Abstract
Determining the genetic architecture of late onset Alzheimer’s disease remains an important research objective. One approach to the identification of novel genetic variants contributing to the disease is the classification of biologically meaningful subgroups within the larger late-onset Alzheimer’s disease phenotype. The occurrence of psychotic symptoms in patients with late-onset Alzheimer’s disease may identify one such group. We attempted to establish methods for the reliable assessment of psychotic symptoms in a large, geographically dispersed collection of families, multiply affected with late onset Alzheimer’s disease, who were participants in the larger National Institute on Aging Late Onset Alzheimer’s Disease Family Study; and to characterize the correlates and familial aggregation of psychosis within this cohort. We found that reliable assessments of psychotic symptoms during in-person or phone interviews were readily implemented. The presence of psychosis in late onset Alzheimer’s disease was significantly associated with degree of cognitive impairment, and significantly, albeit modestly, correlated with the severity of other behavioural symptoms. Psychosis significantly aggregated within late onset Alzheimer’s disease families suggesting that it may identify a genetically determined subgroup. Future studies should examine the linkage and association of psychosis with genetic variation within these families.
Keywords: Alzheimer’s disease, family study, genetics, psychiatric comorbidity
Introduction
The aetiology of Alzheimer’s disease is unknown, although significant strides have been made using gene mapping efforts. Success has been most notable for the highly heritable early onset form (Goate et al., 1991; Levy-Lahad et al., 1995; Sherrington et al., 1995), which comprises a minority (∼1%) of the entire population of Alzheimer’s disease cases. In contrast, the genetic architecture of late onset Alzheimer’s disease is less clear. The association of late onset Alzheimer’s disease with the ε4 variant of apolipoprotein E is well established (Farrer et al., 1997). More recently, multiple replicated associations of late onset Alzheimer’s disease with genetic variation in sortilin-related receptor (SORL)-1 (Rogaeva et al., 2007; Bettens et al., 2008; Li et al., 2008; Feulner et al., 2009; Kimura et al., 2009; Kolsch et al., 2009; Tan et al., 2009), and genome-wide associations with clusterin, phosphatidylinositol-binding clathrin assembly protein (PICALM), and complement receptor 1 have been reported (Harold et al., 2009; Lambert et al., 2009). However, the need to identify other genes responsible for late onset Alzheimer’s disease remains.
One approach to improving the detection of genetic variants associated with late onset Alzheimer’s disease is to identify subgroups within the late onset Alzheimer’s disease phenotype for mapping liability genes (Kehoe et al., 1999; Pericak-Vance et al., 2000). One such subgroup are those individuals who develop psychotic symptoms during the progression of Alzheimer’s disease. Psychosis is frequent in late onset Alzheimer’s disease, with a median prevalence across studies of 41% (Ropacki and Jeste, 2005). Evidence indicates that psychosis is a marker for more severe cognitive impairments and a more rapidly progressive phenotype of late onset Alzheimer’s disease. Psychosis has been associated with more severe cognitive and functional deficits in subjects with late onset Alzheimer’s disease matched on other clinical characteristics (reviewed in Sweet et al., 2003; Ropacki and Jeste, 2005). Similarly, studies indicate that late onset Alzheimer’s disease with psychosis is associated with more rapid cognitive and functional deterioration (Ropacki and Jeste, 2005; Scarmeas et al., 2005). Of relevance for detection of genetic associations, we and others have shown psychosis to aggregate in late onset Alzheimer’s disease families, with an estimated heritability of 61% (Sweet et al., 2002; Bacanu et al., 2005; Hollingworth et al., 2007). Moreover, there is little evidence that psychosis in late onset Alzheimer’s disease is associated with the ε4 variant of apolipoprotein E (DeMichele-Sweet and Sweet, 2009), suggesting that this phenotype may have particular utility for the identification of novel genetic associations in late onset Alzheimer’s disease.
To expand the resources needed to identify additional genes that contribute to the risk for late onset Alzheimer’s disease, the National Institute on Aging launched the Genetics Initiative for Late Onset Alzheimer’s Disease Family Study. The goal of this study was to identify and recruit families with two or more siblings affected with late onset Alzheimer’s disease and unrelated subjects similar in age and ethnic background, but without dementia. We recently described the families and the results of linkage, family-based association and case–control analyses from an initial genome-wide scan using approximately 6000 single-nucleotide polymorphic markers (Lee et al., 2008). We now describe the initial efforts to characterize late onset Alzheimer’s disease subjects within these families for psychotic symptoms, and provide initial evidence for the aggregation of psychosis within the late onset Alzheimer’s disease families in this cohort.
Methods
Subjects and setting
Recruitment for the National Institute on Aging Genetics Initiative has been described previously (Lee et al., 2008). In brief, 18 Alzheimer’s disease centres throughout the United States participated, each of which had received approval by their Institutional Review Board. The recruitment criteria included a family with multiple members affected with late onset Alzheimer’s disease that could provide clinical information and a biological sample for DNA extraction. The proband had to have a diagnosis of definite or probable Alzheimer’s disease (McKhann et al., 1984) with onset after 60 years of age and a full sibling with definite, probable or possible Alzheimer’s disease with onset after 60 years of age. A third biologically related family member was required, who could have been a first-, second- or third-degree relative of the affected sibling pairs and ≥60 years if unaffected or ≥50 years if diagnosed as having Alzheimer’s disease or mild cognitive impairment (Petersen et al., 1999). Unaffected persons were required to have had documented cognitive testing and clinical examination results to verify the clinical designation.
