Abstract
A dramatic increase in the use and dependence of prescription opioids has occurred within the last 10 years. The consequences of long-term prescription opioid use and dependence on the brain are largely unknown, and any speculation is inferred from heroin and methadone studies. Thus, no data have directly demonstrated the effects of prescription opioid use on brain structure and function in humans. To pursue this issue, we used structural magnetic resonance imaging, diffusion tensor imaging and resting-state functional magnetic resonance imaging in a highly enriched group of prescription opioid-dependent patients [(n = 10); from a larger study on prescription opioid dependent patients (n = 133)] and matched healthy individuals (n = 10) to characterize possible brain alterations that may be caused by long-term prescription opioid use. Criteria for patient selection included: (i) no dependence on alcohol or other drugs; (ii) no comorbid psychiatric or neurological disease; and (iii) no medical conditions, including pain. In comparison to control subjects, individuals with opioid dependence displayed bilateral volumetric loss in the amygdala. Prescription opioid-dependent subjects had significantly decreased anisotropy in axonal pathways specific to the amygdala (i.e. stria terminalis, ventral amygdalofugal pathway and uncinate fasciculus) as well as the internal and external capsules. In the patient group, significant decreases in functional connectivity were observed for seed regions that included the anterior insula, nucleus accumbens and amygdala subdivisions. Correlation analyses revealed that longer duration of prescription opioid exposure was associated with greater changes in functional connectivity. Finally, changes in amygdala functional connectivity were observed to have a significant dependence on amygdala volume and white matter anisotropy of efferent and afferent pathways of the amygdala. These findings suggest that prescription opioid dependence is associated with structural and functional changes in brain regions implicated in the regulation of affect and impulse control, as well as in reward and motivational functions. These results may have important clinical implications for uncovering the effects of long-term prescription opioid use on brain structure and function.
Keywords: prescription opioids, chronic pain, addiction, functional connectivity, morphology
Introduction
Opioid dependence is a resurgent public health care problem in the United States (March, 2004). Use of short- and long-acting opioids alter physiological and behavioural functions (Stimmel and Kreek, 2000). Most clinical research on neurobiological aspects of opioid dependence on brain systems thus far has been focused on illicit opioids (i.e. heroin) and the implicated cortical and subcortical limbic/paralimbic brain structures involved in emotion, reward, motivation and impulse control. In heroin addicts, there have been reports of alterations in grey and white matter morphometric (Ryan et al., 2005; Offiah and Hall, 2008) and functional (Ma et al., 2009) properties of the brain. These observations point to altered functional organization that may contribute to a better understanding of drug salience and weakened cognitive controls in the dependent state. Furthermore, preclinical studies of opioid-induced effects on neural systems support the hypothesis that clinical changes in brain function and structure (e.g. altered dendritic spine density and neuronal apoptosis) are a consequence of chronic drug exposure (Robinson and Kolb, 1999; Luo et al., 2004; Xi et al., 2004; Liao et al., 2005; Cunha-Oliveira et al., 2007; Shabat-Simon et al., 2008).
One clinical characteristic that has not yet been accounted for is the impact of long-term use of prescription opioid analgesics (e.g. hydrocodone, codeine and oxycodone). In contrast to dependence on illicit opioids, the misuse of prescription opioids may cause a different illness course (Hall et al., 2008; Pletcher et al., 2008), which may occur more prevalently in certain sociodemographic backgrounds (Compton and Volkow, 2006). Moreover, this class of drugs exhibits a well-defined purity and composition of the pharmacological agents (Klemenc, 2000; Risser et al., 2007), which is not the case for illicit opioids. Publication of the pain experts’ guidelines in the late 1990s (1997) (advocating more liberal and widespread use of prescription opioids) led to a shift in opioid dependency from illicit drugs to those requiring a prescription. Inquiry into the effects of long-term prescription opioid use is therefore highly relevant and timely due to the increased incidence of dependency on these drugs.
Our group participated in the multi-site Prescription Opioid Addiction Treatment Study, sponsored by the National Institute on Drug Abuse Clinical Trials Network (NIDA-CTN). This project examined different combinations of pharmacological and behavioural treatment strategies for patients dependent on prescription opioids (NIDA U10DA015831). McLean Hospital led the study and served as one of the ten recruitment sites. From this population, we selected and characterized a homogeneous group of individuals with prescription opioid dependence that did not have a dependence on alcohol or other drugs, comorbid psychiatric or neurological disease or chronic pain. We used a combination of structural MRI, diffusion tensor imaging and resting state functional magnetic resonance imaging (fMRI) to evaluate potential morphological and functional abnormalities in this cohort of prescription opioid-dependent subjects versus matched comparison subjects. By utilizing a combination of structural and functional brain imaging techniques, the correlation between morphological changes and functional connectivity could be determined for the prescription opioid-dependent group.
In the present work, the a priori emphasis was given to a few clearly identifiable key anatomical structures mediating reward, motivation and interoceptive processing, including cortical (insula) and subcortical (amygdala and nucleus accumbens) structures (Carlezon and Thomas, 2009). These regions may be targets for morphological and functional drug-induced plasticity (Langleben et al., 2008; Liu et al., 2009) as a result of exogenous opioid exposure (Contet et al., 2004; Befort et al., 2008). To our knowledge, this is the first study to evaluate potential morphology and functional connectivity changes in the brains of prescription opioid-dependent patients.
Materials and methods
Psychiatric evaluations including a psychiatric history conducted by a physician and the Composite International Diagnostic Interview for depression and post-traumatic stress disorder performed during patient recruitment suggested that these subjects did not have premorbid or active comorbid psychiatric conditions. However, other factors (e.g. genetic) may have predisposed these subjects to the effects of opioids on brain morphology and connectivity, a conundrum that is common in cross-sectional designs (see Supplementary material ‘Caveats’).
Subjects
Investigators at the McLean Hospital site (one facility of the multi-site Clinical Trials Network study on treatment of prescription opioid dependence) screened 133 candidates during a ∼36-month period preceding this report (Fig. 1). From these 133 patients, 48 candidates were disqualified due to the presence of other psychiatric or medical conditions, thus yielding 85 patients who were enrolled in the Prescription Opioid Addiction Treatment Study at McLean Hospital. Ultimately, 10 [age: 29.4 ± 8.9 years (mean ± SD); gender: seven males, three females] non-smoking prescription opioid-dependent patients, meeting the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision criteria for dependence on opioids were enrolled into the imaging arm of the study (Fig. 1). These 10 patients were free from any other psychiatric disorders (determined by the Composite International Diagnostic Interview) or other medial conditions, including pain. It is noted that 2 of the 10 patients started using prescription opioids in order to treat pain and were prescribed opioids for approximately a 2-week period. However, the two patients continued to use prescription opioids despite no longer having pain. The 10 patients and 10 matched, mentally healthy individuals (age: 29.9 ± 7.9 years; gender: seven males, three females) recruited in the imaging study participated after giving written informed consent to the McLean Hospital Institutional Review Board-approved protocol. The matched healthy controls were chosen based on age (within the same 2–3 years span), gender and handedness. Participants’ demographic characteristics are noted in Table 1.
Figure 1.
Recruitment process of prescription opioid-dependent patients. Patients were recruited from a larger study focusing on treatment strategy for prescription opioid dependence. Implementing strict exclusion criteria for the imaging arm of the study yielded 10 patients who only had a dependence on prescription opioids and who were without other co-morbidities (i.e. dependence on other drugs or chronic pain).
Table 1.
Subject demographics
|
Prescription opioid-dependent subjects |
Healthy controls |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Gender | Age | Race | Handedness | Years of education | Subject | Gender | Age | Race | Handedness | Years of education |
| OD_01 | M | 29 | White | R | 15 | H_01 | M | 28 | White | R | 12 |
| OD_02 | F | 30 | White | R | 13 | H_02 | F | 28 | White | R | 15 |
| OD_03 | M | 20 | White | L | 14 | H_03 | M | 21 | Asian | L | 14 |
| OD_04 | M | 48 | Hispanic | R | 9 | H_04 | M | 47 | White | R | 18 |
| OD_05 | F | 38 | White | R | 16 | H_05 | F | 38 | White | R | 15 |
| OD_06 | M | 33 | White | L | 12 | H_06 | M | 35 | Hispanic | L | 12 |
| OD_07 | F | 22 | White | R | 15 | H_07 | F | 23 | White | R | 13 |
| OD_08 | M | 30 | White | L | 12 | H_08 | M | 29 | White | L | 15 |
| OD_09 | M | 26 | White | R | 15 | H_09 | M | 23 | White | R | 12 |
| OD_10 | M | 18 | White | R | 11 | H_10 | M | 19 | White | R | 10 |
| Mean±SD | 29.4±8.9 | 13.2±2.2 | 29.9±7.9 | 13.6±2.23 | |||||||
OD = opioid-dependent subject; H = age/gender/handedness matched healthy control.
Individuals with dependence on prescription opioids and a concurrent chronic pain condition were excluded from participation in the study. While both opioid analgesics (Becerra et al., 2006; Leppa et al., 2006) and chronic pain (Baliki et al., 2008; Geha et al., 2008; Seifert et al., 2009) may produce partially overlapping brain changes, elucidation of those due to the former but not the latter was the purpose of the present study. Defining each type of change separately provides a solid foundation for future investigations that are aimed at understanding the potential interactions between prescription opioids and chronic pain, with regard to the brain structure and function.
