Abstract
A predominant theory regarding early stroke and its effect on language development, is that early left hemisphere lesions trigger compensatory processes that allow the right hemisphere to assume dominant language functions, and this is thought to underlie the near normal language development observed after early stroke. To test this theory, we used functional magnetic resonance imaging to examine brain activity during category fluency in participants who had sustained pre- or perinatal left hemisphere stroke (n = 25) and in neurologically normal siblings (n = 27). In typically developing children, performance of a category fluency task elicits strong involvement of left frontal and lateral temporal regions and a lesser involvement of right hemisphere structures. In our cohort of atypically developing participants with early stroke, expressive and receptive language skills correlated with activity in the same left inferior frontal regions that support language processing in neurologically normal children. This was true independent of either the amount of brain injury or the extent that the injury was located in classical cortical language processing areas. Participants with bilateral activation in left and right superior temporal-inferior parietal regions had better language function than those with either predominantly left- or right-sided unilateral activation. The advantage conferred by left inferior frontal and bilateral temporal involvement demonstrated in our study supports a strong predisposition for typical neural language organization, despite an intervening injury, and argues against models suggesting that the right hemisphere fully accommodates language function following early injury.
Keywords: stroke, development, plasticity, functional organization, language
Introduction
Adults who sustain damage to classic perisylvian language areas of the left hemisphere show considerable language impairment (Geschwind, 1971; Damasio, 1992; Benson and Ardila, 1996) with highly variable (Kertesz and McCabe, 1977; Lazar and Antoniello, 2008), but often, only at best, moderate recovery (Goodglass, 1993). On the contrary, it is well known that when injury is sustained during the pre- or perinatal period, children typically show remarkable plasticity for language function with low-normal to normal language outcomes (Freud, 1968; Woods and Teuber, 1978; Bates et al., 2001; Stiles et al., 2005). This sparing of function has been taken as evidence for the adaptive, highly plastic capabilities of the developing brain. Furthermore, behavioural studies of children with unilateral brain injury show early deficits in language production and comprehension that are specific to lesion location (Stiles et al., 2005). For example, children with left temporal injuries show the strongest impairments in initial vocabulary production, and children with right hemisphere injuries show the strongest impairments in initial vocabulary comprehension and gesture (Thal et al., 1991; Bates et al., 1997). These lesion site-specific deficits, which differ from those observed in adult patients, are no longer detectable by around age 5 years (Bates et al., 1997; Vicari et al., 2000), suggesting that the immature brain can compensate for such deficits in heterogeneous ways. Taken together, these observations have led some to suggest that children are born with some degree of hemispheric equipotentiality for language and that typical mature neural organization patterns gradually develop as language is acquired (Lashley, 1951; Lenneberg, 1967).
Functional brain imaging is a method that may contribute to understanding the development of neural organization following early injury, but thus far, relatively little research has been conducted using this methodology. Several such studies report either bilateral activity or increased activity in right hemisphere areas homotopic to anterior and posterior left hemisphere language regions (i.e. variously defined regions based on early observations by Broca (1861) and Wernicke (1874); Duncan et al., 1997; Müller et al., 1998, 1999; Booth et al., 1999; Papanicolaou et al., 2001; Staudt et al., 2001, 2002; Jacola et al., 2006; Fair et al., 2006). This evidence has lent credence to the predominant notion that the right hemisphere ‘takes over’ for functions typically processed in left hemisphere regions (Müller et al., 1998a, b, 1999; Hertz-Pannier et al., 2002; Staudt et al., 2002; Lidzba and Staudt, 2008). To support this theory, it has been suggested that children who experience an early injury do not undergo the increasing left lateralization of blood oxygen level dependent activity with age, which commonly occurs in typically developing children (Szaflarski et al., 2006). Thus, a continued bilateral representation for language has been proposed as a potential mechanism supporting recovery in children with early injury (Lidzba and Staudt, 2008). However, a few studies have shown that damage to the left hemisphere does not necessitate recruitment of right hemisphere homologues (Rasmussen and Milner, 1977; Liégeois et al., 2004). For example, using functional MRI, Liégeois and colleagues (2004) showed that even damage to Broca’s area in the left hemisphere did not lead to right lateralization of activity for a verb generation task. Thus, the imaging work to date leaves open the question of whether the right hemisphere has the potential to accommodate completely the maturation of language skills or whether involvement of typical cortical language regions is still an important component of language development, even after early injury. Furthermore, critical for the purposes of the present research, no study to date has directly linked the functional imaging findings to cognitive outcome after pre- or perinatal stroke, often because the sample sizes employed have been too small to capture adequately the variability of outcomes present in this population. In this work, we show for the first time a direct relationship between brain activity and individual language outcomes in people who sustained early brain injury.
Using functional MRI, we examined the brain’s response during a category fluency task (e.g. naming exemplars of animals) in a large sample of participants who had sustained left focal brain lesions as a result of stroke during the pre- or perinatal period (n = 25), as well as in a group of their neurologically intact siblings (n = 27). Category fluency is an ideal task with which to investigate lateralization of brain organization following early brain injury because it elicits a strong and consistent left hemisphere predominance in typical children, with characteristically strong activation of the left relative to right inferior frontal gyrus and activation of left lateral temporal regions (Petersen et al., 1988; Hertz-Pannier et al., 1997; Knecht et al., 2000; Holland et al., 2001; Szaflarski et al., 2006) with some involvement of right lateral temporal homologues (Brown et al., 2005; Szaflarski et al., 2006). We then related lateralization of brain activity to performance on both the category fluency task and to the results of a battery of measures of language performance. Based on previous functional imaging work, we expected right hemisphere activation to predominate in our brain injured cohort and left hemisphere activation to predominate in their siblings. Thus, we first aimed to replicate this finding. Second, we aimed to assess the extent to which this right or left hemisphere activation predicts language outcome in our brain injured cohort, testing the hypothesis that increased right lateralization would correlate with better language functioning, and that other patterns such as increased left lateralization or lack of lateralization would predict worse outcome, as putative right hemisphere compensatory activity would be lower.