Diagnostic assessment
A minimal data set included demographic variables, diagnosis, age at onset, method of diagnosis, Clinical Dementia Rating Scale score (Hughes et al., 1997) and the presence of other relevant health problems. Each centre was required to use standard research criteria for the diagnosis of Alzheimer’s disease (McKhann et al., 1984). Participants with advanced disease or those living in a remote location, who could not complete a detailed in-person evaluation contributed blood samples, and the site investigator conducted a detailed review of the available medical records and informant histories to document the presence or absence of Alzheimer’s disease.
The age at onset for patients with Alzheimer’s disease was the age at which the family first observed memory problems. For deceased family members who had undergone a post-mortem brain evaluation, neuropathological results were used to document the diagnosis. Clinical diagnoses of Alzheimer’s disease have agreed with the autopsy diagnoses for 95% of subjects (Lee et al., 2008).
Assessment of behaviour
Subjects were assessed for psychosis using the Consortium to Establish a Registry for Alzheimer’s Disease Behavioural Rating Scale, 1996 version (Mack et al., 1999). The Behavioural Rating Scale was modified to rate only items addressing psychotic symptoms (items 33–45). In addition, because ratings in the Behavioural Rating Scale focus on the past month, several modifications were made to capture psychotic symptoms more completely. When the informant indicated that the behaviour had occurred prior to the past month, the month when it was most persistent was identified and information on the frequency of the symptom during that month was recorded. We also recorded whether the symptom had ever resulted in the administration of pharmacotherapy. Other behaviour was assessed using the Neuropsychiatric Inventory Questionnaire (Kaufer et al., 2000), modified to be completed as an interview of a knowledgeable informant. Additionally, the rating forms for the Neuropsychiatric Inventory Questionnaire and Behavioural Rating Scale were integrated into a single form for ease of administration and data collection.
For the Behavioural Rating Scale ratings, a delusion was defined as a persistent false belief based on incorrect inference about external reality, resistant to persuasion or contrary evidence, and not attributable to social or cultural mores. Hallucinations were defined as sensory perceptions for which there was no basis in reality. Discrete hypnagogic and hypnopompic hallucinations, as well as symptoms occurring only during an episode of delirium, were not rated. Patients were classified as having psychotic symptoms if they had persistent delusions or hallucinations, operationalized as any one of the Behavioural Rating Scale items, occurring three or more times within a month, at any time during the illness (Wilkosz et al., 2006). Because prior work suggests that the degree of heritability of psychosis in individuals with Alzheimer’s disease is greatest when psychotic symptoms are multiple and/or recurrent (Bacanu et al., 2005), we also classified individuals with regard to the presence of single versus multiple/recurrent symptoms.
Collecting uniform behavioural data is challenging in a study of this nature, given the use of multiple clinical evaluators from multiple, geographically dispersed centres. Furthermore, because family members themselves may be dispersed across the United States, we developed administration procedures that allowed data to be collected by telephone interviews. All clinical evaluators either attended an initial training session conducted by a geriatric psychiatrist investigator (RAS), or reviewed a video recording of this training session. The session addressed administration of the scale and frequently asked questions about questioning and scoring. Ongoing review of questions about assessment and scoring occurred during monthly teleconferences amongst the evaluators and an evaluator experienced in administration of the Consortium to Establish a Registry for Alzheimer's Disease Behavioural Rating Scale (EAW) from the University of Pittsburgh. Other questions were addressed, as needed, via email. Inter-rater reliability of the Behavioural Rating Scale was assessed using a series of six videotaped interviews. A total of 35 evaluators from 15 centres completed this process. An additional four evaluators, from three sites (University of Pittsburgh, University of Alabama, Birmingham and Washington University) did not participate in this process as they had previously demonstrated adequate reliability on the Behavioural Rating Scale psychosis items in a different set of videotaped interviews. In addition, we undertook a comparison of in-person versus phone ratings using the Behavioural Rating Scale in 27 individuals from five centres. The order of in-person versus phone interviews was randomized. Finally, all clinical evaluators had also been certified as reliable on the Neuropsychiatric Inventory Questionnaire by the National Alzheimer’s Coordinating Centre following their established online procedures.
Statistical analysis
All analyses were conducted using the Statistical Package for the Social Sciences, release 17.0.0. Unless otherwise specified, all analyses of the association of psychosis contrasted individuals with no psychotic symptoms, a single psychotic symptom and multiple/recurrent psychotic symptoms. Multinomial logistic regression used the NOMREG command with stepwise selection criteria using the likelihood ratio with an entry probability of 0.05 and removal probability of 0.1. Generalized Estimating Equations analysis assumed a multinomial distribution, a cumulative logit link and an exchangeable correlation structure within families. Inter-evaluator reliability analysis and in person versus phone test–retest reliability analysis used intra-class correlation coefficients with random effects for evaluators and subjects, and tested absolute agreement.
