Abstract
Neuropsychiatric disorders are often characterized by impaired insight into behaviour. Such an insight deficit has been suggested, but never directly tested, in drug addiction. Here we tested for the first time this impaired insight hypothesis in drug addiction, and examined its potential association with drug-seeking behaviour. We also tested potential modulation of these effects by cocaine urine status, an individual difference known to impact underlying cognitive functions and prognosis. Sixteen cocaine addicted individuals testing positive for cocaine in urine, 26 cocaine addicted individuals testing negative for cocaine in urine, and 23 healthy controls completed a probabilistic choice task that assessed objective preference for viewing four types of pictures (pleasant, unpleasant, neutral and cocaine). This choice task concluded by asking subjects to report their most selected picture type; correspondence between subjects’ self-reports with their objective choice behaviour provided our index of behavioural insight. Results showed that the urine positive cocaine subjects exhibited impaired insight into their own choice behaviour compared with healthy controls; this same study group also selected the most cocaine pictures (and fewest pleasant pictures) for viewing. Importantly, however, it was the urine negative cocaine subjects whose behaviour was most influenced by insight, such that impaired insight in this subgroup only was associated with higher cocaine-related choice on the task and more severe actual cocaine use. These findings suggest that interventions to enhance insight may decrease drug-seeking behaviour, especially in urine negative cocaine subjects, potentially to improve their longer-term clinical outcomes.
Keywords: cocaine addiction, insight, choice behaviour, neuropsychology, urine status
Introduction
Drug addiction is characterized by a transition from volitional drug use to drug use that becomes increasingly habitual and compulsive. An influential theoretical account has suggested that this transition occurs as control over drug-seeking and drug-taking behaviour shifts from prefrontal cortical to striatal (especially dorsal striatal) regions, such that drug-seeking behaviour becomes a stimulus-response habit that is triggered and maintained by drug-associated stimuli (Everitt et al., 2008). Thus, drug-seeking behaviour may become increasingly controlled by motivational processes of which the addicted individual is largely unaware. Such impaired insight into behaviour, encompassing denial of (or failure to recognize) the severity of illness, compromised control of action, or unawareness of one’s social impairments (Damasio, 1994; Bechara, 2004), has been studied in various classical neuropsychiatric disorders (e.g. schizophrenia, mania, other mood disorders) (Orfei et al., 2008). These disorders show deficits in similar brain regions to those identified to underlie core addiction clinical symptomatology (Goldstein and Volkow, 2002), leading us to suggest that impaired insight may also be a hallmark of drug addiction (Goldstein et al., 2009).
This suggestion of an insight impairment in drug addiction has received indirect support from previously identified dissociations between subjective and objective markers of behaviour in cocaine addicted individuals (Goldstein et al., 2007, 2008; Hester et al., 2007; Moeller et al., 2009). For example, one study reported that while healthy control subjects showed incentive-related performance enhancements that were correlated with their self-reports of task engagement, these same correlations did not reach significance in the cocaine subjects (Goldstein et al., 2007). In a follow-up study, which used the same sustained attention task to examine modulation of the event-related potential P300 component by expected monetary reward, healthy control subjects, but not cocaine subjects, showed significant correlations between monetary-driven P300 amplitudes and their respective behavioural performance responses (Goldstein et al., 2008). Other laboratories have reported similar neural-behaviour dissociations in other populations of substance abusers (e.g. smokers; Chiu et al., 2008). In a recent study, we developed a probabilistic learning task in which cocaine subjects and healthy control subjects chose between viewing pleasant, unpleasant, neutral or cocaine images under uncertain task contingencies (Moeller et al., 2009). Our results revealed that the objective choice of the cocaine subjects to view the cocaine images was not fully explained by their self-reported preference (value ratings) for these same images (Moeller et al., 2009). Nevertheless, although these studies are collectively suggestive of an insight deficit, they have not tested this novel hypothesis directly.
Here, to provide more direct evidence of an insight deficit in addiction, we used this same probabilistic choice task to study correspondence between subjects’ self-reported choice behaviour (reported here for the first time) with their actual, objective choice behaviour. We also examined the relation of this putative insight deficit to actual cocaine use, thereby examining the potential contribution of insight to drug seeking. We hypothesized that, compared with healthy controls, cocaine subjects will show impaired insight into their behaviour, manifested as incorrect identification of the picture category (cocaine, pleasant, unpleasant or neutral) chosen to be viewed most often. Moreover, drawing on research that has suggested a beneficial effect of insight (awareness of alcohol use severity) on clinical outcome (1-year abstinence) (Kim et al., 2007), we further hypothesized that impaired insight among the cocaine subjects will be associated with heightened cocaine-related choice on the task (especially when compared with the other affectively valenced pictures) and frequency/severity of actual cocaine use. Therefore, throughout our analyses we inspected the potential modulating influence of recent cocaine use on our main dependent variables (insight, cocaine choice behaviour and actual cocaine use). We indexed recent cocaine use on the basis of cocaine urine screening conducted on study day, an assessment that is not subject to self-report errors. These analyses with urine status, also conducted for the first time in the current study, were also undertaken because recent cocaine use may independently (or in conjunction with insight) enhance cocaine-related choice behaviour. This hypothesis is suggested by correlations obtained in our previous study between cocaine choice behaviour and frequency of recent cocaine use (Moeller et al., 2009), and by elevated cocaine choice after administration of priming doses of cocaine (i.e. small doses administered before the opportunity for drug seeking) to cocaine subjects (Donny et al., 2004). Recent cocaine use (and a positive urine status in particular) indeed predicts poor prognosis in treatment outcome studies (Poling et al., 2007; Ahmadi et al., 2009), possibly due to an underlying cognitive deficit (Woicik et al., 2009).
Materials and methods
Subjects
Sixty five subjects (42 cocaine subjects and 23 healthy control subjects) were recruited through advertisements in local newspapers, word-of-mouth and local treatment facilities. Forty of these subjects (20 cocaine and 20 controls) were included in our previous report (Moeller et al., 2009), but all analyses in the current study are unique. All subjects were right-handed native English speakers and free of any medication. Initial telephone screening and on-site medical and neurological evaluations ensured that subjects met the following criteria: (i) absence of head trauma with loss of consciousness; (ii) absence of current neurological or medical disease that required hospitalization or regular monitoring; and (iii) except for cocaine in the cocaine subjects, negative urine screens for all other drugs or their metabolites. All subjects provided written informed consent in accordance with the local Institutional Review Board.
