Fischbach M., Chu F., Losick R., Kolter R. 2009. Structurally diverse natural products that cause potassium leakage trigger multicellularity in Bacillus subtilis. Proc. Natl. Acad. Sci. U. S. A. 106:280–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chu F., Kearns D., Branda S., Kolter R., Losick R. 2006. Targets of the master regulator of biofilm formation in Bacillus subtilis. Mol. Microbiol. 59:1216–1228 [DOI] [PubMed] [Google Scholar]
- 17. Kearns D., Chu F., Branda S., Kolter R., Losick R. 2005. A master regulator for biofilm formation by Bacillus subtilis. Mol. Microbiol. 55:739–749 [DOI] [PubMed] [Google Scholar]
- 18. Laub M., Goulian M. 2007. Specificity in two-component signal transduction pathways. Annu. Rev. Genet. 41:121–145 [DOI] [PubMed] [Google Scholar]
- 19. Perego M., Hoch J. 1996. Protein aspartate phosphatases control the output of two-component signal transduction systems. Trends Genet. 12:97–101 [DOI] [PubMed] [Google Scholar]
- 20. Wolanin P., Thomason P., Stock J. 2002. Histidine protein kinases: key signal transducers outside the animal kingdom. Genome Biol. 3:REVIEWS3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. López D., Vlamakis H., Losick R., Kolter R. 2009. Paracrine signaling in a bacterium. Genes Dev. 23:1631–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bischofs I., Hug J., Liu A., Wolf D., Arkin A. 2009. Complexity in bacterial cell-cell communication: quorum signal integration and subpopulation signaling in the Bacillus subtilis phosphorelay. Proc. Natl. Acad. Sci. U. S. A. 106:6459–6464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Perego M., Brannigan J. 2001. Pentapeptide regulation of aspartyl-phosphate phosphatases. Peptides 22:1541–1547 [DOI] [PubMed] [Google Scholar]
- 24. Forst S., Roberts D. 1994. Signal transduction by the EnvZ-OmpR phosphotransfer system in bacteria. Res. Microbiol. 145:363–373 [DOI] [PubMed] [Google Scholar]
- 25. Kostakioti M., Hadjifrangiskou M., Pinkner J., Hultgren S. 2009. QseC-mediated dephosphorylation of QseB is required for expression of genes associated with virulence in uropathogenic Escherichia coli. Mol. Microbiol. 73:1020–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ruiz N., Silhavy T. 2005. Sensing external stress: watchdogs of the Escherichia coli cell envelope. Curr. Opin. Microbiol. 8:122–126 [DOI] [PubMed] [Google Scholar]
- 27. Raivio T., Silhavy T. 1997. Transduction of envelope stress in Escherichia coli by the Cpx two-component system. J. Bacteriol. 179:7724–7733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wolfe A., Parikh N., Lima B., Zemaitaitis B. 2008. Signal integration by the two-component signal transduction response regulator CpxR. J. Bacteriol. 190:2314–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fleischer R., Heermann R., Jung K., Hunke S. 2007. Purification, reconstitution, and characterization of the CpxRAP envelope stress system of Escherichia coli. J. Biol. Chem. 282:8583–8593 [DOI] [PubMed] [Google Scholar]
- 30. Alves R., Savageau M. 2003. Comparative analysis of prototype two-component systems with either bifunctional or monofunctional sensors: differences in molecular structure and physiological function. Mol. Microbiol. 48:25–51 [DOI] [PubMed] [Google Scholar]
- 31. Schultz G., Wysocki A. 2009. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 17:153–162 [DOI] [PubMed] [Google Scholar]
- 32. Aguilar C., Vlamakis H., Losick R., Kolter R. 2007. Thinking about Bacillus subtilis as a multicellular organism. Curr. Opin. Microbiol. 10:638–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yasbin R., Young F. 1974. Transduction in Bacillus subtilis by bacteriophage SPP1. J. Virol. 14:1343–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fujita M., Losick R. 2005. Evidence that entry into sporulation in Bacillus subtilis is governed by a gradual increase in the level and activity of the master regulator Spo0A. Genes Dev. 19:2236–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.