Results
Reliability of psychosis assessments
Reliability of the classification of psychosis by the 35 evaluators in the videotaped interviews was excellent. Of the six videotaped subjects, three had no psychotic symptoms, one had a single symptom and two had multiple psychotic symptoms. Single measure intraclass correlation coefficient was 0.968 (P < 0.001), the average measure intraclass correlation coefficient was 0.999 (P < 0.001). For the 27 subjects for whom test–retest reliability was assessed, the in-person and phone Behavioural Rating Scale assessments were completed a mean (SD) of 3.7 (2.9) days apart (range 0–8 days). At the in-person interview, 18 subjects had no psychotic symptoms, five had one symptom and four had multiple symptoms. The corresponding values during phone evaluation were 19, 5 and 3. As a result, test–retest reliability was excellent, with a single measure intraclass correlation coefficient of 0.930 (P < 0.001) and an average measure intraclass correlation coefficient of 0.963 (P < 0.001).
Characterization of psychosis in family cohort
A total of 478 unique subjects diagnosed with a dementia completed at least one behavioural assessment. Characteristics of the subjects are presented in Table 1. Nearly all subjects received a diagnosis of Alzheimer’s disease. Nearly half had reached a moderate to advanced stage of dementia, as indicated by a Clinical Dementia Rating global score of ≥2, with 92.6% having illness duration of ≥4 years at the time of the behavioural assessment.
Table 1.
Subject characteristics
| Variable | n (%) or mean (SD)/range |
|---|---|
| Age,a years | 81.0 (7.5)/55–104 |
| Age at onset,b years | 73.8 (7.3)/50–93 |
| Sex | |
| M | 178 (37.2) |
| F | 300 (62.8) |
| Diagnosis | |
| Probable Alzheimer’s disease | 396 (83.0) |
| Possible Alzheimer’s disease | 69 (14.5) |
| Definite Alzheimer’s disease | 3 (0.6) |
| Other | 6 (1.3) |
| Unspecified | 4 (0.8) |
| Last available Clinical Dementia Ratingc | |
| 0 | 4 (0.8) |
| 0.5 | 62 (13.0) |
| 1.0 | 923 (19.2) |
| 2.0 | 77 (16.1) |
| 3.0 | 159 (33.3) |
an = 476 for this measure.
bn = 477 for this measure.
cn = 394 for this measure.
A total of 529 psychosis assessments were completed in these 478 subjects. Informants for the Behavioural Rating Scale interviews were predominantly spouses (n = 226, 42.7%), children (n = 216, 40.8%) or other family (n = 55, 10.4%). Most (n = 327, 61.8%) informants had contact with the subject ≥5 days/week. Nearly half of all assessments (255, 48.2%) were conducted via telephone interview of the informant.
Psychotic symptoms were present in 239 (50.0%) of the 478 subjects. Only a single psychotic symptom was present in 68 (14.2%) subjects. Multiple/recurrent psychotic symptoms were present in 171 (35.8%) of subjects. The individual psychotic symptoms present are shown in Table 2. The most common psychotic symptoms were delusional misidentification of people, affecting 23.4% of subjects, followed by paranoid delusions, affecting 21.1% of individuals. The least common psychotic symptom was the delusion that caregivers were impostors, only present in 3.1% of subjects. Visual hallucinations were not infrequent, occurring in 16.7% of subjects and were more common than auditory hallucinations.
Table 2.