Subjects underwent a comprehensive diagnostic interview conducted by a licensed clinical psychologist (see Supplementary material for a complete listing of interview components). This interview confirmed that all cocaine subjects met criteria for current cocaine dependence (n = 36), cocaine dependence in partial remission (n = 5), or cocaine dependence in fully sustained remission (n = 1) (see Supplementary material for comorbidities, including any additional substance abuse disorders, among these individuals). A triage urine panel for drugs of abuse (Biopsych), a reliable and valid method of detecting cocaine metabolites (Wu et al., 1993), was conducted on study day in all subjects. This test, which confirms drug use (encompassing cocaine, marijuana, opioids, benzodiazepines, phencyclidine, amphetamine/methamphetamine and barbiturates) up to 72 h after administration, divided the cocaine subjects into two subgroups: individuals with cocaine use disorder testing positive for cocaine (CUD+: n = 16, none of whom were seeking treatment; none of whom were intoxicated at study time as confirmed by the diagnostic interview) and individuals with cocaine use disorder testing negative for cocaine (CUD–: n = 26, 12 of whom were currently abstaining and 14 of whom were currently seeking treatment). Table 1 presents complete demographic information for all subjects, split by study group; we accounted for the potentially confounding influence of variables that differed between the groups as described in the ‘Statistical analyses’ and ‘Results’ sections.
Table 1.
Demographic characteristics and drug use by study group
| Urine positive cocaine subjects (n = 16) | Urine negative cocaine subjects (n = 26) | Control subjects (n = 23) | |
|---|---|---|---|
| Gender (male/female) | 15/1 | 24/2 | 18/5 |
| Ethnicity (African-American/Caucasian/other) | 11/2/3 | 17/6/3 | 12/10/1 |
| History of cigarette smoking (current or past/never)a | 15/1b | 22/4b | 4/19c,d |
| Daily frequency of smoking (for current smokers; n = 35) | 6.6 ± 5.4 | 6.1 ± 4.7 | 3.3 ± 5.8 |
| Hours since last cigarette (for current smokers; n = 35) | 14.2 ± 16.2 | 24.6 ± 37.3 | 13.8 ± 9.3 |
| Education (years) | 13.0 ± 1.6 | 13.1 ± 2.9 | 13.9 ± 2.2 |
| Age (years)e | 48.0 ± 5.3d | 42.1 ± 9.1c | 42.7 ± 6.3 |
| Socio-economic status | 33.0 ± 9.9 | 33.6 ± 11.1 | 32.0 ± 10.6 |
| Non-verbal intelligence: Wechsler Abbreviated Scale of Intelligence: Matrix Reasoning scaled score | 8.7 ± 3.9 | 10.7 ± 4.5 | 11.3 ± 2.6 |
| California Verbal Learning Test II: total recall (trials 1–5)f,g | 41.4 ± 10.4b | 44.6 ± 8.5 | 51.2 ± 11.0c |
| California Verbal Learning Test II: long delay free recall | 8.2 ± 3.7 | 9.3 ± 3.0 | 10.8 ± 3.5 |
| California Verbal Learning Test II: long delay cued recall | 9.9 ± 3.5 | 10.1 ± 3.0 | 11.8 ± 3.1 |
| California Verbal Learning Test II: recognition hits | 14.5 ± 1.6 | 14.3 ± 1.8 | 14.7 ± 1.6 |
| Symbol Digits Modality Test | 44.4 ± 12.7 | 47.2 ± 10.5 | 47.8 ± 9.4 |
| Self-reported state depressionh | 8.9 ± 7.6b | 8.5 ± 7.6b | 1.5 ± 2.8c,d |
| Age at onset of cocaine use | 28.3 ± 4.2 | 26.0 ± 7.7 | – |
| Duration of use (years) | 18.3 ± 8.0 | 15.1 ± 7.0 | – |
| Frequency of use (days/week): last 30 daysi | 4.0 ± 2.6d | 1.8 ± 2.1c | – |
| Current use in $ per use (min–max, median): last 30 days | 10–175, 50 | 0–600, 0 | – |
| Duration of current abstinence (days) (min–max, median)j | 0–4, 2.5d | 4–1825, 31c | – |
| Total score on the Cocaine Selective Severity Assessment Scale (measure of withdrawal symptoms) (0–126) | 14.7 ± 8.4 | 17.1 ± 11.5 | – |
| Severity of Dependence Scale (0–15) | 8.3 ± 8.7 | 8.5 ± 3.4 | – |
| Cocaine Craving Questionnaire (0–45) | 18.7 ± 14.3 | 10.6 ± 9.8 | – |
M ± SD.
a χ2 = 32.3, df = 2, n = 65, P < 0.01.
b Mean value significantly differs from that of controls.
c Mean value significantly differs from that of urine positive cocaine subjects.
d Mean value significantly differs from that of urine negative cocaine subjects.
e F = 3.6, df = 2,62, P < 0.05.
f F = 4.9, df = 2,60, P < 0.05.
g We recently reported that urine positive cocaine subjects perform better than urine negative cocaine subjects on selected neuropsychological tests that included learning, memory, and executive functioning (Woicik et al., 2009). We attribute these differences in results to sampling issues, particularly as they pertain to the inclusion of treatment-seeking individuals in the current but not prior study.
h Kruskal–Wallis H = 22.1, P < 0.001.
i t = 3.8, df = 40, P < 0.001.
j Mann–Whitney U-test, Z = −5.0, P < 0.001.
Stimuli
Our tasks used 90 pictures (30 pleasant, 30 unpleasant, 30 neutral) selected from the International Affective Picture System (Lang et al., 1998), and 30 cocaine pictures that were matched to these International Affective Picture System pictures on size and ratio of human to non-human content. The cocaine pictures depicted images of cocaine and individuals preparing, using or simulating use of cocaine (e.g. snorting or smoking), collected from freely available online sources and adapted as still images from a cocaine video used previously in our laboratory (Volkow et al., 2006).
Probabilistic choice task
In the probabilistic choice task, selections were indicated via a single button press for pictures hidden under flipped-over cards, arranged in four decks. Choice of a particular ‘deck’ enlarged the corresponding image that covered the entire screen for 2000 ms of passive viewing. On each subsequent trial, subjects could select again from the same deck or select from a different deck. Each deck was pseudorandomly sorted, containing 26 pictures (87%) of one picture category (e.g. cocaine), two pictures of another category (e.g. pleasant) and one picture of each of the remaining two categories (e.g. unpleasant or neutral). No picture repetitions occurred between the four decks. Subjects completed four runs, each terminating when they had selected from a particular deck for a total of eight times (the eight selections did not need to be consecutive for completion of a run). We summed the total number of cards selected per picture category across the four runs. Complete information on this task, including a figure depicting example trials, can be found in our previous report (Moeller et al., 2009).
