Abstract
Background: Limited data suggest that moderate alcohol drinkers may have better lung airways function than abstainers. Because few studies have fully accounted for confounders (including smoking and coronary disease), and some might have been biased by the inclusion with nondrinkers of alcohol drinkers who quit because of illness, we performed a cross-sectional analysis in a large free-living population.
Methods: We studied the relation between alcohol and airways function in 177,721 members of a comprehensive health plan. An item on a questionnaire administered as part of a health examination asked for “usual number of drinks in the past year.” Respondents were asked to lump “wine, beer, whiskey, and cocktails” together. Health history queries included 47 items indicative of possible cardiorespiratory (CR) illness; participants with one or more positive response (61.0%) were classified as “CR yes.” Lung function measurements were part of the health examination; we studied one-second forced expiratory volume (FEV1), forced vital capacity (FVC), and FEV1/FVC by analysis of covariance and FEV1/FVC <0.7 by logistic regression. Nondrinkers were the referent for alcohol categories; covariates were age, sex, ethnicity, smoking, education, body mass index, and CR composite yes/no.
Results: For each measure studied, persons reporting two or fewer drinks per day or three to five drinks per day had better airways function than nondrinkers (p < 0.001), but heavier drinkers had worse function. This J-shaped relation was consistent across multiple strata, including CR “yes” or “no.”
Conclusion: Independent of smoking and evident lung or heart disease, light to moderate drinkers of alcohol had better FEV1, FVC, and FEV1/FVC than abstainers did. Although this association does not prove causality, drinking moderate amounts of alcoholic beverages may have some benefit for lung function.
Introduction
Alcohol reaches the airway passages both by the bronchial circulation and by direct inhalation.1 Thus, an effect on airway flow is plausible with implications for bronchial asthma and chronic obstructive pulmonary disease (COPD). It has long been thought that alcohol might be beneficial in persons with asthma.2 Some data, mostly from small studies, suggest that low doses of alcohol may have a bronchodilator effect by relaxing smooth muscle tone.1 Even if ethyl alcohol is beneficial for asthma, nonalcohol components of alcoholic beverages, such as congeners, may cause bronchoconstriction in susceptible persons with extrinsic asthma.1 Also, alcohol metabolites such as acetaldehyde may trigger asthma attacks in persons, mostly Asian Americans, with genetic alcohol dehydrogenase polymorphisms.
Although the analyses were not always clearly independent of smoking, cross-sectional reports consistently show impaired lung airway flow (LAF) in heavy drinkers.3–9 Population studies of chronic LAF among light to moderate drinkers of alcohol show conflicting results. A study of 2539 free-living adults10 found no evidence for an independent association of alcohol intake with LAF. Another study of 3800 study participants in Arizona11 found alcohol-associated decreased LAF independent of smoking status. A survey of 1555 random residents of western New York State showed no overall relation between alcohol and LAF, but function was better among wine drinkers, especially drinkers of white wine.12 A large study compared the prevalence of LAF with alcohol intake in 15,294 representative US adults;13 the data demonstrated no relation with light to moderate intake of alcohol but did show increased airflow obstruction in former heavy drinkers.
Based on the extensive health history inventory … 47 items showed a relation to FEV1/FVC ratio of <0.7.
Limited longitudinal analyses of the relations of alcohol drinking to LAF are also conflicting. A report about 1067 male veterans14 revealed no independent association during a five-year interval. A study of 8765 Danish study participants9 found a relation between alcohol drinking and accelerated loss of LAF. A ten-year study of 378 French policemen15 showed no decline in LAF associated with alcohol intake or a liver enzyme marker. A four-year study of 307 young Dutch persons16 suggested better LAF in relation to alcohol use.
Lifestyle traits related to LAF have important implications for chronic airway diseases, including bronchitis, asthma, and COPD. Perceiving a need for more data about the role of alcohol, we performed an observational analysis among 177,721 persons of known sex, age, ethnicity, and smoking habits.
Methods
Subjects and Data
Since 1945, the Kaiser Permanente Medical Care Program of Northern California has provided comprehensive medical care to patients in the San Francisco Bay Area. Except for underrepresentation of the indigent and very wealthy, the subscribers are socially and ethnically diverse. For much of its existence, the Oakland and San Francisco facilities of the program offered an automated multiphasic health checkup to adult subscribers as a periodic health appraisal17 that included an extensive health history questionnaire. Starting in 1964, the collected data were stored on computers. The examinees passed from station to station, where a variety of tests were administered and measurements were made. From July 1964 through December 1973, the tests included spirometric determination of one-second forced expiratory volume (FEV1) and forced vital capacity (FVC). A Collins spirometer was employed from July 1964 through May 1966, after which date a wedge spirometer was used. These measurements were satisfactorily completed among 177,721 persons of known sex, age, and ethnicity.