Materials and methods
Participants
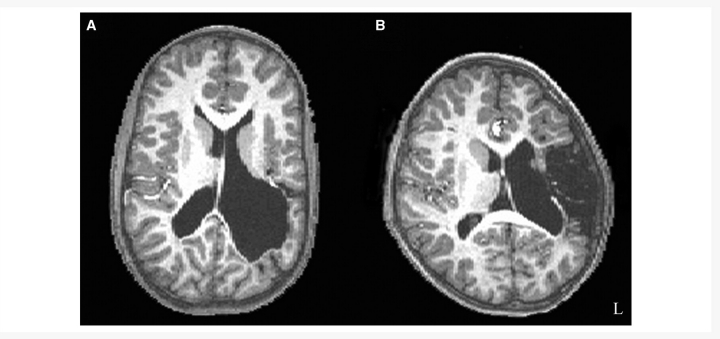
Twenty-five participants (mean age = 14 years 4 months; SD = 6 years 9 months) with pre- or perinatal left hemisphere lesions were recruited through local and national support groups for childhood ‘hemiplegia’ (hemiparesis) as well as by referrals from area neurologists. Patients were recruited irrespective of race, gender, degree of hemiparesis or lesion site. All participants had suffered unilateral brain damage, primarily due to pre- or perinatal stroke, where perinatal stroke is defined as occurring between the 20th week of gestation and the 28th postnatal day. This consensus definition was developed jointly at a workshop sponsored by the National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke of the National Institutes of Health of the United States (Raju et al., 2007). The aetiology of each participant’s lesion was determined by a paediatric neurologist (P.R.H.) as having occurred due to periventricular haemorrhagic (venous) infarction (‘periventricular’; n = 11) or ischaemic infarction of the middle cerebral artery (‘vascular’; n = 14) (Kirton et al., 2008). Periventricular haemorrhagic infarctions predominantly affect white matter, just dorsal and lateral to the external angle of the lateral ventricle (Inder and Volpe, 2000) and tend to occur in the early third trimester of gestation; whereas ischaemic infarctions occur more often in the late third trimester (Staudt et al., 2004). Table 1 shows patient demographics and lesion aetiology, and Fig. 1 gives a representative structural image of a periventricular and vascular case. We excluded cases where brain injury was due to congenital malformations. Twenty-seven healthy sibling controls (mean age = 16 years 3 months; SD = 7 years 11 months) also participated in the study. For participants under the age of eighteen, written informed consent was obtained from a parent or guardian, and the child gave verbal assent. Written informed consent was obtained for adult participants. The Institutional Review Board of the Biological Sciences Division of The University of Chicago approved the study.
Table 1.
Patient demographics and lesion aetiology
| Participant number | Sex | Age at study | Aetiology | Lesion volume (mm3) | Percentage damage to left ROIs |
|---|---|---|---|---|---|
| 103 | F | 19 y 1 m | Vascular | 79 087 | 37 |
| 104 | F | 10 y 9 m | Vascular | 45 239 | 40 |
| 107 | F | 28 y 4 m | Periventricular | 2399 | 0 |
| 108 | M | 26 y 6 m | Vascular | 114 868 | 75 |
| 113 | F | 10 y 11 m | Periventricular | 40 176 | 1 |
| 114 | F | 29 y 10 m | Periventricular | 11 122 | 0 |
| 115 | F | 9 y 9 m | Vascular | 88 899 | 55 |
| 117 | M | 10 y 5 m | Vascular | 5257 | 2 |
| 119 | M | 10 y 7 m | Periventricular | 9761 | 0 |
| 121 | M | 10 y 5 m | Vascular | 80 087 | 68 |
| 124 | F | 18 y 10 m | Vascular | 3443 | 1 |
| 125 | M | 8 y 6 m | Vascular | 62 711 | 43 |
| 128 | M | 11 y 4 m | Vascular | 81 597 | 62 |
| 130 | F | 13 y 7 m | Periventricular | 1153 | 0 |
| 132 | F | 12 y 0 m | Vascular | 839 | 0 |
| 133 | F | 16 y 11 m | Periventricular | 5023 | 0 |
| 134 | F | 7 y 2 m | Periventricular | 37 435 | 1 |
| 135 | F | 18 y 2 m | Vascular | 420 | 0 |
| 136 | M | 14 y 5 m | Periventricular | 48 816 | 0 |
| 137 | M | 13 y 11 m | Periventricular | 61 621 | 21 |
| 143 | M | 7 y 7 m | Vascular | 4502 | 0 |
| 145 | M | 11 y 1 m | Periventricular | 114 090 | 14 |
| 147 | M | 7 y 7 m | Vascular | 88 | 0 |
| 150 | M | 7 y 7 m | Periventricular | 4 | 0 |
| 156 | F | 23 y 8 m | Vascular | 24 735 | 12 |
ROIs = regions of interest; y = years; m = months.
Figure 1.
T1 structural images. (A) A representative participant with periventricular left hemisphere injury. (B) A representative participant with vascular left hemisphere injury. The lesion sizes are approximately equivalent for these two cases.
Behavioural measures
All participants underwent a series of behavioural tests outside the scanner to measure language function. Participants were given assessments of verbal intelligence quotient [Wechsler Intelligence Scale for Children, third edition for children ages 6–16 years (Wechsler, 1974) and the Wechsler Adult Intelligence Scale, third edition for children ages 16 years and older (Wechsler, 1997)], expressive vocabulary (Expressive Vocabulary Test) (Williams, 1997), receptive vocabulary (Peabody Picture Vocabulary Test, Third edition) (Dunn et al., 1997), and expressive and receptive language scores (Clinical Evaluation of Language Fundamentals, Third edition) (Semel et al., 1995). The standardized scores from each of these tests were used as the language measures for each participant. Exploratory analysis revealed strong correlations among the measures. We therefore conducted a principal components analysis on all scores on the language tests. This analysis revealed that all assessments loaded on a single factor representing overall language functioning (referred to as ‘global language score’), and thus we used the factor scores as the outcome measure for language function. Notably, we also analysed the relationship between brain activity and performance on each of the individual language tests that comprise the global language score, and these results, which show the same pattern as the global language score, are included as Supplementary material. As part of this assessment outside the scanner, participants also performed an overt category fluency task in which they had to generate exemplars of a category out loud (e.g. if the category was ‘Animals’, the participant would say ‘dogs’, ‘cats’, ‘chinchillas’, etc.) in order to obtain a behavioural measure of fluency. The behavioural measure was the average number of generated exemplars across four trials that were each 30 s long. Due to equipment error, two siblings only had data recorded across three trials, and the average was taken for these trials only. Performance on the global language score and the category generation measure was compared between: (i) patient and sibling control groups; and (ii) between periventricular and vascular patient groups.
Functional MRI
In the scanner, participants repeated the category fluency task from the behavioural portion except that exemplars were generated covertly in a standard block design paradigm and different categories were presented. The task was designed to be child-friendly to accommodate the younger participants, and was described to the participants as the ‘red light/green light game’. During the task blocks, participants were visually presented with an image of a green stoplight, which they viewed on a projection screen through a mirror. At the start of each block, participants heard the category name through MRI-compatible headphones and were asked to generate covertly examples of that category. During the rest blocks, participants were shown an image of a red stoplight and, prior to scanning, were told to concentrate on their breathing. Participants also performed a story comprehension task, which was not analysed as part of this study.