Behavioural insight measure
We assessed insight with a novel measure reported exclusively in the current study. The probabilistic task concluded with the following question, ‘What kinds of pictures do you think you chose to look at most often?’ (i.e. this question was asked immediately after task completion). Subjects responded by pressing a button corresponding to one of the four picture categories: pleasant, unpleasant, neutral or cocaine. To calculate our measure of insight, we inspected correspondence between the most selected picture category (objectively ascertained by choice behaviour throughout the probabilistic task) and the self-reported most chosen picture category (ascertained at the conclusion of the probabilistic task). If these objective and self-report measures corresponded, subjects were characterized as having unimpaired insight; otherwise, subjects were characterized as having impaired insight.
Additional tasks
Explicit choice task
Immediately before completing the probabilistic task, subjects also completed a task of explicit behavioural choice, which examines choice for the same International Affective Picture System and cocaine pictures but under fully transparent task contingencies (Moeller et al., 2009). In this explicit choice task, subjects chose via continued button pressing between two fully-visible side-by-side images (one image from one picture category and one image from a different picture category; on some trials, images were juxtaposed against a blank screen to allow for comparison with a non-stimulus). Choice for a desired image enlarged this chosen image to fully cover the screen, which subjects could view for the trial duration of 5000 ms by continued button pressing; otherwise, the side-by-side images returned after 500 ms of non-response. We summed the total number of button presses for each picture category across the 70 choice trials.
Picture ratings
Immediately before completing these two choice tasks, subjects underwent recordings of event-related potentials while passively viewing each of these pictures for 2000 ms (results of these event-related potentials will be reported separately). They then rated each picture on pleasantness (‘rate how pleasant or unpleasant you felt about this picture’) and arousal (‘rate how strong of an emotional response you had to this picture’). They also reported how much they liked and wanted cocaine [‘rate how much you like (or do not like) cocaine’ and ‘rate how much you want (or do not want) cocaine’] in response to each picture. Using a computerized version of the Self-Assessment Manikin (Bradley and Lang, 1994), subjects rated each picture from ‘1’ to ‘9’ (‘1’ corresponded to happy/high visceral response manikins, and to high liking and wanting; ‘9’ corresponded to unhappy/no response manikins, and to low liking and wanting). Response options were reversed coded to facilitate interpretation, such that higher numbers reflect higher pleasantness, arousal, liking, and wanting. Results of the explicit choice task and picture ratings are reported in the Supplementary material.
Statistical analyses
To evaluate our first a priori hypothesis (impaired insight in cocaine subjects compared with controls), we conducted chi-square analyses that tested for group differences between cocaine subjects and controls on our insight measure. Based on our prediction that recency of cocaine use may modulate these results, we also conducted this same analysis with the cocaine subjects split into the CUD+ and CUD– subgroups. An additional chi-square analysis was conducted with CUD– split by treatment status, because non-treatment-seeking subjects may be more likely to have impaired insight, especially as it pertains to the more clinically relevant idea of denial of illness. Furthermore, because the measure of insight was partially based on a question obtained after completion of the task, we conducted logistic regression analyses that controlled for learning, memory, and executive and general intellectual functioning to inspect the possibility that our insight measure merely indexed these factors. We accounted for performance on the following tests, which subjects completed as part of a comprehensive neuropsychological battery that has been described in detail elsewhere (Woicik et al., 2009): (i) the Symbol Digits Modality Test (Smith, 1982), a test of incidental memory and executive functioning; (ii) the California Verbal Learning Test II (Delis et al., 2000), a test of verbal learning and memory (we used trials 1–5 learning, short and delayed free and cued recall and recognition hits, which collectively are the most frequently used measures); and (iii) the Matrix Reasoning score from the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999), a measure of non-verbal intelligence. Table 1 presents information on these variables.
Choice behaviour on the probabilistic task was analysed using a 4 (Picture type: pleasant, unpleasant, neutral, cocaine) × 3 (Diagnosis: CUD+, CUD–, control) mixed analysis of variance (ANOVA) (note that the picture choice variables were normally distributed). Subsequently, we conducted an analysis of covariance (ANCOVA) that controlled for the total picture selections across runs to ensure that significant findings were not merely attributable to individual differences in response frequency. The Greenhouse–Geisser correction was used if the assumption of sphericity was not met. A significant Picture type × Diagnosis interaction was followed by tests of between-group linear contrasts, conducted separately for each picture category, but with the specific prediction for the cocaine pictures of CUD+ > CUD– > control (given the literature that suggests elevated cocaine choice after recent use). A significant interaction was also followed by paired t-tests, separately among the three study groups, to inspect within-group differences in picture choice. Age, depression and cigarette smoking history, which significantly differed between the groups (Table 1), were covaried in subsequent ANCOVAs if these measures were significantly associated with our dependent (choice) variables (Stevens, 1992). Associations with age were examined with Pearson correlations; associations with depression, which was not normally distributed, were examined with nonparametric Spearman correlations; and the dichotomous smoking status was inspected with independent t-tests.
Importantly, to evaluate our second a priori hypothesis (impaired insight is associated with enhanced choice for cocaine pictures), we conducted two planned 3 (Diagnosis: CUD+, CUD–, control) × 2 (Insight: unimpaired insight, impaired insight) univariate ANOVAs. The dependent variables for these ANOVAs were change scores that subtracted pleasant or unpleasant choice from cocaine choice for the probabilistic task. These ANOVAs thereby examined whether Insight and urine status interact to influence choice for viewing cocaine pictures, specifically when compared with choice for viewing the other affective non-drug related pictures [note that we also verified these ANOVAs with only the cocaine subjects, as their drug-seeking behaviour was of greater interest for our purposes; we also report in Supplementary material the results of mixed 4 (Picture choice) × 2 (Diagnosis) × 2 (Insight) ANOVAs, which examine how diagnosis and insight interact to influence choice for each picture category including the non-affective neutral pictures].
To evaluate our third a priori hypothesis (impaired insight is associated with enhanced frequency/severity of actual cocaine use), we conducted between-group analyses that examined the potential effect of Insight on the drug use variables listed in Table 1, separately for the CUD+ and CUD– subgroups. Because the drug use variables were not normally distributed, the non-parametric Mann–Whitney U-test was used. In addition, we examined whether the degree of impaired insight relates to these drug use outcomes (as well as to drug-related choice behaviour), specifically for the individuals already characterized as displaying impaired insight for whom such a dimensionality score was calculable (number of selections for the actually most selected picture category minus the number of selections for the self-reported most selected picture category, with a larger score indicative of more impairment). Considering the multiple indices of drug use frequency/severity available for inspection, analyses with these drug use variables were considered significant at P < 0.01 to protect against Type I error (all other analyses were considered significant at P < 0.05). After satisfying this initial criterion, however, we retained significant associations at P < 0.05 when accounting for relevant covariates.