Questionnaire data included demographics, habits, current symptoms, and past health history. One query asked, “In the past year did you drink any alcohol?” and provided check-sheet answer choices of “yes” and “no.” The next item was “If yes, how many alcoholic drinks did you usually have (wine, beer, whisky or cocktails)?” with these four check-sheet options: “2 or less a day, 3 to 5 a day, 6 to 8 drinks a day, total of 9 a day or more.” Responses classified alcohol intake in the 177,721 examinees as “none” (21.4%), two or fewer drinks per day (60.7%), three to five drinks per day (8.0%), and six or more drinks per day (2.3%). The remaining 7.6% gave no response, responded yes but gave no amount, or responded no but gave an amount; these were classified as having “unknown” alcohol data. Table 1 includes distributions of selected traits of the study population.
Table 1.
Unadjusted FEV1 and FVC in liters by alcohola and sex
| Group | Mean FEV1 ± SD | Mean FVC ± SD |
| Men | ||
| All | 3.13 ± 0.91 | 4.07 ± 1.00 |
| No alcohol | 2.90 ± 0.63 | 3.78 ± 1.03 |
| <2 drinks | 3.24 ± 0.89b | 4.18 ± 1.00b |
| 3–5 drinks | 3.09 ± 0.87b | 4.04 ± 0.98b |
| >6 drinks | 2.94 ± 0.86b | 3.93 ± 0.96b |
| Unknown | 3.02 ± 0.90b | 3.96 ± 1.00b |
| Women | ||
| All | 2.24 ± 0.65 | 2.85 ± 0.72 |
| No alcohol | 2.07 ± 0.63b | 2.63 ± 0.70 |
| <2 drinks | 2.33 ± 0.64b | 2.95 ± 0.71b |
| 3–5 drinks | 2.19 ± 0.62b | 2.83 ± 0.70b |
| >6 drinks | 2.09 ± 0.63 | 2.74 ± 0.68b |
| Unknown | 2.16 ± 0.62b | 2.78 ± 0.69b |
a Usual number of drinks per day in the preceding year; unknowns gave no response or a conflicting response.
b p vs nondrinkers <0.001.
FEV1 = one-second forced expiratory volume; FVC = forced vital capacity.
The extensive health history inventory inquired about current or past symptoms or illnesses. We judged 60 items as indicative of possible cardiorespiratory (CR) illness. From these, a composite CR “yes” or CR “no” covariate for analytic models was constructed, as described in the next section.
Analytic Methods
We studied mean values of FEV1, FVC, and FEV1/FVC ratio by analysis of covariance, yielding adjusted means and p values. By logistic regression, we also studied two arbitrary cutoffs of FEV1/FVC ratio, <0.7 (vs ≥0.7) and persons in the lowest decile (vs upper 90%), yielding odds ratios (ORs), 95% confidence intervals (CIs), and p values. Covariates in the analytic models included age (continuous), sex, ethnicity (white, African American, Asian American, other), body mass index ([BMI] <25, 25–29, ≥30 kg/m2), education (no college, some college, college graduate), cigarette smoking (never-smoker, ex-smoker, two to four categories of current smoking in various models), alcohol (nondrinker; two or fewer, three to five, six or more drinks per day), the CR composite (yes/no), and appropriate unknown categories.
To construct the CR composite, we selected 60 potential queries and introduced each into a separate logistic model. There were 47 items that showed a relation (p < 0.05) to FEV1/FVC ratio of <0.7. All of these were included in the composite. The queries involved current or past indicators of possible cardiac or pulmonary conditions (eg, heart attack, angina, stroke, high blood pressure, diabetes, abnormal findings on chest radiographs or electrocardiograms, bronchitis, asthma, emphysema, tuberculosis), symptoms of cardiac or pulmonary conditions (eg, chest pain, shortness of breath). A positive response to any one (or more) of these 47 queries was made by 108,400 (61.0%) study participants; these were classified as CR “yes.” The 69,321 (39.0%) participants with no positive responses to any of these items were classified as CR “no.” The composite included 19 queries about events and symptoms “before one year ago,” 7 related to “the past six months,” and 20 related to “the past year.”
We performed analyses involving all persons and of multiple strata, including the sexes, ethnic groups, smoking categories, and CR-yes and CR-no groups. With the large number of study participants involved, small numeric differences produced impressive p values. Therefore, in this article, we define p < 0.001 as “statistically significant.”
