Abstract
Purpose
We aimed to study the intracellular expression of 5-lipoxygenase (5-LOX), the primary competitor with cyclooxygenase-2 in arachidonic acid metabolism, as inflammatory enzymes may be involved in blocking apoptosis and promoting cancer growth by changing arachidonic acid metabolism within cells. Our purpose was to investigate the possible connection between 5-LOX expression and colon carcinogenesis by characterizing 5-LOX expression in histologically different colonic adenomas, determining the relationship between high expression of 5-LOX and various conventional clinicopathological features of adenomas, and finally characterizing the histological localization of cells with 5-LOX overexpression.
Methods
A total of 111 patients were examined and 120 histologically different colonic adenomas analyzed (including four cases of intramucosal adenocarcinoma in a polyp). Immunohistochemical staining with polyclonal anti-5-LOX antibodies was performed.
Results
There was a significant correlation between high 5-LOX expression and patient age, increased polyp size, high grade of intraepithelial neoplasia, villous and tubulovillous adenoma, and histological epithelial localization.
Conclusions
We observed a strong positive correlation between 5-LOX overexpression and the appearance of typical high-risk factors for malignant transformation in adenomatous polyps. The results support the role of 5-LOX in early stages of colon carcinogenesis.
Keywords: Colonic adenomas, 5-Lipoxygenase, Immunohistochemistry, Colon carcinogenesis
Introduction
Early diagnosis and efficacious treatment of colorectal cancer (CRC) are among the greatest challenges for contemporary gastroenterologists and oncologists. According to current data published by the National Cancer Institute (Bethesda, MD, USA), CRC is the second most common malignancy in both males and females, with high mortality in the USA and Western Europe [1]. It had been proven that over 90% of all CRCs result from adenomatous polyps. Multiple clinical, morphological, and genetic research studies have established the following carcinogenetic sequence of events: normal healthy mucosa → adenoma → adenocarcinoma. Few CRC cases grow de novo as flat mucosal changes [2].
Not every colonic adenoma involves the same risk of malignant transformation. It is believed that only about 5% of all patients with detected adenomas will develop cancer. The commonly recognized risk factors for malignant transformation of colonic adenomas include a polyp size of over 10 mm, villous histological type, high degree of epithelial cell neoplasia, and total number of adenomatous polyps observed during colonoscopy [1, 2].
Living organisms are protected against the development of cancer, among other things, through a natural process of programmed cell death—apoptosis. There is a close relationship between apoptosis and the intracellular concentration of arachidonic acid and its derivatives. An extremely low concentration of these compounds is not sufficient to trigger the apoptotic signal cascade, and the “ageing” cell may be susceptible to neoplastic changes. The primary groups of eicosanoids are prostaglandins, prostacyclin, thromboxanes, leucotrienes, and lipoxins. Two enzymatic pathways are necessary for the synthesis of these compounds: the cyclooxygenase (COX) pathway and lipoxygenase (LOX) pathway [3].
5-Lipoxygenase is involved in eicosanoid metabolism. Several isoforms of this enzyme have been previously described. These are designated according to the arachidonic acid derivatives, which are substrates for the specific metabolic reaction catalyzed by them. These include 5-LOX, 8-LOX, 12-LOX, 15-LOX-1, and 15-LOX-2 [4, 5]. It has been shown that both the 5-LOX and 12-LOX pathways play a significant role in promoting tumor growth [6–9].
In our study, we have focused on the 5-LOX enzyme—the most metabolically active LOX isoform. 5-Lipoxygenase overexpression has been demonstrated in several human cancers [10–15]. Soumaoro et al. found that 5-LOX expression in human colorectal cancer correlated with tumor size, depth, and vessel invasion [16]. Little is known about the role of 5-LOX and other enzymes of this group in oncogenesis; thus, there is a need to continue and extend research into the origins of malignant changes in the large bowel. The aim of our study is to determine the expression of 5-LOX in colonic adenomas of different histological structures and to examine the relationship between the expression of this enzyme and conventional clinicopathological factors of adenomas. We also aim to establish the tissue layer of the adenoma in which the high expression of 5-LOX was most frequently seen.