T1-weighted volumetric in-plane images (124 axial slices, 1.5 mm × 0.938 mm × 0.938 mm resolution) were collected at 3 Tesla in a GE Signa scanner. These images provided the anatomical landmarks on which to superimpose the functional data. T2* gradient echo functional images were acquired in the sagittal plane using spiral acquisition (Noll et al., 1995) with repetition time = 2000 ms, echo time = 25 ms, flip angle = 30°, 3.75 mm × 3.75 mm × 5 mm voxel size. There were 30 slices covering the whole brain. Functional imaging data were analysed with the Analysis of Functional Neuroimaging software package (Cox, 1996) using multiple linear regression. Regressors were waveforms with similarity to the haemodynamic response, generated by convolving a gamma-variant function with the onset time and duration of the blocks of interest. We also included six regressors for motion parameters derived from the spatial registration procedure. For each voxel, output of the regression included a beta estimate, corresponding to percent signal change, and a t-statistic assessing the reliability of the beta estimate. Functional data were spatially smoothed with a 6 mm Gaussian full width at half maximum kernel. Active voxels were clustered using Monte Carlo simulation (Analysis of Functional Neuroimaging program AlphaSim) specifying an individual voxel probability of P < 0.001 to determine a family-wise error rate of P < 0.05.
Regions of interest
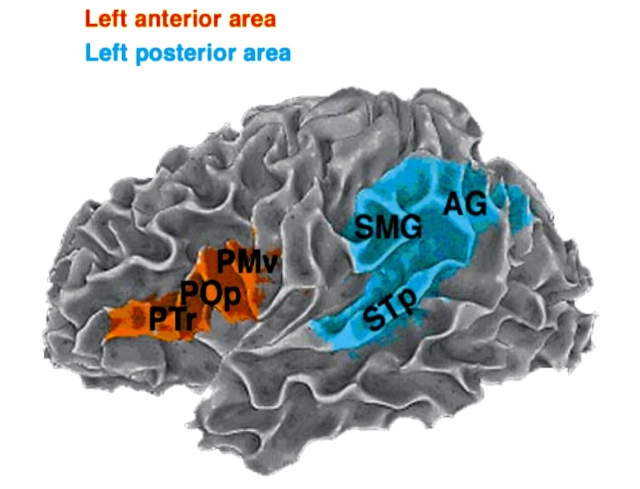
Due to the fact that anatomical lesions can cause distortion during spatial normalization (Crinion et al., 2007), images were neither co-registered nor converted to stereotaxic space, and instead an anatomical region of interest approach was used. Regions were defined a priori and drawn manually on the T1-weighted structural image and checked by a trained anatomist (A.S.). Two traditional language regions were identified in each hemisphere. The anterior language region consisted of pars triangularis and pars opercularis of the inferior frontal gyrus and the ventral premotor region of the precentral gyrus and sulcus. The posterior language region consisted of posterior superior temporal gyrus and sulcus, supramarginal gyrus and angular gyrus (see the surface representation with the pial surface removed for better representation of the sulci on an exemplary subject in Fig. 2). When anatomical landmarks normally used to identify the region were not present due to the missing tissue in the area of the lesion, the characteristics of the homotopic region from the opposite hemisphere and other anatomical landmarks from both hemispheres were used to help demarcate the desired region in the injured hemisphere.
Figure 2.
Regions of interest encompassing areas traditionally associated with language processing in a representative subject. This image shows the white matter surface representation, with the pial surface removed for better representation of sulci. PTr = pars triangularis; POp = pars opercularis; PMv = ventral premotor region; SMG = supramarginal gyrus; AG = angular gyrus; STp = posterior superior temporal gyrus and sulcus.
Laterality index
A laterality index (LI) was computed for active voxels separately within anterior and posterior language regions. LI was defined as the number of active voxels in the left hemisphere region minus number of active voxels in the right hemisphere region divided by the total number of active voxels:
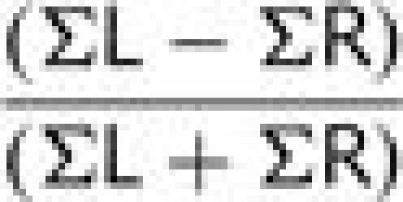 |
This formula generated values of laterality ranging from −1 (maximally right lateralized with no left-sided activity) to 1 (maximally left lateralized with no right-sided activity). LIs close to zero (i.e. LI < 0.1) were considered to represent a bilateral pattern activity (based on the method of Holland et al. (2001); e.g. a subject with LI > 0.1 was categorized as left lateralized, while a subject with LI < −0.1 was categorized as right lateralized). An anterior LI could not be computed for six early injury participants because there were no active voxels that survived the cluster correction in either left or right anterior regions. Similarly, a posterior LI could not be computed for six early injury participants and two siblings because there were no active voxels in either left or right posterior regions.
LIs were compared separately in anterior and posterior regions (i) patient and sibling control groups; and (ii) between periventricular and vascular patient groups using independent-samples t-tests.
Statistical analyses
The relationships between patterns of laterality and global language performance as well as category fluency score were examined by comparing the individual anterior LIs for members of each participant group to each behavioural measure and the individual posterior LIs in each participant group to each behavioural measure using partial correlations. In the first partial correlation assessing the relationship between laterality and behaviour, lesion size was entered as the control variable and partialled out. In the second partial correlation assessing the relationship between laterality and behaviour, the extent of damage affecting language-related regions was entered as the control variable and partialled out. Lesion size and fraction of language areas damaged were highly correlated (r = 0.81), and thus were partialled out separately to avoid multicollinearity. Finally, we statistically verified the observed quadratic relationship between the posterior LIs and behaviour by squaring each subject’s posterior LI and correlating this output with behavioural scores.
Determination of lesion size and extent of language area damage
In order to determine lesion size, brain lesions were traced on the T1 structural images and checked by a trained anatomist familiar with MRI of brains with injury (A.S.). The size of the lesion was calculated by counting the number of voxels within the lesion. The fraction of language areas damaged was determined by computing the overlap of the brain lesion with both the left anterior and left posterior language regions of interest. This number was entered as the control variable in the partial correlation. Lesion size and the fraction of damage affecting the left regions of interest were also correlated with behavioural outcome measures. Table 1 gives lesion volumes (mm3) for each subject as well as the percentage of damage to the left regions of interest. Lesion size and the percent damage to the left regions of interest were compared between patients with each type of lesion (periventricular or vascular).