Results
Mean Values
Unadjusted mean values for FEV1 and FVC for men and women are presented in Table 1, with evident higher values for light to moderate drinkers of alcohol. Because adjusted models consistently showed similar alcohol relations for these measures and for the FEV1/FVC ratio, we present only data about the mean ratio in other tables. The mean FEV1/FVC ratio for all 177,721 study participants was 0.779, of whom 33,532 (18.9%) had a ratio <0.70 and 17,764 (10.0%) had a ratio <0.63. Data showing adjusted mean FEV1/FVC ratios are presented in Table 2. As expected, the mean FEV1/FVC ratios became lower with increasing age and with increased smoking. The lower mean ratios among those with high BMI, with less education, and with CR history and/or symptoms were also expected. In this study population, men had a lower mean FEV1/FVC than women, and white persons had lower mean ratios than African Americans or Asian Americans. For the alcohol drinking categories, the highest mean FEV1/FVC ratio was among the large number of drinkers reporting having two or fewer drinks per day, followed closely by those reporting having three to five drinks per day. Thus, the mean FEV1/FVC ratios for both abstainers and the heaviest drinkers (six or more drinks per day) were lower than for the intermediate alcohol categories. With the large numbers in this study population, most of the apparently small differences in mean ratios had p values <0.001. The relation of mean FEV1/FVC to reported alcohol intake presented in Table 1 was generally consistent in analyses among multiple stratified groups.
Table 2.
Selected traits of study population and mean FEV1/FVC
| Trait | Number (%) | Mean FEV1/FVC | p < 0.001 |
| All | 177,721 (100) | 0.779 | — |
| Sex | |||
| Women | 96,223 (54.1) | 0.787 | Versus men |
| Men | 81,498 (45.9) | 0.771 | Versus women |
| Age (years) | |||
| <40 | 83,485 (47.0) | 0.794 | Versus each other bracket |
| 40–49 | 36,871 (20.8) | 0.783 | Versus each other bracket |
| 50–59 | 32,001 (18.0) | 0.775 | Versus each other bracket |
| 60–69 | 18,864 (10.5) | 0.760 | Versus each other bracket |
| >70 | 6,500 (3.7) | 0.745 | Versus each other bracket |
| Ethnicity | |||
| White | 136,997 (77.1) | 0.768 | Versus each other ethnicity |
| African American | 26,409 (14.9) | 0.786 | Versus white, other |
| Asian American | 7,248 (4.1) | 0.783 | Versus white |
| Other | 7,067 (4.0) | 0.779 | Versus white, African American |
| Smoking history | |||
| Never | 67,608 (38.0) | 0.790 | Versus each, except unknown |
| Ex-smoker | 30,338 (17.1) | 0.782 | Versus each, except unknown |
| <1 ppd | 37,875 (21.3) | 0.775 | Versus each, except unknown |
| ≥1 ppd | 37,623 (21.2) | 0.763 | Versus each, except unknown |
| Unknown | 4,277 (2.4) | 0.786 | Versus <1 ppd; ≥1 ppd |
| Alcohol in past year—drinks per day | |||
| None | 38,074 (21.4) | 0.779 | Versus each other, except ≥6 |
| ≤2 | 107,827 (60.7) | 0.787 | Versus each other, except 3–5 |
| 3–5 | 14,265 (8.0) | 0.783 | Versus each other, except ≥6 |
| ≥6 | 4,084 (2.3) | 0.773 | Versus 3–5, ≥2 |
| Unknowna | 13,471 (7.6) | 0.772 | Versus each other, except ≥6 |
| Body mass index (kg/m2) | |||
| <25 | 88,146 (49.6) | 0.774 | Versus each other |
| 25–29 | 50,599 (28.5) | 0.783 | Versus each other |
| ≥30 | 15,321 (6.6) | 0.790 | Versus each other |
| Unknown | 23,655 (13.3) | 0.769 | Versus each other |
| Education | |||
| No college | 86,712 (48.8) | 0.773 | Versus each, except unknown |
| Some college | 49,770 (28.0) | 0.780 | Versus each other |
| College graduate | 36,243 (20.3) | 0.786 | Versus each other |
| Unknownb | 4,996 (2.8) | 0.769 | Versus each, except no college |
| Possible baseline cardiorespiratory illnessc | |||
| Yes | 108,400 (61.0) | 0.770 | Versus no |
| No | 69,321 (39.0) | 0.789 | Versus yes |
a Analysis of variance; covariates were age, sex, ethnicity, body mass index, education, smoking, alcohol, and baseline illness composite.
b Nonresponse or conflicting response.
c Any of 47 medical history or symptom items “yes.”
FEV1 = one-second forced expiratory volume; FVC = forced vital capacity; FEV1/FVC = ratio of FEV1 to FVC; ppd = pack(s) per day.