Frequencies of individual psychotic symptoms in the 478 subjects with dementia
| Behavioural Rating Scale item number (Mack et al., 1999) | Symptom | Absent n (%) | Present n (%) |
|---|---|---|---|
| 33 | Misidentifies people | 366 (76.6) | 112 (23.4) |
| 34 | Misidentifies self | 453 (94.8) | 25 (5.2) |
| 35 | Misidentifies things | 415 (86.8) | 63 (13.2) |
| 36 | Paranoid | 377 (78.9) | 101 (21.1) |
| 37 | Infidelity | 458 (95.8) | 20 (4.2) |
| 38 | Abandonment | 447 (93.5) | 31 (6.5) |
| 39 | Imposters | 463 (96.9) | 15 (3.1) |
| 40 | Television is real | 441 (92.3) | 37 (7.7) |
| 41 | Other people in home | 416 (87.0) | 62 (13.0) |
| 42 | Dead still alive | 387 (81.0) | 91 (19.0) |
| 43 | House is not home | 395 (82.6) | 83 (17.4) |
| 44 | Auditory hallucination | 412 (86.24) | 66 (13.8) |
| 45 | Visual hallucination | 398 (83.3) | 80 (16.7) |
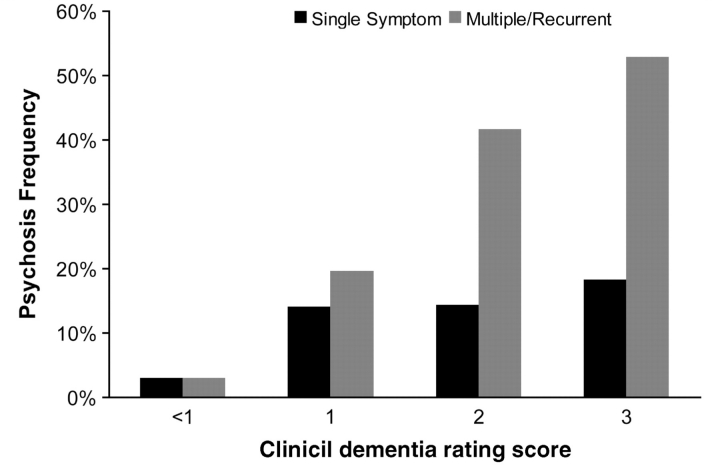
We next examined the demographic and clinical correlates of psychosis in these subjects. Psychotic symptoms were significantly associated with increasing age [F(2,473) = 5.7, P = 0.004], but not with age of onset [F(2,474) = 1.1, P = 0.32]. Psychotic symptoms were also significantly associated with female sex (χ2 = 11.7, df = 2, P = 0.003). For females, 47 (15.7%) had one psychotic symptom and 121 (40.3%) had multiple/recurrent psychotic symptoms. The corresponding numbers for male subjects were 21 (11.8%) and 50 (28.1%). Psychotic symptoms were also associated with greater impairment, as reflected in the Clinical Dementia Rating score (Fig. 1, χ2 = 93.6, df = 8, P < 0.001). There was a trend for psychotic symptoms to be more frequent with the conduct of telephone interviews (χ2 = 5.5, df = 2, P = 0.06), however, this appeared to be due to confounding by dementia severity as the association was not significant after including Clinical Dementia Rating score with interview type in a multiple regression model (χ2 = 3.4, df = 2, P = 0.18). Finally, because all subjects had also been rated for other behavioural symptoms on the Neuropsychiatric Inventory Questionnaire, we evaluated the correlation of these symptoms’ severities with psychotic symptoms, adjusting for any possible confounding due to a general increase in behavioural symptoms with dementia stage by examination of partial correlations after accounting for Clinical Dementia Rating score. Psychotic symptoms demonstrated small but significant correlations with the severity of all ten measured behaviours (agitation, depressed mood, anxiety, elated mood, apathy, disinhibition, irritability, motivation, sleep and appetite, all 0.112 ≤ r < 0.235, all P ≤ 0.037).
Figure 1.
Rates of psychosis, defined by the occurrence of a single psychotic symptom and by the occurrence of multiple and/or recurrent psychotic symptoms, by level of impairment on the Clinical Dementia Rating Scale.
Familial aggregation of psychosis
We identified 143 families in which two or more members were diagnosed with a dementia and characterized for the presence or absence of psychosis. The distribution of affected (by late onset Alzheimer’s disease) relative pairs is shown in Table 3. Because individuals were ascertained in the Family Study without regard to psychosis, for these analyses we arbitrarily identified the proband by ranking individuals within the family by Clinical Dementia Rating, presence of psychosis and arbitrarily assigned identification number and selected them in order of descending severity to ensure that the proband would have the greatest likelihood of an accurate phenotypic characterization (because psychotic symptoms often do not emerge until middle stages of illness) (Drevets and Rubin, 1989; Lopez et al., 2003). There was a highly significant association of psychotic symptoms in the proband with the presence of psychosis in the family member (Table 4, χ2 = 15.8, df = 4, P = 0.003). Consistent with our prior study (Bacanu et al., 2005), the association was strongest in comparing individuals with multiple/recurrent symptoms to those with no symptoms [odds ratio (95% CI) 3.80 (1.54–9.40); χ2 = 9.0, df = 1, P = 0.003]. In contrast, the association was weakened when the presence of any psychotic symptoms was compared to absence of symptoms [odds ratio 2.01 (0.99–4.09); χ2 = 3.8, df = 1, P = 0.052].
Table 3.
Families with two or more individuals diagnosed with dementia and characterized for the presence of psychosis
| Number of individuals within family | Number of families n (%) |
|---|---|
| 2 | 97 (67.8) |
| 3 | 44 (30.8) |
| 4 | 2 (1.4) |
| Total | 143 (100) |
Table 4.