The diagnosis of opioid dependence was performed using the Composite International Diagnostic Interview (Kessler, 1999). Addiction Severity Index for each subject was also evaluated (McLellan et al., 1980). Prescription opioid-dependent subjects with other comorbid Axis I psychiatric diagnoses or with a history of chronic pain in the past, 3 months as assessed with the Brief Pain Inventory (Cleeland and Ryan, 1994), were excluded. We further excluded subjects who had used any potentially confounding medications or drugs within the previous 3 months. These confounding medications included psychostimulants, cannabinoids, dopaminergic or antidopaminergic agents including antipsychotics, mood stabilizers or antidepressants with prominent catecholaminergic effects (e.g. tricylclics, bupropion, mirtazapine, venlafaxine and duloxetine), non-steroidal anti-inflammatory drugs and methadone. Further details on the screening process and inclusion/exclusion criteria for prescription opioid-dependent subjects are available in the Supplementary material.
Preimaging assessment of opioid-dependent patients and healthy controls
All patients and healthy subjects included in the study were in good physical health and had no significant medical history, as determined by medical and neurological screening. The opioid-dependent subjects were active users of opioid analgesics. Subjects used prescription opioids for periods of 9 months to 8 years (3.5 ± 2.2 years, mean ± SD), and the average Addiction Severity Index drug composite score was 0.30 ± 0.05 (range 0–1), suggesting a mild to moderate severity of addiction. Prescription opioids that were commonly consumed by patients included oxycodone and hydrocodone (Table 2). The most commonly misused drug in the 30 days prior to imaging was oxycodone, with 9 of 10 patients consuming this drug alone or in combination with other prescription opioids (Table 2).
Table 2.
Prescription opioids consumed
| Subject | Years of dependence | Prescription opioids consumed | Prescription opioids consumed in past 30 days |
|---|---|---|---|
| OD_01 | 1 | Hydrocodone, oxycodone, Acetaminophen/hydrocodone | Oxycodone |
| OD_02 | 4 | Oxycodone | Oxycodone |
| OD_03 | 5 | Hydrocodone, oxycodone | Hydrocodone, oxycodone |
| OD_04 | 8 | Morphine, oxycodone | Morphine, oxycodone |
| OD_05 | 3 | Hydrocodone, oxycodone, tramadol | Tramadol |
| OD_06 | 4 | Oxycodone | Oxycodone |
| OD_07 | 2 | Oxycodone | Oxycodone |
| OD_08 | 2 | Hydrocodone, oxycodone | Oxycodone |
| OD_09 | 5 | Oxycodone, methylmorphine, | Oxycodone, methylmorphine |
| OD_10 | 0.5-1 | Acetaminophen/hydrocodone, oxycodone, hydrocodone, buprenorphine, hydromorphone, buprenorphine/naloxone, morphine, methylmorphine | Oxycodone, hydrocodone, buprenorphine |
OD = Opioid-dependent subject.
To determine if any patient or healthy subject had an altered sensitivity to painful stimuli, all subjects enrolled into the study underwent Quantitative Sensory Testing to determine levels of pain sensitivity (Pud et al., 2006) and Brief Pain Inventory was obtained. Details of Quantitative Sensory Testing measures are noted in the Supplementary material.
Magnetic resonance imaging data acquisition and analysis
Data acquisition
All data were collected on a 3 Tesla Siemens Trio scanner with an eight-channel phased array head coil (Erlangen, Germany). Two high-resolution, T1-weighted structural MRI datasets were collected from each patient and control subject using a 3D magnetization-prepared rapid gradient echo pulse sequence. diffusion tensor imaging data were collected using a single-shot twice-refocused echo planar imaging pulse sequence, while the resting-state (fMRI) data were collected using a gradient echo- planar imaging pulse sequence. During the ∼7.5 min resting-state fMRI acquisition period, subjects were asked to remain awake with their eyes open.
MRI data analysis
Structural MRI: subcortical volume segmentation
Volumetric segmentation was performed with FreeSurfer image analysis software (http://surfer.nmr.mgh.harvard.edu). For sub-cortical volumetric analysis, FreeSurfer enabled segmentation and labelling of subcortical tissue classes (Fischl et al., 2002, 2004). The initial processing steps included: (i) motion correction and averaging of the two volumetric T1-weighted magnetization-prepared rapid gradient echo images; (ii) removal of non-brain tissue (Segonne et al., 2004); (iii) automated Talairach transformation; (iv) segmentation of the subcortical white matter and deep grey matter volumetric structures (Fischl et al., 2002, 2004); (v) intensity normalization (Sled et al., 1998); (vi) tessellation of the grey matter/white matter boundary; (vii) automated topology correction (Fischl et al., 2001; Segonne et al., 2007); (viii) surface deformation (Dale et al., 1999; Fischl and Dale, 2000); and (x) registration of the subjects’ brains to a common spherical atlas. Once the volumes of the subcortical structures were calculated, statistically significant differences between the two groups were assessed using an unpaired t-test. High test-retest reliability of the implemented analysis method has been previously shown (Dickerson et al., 2008; Wonderlick et al., 2009). To assess the effects of the duration of prescription opioid dependence on volumetric loss or gain, the duration of prescription opioid dependence and age of each patient were included as regressors for a separate opioid-dependent specific group analysis.
Diffusion tensor imaging: anisotropy in white matter
Single-subject and group-level diffusion tensor imaging analyses were performed using FMRIB Software Library (FSL) (www.fmrib.ax.ac.uk/fsl). For each individual subject, the diffusion tensor imaging dataset was initially corrected for eddy current distortion and head motion (Jenkinson and Smith, 2001; Jenkinson et al., 2002). For both eddy current distortion and head motion corrections, an automated affine registration algorithm was implemented in which the skull-stripped non-diffusion weighted volume was used as the reference volume (Jenkinson and Smith, 2001; Jenkinson et al., 2002; Smith, 2002). A diffusion tensor for each voxel was calculated using a least squares fit of the tensor model to the diffusion tensor imaging data. From the diffusion tensors, the eigenvalues of each tensor, which represent the magnitude of the three main diffusion directions, and fractional anisotropy (FA) values, were calculated for each voxel. FA maps were created for each patient and control subject. To determine group-level differences in FA between the opioid-dependent subjects and healthy controls, we performed tract-based spatial statistics (TBSS, corrected for multiple comparisons) (Smith et al., 2006). A multiple comparison correction within TBSS analysis was performed by voxel-wise permutation (5000 permutations) testing, in which inference was performed using cluster-size thresholding. Significant voxels within the FA skeleton were those that survived an initial t-statistic threshold at t > 3. From the 5000 permutations, a null distribution of the cluster-size statistic was generated. Significant clusters were subsequently thresholded at P < 0.05. The TBSS method enabled a whole brain, voxel-wise comparison of FA values between the two subject cohorts. In a comparison of FA between the opioid-dependent and healthy control groups, subject-specific ages were used as a regressor in group analysis. For the prescription opioid-dependent group, the effects of the duration (years) of prescription opioid dependence on white matter integrity was assessed.
A detailed description of the MRI pulse sequence parameters and implemented methods for subcortical volume and diffusion tensor imaging analyses can be found in the Supplementary material.
Functional MRI: resting state networks and functional connectivity
Preprocessing
Initial preprocessing steps for single-subject resting-state fMRI data were performed using FMRIB Software Library (FSL) (http://www.fmrib.ax.ac.uk/fsl). For each subject’s resting-state fMRI dataset, the following preprocessing steps were taken: (i) motion correction using FMRIB’s Linear Motion Correction (MCFLIRT); (ii) skull-stripping using the Brain Extraction Tool; (iii) spatial smoothing with a 5 mm full-width at half maximum spatial filter; and (iv) co-registration and transformation of the resting-state fMRI dataset to the 2 × 2 × 2 mm3 Montréal Neurological Institute (MNI)-152 template brain using FMRIB’s Linear Image Registration Tool.
General Linear Model-based functional connectivity analysis
Each preprocessed resting state network dataset was bandpass filtered to retain signal between 0.01 and 0.1 Hz; thus removing linear drift artefacts and high frequency noise. Filtered datasets were within the MNI152 space, thus allowing timecourses from regions of interest to be extracted. Regions of interest were labelled using the Anatomical Automatic Labelling atlas (Tzourio-Mazoyer et al., 2002). However, the three subdivisions of the amygdala, centromedial, laterobasal and superficial were based on the Juelich probabilistic brain atlas (Amunts et al., 2005). A characterization of functional connectivity of these three amygdala subdivisions in healthy humans has previously been reported (Roy et al., 2009). Timecourses from the structures consisting of segments within the left and right cerebral hemispheres were averaged. We sought to characterize and compare individually the functional connectivity of the insula, amygdala and nucleus accumbens with the rest of the brain in the opioid-dependent and healthy control groups. Furthermore, we sought to characterize individually functional connectivity involving the centromedial, laterobasal and superficial regions of the amygdala. The more specific functional connectivity analysis enabled a determination of whether (and if so, to what degree) there were specific amygdala–cortical or amygdala–subcortical connections that were compromised due to chronic opioid use. Furthermore, given the distinct functional roles of the anterior and posterior insula, timecourses were extracted from both insular subdivisions (Blomfield et al., 1976; Ackermann and Riecker, 2004; Craig, 2009).