Results
Comparisons of patients and sibling controls on behavioural measures showed that siblings performed significantly better on our global language measure [t(50) = 3.15, P < 0.01; Cohen’s d = 0.85, observed power = 0.87] and category fluency measure [t(50) = 2.63, P < 0.05; Cohen’s d = 0.72, observed power = 0.73]. However, the patients performed within low-normal levels, based on the normative values for each of the individual language tests that comprise the global language score. These data are shown in Table 2 as standardized scores (mean = 100, SD = 15). These results are consistent with other findings of language functioning after early lesions (Levine et al., 1987; Reilly et al., 1998; Rowe et al., 2009). Comparisons between patients with either type of injury (periventricular or vascular) revealed no significant differences in performance on either the global language score [t(23) = −0.68, P > 0.05] or category fluency [t(23) = −0.03, P > 0.05].
Table 2.
Group performance on language measures
| Language measure |
Sibling controls |
Early left injury |
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Global language score tests | ||||
| Peabody Picture Vocabulary Test | 103.5 | 14.5 | 94.8 | 16.6 |
| Expressive Vocabulary Test | 101.3 | 12.8 | 86.6 | 20.2 |
| Verbal intelligence quotient | 105.6 | 14.0 | 90.5 | 20.8 |
| Receptive language | 98.6 | 20.1 | 84.2 | 21.1 |
| Expressive language | 97.1 | 12.5 | 84.2 | 21.8 |
| Category fluency task | 11.4 | 2.6 | 9.3 | 3.2 |
Lesion size was not significantly associated with either performance on the global language score [r(23) = −0.17, P > 0.05] or the category fluency measure [r(23) = −0.2, P > 0.05]. The extent to which the lesion damaged anterior and posterior regions of interest was also not significantly associated with performance on the global language score [r(23) = −0.31, P > 0.05] or category fluency [r(23) = −0.23, P > 0.05]. Comparisons between patients with either type of injury (periventricular or vascular) revealed no significant differences in lesion size [t(23) = 0.77, P > 0.05]. In this sample, though, people with vascular injuries showed more damage to the left hemisphere regions of interest [t(23) = 2.764, P < 0.05].
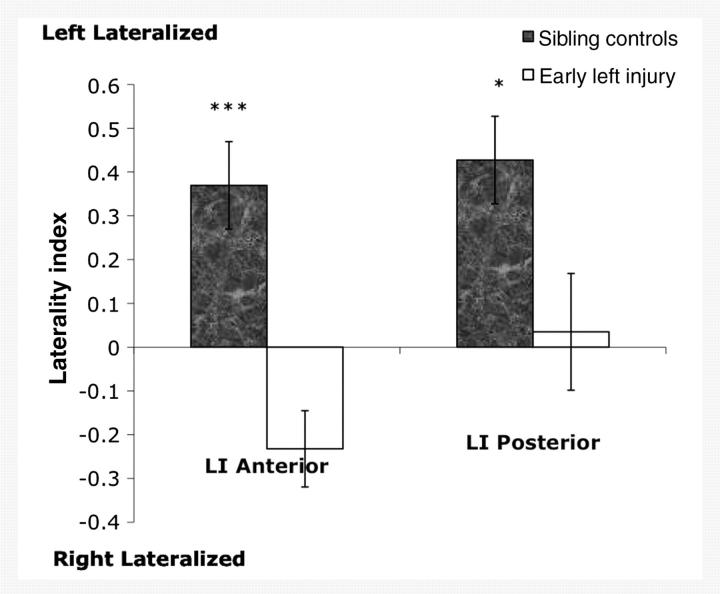
The functional imaging findings for healthy siblings replicated prior work showing left lateralization of activity in anterior (LI = 0.48, SD = 0.38) and posterior regions (LI = 0.50, SD = 0.45) during category fluency (Petersen et al., 1988; Hertz-Pannier et al., 1997; Knecht et al., 2000; Holland et al., 2001; Brown et al., 2005; Szaflarski et al., 2006). In contrast, patients with early injury showed a more right lateralized pattern of activity (LI = −0.23, SD = 0.76) in the anterior regions and a bilateral pattern of activity (LI = 0.03, SD = 0.76) in the posterior regions (Fig. 3). Direct comparison of the patient and control groups revealed a significant difference in lateralization in anterior regions [t(44) = 4.21, P < 0.001, Cohen’s d = 1.19, observed power = 0.984] and posterior regions [t(42) = 2.57, P < 0.05; Cohen’s d = 0.75, observed power = 0.709]. Degree of lateralization in all early injury participants was not significantly correlated with lesion size [anterior laterality r(17) = 0.28, P > 0.05; posterior laterality r(17) = 0.11, P > 0.05] or the extent to which the language regions were damaged [anterior laterality r(17) = −0.08, P > 0.05; posterior laterality r(17) = −0.13, P > 0.05]. Comparisons between patients with periventricular and vascular lesions revealed significantly more left lateralization in anterior regions for patients with periventricular lesions [t(17) = −2.953, P < 0.01], but no significant differences in posterior laterality [t(17) = −1.127, P > 0.05]. Participants who had no activation in either anterior region (n = 6 for early injury participants) or either posterior region (n = 6 for early injury participants; n = 2 for controls) were not included in this or subsequent analyses since it was not possible to compute a LI with a total of zero active voxels in the denominator of the equation.
Figure 3.
Laterality indices in anterior and posterior regions of interest. Sibling controls show a left lateralized pattern of activity in both regions. Participants with early left injury show a right lateralized pattern in anterior regions and a bilateral pattern in posterior regions. Laterality differences between patients and controls are significant in anterior and posterior regions. Error bars represent standard error of the mean. ***P < 0.001; *P < 0.05.
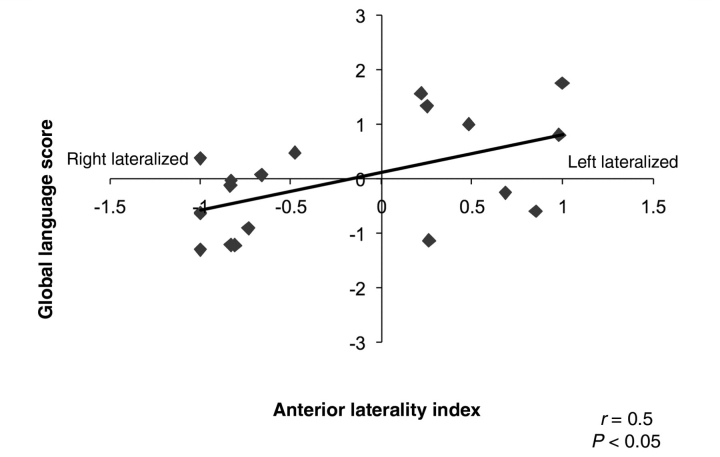
The main focus of this study was to relate LIs in anterior and posterior regions to language functioning. Here we controlled for the size of the lesion and the amount of damage affecting typical language regions. In the patient group, anterior laterality did not significantly correlate with category fluency score [r(16) = 0.37, P > 0.05 controlling for lesion size; r(16) = 0.32, P > 0.05 controlling for the fraction of language areas damaged; Fig. 4], but increased lateralization towards left anterior language regions was correlated with better global language functioning [r(16) = 0.55, P < 0.05 controlling for lesion size; r(16) = 0.54, P < 0.05 controlling for fraction of language areas damaged]. For siblings, we did not find a significant correlation between anterior laterality and language function or category fluency [r(25) = 0.09, P > 0.05 and r(25) = −0.24, P > 0.05 for global language functioning and category fluency, respectively].