Logistic Models for Low FEV1/FVC Ratio
Adjusted logistic models with the FEV1/FVC ratio dichotomized as <0.7 and ≥0.7 showed that compared with nondrinkers, persons reporting two or fewer drinks per day and those reporting three to five drinks per day were less likely to have low ratios (Table 3). This finding was consistent in most subgroup analyses, with persons younger than 40 years being a noteworthy exception. In the various age strata, the largest apparent reduction (35%) in low FEV1/FVC ratio among the light drinkers was in those who were 60 to 69 years old.
Table 3.
Adjusteda odds ratio of FEV1/FVC <0.7 in selected groups according to alcohol intake
| Group | n (percentage of total 177,721) with FEV1/FVC <0.7 | ≤2 drinks per day | 3–5 drinks per day | ≥6 drinks per day | ||
| OR versus nondrinkers (nondrinkers were referent: OR = 1.00) | ||||||
| All | 33,532 (18.9) | 0.82b | 0.88b | 1.04 | ||
| Men | 16,694 (20.5) | 0.83b | 0.87b | 1.08 | ||
| Women | 16,838 (17.6) | 0.81b | 0.91 | 0.91 | ||
| Age <40 years | 11,237 (13.5) | 0.96 | 1.08 | 1.35b | ||
| Age 40–49 years | 5,809 (15.8) | 0.83b | 0.93 | 1.02 | ||
| Age 50–59 years | 5,629 (17.6) | 0.69b | 0.83 | 1.05 | ||
| Age 60–69 years | 4,217 (22.4) | 0.65b | 0.66b | 0.91 | ||
| Age >70 years | 1,730 (26.6) | 0.73b | 0.80 | 1.45 | ||
| White | 26,991 (19.7) | 0.83b | 0.88b | 1.05 | ||
| African American | 4,105 (15.5) | 0.83b | 0.91 | 0.98 | ||
| Asian American | 1,197 (16.5) | 0.86 | 0.92 | 1.20 | ||
| Other ethnicity | 1,239 (17.5) | 0.88 | 1.02 | 1.37 | ||
| Never-smoker | 10,620 (15.7) | 0.83b | 0.92 | 1.00 | ||
| Ex-smoker | 5,561 (18.3) | 0.75b | 0.77 | 0.98 | ||
| Smoke <1 ppd | 7,241 (19.1) | 0.89 | 0.92 | 1.06 | ||
| Smoke ≥1 ppd | 9,384 (24.9) | 0.87b | 0.94 | 1.22 | ||
aVersus FEV1/FVC ≥0.7 by logistic regression among 177,721 examinees for age, ethnicity, body mass index, education, smoking, alcohol, and cardiorespiratory composite.
bp < 0.001.
FEV1/FVC = ratio of one-second forced expiratory volume to forced vital capacity; OR = odds ratio; ppd = pack(s) per day.
Using the more stringent cutoff point of lowest 10% of ratio, with FEV1/FVC <0.63, the U-shaped relation of alcohol drinking to reduced FEV1/FVC ratio had a slightly deeper nadir. With this definition, the ORs (95% CI) versus nondrinkers were as follows: two or fewer drinks per day = 0.79 (0.75–0.82; p < 0.0001); three to five drinks per day = 0.87 (0.81–0.94; p = 0.0002); and six or more drinks per day = 1.11 (0.99–1.23; p = 0.07). The relations of selected covariates to OR of FEV1/FVC ratio <0.7 are shown in Table 4. The strongest relations were with increasing age (ORs were more than double at ≥70 years vs <40 years), smoking (ORs were 71% higher for smokers of one or more pack per day vs never-smokers), and for the CR composite (ORs increased 45% for CR “yes” vs “no”).
Table 4.
Adjusteda odds ratio of FEV1/FVC <0.7 for selected covariate relations
| Covariate (referent) | Odds ratio | 95% confidence interval | p value |
| Men (women) | 1.22 | 1.19–1.26 | <0.0001 |
| Age 50–59 years (<40 years) | 1.29 | 1.25–1.34 | <0.0001 |
| Age 60–69 years (<40 years) | 1.76 | 1.69–1.83 | <0.0001 |
| Age ≥70 years (<40 years) | 2.27 | 2.14–2.41 | <0.0001 |
| African American (white) | 0.80 | 0.77–0.83 | <0.0001 |
| Asian American (white) | 0.89 | 0.83–0.95 | 0.0003 |
| Other ethnicity (white) | 0.91 | 0.85–0.97 | 0.002 |
| College graduate (no college) | 0.82 | 0.79–0.84 | <0.0001 |
| Ex-smoker (never) | 1.15 | 1.11–1.20 | <0.0001 |
| Smoke <1 ppd (never) | 1.34 | 1.29–1.39 | <0.0001 |
| Smoke ≤1 ppd (never) | 1.71 | 1.65–1.77 | <0.0001 |
| Compositeb “yes” (“no”) | 1.45 | 1.41–1.48 | <0.0001 |
a Versus FEV1/FVC ≥0.7 by logistic regression models for age, ethnicity, body mass index, education, smoking, alcohol, and cardiorespiratory composite.
b “Yes” if positive to any of 47 history and/or symptom items.