Results
Insight into picture choice
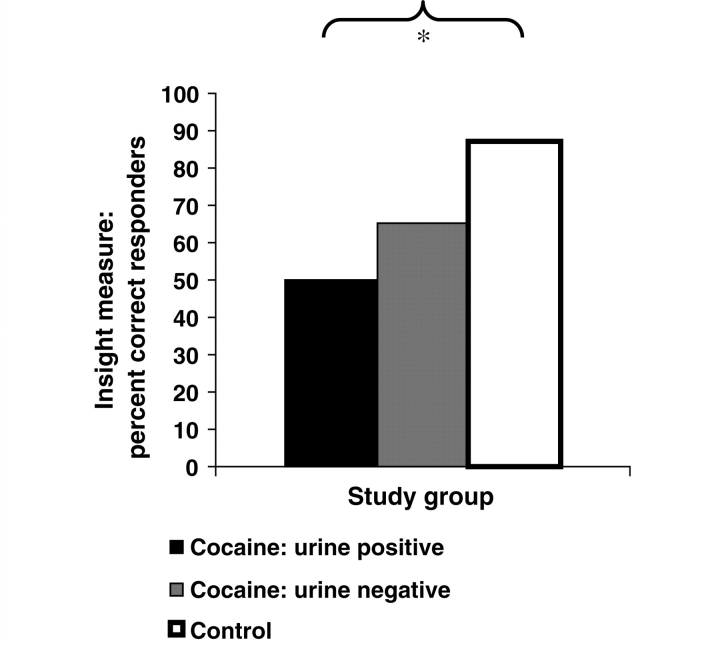
Consistent with our first a priori hypothesis, chi-square analysis showed that cocaine subjects exhibited impaired insight into Picture choice compared with healthy controls [χ2(1, n = 65) = 5.3, P < 0.05]. Further analyses with the cocaine subjects split into the CUD+ and CUD– subgroups still revealed between-group differences on our Insight measure [χ2(2, n = 65) = 6.4, P < 0.05], driven by impaired insight into Picture choice in CUD+ (all non-treatment-seeking) compared with controls [χ2(1, n = 39) = 6.4, P < 0.05] (Fig. 1); CUD– did not differ from either CUD+ [χ2(1, n = 42) = 1.0, P > 0.3] or controls [χ2(1, n = 49) = 3.1, P > 0.08]. Within the CUD– subgroup, however, there were no differences between treatment-seekers and non-treatment-seekers on Insight [χ2(1, n = 26) = 2.3, P > 0.1], supporting the idea of retaining CUD– as a single subgroup that is not further subdivided by treatment status.
Figure 1.
Percentage of individuals displaying correspondence between their self-reported most selected picture category and their objectively most selected picture category (our measure of insight), for each study group. The significant comparison between urine positive cocaine subjects (n = 16) and healthy controls (n = 23) is flagged with an asterisk (P < 0.01). Urine negative cocaine subjects (n = 26) did not significantly differ from the other two study groups.
There were no differences between the individuals with unimpaired insight and those with impaired insight on age [t(63) = 1.4, P > 0.1], depression (Mann–Whitney U-test, Z = –1.5, P > 0.1), cigarette smoking history [χ2(1, n = 65) = 3.6, P > 0.05] or non-verbal intelligence [t(20.0) = 1.8, P > 0.09]. Nevertheless, group differences were observed on the Symbol Digits Modality Test [t(61) = 2.3, P < 0.05] and the California Verbal Learning Test [all variables except recognition hits, ts(61) > 2.0, P < 0.05]; we therefore controlled for these variables (individually, due to concerns of multicollinearity especially among the California Verbal Learning Test variables) in logistic regression analyses. Results of these logistic regressions showed that Diagnosis still predicted Insight after controlling for Symbol Digits Modality Test [χ2(1, n = 65) = 4.1, P < 0.05] and recognition hits [χ2(1, n = 65) = 4.1, P < 0.05], with similar trends emerging for the remaining California Verbal Learning Test variables [χ2(1, n = 65) > 2.7, P ≤ 0.09)]. Note that these results did not change when we examined the combined/synergistic effects of the Symbol Digits Modality Test and California Verbal Learning Test variables, as ascertained with follow-up logistic regressions that simultaneously controlled for these variables (the California Verbal Learning Test variables were still entered individually to avoid multicollinearity). It is also important to note that while these latter California Verbal Learning Test variables attenuated the effect of insight below nominal significance, follow-up mediation analyses, statistically corrected for dichotomous outcomes (MacKinnon and Dwyer, 1993), indicated that these variables did not mediate the association between Diagnosis and Insight (all Sobel’s Z < 1.68, P > 0.09).
Modulation of cocaine choice behaviour by urine status
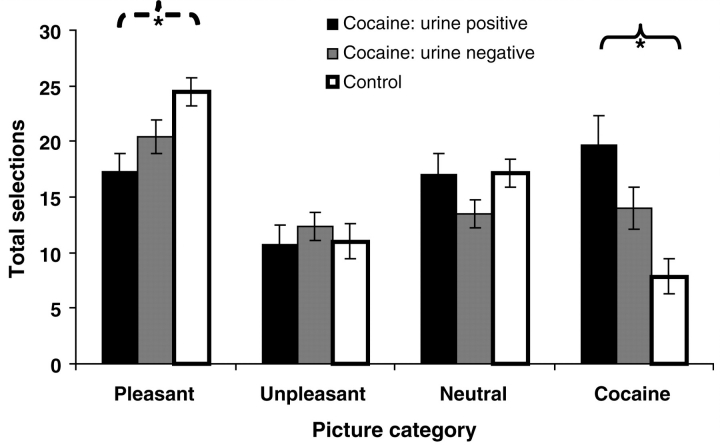
Results of the 4 (Picture type) × 3 (Diagnosis) mixed ANOVA revealed a main effect of Picture type (pleasant > all other picture categories) [F(2.2, 137.7) = 22.8, P < 0.001] but no main effect of Diagnosis [F(2, 62) = 0.3, P > 0.7]. The Picture type × Diagnosis interaction was also significant [F(4.4, 137.7) = 7.7, P < 0.001], driven by between-group differences in selections of cocaine pictures [F(2, 62) = 7.8, P < 0.01] and pleasant pictures [F(2, 62) = 5.1, P < 0.01], but not in selections of unpleasant or neutral pictures [Fs(2, 62) < 2.5, P > 0.09]. Specifically, the between-group linear contrast reached significance for cocaine choice as predicted (CUD + > CUD– > control) [F(1, 62) = 15.3, P < 0.001] (Fig. 2); a reversed linear contrast also emerged for pleasant pictures (CUD + < CUD– < control) [F(1, 62) = 9.9, P < 0.01] (Fig. 2). Within-group comparisons showed that CUD + selected more cocaine pictures than unpleasant pictures [t(15) = 3.4, P < 0.01], whereas CUD– and controls did not [controls selected fewer cocaine pictures than unpleasant pictures; t(22) = 3.1, P < 0.01]. Furthermore, whereas CUD + did not differ in selections of cocaine versus pleasant pictures [t(15) = 0.7, P > 0.4], the other study groups selected fewer cocaine pictures than pleasant pictures [CUD–: t(25) = 2.2, P < 0.05; control: t(22) = 10.4, P < 0.001]. The Picture type × Diagnosis interaction remained significant after accounting for all relevant covariates (P < 0.01).