Figure 4.
The relationship between laterality in anterior regions and language score in the early left injury group. Increasing left lateralization of activity is related to better overall language functioning. No significant relationship was found between laterality and behaviour in siblings. LI ranges from −1.0 (predominantly right lateralized) to +1.0 (predominantly left lateralized).
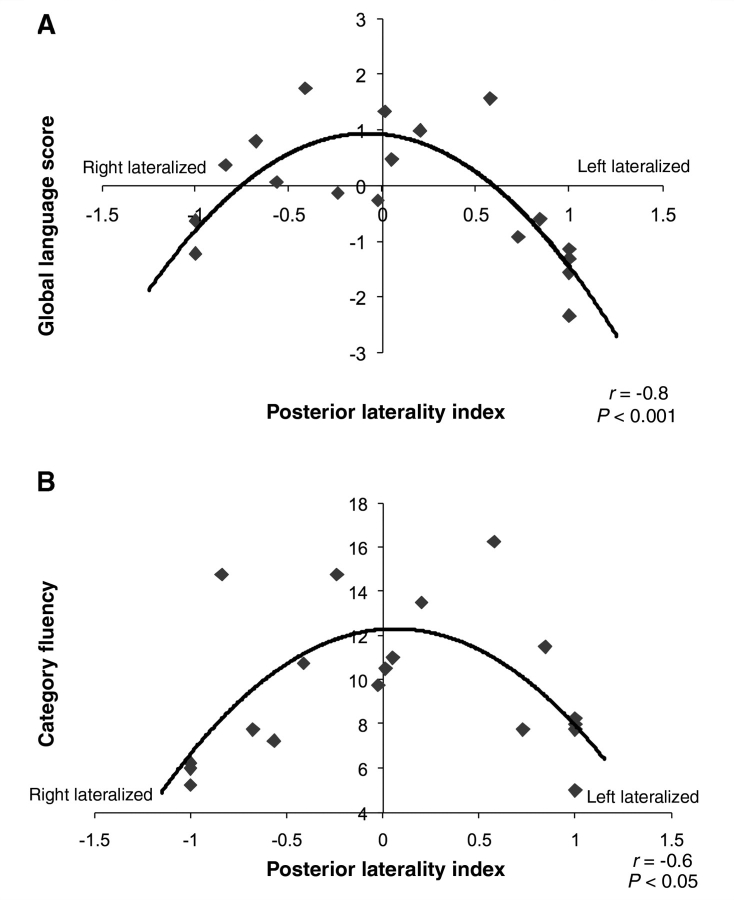
For posterior regions, visual inspection of the graph suggested a curvilinear relation between laterality and behavioural outcome for the participants with brain injury, and this was statistically verified. Laterality was curvilinearly related to both better global language functioning [r(16) = −0.77, P < 0.001 controlling for lesion size; r(16) = −0.76, P < 0.001 controlling for the fraction of language areas damaged] and category fluency [r(16) = −0.59, P < 0.01 controlling for lesion size; r(16) = −0.58, P < 0.05 controlling for the fraction of language areas damaged]. That is, a more even distribution of activity in left and right posterior regions correlated with better language scores, whereas either left- or right-sided dominance correlated with reduced performance (Fig. 5). We did not find a relationship between laterality in posterior regions and language functioning in siblings [r(23) = 0.07, P > 0.05 and r(23) = −0.24, P > 0.05] for either global language functioning or for category fluency, respectively. These results are corroborated by a recent study (Elkana et al., 2010), which also did not find a relationship between laterality and language function in neurologically normal adults.
Figure 5.
The relationship between laterality in posterior regions and behavioural measures in the early left injury group. (A) Balanced activity in both hemispheres is related to better overall language functioning compared to a predominant recruitment of either left or right hemisphere regions. (B) Similarly, balanced activity in both hemispheres is related to increased category generation ability. No significant relationship was found between laterality and behaviour in siblings. LI ranges from −1.0 (predominantly right lateralized) to +1.0 (predominantly left lateralized).
Discussion
Prior empirical work investigating the organization of language in the brain following early injury has shown, for verbal fluency tasks, a predominately right hemisphere, or in some cases, bilateral representation for language, which differs from the typical pattern of left hemisphere lateralization (Petersen et al., 1988; Hertz-Pannier et al., 1997; Knecht et al., 2000; Holland et al., 2001; Brown et al., 2005; Szaflarski et al., 2006). This has led to the suggestion that early left hemisphere lesions trigger compensatory processes that allow the right hemisphere to assume dominant language functions, which is proposed to mediate generally good language development in this population. In the present study, we replicated these findings showing left hemisphere lateralization in typical individuals and right hemisphere lateralization in individuals with early brain injury for anterior regions. For posterior language regions, we also found left hemisphere lateralization for typical individuals, but bilaterality in brain injured participants. At first glance, this would seem to support the current model suggesting some right hemisphere ‘take over’ of language function, which as noted, has been taken to be the mechanism underlying the improved language performance in these individuals. However, when we relate these patterns of brain activity to language outcome, the data tell a different story. That is, our results indicate that, despite an early left hemisphere injury, participants who have better language outcome show a functional organization for language that favours (i) left over right activity in frontal brain regions and (ii) a bilateral pattern of activity in right and left temporal-parietal regions. In particular, such a prominent involvement of left frontal regions despite early left hemisphere injury is supportive of an ontogenetic predisposition of these areas for the maturation of language functions.
Overall, the present results contradict the longstanding belief that language development after early injury takes place by a central mechanism of compensatory activity in the right hemisphere (Lidzba and Staudt, 2008). In contrast, participants with more complete right hemisphere lateralization in either frontal or temporal-parietal regions actually showed poorer language outcomes. Our results also do not support the idea that lateralization of the developing cortical circuitry is equipotential (Lashley, 1951; Lenneberg, 1967). Instead, they show that the left hemisphere (particularly left frontal regions) plays a critical role, even following extensive damage to left hemisphere language regions. In the majority of our sample, damage to cortical language regions was quite extensive, impacting as much as 75% of our predefined language regions of interest (Table 1). However, our analysis revealed that the relationship between laterality and language function occurs across a range of lesion sizes. We also find that this relationship occurs whether language areas are damaged or not, corroborating previous findings that the proximity of the lesion to classic language areas is not a good predictor of the pattern of lateralization (Liégeois et al., 2004). In fact, left lateralization patterns are often observed after lesions close to or even within Broca’s area (Liégeois et al., 2004).