FEV1/FVC = ratio of one-second forced expiratory volume to forced vital capacity; ppd = pack(s) per day.
Stratified Models for Cardiorespiratory Composite “Yes” or “No”
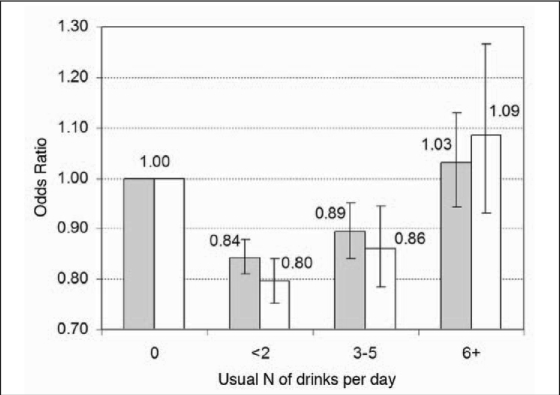
Figure 1 and Table 5 show the ORs of FEV1/FVC ratio <0.7 stratified into CR composite “yes” and “no” groups. Table 5 presents data for the alcohol categories of two or fewer and three to five drinks per day in a number of selected groups. The reduced proportions of low ratios among drinkers were slightly greater in the groups without the CR composite. Thus, study participants without a history of possible baseline cardiovascular or lung problems had slightly deeper nadirs to the curve relating alcohol drinking to low FEV1/FVC ratios, but this disparity was substantially due to the data for women. For men, the reduction in OR for low FEV1/FVC at two or fewer drinks per day was 17% in both strata; whereas in women, it was 24% lower among those in the CR “no” group and 16% lower in the CR “yes” group. Other strata showing deeper U curves within the CR “no” stratum were persons ≥50 years old, never-smokers, and study participants with a BMI <25 kg/m2.
Figure 1.
Odds ratios (OR) for the ratio of one-second forced expiratory volume to forced vital capacity at <0.7 according to reported alcohol intake, with study participants stratified according to cardiorespiratory (CR) composite status. CR status “yes” participants (n = 108,400; shaded bars) responded positively to one or more of 47 items indicating possible cardiovascular or pulmonary disease. CR status “no” participants (n = 69,321; clear bars) responded positively to none of the 47 items. OR were determined by logistic regression in models that included age, ethnicity, body mass index, education, smoking, and alcohol intake. Alcohol abstainers were the referent for other alcohol categories.
Table 5.
Adjusteda odds ratio of FEV1/FVC <0.7 for drinkers reporting ≥2 or 3–5 drinks per day stratified by cardiorespiratory history
| History “Yes”b | History “No”b | |||
| Group | ≤2 drinks per day vs nondrinkers | 3–5 drinks per day vs nondrinkers | ≤2 drinks per day vs nondrinkers | 3–5 drinks per day vs nondrinkers |
| All | 0.84c | 0.89c | 0.79c | 0.86 |
| Men | 0.83c | 0.86c | 0.83c | 0.90 |
| Women | 0.84c | 0.95 | 0.76c | 0.83 |
| Age <40 years | 0.95 | 0.98 | 0.86 | 0.98 |
| Age 40–49 years | 0.82c | 0.91 | 0.87 | 0.90 |
| Age 50–59 years | 0.80c | 0.88 | 0.71c | 0.75 |
| Age 60–69 years | 0.76c | 0.75c | 0.67c | 0.74 |
| Age ≥70 years | 0.71c | 0.73 | 0.81 | 1.01 |
| White | 0.85c | 0.90 | 0.80c | 0.84 |
| African American | 0.85c | 0.90 | 0.81c | 1.00 |
| Asian American | 0.92 | 1.00 | 0.77 | 0.81 |
| Other ethnicity | 0.87 | 0.93 | 0.93 | 1.42 |
| Never-smoker | 0.86c | 0.89 | 0.78c | 0.97 |
| Ex-smoker | 0.73c | 0.77c | 0.79c | 0.76 |
| Smoke <1 ppd | 0.90 | 0.96 | 0.86 | 0.85 |
| Smoke ≥1 ppd | 0.88 | 0.94 | 0.86 | 0.94 |
| BMI <25 kg/m2 | 0.90c | 0.96 | 0.77c | 0.87 |
| BMI 25–29 kg/m2 | 0.77c | 0.88 | 0.79c | 0.83 |
| BMI ≥30 kg/m2 | 0.80 | 0.90 | 0.87 | 0.65 |
a Versus FEV1/FVC ≥0.7 by logistic regression models for age, ethnicity, BMI, education, smoking, alcohol, and CR composite.
b Reply of yes to any of 47 history and/or symptom items. The “yes” group included 108,400 study participants—22,993 (21%) with a ratio of <0.7; the “no” group included 69,237 study participants—10,539 (15%) with a ratio of <0.7.
c p < 0.001.