Figure 2.
Results of the probabilistic task, showing total picture selections for each of the four picture categories (pleasant, unpleasant, neutral, cocaine) for urine positive cocaine subjects (n = 16), urine negative cocaine subjects (n = 26) and healthy controls (n = 23). Significant linear contrasts for both cocaine selections (urine positive > urine negative > control) and pleasant selections (urine positive < urine negative < control) are flagged with asterisks (cocaine selections: solid brackets; pleasant selections: dashed brackets) (P < 0.05). Error bars represent standard error of the mean. Here we only flag the significant linear contrasts, which directly pertain to our a priori hypotheses; a complete listing of all significant between- and within-group comparisons, as well as means and standard deviations for each study group, can be found in Supplementary Table 1.
Relation of insight and recent cocaine use to cocaine-related picture choice
Results of the two 3 (Diagnosis: CUD +, CUD–, control) × 2 (Insight: unimpaired insight, impaired insight) ANOVAs revealed the expected main effects of Diagnosis on choice behaviour [Fs(2, 59) > 6.1, P < 0.01]. Although the main effects of Insight did not reach significance [Fs(1, 59) < 2.8, P > 0.1], both Diagnosis × Insight interactions were detected [cocaine > pleasant selections: F(2, 59) = 3.5, P < 0.05; and a similar trend for cocaine > unpleasant selections: F(2, 59) = 2.8, P < 0.08] (Fig. 3A and B, respectively). Follow-up comparisons showed that cocaine-related choice in CUD– was higher among the individuals with impaired insight [cocaine > pleasant selections: t(24) = 2.6, P < 0.05; cocaine > unpleasant selections: t(24) = 2.2, P < 0.05]. These same comparisons were not significant in the other study groups [CUD +: ts(14) < 0.7, P > 0.4; controls: ts(21) < 1.7, P > 0.1]. Adjusting for relevant covariates did not attenuate these interactions (cocaine > pleasant: P < 0.05; cocaine > unpleasant: P < 0.09). These same interactions also emerged with 2 (Urine status: CUD +, CUD–) × 2 (Insight: unimpaired insight, impaired insight) ANOVAs [cocaine > pleasant selections: F(1, 38) = 5.3, P < 0.05; and a similar trend for cocaine > unpleasant selections: F(1, 38) = 4.0, P < 0.06], buttressing the validity of Insight as particularly relevant to the CUD– subjects.
Figure 3.
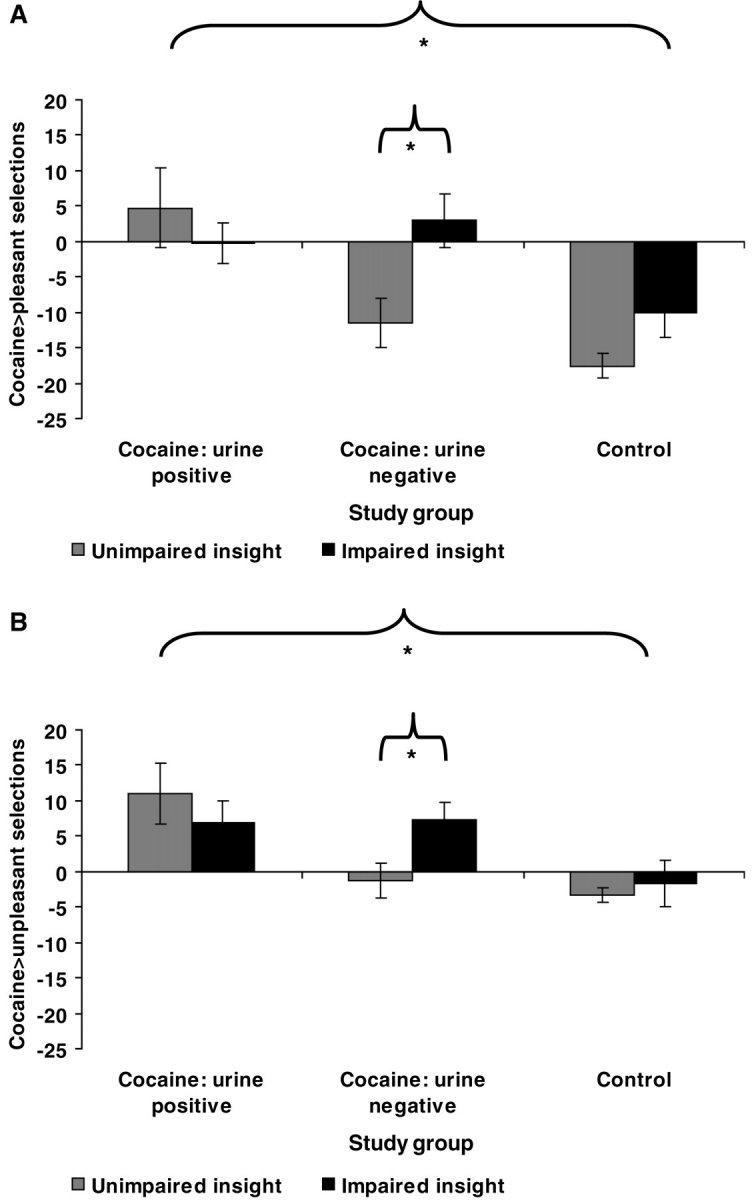
Relation of behavioural Insight to (A) cocaine > pleasant selections and (B) cocaine > unpleasant selections on the probabilistic task for unimpaired insight and impaired insight urine positive cocaine subjects (unimpaired insight: n = 8; impaired insight: n = 8), urine negative cocaine subjects (unimpaired insight: n = 17; impaired insight: n = 9), and healthy controls (unimpaired insight: n = 20; impaired insight: n = 3). Error bars represent standard error of the mean. Significant linear contrasts for Diagnosis (urine positive > urine negative > control) are indicated by an asterisk, as are the specific Insight comparisons within the urine negative subjects (P < 0.05), which pertain to our a priori hypotheses. A complete listing of all significant between- and within-group comparisons, as well as means and standard deviations for the probabilistic task split by study group and insight, can be found in Supplementary Table 2.