The finding that activity in left inferior frontal regions is important for improved language outcome despite intervening left injury suggests an early bias for certain aspects of language organization to occur in the left hemisphere, which is corroborated by findings showing an early left specialization for language function. For example, both neonates (Peña et al., 2002) and 3-month-olds (Dehaene-Lambertz et al., 2002, 2006) have leftward asymmetry of blood oxygen level dependent activation during speech perception in both Broca’s and Wernicke’s areas. A similar study using magnetoencephalography showed activation patterns in left superior temporal and inferior frontal regions in infants during speech perception (Imada et al., 2006). This early left hemisphere bias for language may suggest a strong genetic predisposition (Dehaene-Lambertz et al., 2006) that could explain a pattern of neural organization which persists even after early injury.
Because the distribution of activity between left and right hemispheres in the infant brain is not the same as in the adult brain, relating the findings of studies with infants to the neurobiological organization of language in adults and in older children must be done with caution. Furthermore, there is evidence that language lateralization is an extended process that takes place throughout the course of development (Szaflarski et al., 2006). Of relevance to our results are the findings that this developmental trajectory leads to a left hemispheric predominance in left anterior regions (Brown et al., 2005; Szaflarski et al., 2006), reflecting the specialization of the left inferior frontal cortex for certain functions that are critical for language such as cognitive control (Badre, 2008) and articulation (Brown et al., 2009). The fact that activity in left inferior frontal regions relates to superior language skill in people with brain injury suggests the importance of the maintenance of the typical pattern of left lateralized activity in inferior frontal brain regions after early injury.
Although we found left lateralized activity in inferior frontal brain regions is related to better language outcome, this was not the case for posterior language regions. Instead, activity in bilateral temporal and inferior parietal regions related to improved outcome, suggesting that in the injured brain some brain functions associated with language are mediated in a more distributed manner incorporating both hemispheres. Note that we found a relative left lateralization in posterior regions in our sample of typical participants, which suggests that bilateral representation for category fluency is not the typical pattern in people without brain injury. In this context, right temporal and parietal activation may in fact reflect some compensatory organization following early injury, i.e. that a different distribution of brain activity than that found in typical participants of the same age supports better language skills after early injury. Such a finding is consistent with several behavioural studies looking at early language development after pre- or perinatal injury (Bates et al., 1997; Vicari et al., 2000). For example, children with early left temporal injuries show initial delays in vocabulary and grammar production, but these deficits ameliorate over time, possibly suggesting a reliance on right temporal-parietal regions as a mechanism for compensation.
However, it is also possible that, rather than compensatory organization, this pattern of bilateral activity reflects some degree of functional remediation, or local neural repair. That is, bilateral representation in the posterior regions may reflect the neural organization of typically developing children, which is maintained after injury. More specifically, although we show left lateralization in posterior regions to be the typical pattern in neurologically normal adults, developmental studies with children ages 5–12 years have shown evidence that activity in posterior regions does not significantly shift leftward over this period of development as it does in anterior regions (Szaflarski et al., 2006). Given our results, the eventual shift to left lateralized organization presumably occurs later in the developmental process. In this context, the advantage to development of superior language skill conferred by balanced contributions from both hemispheres in posterior regions may be due to the fact that this balanced pattern maintains the characteristic immature, yet still normal, pattern reflected by younger typically developing children.
For posterior language regions, it is difficult, given the present data, to argue forcefully in favour of one process over the other (i.e. compensatory organization or functional remediation). In order to adjudicate between these two explanations, we would at the very least need to have a more comprehensive language history of the stroke cohort, and longitudinal data would be necessary. One recent investigation of older children with injury, however, presents some preliminary data against any compensatory organization to the right hemisphere. This study (Elkana et al., 2010) examined the relationship between language laterality and a battery of language tasks outside the scanner in a small sample (n = 7; ages 5–17 years) of participants who sustained later childhood injuries; that is, after language acquisition but before complete maturation of the brain. In general, these authors found that more left lateralization of activity in anterior and posterior language regions of interest similar to those we identified correlated with better language outcomes. Considered with our findings, these results suggest again that more consistent right hemisphere activation in both anterior and posterior language regions is associated with poorer behavioural outcome, which argues against right hemisphere compensation as an explanation for our finding that bilateral activity in posterior regions is associated with better language skills. We suggest that when an early brain injury takes place among the backdrop of normally occurring developmental processes, it does not drastically alter these processes in cases where better language development occurs. Instead, for both language regions but particularly for anterior regions, our findings are consistent with more recent adult stroke literature suggesting that the left hemisphere plays an important role in stroke recovery throughout the lifespan (Meinzer et al., 2008).
Finally, it must be noted that the category fluency task we used should not be interpreted as assessing all aspects of language processing. While it is true that the task consistently recruits inferior frontal, posterior superior and middle temporal and inferior parietal regions (Petersen et al., 1988; Hertz-Pannier et al., 1997; Knecht et al., 2000; Holland et al., 2001; Szaflarski et al., 2006), and it is true that we find this brain activity is related to a variety of measures of language function, we must keep in mind that other language tasks may evoke different patterns of neural activity and therefore elicit different relationships to behaviour. We specifically chose category fluency as the index of language function because it reliably elicits activity in brain areas known to be involved in receptive and productive language processes. Thus, any deviations from the typical brain response can arguably be attributed to the brain’s response to early injury.
In conclusion, while there are a number of potential brain organizations for implementing language after early left hemisphere brain injury, there are certain patterns of neural activation that are associated with better language functioning. In particular, for anterior language regions the present results show that the patterns of neural activation associated with better functioning are those which maintain the patterns that have been identified in typically developing young children. For posterior language regions, a more balanced or bilateral pattern of activity is associated with better outcome, and more lateralized activity (whether left or right) is associated with poorer outcome. Notably, these results are only apparent when we relate brain activation to behaviour. Our findings thus underscore the importance of relating neural activity to language at the individual level in heterogeneous brain injured populations because it allows us to draw specific conclusions that are relevant to behavioural outcomes. The fact that our findings hold across a wide range of lesion sizes and amounts of damage to classical cortical language areas supports a strong predisposition for a specific neural organization for language, one that perseveres in the face of early injury.