Relationship between psychotic symptoms in probands and family members
| Proband psychosis |
||||
|---|---|---|---|---|
| Family member psychosis | None n (%) | Single symptom n (%) | Multiple/recurrent symptoms n (%) | Total n (%) |
| None | 43 (76.8) | 29 (78.4) | 55 (56.1) | 127 (68.4) |
| Single symptom | 6 (10.7) | 5 (13.5) | 9 (9.2) | 20 (10.2) |
| Multiple/recurrent symptoms | 7 (12.5) | 3 (8.1) | 34 (34.7) | 44 (21.4) |
| Total, n (%) | 56 (29.3) | 37 (19.4) | 98 (51.3) | 191 (100) |
We looked to confirm the above familial aggregation in relatives in a multinomial regression model including main effects of age, sex, Clinical Dementia Rating and proband psychosis status. These analyses included fewer families due to missing Clinical Dementia Rating information in some individuals. Despite this reduced power, proband psychosis status continued to make a significant contribution to the prediction of psychosis in relatives affected by late onset Alzheimer’s disease (χ2 = 12.4, df = 4, P = 0.015). Clinical Dementia Rating was also significantly associated with family member psychosis in this model (χ2 = 21.3, df = 2, P < 0.001), as was female sex (χ2 = 8.4, df = 2, P = 0.015). Age was not significantly associated with psychotic symptoms in this model. Similar results were obtained in a Generalized Estimating Equations model with age, sex, Clinical Dementia Rating and proband psychosis as predictor variables except the effect of sex was no longer significant (proband psychosis status Wald χ2 = 6.9, df = 2, P = 0.032; Clinical Dementia Rating Wald χ2 = 16.9, df = 1, P < 0.001).
Discussion
We assessed psychotic symptoms in a cohort of individuals with dementia, recruited as part of the National Institute on Aging Late Onset Alzheimer’s Disease Family Study on the basis of having multiple family members diagnosed with late onset Alzheimer’s disease. We found that psychotic symptoms could be reliably assessed by multiple evaluators across sites, and via telephone, facilitating characterization of geographically remote family members. In several key regards, the Family Study members assessed for this report appeared typical of individuals with late onset Alzheimer’s disease not recruited on the basis of familial status, including the frequency of individuals with psychotic symptoms, the frequencies of individual psychotic symptoms and the clinical correlates of psychotic symptoms (Ropacki and Jeste, 2005). Finally, we provide independent evidence of the familial aggregation of psychosis within late onset Alzheimer’s disease subjects, suggesting that the late onset Alzheimer’s disease plus psychosis phenotype may fruitfully be analysed for linkage and association to genetic variation within the Family Study cohort.
Ropacki and Jeste (2005) recently conducted a comprehensive review of 55 studies comprising 9749 subjects evaluated for psychosis in late onset Alzheimer’s disease and reported in the literature from 1990 to 2003. They found that the median prevalence of psychosis (typically defined by the presence of one or more psychotic symptoms and thus most comparable to our combined single and multiple/recurrent groups) across studies was 41.1%, a rate highly congruent with that in the current study. The most common psychotic symptom reported in most (50.9%) studies was paranoid delusions (delusions of theft). Other, predominantly misidentification, delusions occurred with a median prevalence of 25.6%. Hallucinations had a median prevalence of 18.7% (visual) and 9.2% (auditory). While the current study differs somewhat from these estimates, it would be premature to conclude that these differences reflect the presence or absence of familial late onset Alzheimer’s disease for several reasons. First, estimates of specific symptom prevalence reported in the studies reviewed by Ropacki and Jeste (2005) varied widely, with our results falling well within the ranges of reported symptom frequencies. Though this variation could reflect a number of factors, one important factor is the rating instrument used to assess for psychosis. For example, the Behavioural Rating Scale specifically queries a number of misidentification delusions, whereas other instruments, such as the Neuropsychiatric Inventory Questionnaire, do not. Second, the review of studies of psychosis in late onset Alzheimer’s disease revealed several clinical predictors of psychosis prevalence (Ropacki and Jeste, 2005). Because these predictors may themselves vary in frequency across studies, they will contribute to some variation in psychosis rates. Finally, the individuals in the reports reviewed by Ropacki and Jeste (2005) were admixed with regard to family history of late onset Alzheimer’s disease. In fact, in the 4 of 55 studies which evaluated whether family history of late onset Alzheimer’s disease was associated with the presence of psychosis, no association was found (Ropacki and Jeste, 2005). Thus, on the whole, it appears that psychosis, as it presents in individuals with late onset Alzheimer’s disease in the Family Study cohort, may be representative of the psychosis syndrome in unselected late onset Alzheimer’s disease groups.
In support of this interpretation, increasing cognitive impairment was the strongest clinical correlate of psychosis within our subjects. Greater cognitive impairment is by far the most consistent correlate of psychosis in late onset Alzheimer’s disease cohorts not selected on the basis of family history (Ropacki and Jeste, 2005), and a predictor of psychosis status within family cohorts (Bacanu et al., 2005). In contrast to cognitive impairment, Ropacki and Jeste (2005) reported that only 7 out of 24 studies found a significant association between psychosis and sex, and only 12 out of 25 found a significant association between psychosis and age, consistent with the variable (across analyses) associations of these factors with psychosis in our cohort. Psychotic symptoms were also correlated with the severity of other behavioural symptoms in our subjects. This is congruent with prior reports demonstrating the associations of psychosis with agitation (Lopez et al., 2003), aggression (reviewed in Sweet et al., 2003), and depressive symptoms (Lyketsos et al., 2001; Bassiony et al., 2002; Wilkosz et al., 2006). Perhaps reflecting, in part, these correlations, psychotic symptoms in late onset Alzheimer’s disease subjects are associated with increased caregiver distress (Kaufer et al., 1998).