Relation of insight and recent cocaine use to drug use
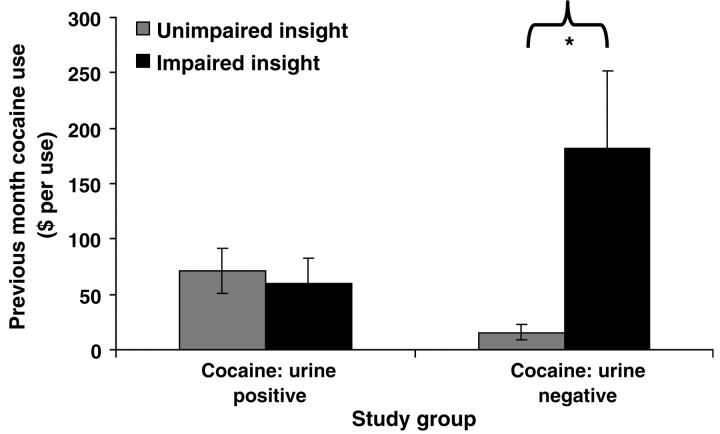
Non-parametric comparisons showed that CUD– with impaired insight spent more money on cocaine per use in the previous month than CUD– with unimpaired insight (Z = –2.7, P < 0.01) (Fig. 4); in contrast, Insight did not impact drug spending within the CUD+ group (Z = –0.8, P > 0.4). This effect of Insight on cocaine spending in CUD– remained significant after accounting for all relevant covariates (P < 0.05). Associations with the other drug use variables listed in Table 1 did not reach the nominal significance level (P < 0.01).
Figure 4.
Cocaine use (in dollars per use) over the previous 30 days for unimpaired insight and impaired insight urine positive cocaine subjects (unimpaired insight: n = 8; impaired insight: n = 8) and urine negative cocaine subjects (unimpaired insight: n = 17; impaired insight: n = 9). The significant effect of Insight on cocaine spending uniquely for the urine negative subjects (impaired insight > unimpaired insight) is indicated by an asterisk. Although we report the raw dollar amounts to enhance figure clarity, between-group comparisons used the non-parametric Mann–Whitney U-test due to violations of normality and the presence of outliers. Error bars represent standard error of the mean.
Relation of dimensional insight to cocaine choice behaviour and drug use
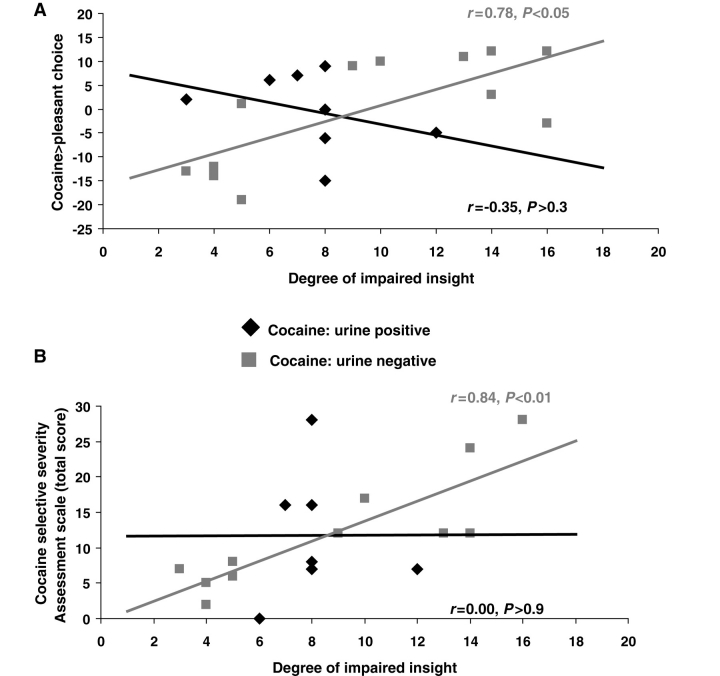
Within those individuals with impaired insight, correlational analyses showed that a higher discrepancy between self-report and behaviour (more impairment) was associated with higher cocaine > pleasant choice (r = 0.52, P < 0.05), with a similar trend for the cocaine > unpleasant choice (r = 0.42, P < 0.07), the former remaining significant after accounting for all relevant covariates (P < 0.05). A higher discrepancy between self-report and behaviour was also associated with higher withdrawal symptoms as assessed by the Cocaine Selective Severity Assessment Scale (Kampman et al., 1998) (r = 0.62, P < 0.01), also remaining significant after accounting for the relevant covariates (P < 0.05). Similarly to the categorical Insight variable, these correlations were driven by CUD–, as revealed for both cocaine > pleasant choice (CUD+: r = –0.35, P > 0.3; CUD–: r = 0.78, P < 0.05; Fig. 5A) and the Cocaine Selective Severity Assessment Scale (CUD+: r = 0.00, P > 0.9; CUD–: r = 0.84, P < 0.01; Fig. 5B).
Figure 5.
Among those individuals with impaired insight, correlations between the magnitude of the discrepancy between self-report and behaviour (our dimensional insight measure) with (A) cocaine > pleasant choice behaviour and (B) total score on the Cocaine Selective Severity Assessment. Separate lines designate urine positive cocaine subjects and urine negative cocaine subjects.
Discussion
The current study directly tested the novel hypothesis of impaired behavioural insight in cocaine addiction, and explored whether such impaired insight relates to cocaine-seeking behaviour and frequency/severity of actual cocaine use. Insight was indicated by categorical correspondence between self-reported behaviour and objective behaviour on a probabilistic-learning choice task (a dimensional insight variable was also examined in follow-up analyses). Cocaine seeking was approximated with tasks of choice for viewing cocaine-related images compared with choice for standardized pleasant, unpleasant and neutral pictures; because these tasks use pictures in lieu of actual cocaine, they are suitable for use even in the treatment-seeking CUD– for whom actual cocaine administration would be unethical. Recency of use was indexed by cocaine metabolites in urine, and frequency and severity of actual cocaine use were ascertained by self-report.
Consistent with our first a priori hypothesis, our results suggested that cocaine addicted individuals are less likely to exhibit insight into their behavioural choice compared with controls. This impairment, which was most pronounced in the CUD+, was attenuated below nominal significance after controlling for other executive functions, speaking to some shared variance between these measures. Nevertheless, non-significant Sobel tests of mediation suggested that these executive functions are not plausible mediators of our effects, consistent with the idea that insight may represent a novel neuropsychological dimension that could underlie clinical symptomatology in drug addiction. A future functional neuroimaging study could probe the neural basis of this insight deficit in CUD+, in which we would anticipate crucial roles for the anterior insula and anterior cingulate cortices, implicated in interoception and behavioural monitoring, respectively (Craig, 2009). These regions indeed predicted diminished awareness of task-related errors in cannabis users (Hester et al., 2009). Other regions (e.g. orbitofrontal cortex, dorsal striatum; Goldstein et al., 2009) and pathways (e.g. somatosensory; Khalsa et al., 2009) may also be involved.