Materials and methods
Tissue material
In total, 120 colonic adenomas were examined after removal from 111 patients. Fifty-two (43.3%) adenomas were extracted from 48 women (aged 36–81 years, median =64.5, SD = ±11.2), and 68 (56.7%) adenomas were extracted from 63 men (aged 42–85 years; median =63.0, SD = ±10.7). Polypectomy procedures were carried out in the Gastroenterology Department of Pomeranian Medical University in Szczecin, Poland. This study did not include hyperplastic polyps or serrated adenomas (due to their differing pathogenic origins). Polyps obtained from patients with an inflammatory bowel disease and polyposis syndrome were excluded. All collected adenomas were fixed immediately with a 10% solution of formalin and embedded in paraffin in the Pathology Department of Medical Faculty, Pomeranian Medical University in Szczecin, Poland. Primary adenoma sections were cut from paraffin blocks for microscopic slides and stained with hematoxylin and eosin and then were examined and classified into different groups by a pathologist, according to the features listed in Tables 1 and 2.
Table 1.
Clinicopathological variables of examined adenomas
| Clinicopathological variables | n (%) |
|---|---|
| Age of patientsa, years | |
| <60 | 48 (40%) |
| ≥60 | 72 (60%) |
| Adenoma type | 116 (97%) |
| Tubular | 55 (46%) |
| Tubulovillous | 49 (41%) |
| Villous | 12 (10%) |
| Adenocarcinoma (intraepithelial) | 4 (3%) |
| Size (mm) | |
| <10 | 60 (50%) |
| ≥10 | 60 (50%) |
| Grade of intraepithelial neoplasiab | |
| Low | 49 (42%) |
| Moderate | 33 (28%) |
| High | 34 (30%) |
| Adenoma location | |
| Proximalc | 34 (28%) |
| Cecum | 6 (5%) |
| Ascending colon | 15 (12.5%) |
| Transverse colon | 13 (11%) |
| Distal | 86 (72%) |
| Descending colon | 11 (9%) |
| Sigmoid colon | 39 (32.5%) |
| Rectum | 36 (30%) |
| Number of adenomas during colonoscopy (in 111 patients) | |
| 1 | 49 (44%) |
| 2–5 | 50 (45%) |
| 6–10 | 5 (4.5%) |
| >10 | 7 (6.5%) |
aTotal of 120 adenomas from 111 patients
bWithout four intraepithelial adenocarcinomas
cWith regard to splenic flexure
Table 2.
Clinicopathological variables and 5-LOX expression of examined polyps
| Clinicopathological variables | n (patients) | 5-LOX expression | p value | |
|---|---|---|---|---|
| Normal | Overexpression | |||
| Age of patients, years | ||||
| <60 | 48 | 22 | 26 | 0.01 |
| ≥60 | 72 | 17 | 55 | |
| Gender | ||||
| Females | 48 | 13 | 35 | NS |
| Males | 63 | 19 | 44 | |
| Size (mm) | ||||
| <10 | 60 | 33 | 27 | <0.05 |
| ≥10 | 60 | 6 | 54 | |
| Grade of intraepithelial neoplasia | ||||
| Low | 49 | 17 | 32 | 0.02 |
| Moderate | 33 | 15 | 18 | |
| High | 34 | 6 | 28 | |
| Adenoma location | ||||
| Proximal | 34 | 14 | 20 | NS |
| Distal | 86 | 25 | 61 | |
| Number of adenomas during colonoscopy | ||||
| 1 | 49 | 14 | 35 | NS |
| 2–5 | 50 | 15 | 35 | |
| 6–10 | 5 | 1 | 4 | |
| >10 | 7 | 2 | 5 | |
| Tissue layer with an advantage of 5-LOX overexpressed cells | ||||
| Ea | 84 | 20 | 64 | <0.002 |
| Bb | 25 | 11 | 14 | |
| TSc | 11 | 8 | 3 | |
| Adenoma type | ||||
| Tubular | 55 | 28 | 27 | 0.001 |
| Tubulovillous | 49 | 10 | 39 | |
| Villous | 12 | 0 | 12 | |
aEpithelium
bBalance between epithelium and tissue stroma
cTissue stroma
Immunohistochemistry
Microscopic sections were cut from formalin-fixed adenoma tissues embedded in paraffin. Sections were deparaffinized at 68°C overnight, washed with xylene at the same temperature, and then dehydrated in alcohol. Cell antigens were recovered by boiling thrice for 7 min in citrate buffer (pH 6.0). Slides were washed in phosphate-buffered saline (PBS) solution (pH 7.4), endogenous peroxidase was blocked with a peroxidase blocking reagent (DAKOCytomation S2001), and then slides were washed again in PBS solution (pH 7.4). Next, tissues were exposed to a diluent (Antibody Diluent DAKOCytomation S0809) and incubated at room temperature in a primary rabbit polyclonal antibody anti-5-LOX (Cayman Chemical 160402) solution. All slides were washed in PBS solution (pH 7.4) for the third time and incubated with the secondary antibody (LAB + Kit, HRP DAKOCytomation K0690). After washing with PBS solution (pH 7.4), immunoreaction was visualized by using chromogene (AEC+ High Sensitivity Substrate Chromogene DAKOCytomation K3469). All slides were then washed with double-distilled water. Tissues were then stained with Meyer’s hematoxylin and covered with a mounting medium (Faramount Aqueous Mounting Medium DAKOCytomation S3025).