With the analysis of the current cohort, three independent cohorts have now found evidence for familial aggregation of psychosis in late onset Alzheimer’s disease (Sweet et al., 2002; Hollingworth et al., 2007). Even the one smaller study which did not find evidence of significant familial aggregation found the pair-wise concordance for psychosis amongst late onset Alzheimer’s disease sibships (i.e. frequency of pairs in which both siblings were positive for psychosis) was 0.21, a value that was modestly higher than the concordance rate of 0.17 expected by chance alone (Tunstall et al., 2000). Thus the majority of evidence supports the familiality of psychosis in late onset Alzheimer’s disease. In fact, the odds ratio of 3.80 for multiple/recurrent psychosis in family members of probands with late onset Alzheimer’s disease plus psychosis in the current study is remarkably similar to the values in these prior reports, which ranged from 3.18 to 5.42 (Sweet et al., 2002; Bacanu et al., 2005; Hollingworth et al., 2007).
Evidence of familial aggregation of psychosis in Alzheimer’s disease suggests that this phenotype is under genetic control. An important question then is in what way genetic factors might lead to psychosis in Alzheimer’s disease. Two alternative pathways seem likely. In one, genetic variants may lead to psychosis by modifying the effects of Alzheimer’s pathology that develops due to other genetic and environmental influences. Such genetic variants would best be identified by association studies contrasting individuals with late onset Alzheimer’s disease with, and without, psychosis. The evidence from such studies to date is limited (reviewed in DeMichele-Sweet and Sweet, 2009), although there is some support for two genes [neuregulin-1(NRG1), catechol-O-methyl transferase (COMT)] that have also been suggested as putative risk genes for schizophrenic psychosis, possibly indicating these genes may modify neurodevelopmental and neurodegenerative processes to yield psychotic symptoms. In the alternate pathway, genes would increase the liability to a form of Alzheimer’s disease characterized by the occurrence of psychosis during the illness. Currently, there is little evidence by which to accept or reject this model. The one gene known to influence risk of late onset Alzheimer’s disease, apolipoprotein E, does not appear to be associated with psychosis (DeMichele-Sweet and Sweet, 2009). Other genes more recently associated with late onset Alzheimer’s disease, such as SORL1, clusterin, complement receptor 1 and PICALM (Rogaeva et al., 2007; Harold et al., 2009; Lambert et al., 2009) have not been studied for association with psychosis. Genetic variation in this pathway may best be detected through linkage analysis of families with multiple individuals affected by late onset Alzheimer’s disease and psychosis, or through association studies contrasting individuals with late onset Alzheimer’s disease and psychosis to those without psychosis complicating their Alzheimer’s disease course.
We evaluated the occurrence of psychotic symptoms in a cohort recruited as part of the National Institute on Aging Late Onset Alzheimer’s Disease Family Study. Reliable assessment of symptoms was readily implemented. Psychotic symptoms showed evidence of aggregation within families, and were associated with greater burden of cognitive impairment and behavioural symptoms. Future studies should examine the linkage and association of psychotic symptoms to genetic variation within these families.
Funding
Supported by the following grants from the National Institute on Aging: U24AG026395 (NIA-LOAD Family Study); U24AG021886 (National Cell Repository for Alzheimer’s Disease); R01AG027224 and P50AG005133 University of Pittsburgh; P30AG10161 Rush University Medical Center; P30AG013846 Boston University; P50AG08702 Columbia University; P30AG028377 Duke University; P30AG010133 Indiana University; P50AG05134 Massachusetts General Hospital; P50AG165574 Mayo Clinic, Rochester and Mayo Clinic, Jacksonville; P01AG05138, P01AG02219, and P50AG05138 Mount Sinai School of Medicine; P30AG13854 Northwestern University Medical School; P30AG008017 Oregon Health and Science University; P50AG016582 University of Alabama at Birmingham; P50AG016579 David Geffen School of Medicine, University of California, Los Angeles; P30AG028383 University of Kentucky, Lexington; P30AG10124 University of Pennsylvania; P50AG05142 University of Southern California; P30AG12300 The University of Texas Southwestern Medical Center; P50AG05136 University of Washington; and P50AG05681 and P01AG03991 Washington University School of Medicine.
Acknowledgements
Susan LaRusse Eckert, MS and Stephanie Doan, MPH (Columbia University) and Michele Goodman and Kelley Faber, MS (Indiana University), helped coordinate the project across the United States. Creighton H. Phelps, PhD, Marcelle Morrison-Bogorod, PhD and Marilyn Miller, PhD, at the NIA provided guidance.