BMI = body mass index; FEV1/FVC = ratio of one-second forced expiratory volume to forced vital capacity; ppd = pack(s) per day.
Discussion
The main finding of our study is that light to moderate drinkers of alcohol have better LAF than alcohol abstainers. Although this difference is modest in absolute magnitude, the p values, the consistency in stratified subgroup analyses, and the independence from strictly defined baseline illness make a chance difference unlikely. However, because these data are cross-sectional, interpretation of better LAF as a possible causal benefit of alcohol drinking requires great caution.
One important problem of alcohol categorization relevant to this study, known as the “sick quitter” hypothesis,18 is present in analyses that use all nondrinkers as the referent group. Such categorization fails to separate ex-drinkers from lifelong abstainers. Because ex-drinkers include some who quit drinking because of alcohol-related or other medical problems, this could increase the likelihood of illness among the non-drinker category and make light to moderate drinkers spuriously appear healthier. This issue has been raised for observational studies that show less coronary artery disease risk among light to moderate drinkers than among abstainers.18 Although the alcohol-coronary instance is refuted by studies that use lifelong abstainers as the referent,19 alcohol data for the present analysis of LAF does not enable such direct refutation.
… light to moderate drinkers of alcohol have better lung airway flow than alcohol abstainers.
We attempted to deal with the “sick quitter” problem by studying the subcohort with no evidence of CR disease. A caveat is that because many healthy persons have nonspecific CR symptoms, a proportion of persons in the CR “yes” group were probably free of actual CR disease. In creating this CR composite, we intended to be inclusive in order to derive a group truly free of CR disease. We reason that the “no CR” group, with negative responses to all queries, was unlikely to have quit drinking because of cardiovascular or lung disease. Thus, the better LAF in light to moderate drinkers in this subgroup adds substantial credibility to a possible lung function benefit of light drinking.
The broadness of the category of two or fewer drinks per day precludes ascertainment of a possible threshold. These persons composed more than half of study participants, and, even assuming truthful reporting, include a range from occasional drinking (less than one drink per month) to intake of two large drinks daily. Furthermore, the group almost surely includes heavier drinkers who underreport. By inclusion of some heavy drinkers as “light to moderate” drinkers, underreporting, in a situation where light but not heavy drinking has a possible benefit, diminishes the apparent benefit. In this connection, the decreased prevalence of impaired LAF among those reporting having three to five drinks per day strengthens the validity of our main finding.
These measurements were performed with equipment that was technically inferior to more modern lung-testing machines. Thus, technical factors might be partially responsible for the relatively low FEV1 and FVC numbers we obtained (Table 1). However, the implausibility of a systematic relation of technical test aspects to alcohol drinking habits leaves these data valid for the analyses we did.
The FEV1/FVC ratio is widely used as a screen for COPD. Because COPD is primarily a disease of smokers, the strong relationship between smoking and drinking20,21 makes it difficult to eliminate confounding when analyzing the possible role of alcohol in this condition. Thus, the lesser likelihood of a low FEV1/FVC among never-smokers in our data (Table 4) indicates independence of the finding from confounding by smoking.
A few reports have suggested a possible benefit by light to moderate alcohol intake for COPD. A retrospective autopsy study among male veterans showed an inverse relationship of alcohol consumption to emphysema.22 The Lung Health Study in 5887 Canadian smokers with airways obstruction23 found a significant protective effect of moderate drinking in men, but not women, for both hospitalizations and deaths. A 20-year mortality study among 2953 middle-aged men from several European countries24 showed a U-shaped relation between alcohol and COPD mortality.
Speculative mechanisms of potential benefit for LAF by moderate alcohol drinking include anti-inflammatory effects,22 improved mucociliary clearance,1,25 direct bronchodilation,1 and antioxidant effects.12 Antioxidants in alcoholic beverages are most plentiful as nonalcohol phenolics, especially in red wine.26,27 A report of possible specific benefit for LAF by wine drinking12 found slightly more benefit for white than for red wine. We have no data in our study cohort about beverage choice.