Microscopic evaluation
An assessment of all tissue samples was performed using the OLYMPUS BX41 light microscope. Every microscopic image, in exactly the same field of view for all slides, was examined simultaneously by two of the authors (MPW and BK) using two independent vision tracks. Both the percentage of 5-LOX-positive cells (quantity score) and the intensity of chromogene staining of these cells (intensity score) were assessed in all tissues. In addition, the location of stained cells was evaluated in terms of their comparative presence in the epithelium or tissue stroma. The scoring system proposed by Soslow with our own modification was used for the classification of tissue images in terms of examined enzyme expression [17]. The field of view under a microscope is divided into four parts with all points from each quadrant noted and analyzed individually. The final points from both assessing doctors are then averaged to promote more highly objective results, which was the aim of our modification of the system. Typical microscopic images of high 5-LOX expression are shown in Figs. 1 and 2. Each of the polyps was scored for each quadrant as follows: staining intensity from the most intense-colored slide’s field of view, enlarged to ×400, was compared to the control slide using a scale of 0–3 points (“0”—negative, “1”—weak, “2”—moderate, “3”—strong) and the percentage of 5-LOX-positive cells was measured on a scale of 0–4 points (“0” = <1%, “1” = 1–25%, “2” = 26–50%, “3” = 51–75%, “4” = 76–100%). The total score from the eight fields of view obtained from both examiners for each of the two categories mentioned above were then averaged to obtain individual end-values. It should be noted that the results generated by the two investigators did not differ significantly; according to current literature, the values obtained in this scoring system by the individual evaluators do not vary significantly from the results obtained by means of a computerized image analyzer [18]. In the present scoring system, the intensity of staining points (0–3) and percentage of 5-LOX-positive cell points (0–4) for all of the slides were multiplied with each other, resulting in final scores ranging from 0 to 12. These values correspond to the expression of the examined enzyme in the tissue; thus, a result of 0–4 points means a total lack or low expression of the enzyme (negative or questionable result = normal/no overexpression) and a result of 5 or greater is considered to be unambiguously positive for overexpression of the enzyme. Finally, the layer of tissue in which 5-LOX expression was the highest was determined: (a) epithelium, (b) tissue stroma, and (c) a similar expression in these two layers (balanced expression).
Fig. 1.
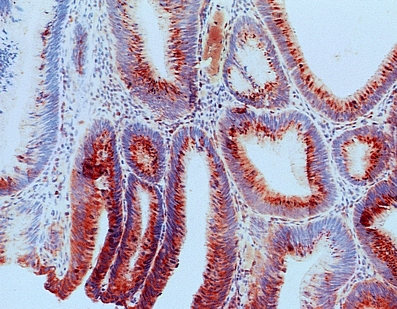
Cells with overexpression of 5-LOX (marked with red chromogene) in tubulovillous adenomas with intraepithelial moderate/high-grade focal neoplasia—positive epithelium [259 × 202 mm (72 × 72 DPI)]
Fig. 2.