Funding
National Institutes of Health National Research Service Award grant #F32DC008909, and National Institutes of Child Health and Human Development of the National Institutes of Health, grant 1 P01 HD40605 and grant #RO1-NS-54942.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Acknowledgements
The authors wish to thank Aaron R. Beharelle, Uri Hasson, Nameeta Lobo, Robert Lyons, Xander Meadow, Jeremy Skipper, Linda Whealton Suriyakham, Helen Wier, Lauren Wineburgh and Nick Wymbs for their help in data collection, analysis and editing of this manuscript.
Glossary
Abbreviations
- LI
laterality index
References
- Badre D. Cognitive control, hierarchy, and rostro-caudal organization of the frontal lobes. Trends Cogn Sci. 2008;12:193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Bates E, Reilly J, Wulfeck B, Dronkers N, Opie M, Fenson J, et al. Differential effects of unilateral lesions on language production in children and adults. Brain Lang. 2001;79:223–65. doi: 10.1006/brln.2001.2482. [DOI] [PubMed] [Google Scholar]
- Bates E, Thal D, Aram D, Nass R, Trauner D. From first words to grammar in children with focal brain injury. Dev Neuropsychol. 1997;13:275–343. [Google Scholar]
- Benson DF, Ardila A. Aphasia. New York: Oxford University Press; 1996. [Google Scholar]
- Booth JR, Macwhinney B, Thulborn K, Sacco K, Voyvodic J, Feldman HM. Functional organization of activation patterns in children: whole brain fMRI imaging during three different cognitive tasks. Progr Neuropsychopharmacol Biol Psychiatry. 1999;23:669–82. doi: 10.1016/s0278-5846(99)00025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broca PP. Loss of speech, chronic softening and and partial destruction of the anterior left lobe of the brain. Bull de la Société Anthropologique. 1861;2:235–8. [Google Scholar]
- Brown S, Laird A, Pfordresher P, Thelen S, Turkeltaub P, Liotti M. The somatotopy of speech: phonation and articulation in the human motor cortex. Brain and Cogn. 2009;70:31–41. doi: 10.1016/j.bandc.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cereb Cortex. 2005;15:275–90. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crinion J, Ashburner J, Leff A, Brett M, Price C, Friston K. Spatial normalization of lesioned brains: performance evaluation and impact on fMRI analyses. Neuroimage. 2007;37:866–75. doi: 10.1016/j.neuroimage.2007.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR. Aphasia. N Engl J Med. 1992;326:531–9. doi: 10.1056/NEJM199202203260806. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002;298:2013–5. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Hertz-Pannier L, Dubois J, Mériaux S, Roche A, Sigman M, et al. Functional organization of perisylvian activation during presentation of sentences in preverbal infants. Proc Natl Acad Sci USA. 2006;103:14240–5. doi: 10.1073/pnas.0606302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Hertz-Pannier L, Dubois J. Nature and nurture in language acquisition: anatomical and functional brain-imaging studies in infants. Trends Neurosci. 2006;29:367–73. doi: 10.1016/j.tins.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Duncan JD, Moss SD, Bandy DJ, Manwaring K, Kaplan AM, Reinian EM, et al. Use of positron emission tomography for presurgical localization of eloquent brain areas in children with seizures. Pediatr Neurosurg. 1997;26:144–56. doi: 10.1159/000121180. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody Picture Vocabulary Test-Third Edition (PPVT-3) Circle Pines, MN: AGS Publishing; 1997. [Google Scholar]
- Elkana O, Frost R, Kramer U, Ben-Bashat D, Hendler T, Schmidt D, et al. Cerebral reorganization as a function of linguistic recovery in children: an fMRI study. Cortex. 2009 doi: 10.1016/j.cortex.2009.12.003. December 9 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Fair DA, Brown TT, Petersen SE, Schlaggar BL. FMRI reveals novel functional neuroanatomy in a child with perinatal stroke. Neurology. 2006;67:2246–9. doi: 10.1212/01.wnl.0000249348.84045.0e. [DOI] [PubMed] [Google Scholar]
- Freud S. Translated by LA Russin. Coral Gables, FL: University of Miami; 1968. Infantile Cerebral Paralysis (Infantile Cerebrallähmung, 1897) [Google Scholar]
- Geschwind N. Aphasia. N Engl J Med. 1971;284:654–6. doi: 10.1056/NEJM197103252841206. [DOI] [PubMed] [Google Scholar]
- Goodglass H. Understanding aphasia. San Diego, CA: Academic Press; 1993. [Google Scholar]
- Hertz-Pannier L, Chiron C, Jambaqué I, Renaux-Kieffer V, Van de Moortele P-F, Delalande O, et al. Late plasticity for language in a child's non-dominant hemisphere: a pre- and post-surgery fMRI study. Brain. 2002;125:361–72. doi: 10.1093/brain/awf020. [DOI] [PubMed] [Google Scholar]
- Hertz-Pannier L, Gaillard WD, Mott SH, Cuenod CA, Bookheimer SY, Weinstein S, et al. Noninvasive assessment of language dominance in children and adolescents with functional MRI: a preliminary study. Neurology. 1997;48:1003–12. doi: 10.1212/wnl.48.4.1003. [DOI] [PubMed] [Google Scholar]
- Holland SK, Plante E, Weber Byars A, Strawsburg RH, Schmithorst VJ, Ball WS., Jr Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 2001;14:837–43. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Imada T, Zhang Y, Cheour M, Taulu S, Ahonen A, Kuhl PK. Infant speech perception activates Broca's area: a developmental magnetoencephalography study. Neuroimage. 2006;17:957–62. doi: 10.1097/01.wnr.0000223387.51704.89. [DOI] [PubMed] [Google Scholar]
- Inder TE, Volpe JJ. Mechanisms of perinatal brain injury. Semin Neonatol. 2000;5:3–16. doi: 10.1053/siny.1999.0112. [DOI] [PubMed] [Google Scholar]
- Jacola LM, Schapiro MB, Schmithorst VJ, Byars AW, Strawsburg RH, Szaflarski JP, et al. Functional magnetic resonance imaging reveals atypical language organization in children following perinatal left middle cerebral artery stroke. Neuropediatrics. 2006;37:46–52. doi: 10.1055/s-2006-923934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A, McCabe P. Recovery patterns and prognosis in aphasia. Brain. 1977;100:1–18. doi: 10.1093/brain/100.1.1. [DOI] [PubMed] [Google Scholar]
- Kirton A, deVeber G, Pontigon A-M, Macgregor D, Shroff M. Presumed perinatal ischemic stroke: vascular classification predicts outomces. Ann Neurol. 2008;63:436–43. doi: 10.1002/ana.21334. [DOI] [PubMed] [Google Scholar]