Although benefit by alcohol is one possible explanation for our data, the numerous well-established harmful effects of heavy drinking include impaired lung defenses,1,28,29 with resultant increased susceptibility to infections. This disparity between the possible effects of moderate and heavy drinking must be kept in mind when considering advice to individuals or the general public.
Conclusion
Our study in a large, free-living, multiethnic population found better LAF among light to moderate drinkers than among abstainers, independent of smoking and evident lung or heart disease. Drinking moderate amounts of alcoholic beverages may have some benefit for lung function.
Disclosure Statement
This work was supported by a Community Budget grant from the Kaiser Permanente Medical Care Program.
Acknowledgments
Katharine O'Moore-Klopf, ELS, of KOK Edit provided editorial assistance.
Soul of the healer
“The Cafe” Oil on canvas 24 × 36” By C Shore
Ms Shore studies under the tutelage of Richard Morris, at the University of California Los Angeles, at international workshops, and with Nan Rae Parker at her Pasadena Studio. Ms Shore is a Kaiser Permanente member. She has been an educator in the Los Angeles Unified School District, employing all forms of art within the classroom. She found that being bilingual and using art throughout the curriculum were successful tools to teach subject matter to limited English proficient students. Ms Shore continues to advance her education in art and language.
More of Ms Shore's art may be seen on her Web site: www.fineartbycarolyn.com.
References
- 1.Sisson JH. Alcohol and airways function in health and disease. Alcohol. 2007 Aug;41(5):293–307. doi: 10.1016/j.alcohol.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayres J, Ancic P, Clark TJ. Airways responses to oral ethanol in normal subjects and in patients with asthma. J R Soc Med. 1982 Sep;75(9):699–704. doi: 10.1177/014107688207500905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banner AS. Alcohol and the lung. Chest. 1980 Apr;77(4):460–1. doi: 10.1378/chest.77.4.460. [DOI] [PubMed] [Google Scholar]
- 4.Hrubec Z, Cederlof R, Friberg L, Horton R, Ozolins G. Respiratory symptoms in twins: effects of residence-associated air pollution, tobacco and alcohol use, and otherfactors. Arch Environ Health. 1973 Sep;27(3):189–95. doi: 10.1080/00039896.1973.10666350. [DOI] [PubMed] [Google Scholar]
- 5.Emirgil C, Sobol BJ, Heymann B, Shibutani K. Pulmonary function in alcoholics. Am J Med. 1974 Jul;57(1):69–77. doi: 10.1016/0002-9343(74)90770-0. [DOI] [PubMed] [Google Scholar]
- 6.Emirgil C, Sobol BJ. Pulmonary function in former alcoholics. Chest. 1977 Jul;72(1):45–51. doi: 10.1378/chest.72.1.45. [DOI] [PubMed] [Google Scholar]
- 7.Lyons DJ, Howard SV, Milledge JS, Peters TJ. Contribution of ethanol and cigarette smoking to pulmonary dysfunction in chronic alcoholics. Thorax. 1986 Mar;41(3):197–202. doi: 10.1136/thx.41.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garshick E, Segal MR, Worobec TG, Salekin CM, Miller MJ. Alcoholconsumption and chronic obstructive pulmonary disease. Am Rev Respir Dis. 1989 Aug;140(2):373–8. doi: 10.1164/ajrccm/140.2.373. [DOI] [PubMed] [Google Scholar]
- 9.Lange P, Groth S, Mortensen J, et al. Pulmonary function is influenced by heavy alcohol consumption. Am Rev Respir Dis. 1988 May;137(5):1119–23. doi: 10.1164/ajrccm/137.5.1119. [DOI] [PubMed] [Google Scholar]
- 10.Cohen BH, Celentano DD, Chase GA, et al. Alcohol consumption and airway obstruction. Am Rev Respir Dis. 1980 Feb;121(2):205–15. doi: 10.1164/arrd.1980.121.2.205. [DOI] [PubMed] [Google Scholar]
- 11.Lebowitz MD. Respiratory symptoms and disease related to alcohol consumption. Am Rev Respir Dis. 1981 Jan;123(1):16–9. doi: 10.1164/arrd.1981.123.1.16. [DOI] [PubMed] [Google Scholar]
- 12.Schünemann HJ, Grant BJ, Freudenheim JL, et al. Evidence for a positive association between pulmonary function and wine intake in a population-based study. Sleep Breath. 2002 Dec;6(4):161–73. doi: 10.1007/s11325-002-0161-6. [DOI] [PubMed] [Google Scholar]