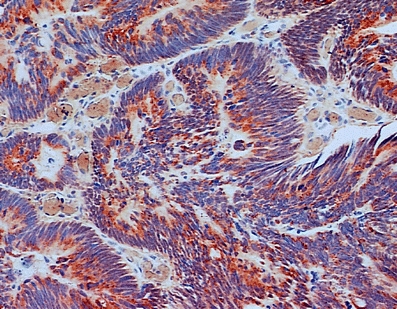
Cells with overexpression of 5-LOX (marked with red chromogene) in early intramucosal adenocarcinomas inside the polyp—positive epithelium
Statistics
All relations (p values) between 5-LOX expression and characteristic features of examined adenomas were calculated using Fisher’s exact method. In addition, the relationship between the adenomas’ size and 5-LOX expression was examined using the U Mann–Whitney nonparametric method; p < 0.05 was considered statistically significant.
Results
Eighty-one (67.5%) out of the 120 examined adenomas showed overexpression of 5-LOX. Thirty-nine (32.5%) adenomas showed either weak or no expression of 5-LOX. There were significant statistical differences between 5-LOX expression and the following parameters: patient age, those who had undergone endoscopic polypectomy, histological type of examined adenomas, grade of intraepithelial neoplasia in adenomas’ epithelium, size of adenomas, adenomas’ location in the large bowel, and tissue layer inside the adenoma.
Age of patients with colorectal adenomas
5-Lipoxygenase overexpression was observed in 55 (76.4%) adenomas taken from patients 60 years or older and in 26 (54.2%) adenomas taken from patients under 60 years of age; this difference was statistically significant (p < 0.0002).
Histological type of adenomas
Seventy-eight (67.2%) adenomas and three (75%) early adenocarcinomas in the polyps showed a high expression of 5-LOX. High enzyme expression was seen in 12 (100%) villous adenomas, 39 (79.6%) tubulovillous adenomas, and 27 (49.1%) tubular adenomas. Statistically significant differences of 5-LOX overexpression were observed between villous adenomas as compared to tubular adenomas (p < 0.001) and between tubulovillous adenomas as compared to tubular adenomas (p = 0.001). There was no significant difference between villous and tubulovillous adenomas.
Grade of adenomas’ intraepithelial neoplasia
5-Lipoxygenase overexpression was seen in 28 (82.4%) adenomas with high-grade intraepithelial neoplasia and in 50 (61%) adenomas with a low or moderate grade of intraepithelial neoplasia in the epithelium. A statistically significant difference was observed between different grades of intraepithelial neoplasia—5-LOX overexpression was found to be more common in the epithelium of polyps with a high-grade neoplastic change compared to those with lesser intraepithelial neoplasia (p = 0.026). Four cases of early adenocarcinoma in polyps were analyzed together with the group of high-grade intraepithelial neoplasia adenomas due to the very small number of early adenocarcinomas. All other relationships were of no statistical significance.
Size of adenomas
5-Lipoxygenase overexpression was observed in 54 (90%) adenomas with a size of 10 mm or greater and in 27 (45%) adenomas of a size less than 10 mm; the difference in 5-LOX expression between the groups was statistically significant (p < 0.000001). This relationship of 5-LOX overexpression and the size of the adenoma were additionally analyzed with the U Mann–Whitney test, confirming the strong statistical difference (p < 0.000001).
Adenomas’ location in the large bowel
5-Lipoxygenase overexpression was observed in four (66.7%) adenomas removed from the cecum, in ten (66.7%) adenomas from the ascending colon, in six (46.2%) adenomas from the transverse colon, in nine (81.8%) adenomas from the descending colon, in 32 (82.1%) adenomas from the sigmoid colon, and in 20 (55.6%) adenomas from the rectum. Statistically, significant differences of 5-LOX overexpression were seen in the group of transverse colon polyps as compared to those taken from the sigmoid colon (p = 0.01). There was also a significantly more frequent appearance of 5-LOX overexpression among adenomas from the sigmoid colon when compared to the group from the rectum (p = 0.01). Other relationships were not significant.
Tissue layer location of 5-lipoxygenase overexpressed cells in adenomas
In the group in which all 81 examined adenomas had high 5-LOX expression, 64 (79%) had the 5-LOX overexpressed cells located only in the epithelium; in 14 (17.3%) adenomas, we observed 5-LOX-positive cells exhibiting a balanced location—both in the epithelium and in the tissue stroma. In three (7.4%) adenomas, the overexpressed cells were mainly located in the tissue stroma. Each of these differences was statistically significant (for all relations; p < 0.002).