Six sets of General Linear Model (GLM) seed-region analyses were performed for each subject. The following six resting-state, subject-specific timecourses were individually used as explanatory variables in each GLM analysis: (i) anterior insula, (ii) posterior insula, (iii) nucleus accumbens, (iv) centromedial amygdala, (v) laterobasal amygdale and (vi) superficial amgydala. In each of the six GLM analyses, subject-specific timecourses from the white matter and cerebral spinal fluid were used as confound explanatory variables. It is noted that the averaged anterior or posterior insula timecourses were extracted from anatomically defined volumes that were larger in comparison to the amygdala and nucleus accumbens volumes. The larger insular volumes could have yielded less specific timecourses, and in turn affected the results of both functional connectivity analysis methods. Furthermore, a seed serving as a negative control was placed in the precentral gyrus (bilateral). The choice of precentral gyrus was due to the fact that, based on previous pharmacological MRI, direct opioid effects in healthy subjects do not have a particularly strong effect on this region (effects solely due to drug administration, no pain or sensory stimulation given). This is in contrast to regions such as the amygdala, putamen, hippocampus or anterior cingulate cortex. For example, Becerra et al. (2006) reported little or no functional effect within the precentral gyrus subsequent to morphine infusion. Furthermore, Leppa et al. (2006) showed little or no functional effect within the precentral gyrus subsequent to remifentanil infusion. Once individual GLM analyses were completed for each region of interest, an unpaired mixed-effects group analysis was performed. A Gaussian mixture modelling approach was used to threshold group comparison statistical maps (Opioid-dependent subjects > Control subjects; Control subjects > Opioid-dependent subjects) as well as within-group average maps defining the effects of the duration of prescription opioid dependence on functional connectivity (Pendse et al., 2009). The Gaussian mixture modelling approach corrects for multiple comparisons and is similar to other dynamic statistical thresholding techniques such as false discovery rates. For functional connectivity analysis, subject-specific ages were used as regressors in group analysis. The dependence between functional connectivity and duration of prescription opioid dependence was also assessed in separate group-level analysis. In order to quantify functional connectivity changes, parameter estimates extracted from group-level results were examined. In GLM-seed region functional connectivity analysis the parameter estimates define the degree to which the features of a voxel-based timecourse are similar, in terms of shape and amplitude to the explanatory variable (i.e. average timecourse of the seed region, e.g. anterior insula). The lower the parameter estimates, the poorer the fit of the voxel timecourses by the seed region timecourse.
Results
Pre-imaging evaluation of opioid-dependent and healthy control subjects
Pain measures
At the time of physical examination immediately prior to scanning, no significant hypo- or hyperalgesia was detected during examination for any of the opioid-dependent subjects or healthy controls. Pain intensity ratings (Visual Analogue Scale: 0 = no pain and 10 = maximum pain) based on the Brief Pain Inventory were 1.1 ± 2.3 and 1.0 ± 2.3 (mean ± SD) for the opioid-dependent subjects and healthy controls, respectively. These levels were consistent with pain ratings in the past 24 h. None of the opioid-dependent subjects were receiving other clinically used pain medications (i.e. membrane stabilizers, antidepressants or anticonvulsants), nor were they being treated by a physician for pain.
Pain measures also included measures of suprathreshold hot and cold temperatures that corresponded to a pain level of 7/10 (Visual Analogue Scale: 0 = no pain, 10 = maximum pain) for both opioid-dependent subjects and healthy control subjects. Temperatures recorded were 48.5 ± 1.1 and 48.0 ± 1.1°C (mean ± SD) for heat and 3.9 ± 9.1 and 3.1 ± 9.1°C for cold, for the opioid-dependent subjects and healthy control subjects, respectively. These data are essentially identical for these measures and suggest that the opioid-dependent group was not hypersensitive to the thermal stimuli used.
Depression ratings
Beck Depression Index ratings for subjects were all <10, with an average of 0.6 ± 1.1 (mean ± SD) and 1.6 ± 3.6 for opioid-dependent subjects and healthy controls, respectively. This difference between the two groups was not statistically significantly (unpaired t-test).
Toxicology screen
None of the patients or healthy control subjects tested positive for drugs of abuse (barbiturates, benzodiazepines, amphetamine, cocaine, tetrahydrocannabinol, phencyclidine or morphine) immediately before scanning (confirmed by a urine toxicology screen, using the 7 Drug InstaStrip Drug Screen Test, Cortez Diagnostics, Calabas, CA).
Imaging evaluation
Morphometric analyses
Structural MRI: subcortical volume analysis
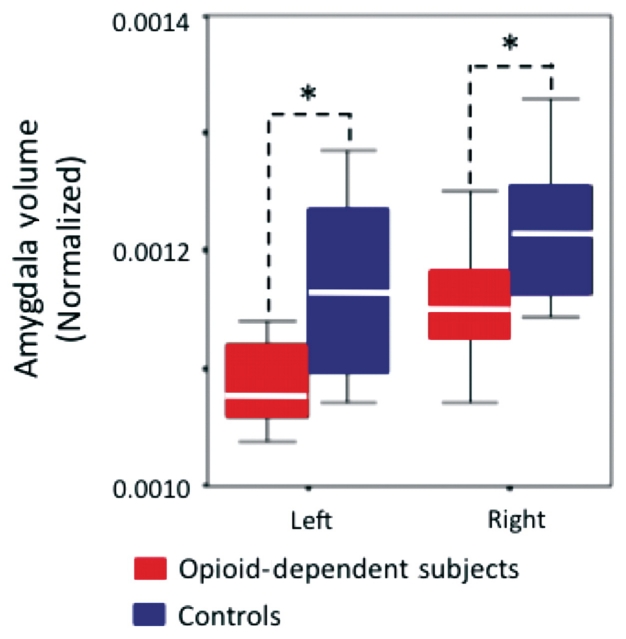
There was a significant (P < 0.05) reduction of the volume in the amygdala in both hemispheres in the opioid-dependent subject group as compared to the healthy control group. For this comparison, the volume of the amygdala was normalized to the total intracranial volume. The results are presented in Fig. 2. No significant difference was found between the opioid-dependent and healthy control groups when comparing the volume of the other subcortical structures. In addition, we found no significant correlation with amygdala volume and duration of prescription opioid dependence.
Figure 2.
Decreases in mean amygdala volume in opioid-dependent subjects. Note that volumes have been normalized to the total intracranial volume to scale for brain volume. White lines represent the mean value for each volumetric measurement, while the length of each box represents the variance. Error bars represent the 95% confidence interval of the mean. *P < 0.05.
Diffusion tensor imaging: anisotropy in white matter
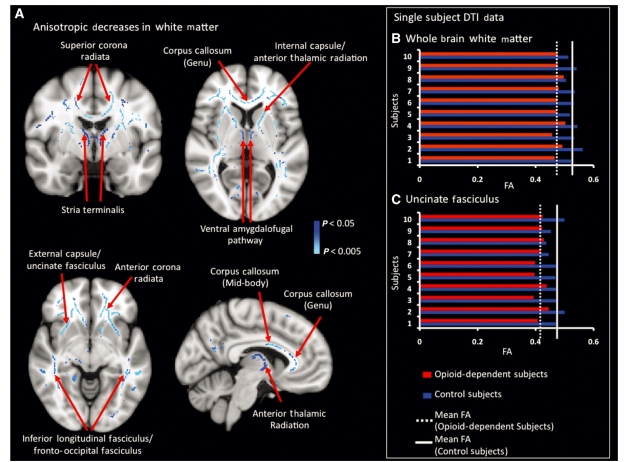
In Supplementary Fig. 1, the mean (n = 20) FA skeleton as well as a comparison of single subject FA maps from both study cohorts are given. Comparison of single subject FA maps qualitatively depicts white matter regions with decreased FA in prescription opioid-dependent patients relative to the respective controls. From group-level TBSS analysis, a decrease in FA in white matter pathways was observed (Fig. 3). In Fig. 3A, the statistical maps superimposed on the standard 1 × 1 × 1 mm3 MNI152 space image indicate significantly decreased FA (P < 0.05, corrected) in the opioid-dependent subject group when compared to the matched healthy control group. As can be seen in the montage of slices, bilateral decreases in FA occurred throughout white matter, particularly in superior and anterior regions of the brain. In group-level TBSS results, decreased FA was found in the following tracts: (i) the three white matter pathways projecting to and/or from the amygdala (stria terminalis, ventral amygdalofugal pathway and uncinate fasciculus); (ii) the internal capsule (corticothalamic and corticospinal pathways) and external capsule (corticocortical and corticostriatal pathways); and (iii) the genu and mid-body of the corpus callosum. Atlas-based images and data were used to confirm the location of these white matter pathways. We did not observe any white matter regions where the FA was significantly greater (P < 0.05, corrected) in the prescription opioid-dependent cohort. No white matter tracts were observed to have a significant correlation between FA and duration of prescription opioid dependence. Furthermore, white matter atlases provided by FSL software (www.fmrib.ox.ac.uk/fsl/) and anatomical atlases were used to define and confirm the location of white matter tracts (Mori et al., 2005). Details of tract definition are shown in Supplementary Fig. 2 and are discussed in the Supplementary material. In Fig. 3, a single-subject comparison of FA values between each opioid-dependent subject and the respective control subject is given for both the uncinate fasciculus (Fig. 3C) as well as for all white matter regions in the brain showing significantly decreased FA in the opioid dependent group (Fig. 3B). Initially, a mask was created by setting the threshold level of the corrected TBSS statistical map shown in Fig. 3A at P < 0.01. This mask was used to extract FA values from each subject in all voxels within the FA skeleton surviving the P < 0.01 threshold. Furthermore, single-subject FA values specific to the uncinate fasciculus were extracted using an atlas-based (JHU-ICBM white matter atlas) mask for the uncinate fasciculus. Comparison of single-subject FA values for both whole brain white matter and the uncinate fasciculus consistently showed opioid-dependent subjects to have lower FA values than their healthy counterparts, with no detectable outliers. No significant correlation with FA values and duration of prescription opioid dependence was observed.
Figure 3.