Results were also consistent with our second and third a priori hypotheses of elevated cocaine-related choice and actual cocaine use among the cocaine subjects with impaired insight. Three lines of evidence supported this conclusion: (i) cocaine-related choice was highest in the CUD+ (as described in the main text as well as in the Supplementary material), who were the least likely to display insight; (ii) CUD– with impaired insight demonstrated cocaine-related choice resembling that of CUD+, while CUD– with unimpaired insight demonstrated cocaine-related choice resembling that of controls; and (iii) CUD– with impaired insight spent more on cocaine per use in the previous month than CUD– with unimpaired insight. These latter two findings received additional support from analyses with the dimensional insight variable among those characterized by impaired insight. Importantly, these latter two findings suggest that despite showing an overall lower drug-related choice than CUD+, it was the CUD– subgroup whose drug-seeking behaviour was most directly impacted by insight. One possibility for this differential effect in CUD– may pertain to this subgroup’s increased variability in severity of cocaine use [e.g. current amount spent on cocaine per use was more variable in CUD– (SD = 142.0) than in CUD+ (SD = 55.1)], allowing for more of this variability to be captured by insight. A contributing source of this elevated variability in CUD– could involve treatment-seeking status. If insight were related to the more clinically relevant notion of a ‘denial of or failure to recognize the severity of illness,’ it would seem that the non-treatment-seekers would be more likely to have impaired insight than the treatment-seekers. Although our results did not show a significant difference between these subgroups within the CUD– group, such analyses need to be repeated in larger sample sizes, and possibly while also including treatment-seeking CUD+. Taken together, our results suggest that enhancing insight in clinical settings may decrease the chance of uncontrolled drug seeking and relapse to improve treatment outcomes (for discussion of potential interventions see Goldstein et al., 2009) Indeed, a recent study reported that better insight into one’s severity of alcohol use predicted actual abstinence for up to one year after treatment among male alcoholics (Kim et al., 2007).
Results of the probabilistic task further revealed that in addition to demonstrating the highest cocaine-related choice, CUD+ also demonstrated the lowest pleasant-related choice. In tandem, these findings are consistent with self-administration paradigms whereby acute cocaine administration elevates the choice for cocaine over another pleasant reinforcer (money) (Donny et al., 2004). Although in our study the specific cocaine > pleasant within-group comparison did not reach significance in the CUD+, inspection of the means shows that the results were in this hypothesized direction. Consistent with the theoretical perspective that cocaine addicted individuals pursue cocaine and cocaine-related stimuli at the expense of non-drug related goals (Goldstein and Volkow, 2002), we speculate that this trend may have reached significance with a larger sample size or during a more direct drug-related state [e.g. under the influence of methylphenidate (Volkow et al., 1999), in anticipation of consuming a drug (Wertz and Sayette, 2001; Wilson et al., 2004, 2008), or when recalling a drug-specific context (Goldstein et al., in press)]. Overall, these disproportionate drug-seeking effects in the CUD+ may be contributing to the poorer treatment success rates that have been reported in this subgroup (Poling et al., 2007; Ahmadi et al., 2009).
Limitations of the current study include the following: firstly, we cannot rule out the impact of other factors (e.g. greater familiarity with cocaine stimuli, age, depression, cigarette smoking and other relevant psychological variables) on the current results. However, we note from Table 1 that CUD+ and CUD– did not differ on age of cocaine use onset or duration of cocaine use, and the impacts of age, depression and cigarette smoking were partially addressed through statistical controls. Other cognitive and psychological processes [e.g. coping, more direct measures of sustained attention such as the Continuous Performance Test (Connors, 1995)] also remain to be studied. Secondly, previous viewing of these same drug-related stimuli (i.e. when subjects provided their self-report ratings or during the explicit task, which both occurred prior to the probabilistic task; see ‘Materials and methods’ section) could have induced craving in the cocaine subjects (Carter and Tiffany, 1999), and perhaps especially in CUD+ who could be expecting an imminent drug self-administration opportunity (Wertz and Sayette, 2001; Wilson et al., 2004, 2008). Implementing a counterbalanced design in future research could address this concern. Thirdly, socially desirable responding and other demand characteristics could have impacted our results. Nevertheless, although we suspect that the treatment-seeking CUD– would be most motivated to deny interest in viewing cocaine-related stimuli, cocaine-related choice in the CUD– group still surpassed similar choice in healthy controls. Finally, insight is a complex psychological and neurobiological concept that is unlikely to be fully captured with a single task [e.g. there may be multiple forms of anosognosia (Prigatano, in press), which may differentially influence the type and magnitude of symptoms that the addicted individual can perceive]. Future studies could benefit from (i) online probing into insight (e.g. assessing knowledge of behaviour throughout the task) in order to investigate impaired insight in drug addiction within a fully dimensional framework, and further reduce the possibility that a general cognitive deficit or other non-tested cognitive/emotional dimensions can explain the current findings; (ii) cross-validation with other measures [e.g. the Levels of Emotional Awareness Scale (Lane et al., 1990)] and tasks (e.g. not just those with covert stimulus-response contingencies), which could potentially tap into additional insight phenomena; and (iii) use of a drug-seeking task that is completely distinct from the insight task.
In summary, to our knowledge, the current study is the first to target specifically insight in addiction. Using a probabilistic task with non-fully transparent contingencies, our findings revealed that recent cocaine users were less likely to show insight into their behavioural choice compared with controls, and had the highest cocaine-related choice behaviour. Our results further suggested that the abstinent/treatment-seeking CUD– were most directly impacted by insight, such that those with impaired insight exhibited higher cocaine-related choice behaviour and more severe cocaine use than those with unimpaired insight. Thus, our results suggest that drug addicted individuals (and perhaps especially abstinent or treatment-seeking individuals, for whom curtailing drug use is an immediate goal) may derive benefit from interventions to enhance insight, having the potential to pre-empt drug seeking that could derail treatment success.
Funding
National Institute on Drug Abuse (grants 1R01DA023579, R21DA02062 to R.Z.G.) and the General Clinical Research Center (5-MO1-RR-10710).
Conflict of interest
Rita Goldstein received consultation fee from Medical Directions, Inc, and honoraria fee from the Federal Judicial Centre and the Gruter Institute for Law and Behavioural Research.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the contributions of Jonathan Dunning and Greg Hajcak to this research. This manuscript has been authored by Brookhaven Science Associates, LLC under Contract No. DE-AC02-98CHI-886 with the U.S. Department of Energy. The United States Government retains, and the publisher, by accepting the article for publication, acknowledges, a world-wide license to publish or reproduce the published form of this manuscript, or allow others to do so, for the United States Government purposes.