We did not find any significant differences in 5-LOX overexpression related to the gender of the patient or to the total amount of adenomas removed during endoscopic polypectomy. Table 2 shows all primary results.
Discussion
The exact role played by 5-LOX, the main metabolic competitor for COX-2, in the mechanism of colonic polyp formation and transformation to CRC is unknown. Soumaoro et al. found a relationship between 5-LOX overexpression in colon cancer and poor prognosis [16]. Recently, it was shown that 5-LOX is overexpressed in human tubular adenomas of the colon and cancer specimens, whereas it is only seen in scattered cells of normal colonic mucosa [19].
This study, which includes 120 colonic adenomas, comprises one of the largest 5-LOX expression analyses to date. High expression of 5-LOX was shown in 67.5% of cases. In this study, 5-LOX overexpression was associated with histological structure type, degree of intraepithelial neoplasia in epithelial cells, size and location of adenomas in the large bowel, and patient age at endoscopic polypectomy. As we have demonstrated in our previous studies, high 5-LOX expression was associated with the most advanced neoplastic changes, including early adenocarcinomas in adenomatous polyps (three out of four cancers showed a high expression of 5-LOX).
Analyzing the expression of 5-LOX in the group of all adenomas, we observed the highest expression of 5-LOX in all villous adenomas, which is widely recognized as one of the high-risk features associated with malignant transformation. Among tubulovillous and tubular adenomas, the phenomenon of high expression of this enzyme was seen less frequently (in 79% and 49% of cases, respectively). Similar results regarding the expression of COX-2 were obtained by Fujita et al., who observed a high expression of this enzyme in tubulovillous adenoma cells compared to tubular adenomas [20].
The relationship between 5-LOX expression and the presence of intraepithelial neoplasia in the epithelial cells of adenomas was also found. 5-Lipoxygenase overexpression occurred in almost half of all cases of adenomas with a small to medium degree of intraepithelial neoplasia, but more often it was observed within high-grade neoplastic changes (more than 82% of cases).
A positive correlation between the expression of 5-LOX and the size of the adenoma has also been demonstrated. A vast majority of researchers reported a similar correlation between the size and intensity of COX-2 expression in the evaluation of this enzyme [21–24].
In our study, the gender of patients who underwent polypectomy had no effect on the expression of 5-LOX in the tested colonic adenomas, but there was a difference in the expression of this enzyme relating to the patient’s age: adenomas removed from patients over 60 years of age showed an overexpression of 5-LOX more frequently (three fourth of cases).
We observed the highest concentration of 5-LOX in the epithelium only. 5-Lipoxygenase overexpression was only rarely seen simultaneously in epithelial and stromal cells. The most rare localization of overexpressed 5-LOX cells was mucosal stroma without epithelial expression. In their own studies on COX-2, Bamba et al. [25] and Chapple et al. [5] described the partial presence of high expression of this enzyme in cells of epithelial origin, but both authors stressed the advantages of cells with COX-2 overexpression inside the mucosal stroma. Hardwick et al. [26] and Tanaka et al. [27] agreed and similarly found a strong expression of COX-2 in stromal macrophages. Arnoletti et al. published observations regarding the dominance of high COX-2 expression in stromal cells and additionally, in his opinion, the epithelial localization had a direct relationship with the moment of adenomas’ malignant transformation to cancer [18]. If such a hypothesis is correct, it may be that 5-LOX is an even more sensitive indicator of incidence for adenomas with high risk of malignant transformation, but this clearly requires further research. It is possible that we could use some information from such studies to create concepts for future investigations, e.g., create “easy to perform” tests for detecting adenomas with high risk of malignant transformation or find inflammatory enzyme polymorphisms to help develop chemopreventive drugs.
Conclusions
5-Lipoxygenase, marked with immunohistochemical methods, occurred in all types of adenomas.
It is possible that the occurrence of 5-LOX overexpression in adenomas smaller than 10 mm demonstrates a greater risk of malignant transformation, but this requires further investigation.
In colonic adenomas, high expression of 5-LOX, marked with immunohistochemical methods, may be an additional criterion for assessing the risk of transformation into cancer.