Significant group-level FA decreases in white matter pathways. (A) Significant (P < 0.05, corrected) decreases in FA were observed in the opioid-dependent group as compared to healthy controls. Specific white matter tracts are noted in the figure. Note that the changes were bilaterally present throughout the brain. Further details on white matter pathways showing decreased FA have been given in Supplementary Fig. 3. (B and C) Changes in FA (single subject data): to quantify the difference in FA between opioid-dependent subjects and healthy controls, FA values from each opioid dependent patient were compared with the respective healthy control. (B) To extract subject-specific FA values, a mask was created by thresholding the TBSS-based statistical map shown in (A) at a significance level of P < 0.01. This mask was used to extract FA values from the 4D-skeletonized FA dataset. In each comparison, opioid-dependent subjects consistently possessed lower FA values than healthy controls and no outliers were present. (C) Subject specific FA values are reported that are specific to the uncinate fasciculus. The FA values of the uncinate fasciculus were extracted using an atlas-based (JHU-ICBM white matter atlas) definition of this particular pathway. Changes in FA were not reliant on the duration of opioid dependence.
Functional MRI: functional connectivity during the resting state
Functional connectivity in insula, amygdala and nucleus accumbens
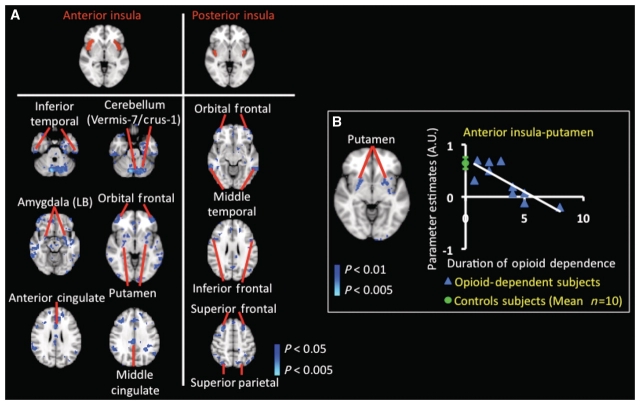
GLM-based functional connectivity analysis revealed decreased connectivity in the opioid-dependent subject group. Decreases in functional connectivity involved the insula (Fig. 4), three amygdalar subdivisions (Fig. 5) and the nucleus accumbens (Fig. 6). No widespread or significant increases in functional connectivity were observed in the opioid-dependent subject group. Moreover, functional connectivity of the anterior insula, laterobasal amygdala and nucleus accumbens were found to be significantly dependent (P < 0.05, corrected) on the duration of prescription opioid dependence. In Fig. 4A, the statistical maps represent regions showing significantly decreased functional connectivity (P < 0.05, corrected) with the anterior and posterior insula. Notably, anterior insula–anterior cingulate and anterior insula–putamen connections were decreased in the opioid-dependent subject group. In comparison to the changes in functional connectivity observed within the anterior insula, the observed decreases were less robust and widespread for the posterior insula.
Figure 4.
Resting-state functional connectivity changes in opioid-dependent subjects in insula. (A) Significant decreases in resting-state functional connectivity in insula: GLM-based seed region connectivity analysis revealed decreased functional connectivity in the opioid-dependent subject group as compared to the healthy control group between the insula and various cortical and subcortical structures. The two insular seed regions are depicted on the MNI standard brain in the top row of A. The functional connectivity difference maps showed significantly (P < 0.05, corrected) decreased connectivity between the anterior insula and both anterior and middle cingulate cortices, putamen and laterobasal (LB) amygdala. Moreover, functional connectivity differences were also observed between the posterior insula and very focused regions of the middle temporal, parietal and frontal cortices. Regions of increased functional connectivity with either insular region in the opioid-dependent group were not observed. (B) Effects of duration (years) of prescription opioid dependence on functional connectivity of the anterior insula: the statistical map in B depicts the specific region of the putamen in which functional connectivity with the anterior insula was significantly anti-correlated (P < 0.01, corrected) with the duration of prescription opioid dependence. Specifically, a longer duration of prescription opioid dependence yielded lower functional connectivity strength between the anterior insula and putamen. This region or volume of the putamen was used as a mask to extract subject-specific parameter estimates. The green circle denotes the control group parameter estimates (mean ± standard error) between the anterior insula and the specific region of the putamen identified in the opioid-dependent group. Significant and robust positive correlation between functional connectivity with the anterior insula and duration of dependence was not detected. Functional connectivity of the posterior insula was not significantly dependent on duration of prescription opioid dependence.
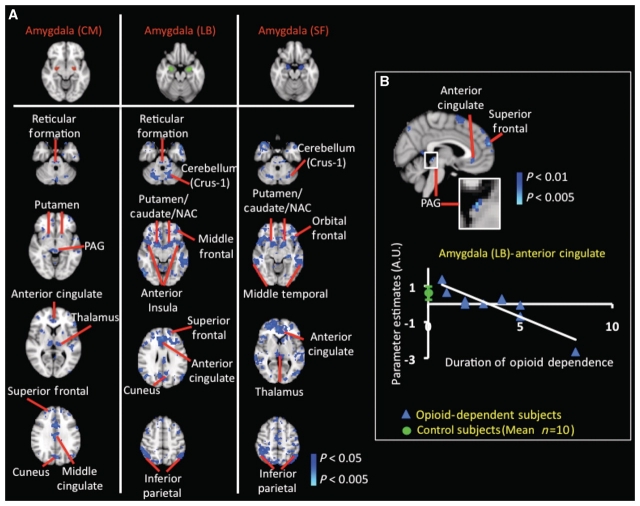
Figure 5.
Resting-state functional connectivity in opioid-dependent subjects in amygdala. (A) Significant decreases in resting-state functional connectivity in amygdala: significant (P < 0.05, corrected) decreases in functional connectivity were observed involving all three subdivisions of the amygdala [centromedial (CM), superficial (SF) and laterobasal (LB)]. Functional connectivity was significantly decreased in the opioid-dependent subject group between the centromedial amygdala and superior frontal and cingulate cortices. The analysis also revealed a significant decrease in functional connectivity between the centromedial amygdala and subcortical structures such as the thalamus (pulvinar and lateral posterior nucleus), putamen and periaqueductal grey matter (PAG). The laterobasal amygdala showed a decrease in functional connectivity with the ventral striatum [putamen, caudate and nucleus accumbens (NAC)], and also with cortical regions such as the anterior insula, frontal, inferior parietal and anterior cingulate cortices. GLM-based seed region analysis results for the superficial amygdala showed similar results to results for the laterobasal amygdalasubdivision. Functional connectivity analysis did not show significant increases in functional connectivity in the opioid-dependent subject group. (B) Effects of duration (years) of prescription opioid dependence on functional connectivity of the laterobasal amygdala: the statistical map in B depicts the specific cortical and subcortical structures that were observed to have a significant anti-correlation (P < 0.05, corrected) between functional connectivity with the laterobasal amygdala and duration of prescription opioid dependence. A significant (P < 0.05, corrected) anti-correlation was detected between the functional connectivity of the (i) laterobasal amygdala–anterior cingulate, laterobasal amygdala–superior frontal; and (iii) laterobasal amygdala–periaqueductal grey functional interactions and the duration of prescription opioid dependence. The region of the anterior cingulate whose functional connectivity was significantly reliant on duration of opioid dependence was used as a mask to extract subject-specific parameter estimates. The green circle in the correlation plot denotes the control group (mean ± standard error) parameter estimate between the laterobasal amygdala and anterior cingulate. Significant and robust dependence between functional connectivity of the centromedial and superficial amygdala and duration of prescription opioid dependence was not observed.
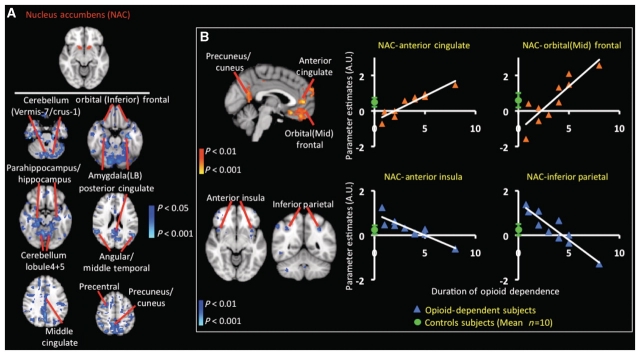
Figure 6.
Resting-state functional connectivity in opioid-dependent subjects in nucleus accumbens. (A) Significant decreases in resting-state functional connectivity in nucleus accumbens (NAC): similar to the functional connectivity analysis results involving the amygdala, GLM-based seed region connectivity analysis demonstrated marked functional changes involving the nucleus accumbens (defined in the top row) and multiple cortical and subcortical structures in the opioid-dependent subject group. Of particular interest was the significant (P < 0.05, corrected) decrease amongst the nucleus accumbens and (i) laterobasal amygdale, (ii) hippocampus, (iii) parahippocampus, (iv) orbital (inferior) frontal and (v) mid-cingulate cortices. Functional connectivity analysis did not show significant increases in functional connectivity in the opioid-dependent subject group. (B) Effects of duration (years) of prescription opioid dependence on functional connectivity of the nucleus accumbens. The statistical maps in B depict the specific cortical and subcortical structures that were observed to have a significant correlation or anti-correlation (P < 0.05, corrected) between functional connectivity with the nucleus accumbens and duration of prescription opioid dependence. Each of the four regions or volumes whose functional connectivity was significantly reliant on duration of opioid dependence was used as a mask to extract subject-specific parameter estimates. The green circle in each correlation plot denotes the control group (mean ± standard error) parameter estimate. A significant (P < 0.05, corrected) correlation was detected between the functional connectivity of the nucleus accumbens–anterior cingulate and nucleus accumben–orbital (mid) frontal cortex connections and the duration of prescription opioid dependence. A significant (P < 0.05, corrected) anti-correlation was detected between the nucleus accumben–anterior insula and nucleus accumben–inferior parietal functional interactions and the duration of prescription opioid dependence. Opioid-dependent subjects with the lowest duration of prescription opioid dependence did not have connectivity strengths similar to the mean connectivity strength for controls. This is true, for example, in the nucleus accumbens–orbital (mid) frontal cortex connections.