- Knecht S, Deppe M, Dräger B, Bobe L, Lohmann H, Ringelstein E. Language lateralization in healthy right-handers. Brain. 2000;123:74–81. doi: 10.1093/brain/123.1.74. [DOI] [PubMed] [Google Scholar]
- Lashley KS. Central mechanisms in behavior. New York: Wiley; 1951. [Google Scholar]
- Lazar RM, Antoniello D. Variability in recovery from aphasia. Curr Neurol Neurosci Rep. 2008;8:497–502. doi: 10.1007/s11910-008-0079-x. [DOI] [PubMed] [Google Scholar]
- Lenneberg EH. Biological foundations of language. New York: Wiley; 1967. [Google Scholar]
- Levine SC, Huttenlocher PR, Banich MT, Duda E. Factors affecting cognitive functioning in hemiplegic children. Dev Med Child Neurol. 1987;29:27–35. doi: 10.1111/j.1469-8749.1987.tb02104.x. [DOI] [PubMed] [Google Scholar]
- Lidzba K, Staudt M. Development and (re)organization of language after early brain lesions: capacities and limitation of early brain plasticity. Brain Lang. 2008;106:167–76. doi: 10.1016/j.bandl.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Liégeois F, Connelly A, Cross JH, Boyd SG, Gadian DG, Vargha-Khadem F, et al. Language reorganization in children wtih early-onset lesions of the left hemisphere: an fMRI study. Brain. 2004;127(Pt 6):1229–36. doi: 10.1093/brain/awh159. [DOI] [PubMed] [Google Scholar]
- Meinzer M, Flaisch T, Breitenstein C, Wienbruch C, Elbert T, Rockstroh B. Functional re-recruitment of dysfunctional brain areas predicts language recovery in chronic aphasia. Neuroimage. 2008;39:2038–46. doi: 10.1016/j.neuroimage.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Müller R-A, Behen ME, Rothermel RD, Muzik O, Chakraborty PK, Chugani HT. Brain organization for language in children, adolescents, and adults with left hemisphere lesions: a PET study. Progr Neuropsychopharmacol Biol Psychiatry. 1999;23:657–68. doi: 10.1016/s0278-5846(99)00024-x. [DOI] [PubMed] [Google Scholar]
- Müller R-A, Rothermel RD, Behen ME, Muzik O, Becker C, Fuerst DR, et al. Determination of language dominance by [15O]-water PET in pediatric patients: a comparison with the Wada test. J Epilepsy. 1998;11:152–61. [Google Scholar]
- Müller R-A, Rothermel RD, Behen ME, Muzik O, Mangner TJ, Chakraborty PK, et al. Brain organization of language after early unilateral lesion: a PET study. Brain Lang. 1998;62:422–51. doi: 10.1006/brln.1997.1931. [DOI] [PubMed] [Google Scholar]
- Noll DC, Cohen JD, Meyer CH, Schneider W. Spiral K-space MR imaging of cortical activity. J Magn Resonan Imaging. 1995;5:49–56. doi: 10.1002/jmri.1880050112. [DOI] [PubMed] [Google Scholar]
- Papanicolaou AC, Simos PG, Breier JI, Wheless JW, Mancias P, Baumgartner JE, et al. Brain plasticity for sensory and linguistic functions: a functional imaging study using magnetoencephalography with children and young adults. J Child Neurol. 2001;16:241–52. doi: 10.1177/088307380101600403. [DOI] [PubMed] [Google Scholar]
- Peña M, Maki A, Kovačić D, Dehaene-Lambertz G, Koizumi H, Bouquet F, et al. Sounds and silence: an optical topography study of language recognition at birth. Proc Natl Acad Sci USA. 2002;100:11702–5. doi: 10.1073/pnas.1934290100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331:585–9. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Raju TNK, Nelson KB, Ferriero D, Lynch JK. Ischemic perinatal stroke: summary of a workshop sponsored by the national institute of child health and human development and the national institute of neurological disorders and stroke. Pediatrics. 2007;120:609–16. doi: 10.1542/peds.2007-0336. [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Milner B. The role of early left-brain injury in determining lateralization of cerebral speech functions. Ann NY Acad Sci. 1977;299:355–69. doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- Reilly JS, Bates EA, Marchman VA. Narrative discourse in children with early focal brain injury. Brain Lang. 1998;61:335–75. doi: 10.1006/brln.1997.1882. [DOI] [PubMed] [Google Scholar]
- Rowe ML, Levine SC, Fisher JA, Goldin-Meadow S. Does linguistic input play the same ole in language learning for children with and without early brain injury? Dev Psychol. 2009;45:90–102. doi: 10.1037/a0012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semel EM, Wiig EH, Secord W. Clinical evaluation of language fundamentals-3. San Antonio, TX: Psychological Corporation; 1995. [Google Scholar]
- Staudt M, Grodd W, Niemann G, Wildgruber D, Erb M, Krägeloh-Mann I. Early left periventricular brain lesions induce right hemispheric organization of speech. Neurology. 2001;57:122–5. doi: 10.1212/wnl.57.1.122. [DOI] [PubMed] [Google Scholar]
- Staudt M, Lidzba K, Grodd W, Wildgruber D, Erb M, Krägeloh-Mann I. Right-hemispheric organization of language following early left-sided brain lesions: functional MRI topography. Neuroimage. 2002;16:954–67. doi: 10.1006/nimg.2002.1108. [DOI] [PubMed] [Google Scholar]
- Stiles J, Reilly J, Paul B, Moses P. Cognitive development following early brain injury: evidence for neural adaptation. Trends Cogn Sci. 2005;9:137–43. doi: 10.1016/j.tics.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, Schmithorst VJ, Byars AW. FMRI study of language lateralization in children and adults. Hum Brain Mapp. 2006;27:202–12. doi: 10.1002/hbm.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DJ, Marchman V, Stiles J, Aram D, Trauner D, Nass R, et al. Early lexical development in children with focal brain injury. Brain Lang. 1991;40:491–527. doi: 10.1016/0093-934x(91)90145-q. [DOI] [PubMed] [Google Scholar]
- Vicari S, Albertoni A, Chilosi AM, Cipriani P, Cioni G, Bates E. Plasticity and reorganization during language development in children with early brain injury. Cortex. 2000;36:31–46. doi: 10.1016/s0010-9452(08)70834-7. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale – III. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children-Revised. San Antonio, TX: The Psychological Corporation; 1974. [Google Scholar]
- Wernicke C. Der Aphasische Symptomencomplex. Breslau: Cohn and Weigert; 1874. [Google Scholar]
- Williams KT. Expressive vocabulary test: American Guidance Service. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- Woods BT, Teuber HL. Changing patterns of childhood aphasia. Ann Neurol. 1978;3:273–80. doi: 10.1002/ana.410030315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.