Acknowledgment
This study was supported by a grant (NN 402 307236) from the Polish Committee for Scientific Research. All authors declare no conflict of interest to disclose.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Abraham J, Allegra CJ, Gulley J. Bethesda handbook of clinical oncology. Philadelphia: Lippincott, Williams & Wilkins; 2005. [Google Scholar]
- 2.Hauser SC, Pardi DS, Poterucha JJ (2006) Mayo Clinic Gastroenterology and Hepatology Board Review. Mayo Clinic
- 3.Stryer L. Biochemistry. New York: Freeman; 1995. [Google Scholar]
- 4.DuBois RN. Review article: cyclooxygenase—a target for colon cancer prevention. Aliment Pharmacol Ther. 2000;14:64–67. doi: 10.1046/j.1365-2036.2000.014s1064.x. [DOI] [PubMed] [Google Scholar]
- 5.Chapple KS, Cartwright EJ, Hawcroft G, Tiwsbury A, Bonifer C, Scott N, Windsor ACJ, Guillou PJ, Markham AF, Coletta PL, Hull MA. Localisation of cyclooxygenase-2 in human sporadic colorectal adenomas. Am J Pathol. 2000;156:545–553. doi: 10.1016/S0002-9440(10)64759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson KM, Seed T, Meng J, Ou D, Alrefai WA, Harris JE. Five-lipoxygenase inhibitors reduce Panc-1 survival: the mode of cell death and synergism of MK886 with gamma linolenic acid. Anticancer Res. 1998;18:791–800. [PubMed] [Google Scholar]
- 7.Anderson KM, Seed T, Vos M, Mulshine J, Meng J, Alrefai WA, Ou D, Harris JE. 5-lipoxygenase inhibitors reduce PC-3 cell proliferation and initiate nonnecrotic cell death. Prostate. 1998;37:61–173. doi: 10.1002/(SICI)1097-0045(19981101)37:3<161::AID-PROS5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh J, Myers CE. Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proc Natl Acad Sci USA. 1998;95:13182–13187. doi: 10.1073/pnas.95.22.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rioux N, Castonguay A. Inhibitors of lipoxygenase: a new class of cancer chemopreventive agents. Carcinogenesis. 1998;19:1393–1400. doi: 10.1093/carcin/19.8.1393. [DOI] [PubMed] [Google Scholar]
- 10.Hennig R, Ding XZ, Tong WG, Schneider MB, Standop J, Friess H, Büchler MW, Pour PM, Adrian TE. 5-Lipoxygenase and leukotriene B(4) receptor are expressed in human pancreatic cancers but not in pancreatic ducts in normal tissue. Am J Pathol. 2002;161:421–428. doi: 10.1016/S0002-9440(10)64198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta S, Srivastava M, Ahmad N, Sakamoto K, Bostwick DG, Mukhtar H. Lipoxygenase-5 is overexpressed in prostate adenocarcinoma. Cancer. 2001;91:737–743. doi: 10.1002/1097-0142(20010215)91:4<737::AID-CNCR1059>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 12.Ohd JF, Nielsen CK, Campbell J, Landberg G, Löfberg H, Sjölander A. Expression of the leukotriene D4 receptor CysLT1, COX-2, and other cell survival factors in colorectal adenocarcinomas. Gastroenterology. 2003;124:57–70. doi: 10.1053/gast.2003.50011. [DOI] [PubMed] [Google Scholar]
- 13.Yoshimura R, Matsuyama M, Mitsuhashi M, Takemoto Y, Tsuchida K, Kawahito Y, Sano H, Nakatani T. Relationship between lipoxygenase and human testicular cancer. Int J Mol Med. 2004;13:389–393. [PubMed] [Google Scholar]
- 14.Yoshimura R, Matsuyama M, Tsuchida K, Kawahito Y, Sano H, Nakatani T. Expression of lipoxygenase in human bladder carcinoma and growth inhibition by its inhibitors. Clin Cancer Res. 2004;10:6703–6709. doi: 10.1158/1078-0432.CCR-0946-03. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Wang S, Wu N, Sood S, Wang P, Jin Z, Beer DG, Giordano TJ, Lin Y, Shih WC, Lubet RA, Yang CS. Overexpression of 5-lipoxygenase in rat and human esophageal adenocarcinoma and inhibitory effects of zileuton and celecoxib on carcinogenesis. J Urol. 2003;170:1994–1999. doi: 10.1097/01.ju.0000080296.54262.c8. [DOI] [PubMed] [Google Scholar]