The functional connectivity between the anterior insula and putamen was found to be significantly dependent (P < 0.01, corrected) on the duration (years) of prescription opioid dependence (Fig. 4B). An anti-correlation (longer duration of prescription opioid dependence correlated with lower functional connectivity) was observed between the anterior insula and putamen. A mask to extract subject-specific parameter estimates was created using the specific region or volume of the putamen shown in Fig. 4B. For comparison purposes, the mean control group parameter estimates (mean ± standard error) between the anterior insula and putamen is also given (green circle). The same mask was used to extract parameter estimates in the control group and the opioid-dependent group. Functional connectivity of the posterior insula was not significantly reliant on the duration of prescription opioid dependence.
The functional changes involving the three subdivisions of the amygdala (Fig. 5) and nucleus accumbens (Fig. 6) were found to be robust and widespread in the prescription opioid-dependent cohort. The three subdivisions of the amygdala showed varying alterations in coherence patterns with subcortical and cortical structures (Fig. 5). For example, the decreased connectivity between the amygdala and (i) periaqueductal grey matter; (ii) thalamus; and (iii) frontal cortex were most specific to the centromedial division of the amygdala, while the laterobasal division of the amygdala showed significant decreases in connectivity with the dorsal and ventral striatum (caudate, putamen and nucleus accumbens). Furthermore, GLM-based connectivity analyses showed a decrease in interactions amongst the superficial amygdala and cortical regions such as the frontal, temporal and parietal cortices. Regarding the nucleus accumbens, connectivity analysis revealed a decrease in functional connectivity with subcortical structures such as the hippocampus, parahippocampus and laterobasal amygdala as well as with frontal, cingulate and parietal cortical regions.
The functional connectivity between the laterobasal amygdala or nucleus accumbens with other subcortical and cortical structures was found to have a strong and significant (P < 0.05, corrected) reliance on the duration of prescription opioid dependence. This relationship was not observed between the centromedial and superficial subdivisions of the amygdala. As in Fig. 4B, for each of the regions or volumes whose functional connectivity with either the laterobasal amygdala or nucleus accumbens was significantly dependent on duration of opioid dependence, the parameter estimates (mean ± SE) of the specific functional interaction for the control group is also given (denoted by a green circle). The connectivity between the laterobasal amygdala and regions such as the anterior cingulate, superior frontal or periaqueductal grey matter, was anti-correlated (a longer duration of prescription opioid dependence yielded lower functional connectivity) with the duration of opioid dependence (Fig. 5B). As an example, single-subject parameter estimates depicting the decrease in functional connectivity with respect to the duration of prescription opioid dependence are given for the laterobasal amygdala and anterior cingulate interaction. The functional connectivity involving the nucleus accumbens was either strongly correlated (a longer duration of prescription opioid dependence yielded higher functional connectivity) with some structures [i.e. anterior cingulate and orbital (mid) frontal] or anti-correlated (i.e. anterior insula and inferior parietal) (Fig. 6B). In functional connectivity group analysis specific to the nucleus accumbens, opioid-dependent subjects with the shortest duration of prescription opioid dependence had dissimilar connectivity strengths to the mean connectivity strength for controls. This is true for the following functional interactions: (i) nucleus accumbens–anterior cingulate, (ii) nucleus accumbens–orbital mid-frontal and (iii) nucleus accumbens–anterior insula and nucleus accumbens–inferior parietal (Fig. 6B). An overlap in mean parameter estimate values between the prescription opioid-dependent and healthy control cohorts would lead to an insignificant difference in a group-level comparison. Thus, significant increases or decreases in function connectivity in the prescription-opioid group (Fig. 6A) were not observed in the specific regions shown in Fig. 6B.
Negative control seed region (precentral gyrus)
Using the precentral gyrus as a negative control seed region, the resulting functional connectivity between the prescription opioid-dependent and healthy control groups was not significantly different (Supplementary Fig. 3). To show this lack of significant difference, within group-average statistical maps for the prescription opioid dependent and healthy control groups are each given. It is noted, however, that the amygdala in the negative control functional connectivity analysis had less functional connectivity in the prescription opioid-dependent group than in healthy control group. This is further evidence that normal functional connectivity of the amygdala is compromised in the prescription opioid-dependent subjects. The functional connectivity results (group-average results) using the laterobasal amygdala as a seed region are given as a comparison for the precentral gyrus functional connectivity results. The difference in functional connectivity between the two groups is much greater in the case where the laterobasal amygdala was used as a seed region.
Dependence between structural and functional connectivity changes in amygdala
The above results suggest that the structural (volume and white matter FA) and functional connectivity properties of the amygdala are compromised in as a result of prescription opioid dependence. Therefore, we aimed to integrate these findings to define whether or not there were dependencies between the observed structural and functional alterations.
It is noted that volumetric analysis was performed on the entire amygdala structure. Thus, additional functional connectivity analysis was performed such that the whole anatomically defined amygdala volume (defined by Anatomical Automatic Labelling atlas) was used as the seed region (Supplementary Fig. 4). A great deal of overlap was observed between results shown in Fig. 5A and Supplementary Fig. 4. The resulting parameter estimates obtained from this latter analysis were correlated with subject-specific amygdala volumes from the opioid-dependent subject group. A significant (P < 0.05, corrected) dependence between amygdala volume and functional connectivity between the amygdala, and structures (i.e. inferior orbital frontal cortex and nucleus accumbens) that are connected to the amygdala, was detected (Fig. 7A). Specifically, the smaller the amygdala volume, the lower the functional connectivity between two structures (i.e. amygdala–inferior orbital frontal cortex and amygdala–nucleus accumbens).
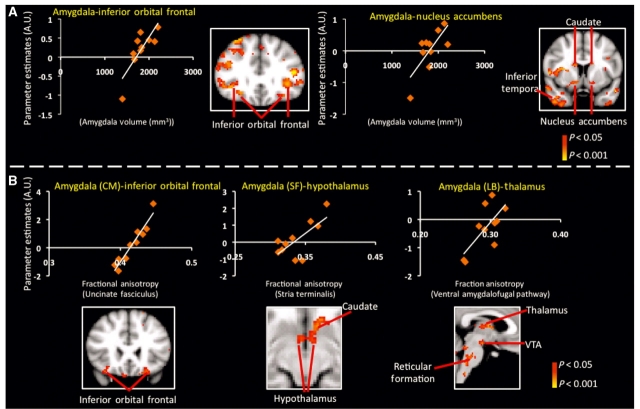
Figure 7.
Dependence between structural and functional connectivity changes in amygdala in prescription opioid-dependent patients. (A) Dependence between amygdala volume and amygdala functional connectivity: significant (P < 0.05, corrected) dependence was detected between amygdala volume and functional connectivity between the amygdala and structures that are connected to the amygdala (i.e. inferior orbital frontal cortex and nucleus accumbens). These structures are part of the reward/addiction circuitry. The entire amygdala volume was used as the seed region in additional functional connectivity analysis. The mixed-effects paired comparison (corrected for multiple comparison) results are given in Supplementary Fig. 4. The parameter estimates obtained from this latter analysis were correlated with subject specific amygdala volumes from the opioid-dependent subject group. (B) Dependence between white matter FA in amygdala pathways and amygdala functional connectivity: significant (P < 0.05, corrected) dependence between white matter integrity as defined by FA of efferent and afferent pathways of the amygdala and functional connectivity of the amygdala subdivisions was observed. Lower functional connectivity values between the amygdala subdivision (e.g. superficial amygdala) and structures that they have structural connectivity with (e.g. hypothalamus) were associated with lower FA of the axonal pathways that connect the two structures (e.g. stria terminalis).
The relationship between functional connectivity of the amygdala subdivision and white matter integrity as defined by FA of efferent and afferent pathways of the amygdala was also sought (Fig. 7B). As described in the Supplementary material, the stria terminalis connects the superficial amygdala and hypothalamus, while the unciuate fasciculus unites the amygdala (as well as hippocampus) and inferior orbital frontal cortex. The laterobasal amygdala is connected to the thalamus via the ventral amygdalofugal pathway. Also, we find that lower functional connectivity between the centromedial amygdala and inferior orbital frontal cortex indicates lower FA of the uncinuate fasciculus (P < 0.05, corrected). We also observed that the lower the functional connectivity between the superficial amygdala and hypothalamus the lower FA of the stria terminalis (P < 0.05, corrected). Lastly, we observed that the lower the functional connectivity between the laterobasal amygdala and thalamus the lower the FA of the ventral amygdalofugal pathway (P < 0.05, corrected). Other regions labelled also possessed functional interactions that were either dependent on amygdala volume (Fig. 7A) or anisotropy of efferent and afferent pathways of the amygdala (Fig. 7B).
Discussion
Summary of results
Ten highly screened prescription opioid-dependent patients (i.e. met strict inclusion criteria including no psychiatric or medical comorbiditiy and no pain; Fig. 1) and 10 matched control subjects were enrolled into the current study. The prescription opioid-dependent cohort demonstrated significant volumetric changes, white matter tract abnormalities and alterations in functional connectivity. Bilateral volumetric loss in the amygdala was observed in the prescription opioid-dependent subject group in comparison to the control subjects. Changes were also observed in white matter; opioid-dependent subjects showed significantly decreased anisotropy in axonal pathways such as the internal and external capsules as well as efferent and afferent pathways of the amygdala (Fig. 3). In comparison to healthy controls, significant functional connectivity decreases in prescription opioid-dependent subjects were found in the brain networks involving the anterior insula, amygdala (three subdivisions) and nucleus accumbens (Figs 4–6A). The functional connectivity was observed to be either significantly correlated or anti-correlated with the duration of prescription opioid dependence (Figs 4–6B). Lastly, the decreases in amygdala volume and decreases in anisotropy of amygdala-specific white matter pathways were correlated with functional connectivity involving the three-amygdala subdivisions we evaluated (Fig. 7).