Glossary
Abbreviations
- CUD+
individuals with cocaine use disorder testing positive for cocaine
- CUD–
individuals with cocaine use disorder testing negative for cocaine
References
- Ahmadi J, Kampman KM, Oslin DM, Pettinati HM, Dackis CA, Sparkman T. Predictors of treatment outcome in outpatient cocaine and alcohol dependence treatment. Am J Addictions. 2009;18:81–6. doi: 10.1080/10550490802545174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Disturbances of emotion regulation after focal brain lesions. Int Rev Neurobiol. 2004;62:159–93. doi: 10.1016/S0074-7742(04)62006-X. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: the self-assessment manikin and the semantic differential. J Behav Therapy Exp Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–40. [PubMed] [Google Scholar]
- Chiu PH, Lohrenz TM, Montague PR. Smokers' brains compute, but ignore, a fictive error signal in a sequential investment task. Nat Neurosci. 2008;11:514–20. doi: 10.1038/nn2067. [DOI] [PubMed] [Google Scholar]
- Connors CK. The Conners Continuous Performance Test. Toronto: Multi-Health Systems. 1995 [Google Scholar]
- Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes' Error: Emotion, Reason, and the Human Brain. New York: Grosset/Putnam; 1994. [Google Scholar]
- Delis DC, Kaplan E, Kramer JH, Ober BA. California Verbal Learning Test: Adult Version. San Antonio, TX: The Psychological Corporation. 2000 [Google Scholar]
- Donny EC, Bigelow GE, Walsh SL. Assessing the initiation of cocaine self-administration in humans during abstinence: Effects of dose, alternative reinforcement, and priming. Psychopharmacology. 2004;172:316–23. doi: 10.1007/s00213-003-1655-z. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–35. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, et al. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? Am J Psychiatry. 2007;164:43–51. doi: 10.1176/appi.ajp.164.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, et al. The Neurocircuitry of Impaired Insight in Drug Addiction. Trends Cogn Sci. 2009;13:372–80. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Parvaz MA, Maloney T, Alia-Klein N, Woicik PA, Telang F, et al. Compromised sensitivity to monetary reward in current cocaine users: an ERP study. Psychophysiology. 2008;45:705–13. doi: 10.1111/j.1469-8986.2008.00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Woicik PA, Moeller SJ, Telang F, Jayne M, Wong C, et al. Liking and wanting of drug and non-drug rewards in active cocaine users: the STRAP-R questionnaire. J Psychopharmacol. 24:257–266. doi: 10.1177/0269881108096982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacol. 2009;34:2450–8. doi: 10.1038/npp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Simoes-Franklin C, Garavan H. Post-error behaviour in active cocaine users: Poor awareness of errors in the presence of intact performance adjustments. Neuropsychopharmacol. 2007;32:1974–84. doi: 10.1038/sj.npp.1301326. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, McGinnis DE, Alterman AI, Weinrieb RM, D'Angelo L, et al. Reliability and validity of the Cocaine Selective Severity Assessment. Addictive Behaviors. 1998;23:449–61. doi: 10.1016/s0306-4603(98)00011-2. [DOI] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Feinstein JS, Tranel D. The pathways of interoceptive awareness. Nat Neurosci. 2009;12:1494–6. doi: 10.1038/nn.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Park BK, Kim GJ, Kim SS, Jung JG, Oh MK, et al. The role of alcoholics' insight in abstinence from alcohol in male Korean alcohol dependents. J Korean Med Sci. 2007;22:132–7. doi: 10.3346/jkms.2007.22.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD, Quinlan DM, Schwartz GE, Walker PA. The levels of emotional awareness scale: a cognitive-developmental measure of emotion. J Personality Assessment. 1990;55:124–34. doi: 10.1080/00223891.1990.9674052. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Gainsville: University of Florida; 1998. The International Affective Picture System (IAPS): photographic slides. [Google Scholar]
- MacKinnon DP, Dwyer JH. Estimating mediated effects in prevention studies. Evaluation Rev. 1993;17:144–58. [Google Scholar]
- Moeller SJ, Maloney T, Parvaz MA, Dunning JP, Alia-Klein N, Hajcak G, et al. Enhanced choice for viewing cocaine pictures in cocaine addiction. Biol Psychiatry. 2009;66:169–76. doi: 10.1016/j.biopsych.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orfei MD, Robinson RG, Bria P, Caltagirone C, Spalletta G. Unawareness of illness in neuropsychiatric disorders: phenomenological certainty versus etiopathogenic vagueness. Neuroscientist. 2008;14:203–22. doi: 10.1177/1073858407309995. [DOI] [PubMed] [Google Scholar]
- Poling J, Kosten TR, Sofuoglu M. Treatment outcome predictors for cocaine dependence. Am J Drug Alcohol Abuse. 2007;33:191–206. doi: 10.1080/00952990701199416. [DOI] [PubMed] [Google Scholar]
- Prigatano GP. Anosognosia: clinical and ethical considerations. Curr Opin Neurol. doi: 10.1097/WCO.0b013e328332a1e7. (in press) [DOI] [PubMed] [Google Scholar]
- Smith A. Symbol Digit Modality Test (SDMT) Manual. Los Angeles: Western Psychological Services; 1982. [Google Scholar]
- Stevens J. 2nd. New Jersey: Lawrence Erlbaum Associates; 1992. Applied multivariate statistics for the social sciences. [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Hitzemann R, Angrist B, Gatley SJ, et al. Association of methylphenidate-induced craving with changes in right striato-orbitofrontal metabolism in cocaine abusers: implications in addiction. Am J Psychiatry. 1999;156:19–26. doi: 10.1176/ajp.156.1.19. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–8. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Wertz JM, Sayette MA. A review of the effects of perceived drug use opportunity on self-reported urge. Exp Clin Psychopharmacol. 2001;9:3–13. doi: 10.1037/1064-1297.9.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Delgado MR, Fiez JA. Effect of smoking opportunity on responses to monetary gain and loss in the caudate nucleus. Journal of Abnormal Psychol. 2008;117:428–34. doi: 10.1037/0021-843X.117.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci. 2004;7:211–4. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woicik PA, Moeller SJ, Alia-Klein N, Maloney T, Lukasik TM, Yeliosof O, et al. The neuropsychology of cocaine addiction: Recent cocaine use masks impairment. Neuropsychopharmacology. 2009;34:1112–22. doi: 10.1038/npp.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu AH, Wong SS, Johnson KG, Callies J, Shu DX, Dunn WE, et al. Evaluation of the triage system for emergency drugs-of-abuse testing in urine. J Anal Toxicol. 1993;17:241–5. doi: 10.1093/jat/17.4.241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.