- 16.Soumaoro LT, Iida S, Uetake H, et al. Expression of 5-lipoxygenase in human colorectal cancer. World J Gastroenterol. 2006;12:6355–6360. doi: 10.3748/wjg.v12.i39.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, Koki AT. COX-2 is expressed in human pulmonary, colonic and mammary tumors. Cancer. 2000;89:2637–2645. doi: 10.1002/1097-0142(20001215)89:12<2637::AID-CNCR17>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 18.Arnoletti JP, Upson J, Babb JS, Bellacosa A, Watson JC. Differential stromal and epithelial localization of cyclooxygenase-2 (COX-2) during colorectal tumorigenesis. J Exp Clin Cancer Res. 2005;24:279–287. [PubMed] [Google Scholar]
- 19.Melstrom LG, Bentrem DJ, Salabat MR, Kennedy TJ, Ding XZ, Struch M, Rao SM, Witt RC, Ternent CA, Talamonti MS, Bell RH, Adrian TA. Overexpression of 5-lipoxygenase in colon polyps and cancer and the effect of 5-LOX inhibitors in vitro and in a murine model. Clin Cancer Res. 2008;14:6525–6530. doi: 10.1158/1078-0432.CCR-07-4631. [DOI] [PubMed] [Google Scholar]
- 20.Fujita M, Fukui H, Kusaka T, Morita K, Fujii S, Ueda Y, Chiba T, Sakamoto C, Kawamata H, Fujimori T. Relationship between cyclooxygenase-2 expression and K-ras gene mutation in colorectal adenomas. J Gastroenterol Hepatol. 2000;15:1277–1281. doi: 10.1046/j.1440-1746.2000.02399.x. [DOI] [PubMed] [Google Scholar]
- 21.Pisano C, Ottaiano A, Tatangelo F, Di Bonito M, Falanga M, Iaffaioli VR, Botti G, Pignata S, Acquaviva AM. Cyclooxygenase-2 expression is associated with increased size in human sporadic colorectal adenomas. Anticancer Res. 2005;25:2065–2068. [PubMed] [Google Scholar]
- 22.Kim SH, Lee JH, Kang KH, Park JH, Park CK, Cho CM, Kweon YO, Kim SK, Choi YH, Bae HI, Kim MS. Cyclooxygenase-2 expression according to size and location of gastric and colorectal tubular adenomas. Korean J Gastroenterol. 2004;44:206–211. [PubMed] [Google Scholar]
- 23.Elder DJE, Baker JA, Banu NA, Moorghen M, Paraskeva C. Human colorectal adenomas demonstrate a size-dependent increase in epithelial cyclooxygenase-2 expression. J Pathol. 2002;198:428–434. doi: 10.1002/path.1232. [DOI] [PubMed] [Google Scholar]
- 24.Azumaya M, Kobayashi M, Ajioka Y, Honma T, Suzuki Y, Takeuchi M, Narisawa R, Asakura H. Size-dependent expression of cyclooxygenase-2 in sporadic colorectal adenomas relative to adenomas in patients with familial adenomatous polyposis. Pathol Int. 2002;52:272–276. doi: 10.1046/j.1440-1827.2002.01350.x. [DOI] [PubMed] [Google Scholar]
- 25.Bamba H, Ota S, Kato A, Adachi A, Itoyama S, Matsuzaki F. High expression of cyclooxygenase-2 in macrophages of human colonic adenoma. Int J Cancer. 1999;83:470–475. doi: 10.1002/(SICI)1097-0215(19991112)83:4<470::AID-IJC6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 26.Hardwick JC, van den Brink GR, Offerhaus GJ, van Deventer SJ, Peppelenbosch MP. NF-kappaB, p38 MAPK and JNK are highly expressed and active in the stroma of human colonic adenomatous polyps. Oncogene. 2001;20:819–827. doi: 10.1038/sj.onc.1204162. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka S, Tatsuguchi A, Futagami S, Gudis K, Wada K, Seo T, Mitsui K, Yonezawa M, Nagata K, Fujimori S, Tsukui T, Kishida T, Sakamoto C. Monocyte chemoattractant protein 1 and macrophage cyclooxygenase 2 expression in colonic adenoma. Gut. 2006;55:54–61. doi: 10.1136/gut.2004.059824. [DOI] [PMC free article] [PubMed] [Google Scholar]