- 13.Sisson JH, Stoner JA, Romberger DJ, et al. Alcohol intake is associated with altered pulmonary function. Alcohol. 2005 May;36(1):19–30. doi: 10.1016/j.alcohol.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Sparrow D. Rosner B, Cohen M, Weiss ST. Alcohol consumption and pulmonary function. A cross-sectional and longitudinal study. Am Rev Respir Dis. 1983 Jun;127(6):735–8. doi: 10.1164/arrd.1983.127.6.735. [DOI] [PubMed] [Google Scholar]
- 15.Zureik M, Kauffmann F, Touboul PJ, Courbon D, Ducimetière P. Association between peak expiratory flow and the development of carotid atherosclerotic plaques. Arch Intern Med. 2001 Jul 9;161(13):1669–76. doi: 10.1001/archinte.161.13.1669. [DOI] [PubMed] [Google Scholar]
- 16.Twisk JWR, Staal BJ, Brinkman MN, Kemper HC, van Mechelen W. Tracking of lung function parameters and the longitudinal relationshipwith lifestyle. Eur Respir J. 1998 Sep;12(3):627–34. doi: 10.1183/09031936.98.12030627. [DOI] [PubMed] [Google Scholar]
- 17.Collen MF, Davis LF. The multitestlaboratory in health care. J OccupMed. 1969 Jul;11(7):355–60. [PubMed] [Google Scholar]
- 18.Shaper AG, Wannamethee G, Walker M. Alcohol and mortality in British men: explaining the U-shaped curve. Lancet. 1988 Dec 3;2(8623):1267–73. doi: 10.1016/s0140-6736(88)92890-5. [DOI] [PubMed] [Google Scholar]
- 19.Klatsky AL, Udaltsova N. Alcoholdrinking and total mortality risk. Ann Epidemiol. 2007 May;17(5 Supp 1):S63–7. [Google Scholar]
- 20.Klatsky AL, Friedman GD, Siegelaub AB. Alcohol consumption before myocardial infarction. Results from the Kaiser-Permanente epidemiologic study of myocardial infarction. Ann Intern Med. 1974 Sep;81(3):294–301. doi: 10.7326/0003-4819-81-3-294. [DOI] [PubMed] [Google Scholar]
- 21.Klatsky AL, Friedman GD, Siegelaub AB. Alcohol and tobacco: relations and possible interactions in health and disease. In: Avogaro P, Sirtori CR, Tremoli E, editors. Metabolic effects of alcohol. The Netherlands: North-Holland Biomedical Press; 1979. pp. 143–54. [Google Scholar]
- 22.Pratt PC, Vollmer RT. The beneficial effect of alcohol consumption on the prevalence and extent of centrilobular emphysema. A retrospective autopsy analysis. Chest. 1984 Mar;85(3):372–7. doi: 10.1378/chest.85.3.372. [DOI] [PubMed] [Google Scholar]
- 23.Murray RP, Connett JE, Tyas SL, et al. Alcohol volume, drinking pattern, and cardiovascular disease morbidity and mortality: is there a U-shaped function? Am J Epidemiol. 2002 Feb 1;155(3):242–8. doi: 10.1093/aje/155.3.242. [DOI] [PubMed] [Google Scholar]
- 24.Tabak C, Smit HA, Rasanen L, et al. Alcohol consumption in relation to 20-year COPD mortality and pulmonary function in middle-aged men from three European countries. Epidemiology. 2001 Mar;12(2):239–45. doi: 10.1097/00001648-200103000-00018. [DOI] [PubMed] [Google Scholar]
- 25.Elliott MK, Sisson JH, Wyatt TA. Effects of cigarette smoke and alcohol on ciliated tracheal epithelium and inflammatory cell recruitment. Am J Respir Cell Mol Biol. 2007 Apr;36(4):452–9. doi: 10.1165/rcmb.2005-0440OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rimm E, Klatsky AL, Grobbee D, Stampfer MJ. Review of moderate alcohol consumption and reduced risk of coronary heart disease: is the effect due to beer, wine, or spirits. BMJ. 1996 Mar 23;312(7033):731–6. doi: 10.1136/bmj.312.7033.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Booyse FM, Parks DA. Moderatewine and alcohol consumption: beneficial effects on cardiovascular disease. Thromb Haemost. 2001 Aug;86(2):517–28. [PubMed] [Google Scholar]
- 28.Bomalaski JS, Phair JP. Alcohol, immunosuppression, and thelung. Arch Intern Med. 1982 Nov;142(12):2073–4. [PubMed] [Google Scholar]
- 29.Happel KI, Nelson S. Alcohol, immunosuppression, and the lung. Proc Am Thorac Soc. 2005;2(5):428–32. doi: 10.1513/pats.200507-065JS. [DOI] [PubMed] [Google Scholar]