The current cross-section study examined patients after they had become dependent on prescription opioids. However, it was not possible to determine the presence or absence of a pre-existing structural or functional condition in the brain that may have caused the dependence of the prescription opioid-dependent subjects. Psychiatric evaluations including a psychiatric history conducted by a physician and the Composite International Diagnostic Interview performed during patient recruitment suggested that these subjects did not have pre-morbid or active co-morbid psychiatric conditions. However, other factors (e.g. genetic) may have predisposed these subjects to the effects of opioids on brain morphology and connectivity, a conundrum that is common in cross-sectional designs (Supplementary material ‘Caveats’). Notwithstanding these concerns, the present work indicates that the brains of prescription opioid dependents are significantly different from matched healthy controls from both a functional and structural standpoint. In support of direct drug effects, functional changes observed were dependent on the duration of prescription opioid use. Moreover, there was a significant dependence between functional connectivity changes and structural changes.
An obvious limitation of the cross-sectional design employed on the present study is in its inability to resolve conclusively the pre-existing versus acquired nature of the observed alterations. Conclusive resolution of this issue may require prospective and/or twin studies. Notwithstanding this limitation, the present work indicates that the brains of patients with prescription opioid dependence are significantly different from the matched healthy controls from both functional and structural standpoints. However, the observed functional connectivity changes were significantly correlated with the duration of prescription opioid dependence (Figs 4B, 5B and 6B), while there was a significant correlation between functional connectivity changes and structural changes. Although such correlation does not prove direct causality, it is compatible with a causal model in which the use of prescription opioids causes cumulative anatomical–functional changes.
Alterations in morphological properties in individuals with prescription opioid dependence
Results show significant and bilateral decreases in the amygdala volume in opioid-dependent individuals versus controls (Fig. 2). The significantly smaller amygdala volumes observed for prescription opioid dependence have also been reported for cocaine and alcohol dependence (Makris et al., 2004; Wrase et al., 2008). Furthermore, antipsychotic drugs have also been observed to alter brain volume (Moncrieff and Leo, 2010). Opioids are specifically known to decrease dendritic spine density, and this decrease could explain the smaller amygdala volumes (Liao et al., 2005, 2007). In the current study, the prescription opioids used composed of mu- and kappa- opioid receptor agonists, shown to produce cortical and sub-cortical dendritic atrophy in non-human primates (Heath et al., 1984).
We are unaware of any prior reports of decreased amygdala volume within an opioid-dependent patient population. The amygdala has been considered as a key brain system involved in addiction (Koob, 2009) and is likely to be involved in drug-associated memory and drug seeking behaviours (Milton et al., 2008). The amygdala is involved in encoding emotional memories and becomes maladaptive following repeated stress that may include withdrawal states or anxiety related to drug seeking (Koob, 2009). In parallel with these volumetric changes, we also observed changes in the efferent and afferent pathways of the amygdala as well as robust functional connectivity changes between the amygdala and other brain structures (see below). Taken together, these changes implicate altered afferent and efferent information processing of the amygdala.
Decreases in white matter FA were observed in a number of pathways, including neuronal circuits involving the amygdala, nucleus accumbens and the insula. Three major pathways (stria terminalis, ventral amygdalofugal and uncinuate fasciculus) that connect the amygdala with other subcortical and cortical structures, including the nucleus accumbens and insula (see details on pathways in the Supplementary material), were observed to have decreased FA. These amygdala-related alterations may, in part, facilitate vulnerability to risky behaviours (Love et al., 2009). In addition, other white matter tracts (external capsule, internal capsule, anterior thalamic radiation and genu and anterior mid-body regions of the corpus callosum) were also noted to have significant bilateral decreases in FA in the opioid-dependent group (see details on pathways in the Supplementary materials).
Previous studies have reported on changes in white matter anisotropy in patients with alcohol dependence (Yeh et al., 2009), heroin dependence (Koussa et al., 2001) or chronic marijuana use (Arnone et al., 2008). The white matter changes observed in the present study parallel these previous studies of drug dependence, implicating changes in common pathways. The effects of prescription opioid dependence on white matter tract integrity may be attributed to a direct deleterious effect of opioids on myelin or other axonal membrane properties (e.g. membrane thickness, axonal diameter) (Tscherne and Wippermann, 1989). Given that cell types such as oligodendrocytes express opioid receptors and blocking opioid receptor activity alters myelin production, it is quite plausible that chronic opioid exposure would induce changes in white matter integrity (Foote, 1987; Persson et al., 2003). Alternatively, long-term hypo- or hyperactivity and hypo- or hypertrophy within neuronal circuits (related to metabolic alterations in the neurons) involved in drug addiction may lead to grey matter changes, which in turn affect white matter tracts (Gilbertson et al., 2002; Roozendaal et al., 2009).
Alterations in functional connectivity in subjects dependent upon prescription opioids
Significant decreases in functional connectivity in cortical (insula) and subcortical (amygdala and nucleus accumbens) structures in the opioid-dependent group were observed. We hypothesized that the insula would show functional differences between the two groups of subjects. We derived this hypothesis based on the recently uncovered key role played by the insula in maintaining smoking addiction—perhaps by mediating conscious urges and emotional decision making (Naqvi et al., 2007; Geha et al., 2008). The structure has been implicated in craving for multiple drugs of abuse (Naqvi et al., 2007; Naqvi and Bechara, 2009). In addition, the insula is a region involved in sensory and emotional processing, including interoceptive processing (Block et al., 1992). Significantly decreased functional connectivity was observed between the anterior insula and both the anterior and middle cingulate cortices as well as the putamen in the opioid-dependent cohort (Fig. 4A). The two systems of insular-cingulate resting-state connectivity have previously been reported. It has been postulated that the two systems integrate interoceptive information with emotional salience as well as awareness (Taylor et al., 2008). The disrupted connectivity observed in this study in the opioid-dependent subject group between the insula and frontal regions may also underlie factors in interoceptive and cognitive processing (Gray and Critchley, 2007; Goldstein et al., 2009).
The functional connectivity analysis indicated a significant decrease in connectivity with the three amygdala subdivisions—centromedial, laterobasal and superficial—to cortical (superior frontal and orbital frontal cortices), subcortical (striatum and thalamus) and brainstem (reticular formation and periaqueductal grey matter) (Fig. 5) structures. The amygdala is considered to play a vital role in addiction, including motivational withdrawal and emotional memories (Koob, 2009). As such, the data may reflect diminished function, which is related to the failure of counter-adaptive processes that are part of an anti-reward system (Koob and Le Moal, 2008). Decreased connectivity to the striatum, including the nucleus accumbens, orbital frontal regions and the periaqueductal grey matter (all part of the classic reward system), were observed. Additionally, the laterobasal amygdala is known to have connections with the nucleus accumbens and is considered to be involved in emotional responses including stress (e.g. withdrawal) and memories relating to these emotions (McGaugh, 2004).
The nucleus accumbens is part of the classic reward circuitry and is involved in reward-seeking behaviour (Carlezon and Thomas, 2009). It is involved in discrimination of the motivational value of conditioned stimuli and in predetermining behaviour for unconditioned stimuli, including repeated use of addictive drugs (Meredith et al., 2008). In functional connectivity analysis (Fig. 6), we observed a decrease in connectivity in patients compared with controls in a number of regions known to have connections with the nucleus accumbens (Zahm, 1999). Functional connectivity with regions involved in cognitive and decision-making processes (superior and orbital frontal cortices), emotion (amygdala, hippocampus), affective processing (mid-cingulate) and brainstem (periaqueductal grey matter) were altered in opioid-dependent patients. Thus, modification in functional connectivity with specific regions may have significant consequences; for example, altered expected outcomes or inappropriate decision making as a result of addictive drug use (Schoenbaum et al., 2006). Further included in the discussion on nucleus accumbens connectivity within the prescription opioid cohort can be found within the Supplementary material.
The anterior insula (Fig. 4B), laterobasal amygdala (Fig. 5B) and nucleus accumbens (Fig. 6B) each displayed changes in functional connectivity with specific structures that were correlated or anti-correlated with the duration of prescription opioid dependence. Some functional interactions, such as the anterior insula–putamen (Fig. 4B) or laterobasal amygdala–anterior cingulate (Fig. 5B) interaction showed that longer prescription opioid consumption lead to a decrease in functional connectivity. Furthermore, the prescription opioid-dependent subjects who consumed opioids for a short period of time (∼1 year) showed connectivity strengths in the same range as control subjects. However, for functional interactions pertaining to the nucleus accumbens–anterior cingulate or nucleus accumbens–orbital (mid) frontal connection (Fig. 6B), functional connectivity of subjects who consumed opioids for ∼1 year were distinct from healthy control subjects. The difference between parameter estimates of short-term (∼1 year) prescription opioid-dependent patients and healthy controls may be due to initial effects of prescription opioid exposure on specific functional circuits or connection (i.e. nucleus accumbens–anterior cingulate or nucleus accumbens–orbital mid-frontal). Upon more persistent consumption of prescription opioids over a longer period of time, the functional connectivity within specific functional circuits may increase or decrease. This phenomenon of adaptive or maladaptive functional properties of specific structures or circuitry is not unique to the present study. Similar alterations in other diseased states have been observed in disorders such as complex regional pain syndrome (Fukumoto et al., 1999).