Glossary
Abbreviations
- PICALM
phosphatidylinositol-binding clathrin assembly protein
- SORL
sortilin-related receptor
Appendix 1
Investigators from the National Institute on Aging Late Onset Alzheimer’s Disease Family Study Group
Robert Green, MD, Neil Kowal, MD and Lindsay Farrer, PhD (Boston University, Boston, MA, USA); Jennifer Williamson, MS and Vincent Santana, MBA (Columbia University, New York, NY, USA); Donald Schmechel, MD and Peter Gaskel, BS (Duke University, Durham, NC, USA); Bernardino Ghetti, MD, Martin R. Farlow, MD, Tatiana Foroud, PhD, Kelly Horner, Kate Kreiner and Heather Prentice (Indiana University, IN, USA); John H. Growdon, MD, Deborah Blacker, MD, ScD, Rudolph E. Tanzi, PhD and Bradley T. Hyman, MD (Massachusetts General Hospital, Boston, MA, USA); Bradley Boeve, MD, Karen Kuntz, RN, Lindsay Norgaard, BS and Nathan Larson, BS (Mayo Clinic, Rochester, MN, USA); Dana Kistler, BSH, Francine Parfitt, MS and Jenny Haddow, BS (Mayo Clinic, Jacksonville, FL, USA); Jeremy Silverman, PhD, Michal Schnaider Beeri, PhD, Mary Sano, PhD, Joy Wang, BA and Rachel Lally, BA (Mount Sinai School of Medicine, NY, USA); Nancy Johnson, PhD, Marcel Mesulam, PhD, Sandra Weintraub, PhD and Eileen Bigio, MD (Northwestern University, Chicago, IL, USA); Jeffery Kaye, MD, Patricia Kramer, PhD and Jessica Payne-Murphy, BA (Oregon Health and Science University, Portland, OR, USA); Holli Jacobs, BA, Jeen-Soo Chang, MD and Danielle Arends, RN (Rush University, Chicago, IL, USA); Lindy Harrell, MD, PhD (University of Alabama, Birmingham, AL, USA); George Bartzokis, MD, Jeffery Cummings, MD, Po H. Lu, PsyD and Usha Toland, MS (University of California, Los Angeles, CA, USA); William Markesberry, MD, Charles Smith, MD and Alise Brickhouse, BA (University of Kentucky, Lexington, KY, USA); John Trojanowski, MD, PhD, Vivianna Van Deerlin, MD, PhD and Elisabeth McCarty Wood, MS (University of Pennsylvania, Philadelphia, PA, USA); Steven DeKosky, MD and Elise Weamer, MPH (University of Pittsburgh, Pittsburgh, Pennsylvania, PA, USA); I. Helena Chui, MD and Arousiak Varpetian, MD (University of Southern California, Los Angeles, CA, USA); Ramon Diaz-Arrastia, MD, PhD, Roger Rosenberg, MD and Barbara Davis, MA (The University of Texas Southwestern Medical Center, Dallas, TX, USA); Thomas Bird, MD, Malia Rumbaugh, MS, Gerard D. Schellenberg, PhD and Murray Raskind, MD (University of Washington, Seattle, WA, USA); and Alison Goate, DPhil, John Morris, MD, Joanne Norton, MSN, RN, Denise Levitch, RN, Betsy Grant, MSW, PhD and Mary Coats, MSN, RN (Washington University, St Louis, MO, USA).
References
- Bacanu SA, Devlin B, Chowdari KV, DeKosky ST, Nimgaonkar VL, Sweet RA. Heritability of psychosis in AlzQheimer disease. Am J Geriatr Psychiatry. 2005;13:624–27. doi: 10.1176/appi.ajgp.13.7.624. [DOI] [PubMed] [Google Scholar]
- Bassiony MM, Rosenblatt A, Baker A, Steinberg M, Steele CD, Sheppard J, et al. The relationship between delusions and depression in Alzheimer's; disease. Int J Geriatr Psychiatry. 2002;17:549–56. doi: 10.1002/gps.641. [DOI] [PubMed] [Google Scholar]
- Bettens K, Brouwers N, Engelborghs S, De Deyn PP, Van BC, Sleegers K. SORL1 is genetically associated with increased risk for late-onset Alzheimer disease in the Belgian population. Hum Mutat. 2008;29:769–70. doi: 10.1002/humu.20725. [DOI] [PubMed] [Google Scholar]
- DeMichele-Sweet MA, Sweet RA. Genetics of psychosis in Alzheimer Disease: a review. J Alzheimers Dis. 2009 doi: 10.3233/JAD-2010-1274. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Rubin EH. Psychotic symptoms and the longitudinal course of senile dementia of the Alzheimer type. Biol Psychiatry. 1989;25:39–48. doi: 10.1016/0006-3223(89)90145-5. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–56. [PubMed] [Google Scholar]
- Feulner TM, Laws SM, Friedrich P, Wagenpfeil S, Wurst SH, Riehle C, et al. Examination of the current top candidate genes for AD in a genome-wide association study. Mol Psychiatry. 2009 doi: 10.1038/mp.2008.141. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's; disease. Nature. 1991;349:704–6. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's; disease. Nat Genet. 2009;41:1088–93. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth P, Hamshere ML, Holmans PA, O'D;onovan MC, Sims R, Powell J, et al. Increased familial risk and genomewide significant linkage for Alzheimer's; disease with psychosis. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:841–48. doi: 10.1002/ajmg.b.30515. [DOI] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1997;140:566–72. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Kaufer DI, Cummings JL, Christine D, Bray T, Castellon S, Masterman D, et al. Assessing the impact of neuropsychiatric symptoms in Alzheimer's; disease: the Neuropsychiatric Inventory Caregiver Distress Scale. J Am Geriatr Soc. 1998;46:210–15. doi: 10.1111/j.1532-5415.1998.tb02542.x. [DOI] [PubMed] [Google Scholar]
- Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–39. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- Kehoe P, Wavrant-De Vrieze F, Crook R, Wu WS, Holmans P, Fenton I, et al. A full genome scan for late onset Alzheimer's; disease. Hum Mol Genetics. 1999;8:237–45. doi: 10.1093/hmg/8.2.237. [DOI] [PubMed] [Google Scholar]
- Kimura R, Yamamoto M, Morihara T, Akatsu H, Kudo T, Kamino K, et al. SORL1 is genetically associated with Alzheimer disease in a Japanese population. Neurosci Lett. 2009;461:177–80. doi: 10.1016/j.neulet.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Kolsch H, Jessen F, Wiltfang J, Lewczuk P, Dichgans M, Teipel SJ, et al. Association of SORL1 gene variants with Alzheimer's; disease. Brain Res. 2009;1264:1–6. doi: 10.1016/j.brainres.2009.01.044. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's; disease. Nat Genet. 2009;41:1094–99. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- Lee JH, Cheng R, Graff-Radford N, Foroud T, Mayeux R. Analyses of the National Institute on Aging Late-Onset Alzheimer's; Disease Family Study: implication of additional loci. Arch Neurol. 2008;65:1518–26. doi: 10.1001/archneur.65.11.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, et al. Candidate gene for the chromosome 1 familial Alzheimer's; disease locus. Science. 1995;269:973–77. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- Li Y, Rowland C, Catanese J, Morris J, Lovestone S, O'D;onovan MC, et al. SORL1 variants and risk of late-onset Alzheimer's; disease. Neurobiol Dis. 2008;29:293–96. doi: 10.1016/j.nbd.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez OL, Becker JT, Sweet RA, Klunk W, Kaufer DI, Saxton J, et al. Psychiatric symptoms vary with the severity of dementia in probable Alzheimer's; disease. J Neuropsychiatry Clin Neurosci. 2003;15:346–53. doi: 10.1176/jnp.15.3.346. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Sheppard J, Steinberg G, Tschanz J, Norton MC, Steffens DC, et al. Neuropsychiatric disturbance in Alzheimer's; disease clusters into three groups; the Cache County study. Int J Geriatr Psychiatry. 2001;16:1043–53. doi: 10.1002/gps.448. [DOI] [PubMed] [Google Scholar]
- Mack JL, Patterson MB, Tariot PN. Behavior Rating Scale for Dementia: development of test scales and presentation of data for 555 individuals with Alzheimer's; disease. J Geriatr Psychiatry Neurol. 1999;12:211–23. doi: 10.1177/089198879901200408. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's; disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's; disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Pericak-Vance MA, Grubber J, Bailey LR, Hedges D, West S, Santoro L, et al. Identification of novel genes in late-onset Alzheimer's; disease. Exp Gerontol. 2000;35:1343–52. doi: 10.1016/s0531-5565(00)00196-0. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai T, Zou F, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39:168–77. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropacki SA, Jeste DV. Epidemiology of and risk factors for psychosis of Alzheimer's; disease: a review of 55 studies published from 1990 to 2003. Am J Psychiatry. 2005;162:2022–30. doi: 10.1176/appi.ajp.162.11.2022. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Brandt J, Albert M, Hadjigeorgiou G, Papadimitriou A, Dubois B, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62:1601–8. doi: 10.1001/archneur.62.10.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's; disease. Nature. 1995;375:754–60. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Nimgaonkar VL, Devlin B, Jeste DV. Psychotic symptoms in Alzheimer disease: evidence for a distinct phenotype. Mol Psychiatry. 2003;8:383–92. doi: 10.1038/sj.mp.4001262. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Nimgaonkar VL, Devlin B, Lopez OL, DeKosky ST. Increased familial risk of the psychotic phenotype of Alzheimer disease. Neurology. 2002;58:907–11. doi: 10.1212/wnl.58.6.907. [DOI] [PubMed] [Google Scholar]
- Tan EK, Lee J, Chen CP, Teo YY, Zhao Y, Lee WL. SORL1 haplotypes modulate risk of Alzheimer's; disease in Chinese. Neurobiol Aging. 2009;30:1048–51. doi: 10.1016/j.neurobiolaging.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Tunstall N, Owen MJ, Williams J, Rice F, Carty S, Lillystone S, et al. Familial influence on variation in age of onset and behavioural phenotype in Alzheimer's; disease. Br J Psychiatry. 2000;176:156–59. doi: 10.1192/bjp.176.2.156. [DOI] [PubMed] [Google Scholar]
- Wilkosz PA, Miyahara S, Lopez OL, DeKosky ST, Sweet RA. Prediction of psychosis onset in Alzheimer disease: the role of cognitive impairment, depressive symptoms, and further evidence for psychosis subtypes. Am J Geriatr Psychiatry. 2006;14:352–56. doi: 10.1097/01.JGP.0000192500.25940.1b. [DOI] [PubMed] [Google Scholar]