Drug dependence is not an instantaneous event; instead, it involves changes in drug-associated behaviours over a period of time (i.e. drug seeking behaviour, risk taking behaviour and compulsive craving). Moreover, the motivation for using addictive drugs (prescription opioids, cocaine, heroin, etc) may change between the initial stages of drug dependence (strong desire to feel euphoria) to later stages of dependence (inability to perform normal day to day functions without the drug). Adaptation and sensitization can also occur over an extended period of time with respect to the neurobiology of drug dependence in structures that are part of the reward circuitry or mesolimbic pathway (Jones and Bonci, 2005; Kauer and Malenka, 2007). Thus, the functional connectivity properties can also change substantially as a function of duration of drug use can also change. Factors such as loss of normal inhibitory or excitatory input to a structure such as the amygdala or nucleus accumbens could determine how the connectivity changes upon continued prescription opioid consumption.
Overall, a decrease in functional connectivity was observed within the prescription opioid dependent cohort. Prescription opioids are known to disrupt normal cellular mechanisms such as inhibition of GABA mediated synaptic transmission (Vaughan et al., 1997) and are known to be CNS depressants (Becerra et al., 2006). Our data suggest that such effects of opioids over a long-term period are likely to underlie the decreased functional connectivity observed in the prescription opioid-dependent cohort. The structures implicated in our study are highly interconnected. Thus, it is not surprising to observe global decreases in functional connectivity amongst them as a result of a likely disruption of their normal functioning due to opioid abuse. More specifically, the functional alterations involving the amygdala and nucleus accumbens were also associated with alterations at the level of other structures, often thought as playing important executive functions (e.g. anterior cingulate), and which entertain mutual relationships of either up or down-regulation with the amygdala and nucleus accumbens. Our results suggest a global effect of network disconnection among most structures that have been implicated in addiction at various levels. They suggest, in line with clinical data and neuropsychology of opiate abuse (Davis et al., 2002; Mintzer et al., 2005), that, through their putative widespread structural and functional effects, prescription opioids are likely to interfere causally, not only with normal reward and interoception, but also with normal decision-making processes and executive control of ones’ ability to resist consuming prescription opioids despite their known harmful potential. A more specific determination of which functional alterations ultimately may have caused changes in processes such as decision-making was beyond the scope of the present study. Future functional imaging studies that include risk behaviour or decision-making tasks will yield a better characterization of which cognitive processes(s) are modified in prescription opioid-dependent patients. Clearly, future research is needed before drawing further conclusions.
Structural and functional connectivity changes in amygdala
A key observation in the current investigation was the significant correlation between functional connectivity and morphological changes. Specifically, in prescription opioid-dependent patients, functional connectivity between the amygdala and the inferior orbital frontal cortex and the nucleus accumbens was significantly correlated with the amygdala volume (Fig. 7A). These two functional links may be clinically meaningful as they play a crucial role in mediating reward, motivation and addictive behaviours.
The functional connectivity of the amygdala was also significantly correlated with the FA of the three axonal pathways that project to and from the amygdala (Fig. 7B). The amygdala has specific subdivisions that are known to possess distinct functional roles (Aggleton, 1993). Moreover, each amygdala subdivision is connected to distinct subcortical and cortical structures via three efferent and afferent axonal pathways (see Supplementary material). In our study, we observed that the FA of the efferent and afferent pathways of the amygdala (i.e. uncinate fasciculus) was significantly correlated with the functional connectivity between the amygdala subdivision (i.e. centromedial amygdala) and a neuronal structure that the amygdala subdivision is structurally connected to (i.e. inferior orbital frontal cortex). The integrative aspect of the dependence between functional connectivity and morphological changes supports the idea that prescription opioids not only affect specific structures, but rather affect entire systems that mediate addiction, reward, motivation, awareness or interoception.
Conclusions
Significant morphological and functional changes were observed in the brains of those dependent upon prescription opioids. Given the high frequency of clinical prescription opioid use for chronic pain and high levels of abuse of these same drugs, there are potentially important implications of this study for understanding what effects prescription opioids may have on the human brain. Further segregation of opioid effects from the addiction effects per se may require study of non-dependent healthy subjects chronically exposed to prescription opioids as well as similar experiments in a preclinical setting using non-human primates. Provided the important public health policy implications of prescription opioid consumption, health policy makers and clinicians alike may adopt the use of structural and functional brain imaging indices as a meaningful marker guiding their therapeutic decisions and assigning their patients the proper level of care.
Funding
Supplemental Grant from National Institute on Drug Abuse U10DA015831 (R.W.); National Institute on Drug Abuse Grant K24 DA022288 (R.W.); L Herlands Fund to D.B. and L.B. (P.A.I.N. Group) and National Institute of Neurological Disorders and Stroke K24 NS064050 (D.B.).
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Glossary
Abbreviations
- FA
fractional anisotropy
- fMRI
functional magnetic resonance imaging
- GLM
General Linear Model
- MNI
Montréal Neurological Institute
- TBSS
tract-based spatial statistics
References
- Wilson PR, Caplan RA, Connis RT, Gilbert HC, Grigsby EJ, Haddox JD, et al. Practice guidelines for chronic pain management A report by the American Society of Anesthesiologists Task Force on Pain Management, Chronic Pain Section. Anesthesiology. 1997;86:995–1004. [PubMed] [Google Scholar]
- Policy OoNDC., editor. National Drug Control Strategy National Data Tables. Washington, DC: March 2004. [Google Scholar]
- Ackermann H, Riecker A. The contribution of the insula to motor aspects of speech production: a review and a hypothesis. Brain Lang. 2004;89:320–8. doi: 10.1016/S0093-934X(03)00347-X. [DOI] [PubMed] [Google Scholar]
- Aggleton JP. The amygdala. New York: Wiley-Liss; 1993. [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol. 2005;210:343–52. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Arnone D, Barrick TR, Chengappa S, Mackay CE, Clark CA, Abou-Saleh MT. Corpus callosum damage in heavy marijuana use: preliminary evidence from diffusion tensor tractography and tract-based spatial statistics. Neuroimage. 2008;41:1067–74. doi: 10.1016/j.neuroimage.2008.02.064. [DOI] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008;28:1398–403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L, Harter K, Gonzalez RG, Borsook D. Functional magnetic resonance imaging measures of the effects of morphine on central nervous system circuitry in opioid-naive healthy volunteers. Anesth Analg. 2006;103:208–16, table of contents. doi: 10.1213/01.ane.0000221457.71536.e0. [DOI] [PubMed] [Google Scholar]
- Befort K, Filliol D, Ghate A, Darcq E, Matifas A, Muller J, et al. Mu-opioid receptor activation induces transcriptional plasticity in the central extended amygdala. Eur J Neurosci. 2008;27:2973–84. doi: 10.1111/j.1460-9568.2008.06273.x. [DOI] [PubMed] [Google Scholar]
- Block CM, Mayo JA, Evans GH. Effects of the Nd:YAG dental laser on plasma-sprayed and hydroxyapatite-coated titanium dental implants: surface alteration and attempted sterilization. Int J Oral Maxillofac Implants. 1992;7:441–9. [PubMed] [Google Scholar]
- Blomfield J, Rush AR, Allars HM. Interrelationships between flow rate, amylase, calcium, sodium, potassium and inorganic phosphate in stimulated human parotid saliva. Arch Oral Biol. 1976;21:645–50. doi: 10.1016/0003-9969(76)90138-2. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(Suppl 1):122–32. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–38. [PubMed] [Google Scholar]
- Compton WM, Volkow ND. Abuse of prescription drugs and the risk of addiction. Drug Alcohol Depend. 2006;83(Suppl 1):S4–7. doi: 10.1016/j.drugalcdep.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Contet C, Kieffer BL, Befort K. Mu opioid receptor: a gateway to drug addiction. Curr Opin Neurobiol. 2004;14:370–8. doi: 10.1016/j.conb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Cunha-Oliveira T, Rego AC, Garrido J, Borges F, Macedo T, Oliveira CR. Street heroin induces mitochondrial dysfunction and apoptosis in rat cortical neurons. J Neurochem. 2007;101:543–54. doi: 10.1111/j.1471-4159.2006.04406.x. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Davis PE, Liddiard H, McMillan TM. Neuropsychological deficits and opiate abuse. Drug Alcohol Depend. 2002;67:105–8. doi: 10.1016/s0376-8716(02)00012-1. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Fenstermacher E, Salat DH, Wolk DA, Maguire RP, Desikan R, et al. Detection of cortical thickness correlates of cognitive performance: Reliability across MRI scan sessions, scanners, and field strengths. Neuroimage. 2008;39:10–8. doi: 10.1016/j.neuroimage.2007.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Foote RW. Fiber tracts that contain more opioid binding sites than gray matter does: a quantitative autoradiographic study in the guinea-pig. Neuroscience. 1987;20:109–16. doi: 10.1016/0306-4522(87)90007-8. [DOI] [PubMed] [Google Scholar]
- Fukumoto M, Ushida T, Zinchuk VS, Yamamoto H, Yoshida S. Contralateral thalamic perfusion in patients with reflex sympathetic dystrophy syndrome. Lancet. 1999;354:1790–1. doi: 10.1016/S0140-6736(99)03746-0. [DOI] [PubMed] [Google Scholar]
- Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008;60:570–81. doi: 10.1016/j.neuron.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–7. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, et al. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009;13:372–80. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MA, Critchley HD. Interoceptive basis to craving. Neuron. 2007;54:183–6. doi: 10.1016/j.neuron.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AJ, Logan JE, Toblin RL, Kaplan JA, Kraner JC, Bixler D, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613–20. doi: 10.1001/jama.2008.802. [DOI] [PubMed] [Google Scholar]
- Heath RG, Fitzjarrell AT, Walker CF. Kappa opiate receptor agonists: effects on behavior and on brain function and structure in rhesus monkeys. Biol Psychiatry. 1984;19:1045–74. [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jones S, Bonci A. Synaptic plasticity and drug addiction. Curr Opin Pharmacol. 2005;5:20–5. doi: 10.1016/j.coph.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–58. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The World Health Organization International Consortium in Psychiatric Epidemiology (ICPE): initial work and future directions – the NAPE Lecture 1998. Nordic Association for Psychiatric Epidemiology. Acta Psychiatr Scand. 1999;99:2–9. doi: 10.1111/j.1600-0447.1999.tb05378.x. [DOI] [PubMed] [Google Scholar]
- Klemenc S. Noscapine as an adulterant in illicit heroin samples. Forensic Sci Int. 2000;108:45–9. doi: 10.1016/s0379-0738(99)00201-7. [DOI] [PubMed] [Google Scholar]
- Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61–75. doi: 10.1016/j.brainres.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koussa S, Tamraz J, Nasnas R. Leucoencephalopathy after heroin inhalation. A case with partial regression of MRI lesions. J Neuroradiol. 2001;28:268–71. [PubMed] [Google Scholar]
- Langleben DD, Ruparel K, Elman I, Busch-Winokur S, Pratiwadi R, Loughead J, et al. Acute effect of methadone maintenance dose on brain FMRI response to heroin-related cues. Am J Psychiatry. 2008;165:390–4. doi: 10.1176/appi.ajp.2007.07010070. [DOI] [PubMed] [Google Scholar]
- Leppa M, Korvenoja A, Carlson S, Timonen P, Martinkauppi S, Ahonen J, et al. Acute opioid effects on human brain as revealed by functional magnetic resonance imaging. Neuroimage. 2006;31:661–9. doi: 10.1016/j.neuroimage.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Liao D, Lin H, Law PY, Loh HH. Mu-opioid receptors modulate the stability of dendritic spines. Proc Natl Acad Sci USA. 2005;102:1725–30. doi: 10.1073/pnas.0406797102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Grigoriants OO, Wang W, Wiens K, Loh HH, Law PY. Distinct effects of individual opioids on the morphology of spines depend upon the internalization of mu opioid receptors. Mol Cell Neurosci. 2007;35:456–69. doi: 10.1016/j.mcn.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liang J, Qin W, Tian J, Yuan K, Bai L, et al. Dysfunctional connectivity patterns in chronic heroin users: an fMRI study. Neurosci Lett. 2009;460:72–7. doi: 10.1016/j.neulet.2009.05.038. [DOI] [PubMed] [Google Scholar]
- Love TM, Stohler CS, Zubieta JK. Positron emission tomography measures of endogenous opioid neurotransmission and impulsiveness traits in humans. Arch Gen Psychiatry. 2009;66:1124–34. doi: 10.1001/archgenpsychiatry.2009.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F, Xi ZX, Wu G, Liu C, Gardner EL, Li SJ. Attenuation of brain response to heroin correlates with the reinstatement of heroin-seeking in rats by fMRI. Neuroimage. 2004;22:1328–35. doi: 10.1016/j.neuroimage.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Ma N, Liu Y, Li N, Wang CX, Zhang H, Jiang XF, et al. Addiction related alteration in resting-state brain connectivity. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Gasic GP, Seidman LJ, Goldstein JM, Gastfriend DR, Elman I, et al. Decreased absolute amygdala volume in cocaine addicts. Neuron. 2004;44:729–40. doi: 10.1016/j.neuron.2004.10.027. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J Nerv Ment Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Baldo BA, Andrezjewski ME, Kelley AE. The structural basis for mapping behavior onto the ventral striatum and its subdivisions. Brain Struct Funct. 2008;213:17–27. doi: 10.1007/s00429-008-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AL, Lee JL, Butler VJ, Gardner R, Everitt BJ. Intra-amygdala and systemic antagonism of NMDA receptors prevents the reconsolidation of drug-associated memory and impairs subsequently both novel and previously acquired drug-seeking behaviors. J Neurosci. 2008;28:8230–7. doi: 10.1523/JNEUROSCI.1723-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintzer MZ, Copersino ML, Stitzer ML. Opioid abuse and cognitive performance. Drug Alcohol Depend. 2005;78:225–30. doi: 10.1016/j.drugalcdep.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Moncrieff J, Leo J. A systematic review of the effects of antipsychotic drugs on brain volume. Psychol Med. 2010;20:1–14. doi: 10.1017/S0033291709992297. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, van Zijl PCM, Nagae-Poetscher LM. MRI Atlas of Human White Matter. Amsterdam: Elsevier; 2005. [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–4. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offiah C, Hall E. Heroin-induced leukoencephalopathy: characterization using MRI, diffusion-weighted imaging, and MR spectroscopy. Clin Radiol. 2008;63:146–52. doi: 10.1016/j.crad.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Pendse G, Borsook D, Becerra L. Enhanced false discovery rate using Gaussian mixture models for thresholding fMRI statistical maps. Neuroimage. 2009;47:231–61. doi: 10.1016/j.neuroimage.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson AI, Thorlin T, Bull C, Zarnegar P, Ekman R, Terenius L, et al. Mu- and delta-opioid receptor antagonists decrease proliferation and increase neurogenesis in cultures of rat adult hippocampal progenitors. Eur J Neurosci. 2003;17:1159–72. doi: 10.1046/j.1460-9568.2003.02538.x. [DOI] [PubMed] [Google Scholar]
- Pletcher MJ, Kertesz SG, Kohn MA, Gonzales R. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299:70–8. doi: 10.1001/jama.2007.64. [DOI] [PubMed] [Google Scholar]
- Pud D, Cohen D, Lawental E, Eisenberg E. Opioids and abnormal pain perception: New evidence from a study of chronic opioid addicts and healthy subjects. Drug Alcohol Depend. 2006;82:218–23. doi: 10.1016/j.drugalcdep.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Risser D, Uhl A, Oberndorfer F, Honigschnabl S, Stichenwirth M, Hirz R, et al. Is there a relationship between street heroin purity and drug-related emergencies and/or drug-related deaths? An analysis from Vienna, Austria. J Forensic Sci. 2007;52:1171–6. doi: 10.1111/j.1556-4029.2007.00507.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Morphine alters the structure of neurons in the nucleus accumbens and neocortex of rats. Synapse. 1999;33:160–2. doi: 10.1002/(SICI)1098-2396(199908)33:2<160::AID-SYN6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–33. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, et al. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–26. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan A, Molloy FM, Farrell MA, Hutchinson M. Fatal toxic leukoencephalopathy: clinical, radiological, and necropsy findings in two patients. J Neurol Neurosurg Psychiatry. 2005;76:1014–6. doi: 10.1136/jnnp.2004.047134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci. 2006;29:116–24. doi: 10.1016/j.tins.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 2007;26:518–29. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–75. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Seifert F, Kiefer G, DeCol R, Schmelz M, Maihofner C. Differential endogenous pain modulation in complex-regional pain syndrome. Brain. 2009;132(Pt 3):788–800. doi: 10.1093/brain/awn346. [DOI] [PubMed] [Google Scholar]
- Shabat-Simon M, Levy D, Amir A, Rehavi M, Zangen A. Dissociation between rewarding and psychomotor effects of opiates: differential roles for glutamate receptors within anterior and posterior portions of the ventral tegmental area. J Neurosci. 2008;28:8406–16. doi: 10.1523/JNEUROSCI.1958-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Stimmel B, Kreek MJ. Neurobiology of addictive behaviors and its relationship to methadone maintenance. Mt Sinai J Med. 2000;67:375–80. [PubMed] [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp. 2008;30:2731–45. doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tscherne H, Wippermann BW. [Current state of functional therapy. Definition and indications] Langenbecks Arch Chir Suppl II Verh Dtsch Ges Chir. 1989:465–8. [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Ingram SL, Connor MA, Christie MJ. How opioids inhibit GABA-mediated neurotransmission. Nature. 1997;390:611–4. doi: 10.1038/37610. [DOI] [PubMed] [Google Scholar]
- Wonderlick JS, Ziegler DA, Hosseini-Varnamkhasti P, Locascio JJ, Bakkour A, van der Kouwe A, et al. Reliability of MRI-derived cortical and subcortical morphometric measures: effects of pulse sequence, voxel geometry, and parallel imaging. Neuroimage. 2009;44:1324–33. doi: 10.1016/j.neuroimage.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Makris N, Braus DF, Mann K, Smolka MN, Kennedy DN, et al. Amygdala volume associated with alcohol abuse relapse and craving. Am J Psychiatry. 2008;165:1179–84. doi: 10.1176/appi.ajp.2008.07121877. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Wu G, Stein EA, Li SJ. Opiate tolerance by heroin self-administration: an fMRI study in rat. Magn Reson Med. 2004;52:108–14. doi: 10.1002/mrm.20119. [DOI] [PubMed] [Google Scholar]
- Yeh PH, Simpson K, Durazzo TC, Gazdzinski S, Meyerhoff DJ. Tract-Based Spatial Statistics (TBSS) of diffusion tensor imaging data in alcohol dependence: abnormalities of the motivational neurocircuitry. Psychiatry Res. 2009;173:22–30. doi: 10.1016/j.pscychresns.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS. Functional-anatomical implications of the nucleus accumbens core and shell subterritories. Ann NY Acad Sci. 1999;877:113–28. doi: 10.1111/j.1749-6632.1999.tb09264.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.