Abstract
OBJECTIVE: To describe the effect of a combination prophylactic regimen of levofloxacin, a synthetic chemotherapeutic antibiotic of the fluoroquinolone drug class, with either penicillin or doxycycline on the changing epidemiology of bacterial infections and antimicrobial resistance patterns of isolated organisms in the allogeneic hematopoietic stem cell transplant (HSCT) patient population.
PATIENTS AND METHODS: We conducted a single-center, retrospective cohort study of all allogeneic HSCT recipients from January 1, 2003, through August 31, 2008, who received prophylactic levofloxacin in combination with penicillin (or with doxycycline in penicillin-allergic patients) from allogeneic stem cell infusion until neutrophil engraftment.
RESULTS: Of the 258 patients who underwent allogeneic HSCT during the study period, 231 received levofloxacin prophylaxis, 76 (33%) of whom developed an infection within 3 months after transplant. Over time, the ratio of gram-positive to gram-negative (GN) infections decreased from 2.11 in 2004, the first year that GN organisms were isolated, to 1.11 in 2008 (P=.20). Emergence of fluoroquinolone-resistant GN bacteria was observed (P=.02), whereas resistance to extended-spectrum β-lactams did not change over time. Combined vancomycin-resistant enterococci colonization and infection rates increased during the study period (P=.04). Clostridium difficile colitis was uncommon.
CONCLUSION: Levofloxacin with penicillin or doxycycline prophylaxis may contribute to the emergence of resistant GN infections in allogeneic HSCT recipients over time. Our findings provide additional support for the current standard of practice of administering empiric monotherapy with an antipseudomonal β-lactam if these patients develop fever or are suspected to have an infection.
Levofloxacin with penicillin or doxycycline prophylaxis may contribute to emergence of resistant gram-negative infections in these recipients over time. These findings support the current practice of administering empiric monotherapy with an antipseudomonal β-lactam in those who develop a fever.
BAL = bronchoalveolar lavage; CoNS = coagulase-negative staphylococci; GN = gram-negative; GP = gram-positive; HSCT = hematopoietic stem cell transplant; MDR = multidrug-resistant; VRE = vancomycin-resistant enterococci
The utility of prophylactic antibiotics during the period of chemotherapy-induced neutropenia has been a source of controversy for decades. Reductions in gram-negative (GN) bacteremia, febrile episodes, and hospitalizations have been documented with the use of a prophylactic regimen.1-6 Newer fluoroquinolones, particularly levofloxacin, have emerged as preferred agents because of their potent activity against GN bacteria, enhanced gram-positive (GP) coverage, excellent oral bioavailability, and favorable patient tolerance. Historically, the National Comprehensive Cancer Network, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America have offered conflicting recommendations regarding the use of a prophylactic antibiotic regimen after allogeneic hematopoietic stem cell transplant (HSCT).7-9 Although this patient population is at high risk of fever and subsequent infection during the neutropenic period, a reduction in infection-related mortality rates has not been clearly demonstrated, and emergence of multidrug-resistant (MDR) organisms has remained a concern. Recently, the American Society for Blood and Marrow Transplantation endorsed fluoroquinolone prophylaxis if neutropenia is anticipated for 7 days or more, again emphasizing the risk of perpetuating fluoroquinolone-resistant bacteria.10
Infections caused by MDR organisms are associated with increased mortality, hospital length of stay, and health care costs.11 Given these concerns, the Infectious Diseases Society of America suggests that, if a prophylactic regimen is used in asymptomatic, afebrile, neutropenic patients, hospital and HSCT center antibiotic-susceptibility profiles should be reviewed routinely.8 In allogeneic HSCT recipients, the patient population at highest risk of developing infection during the period of neutropenia, the effect of fluoroquinolone prophylaxis on infections over time has not been adequately described, nor is there adequate information identifying the evolution of resistance patterns of those organisms isolated.
Since 1998, levofloxacin has been used with penicillin (or doxycycline for penicillin-allergic patients) at Mayo Clinic in Rochester, MN, as prophylaxis during the neutropenic period after HSCT. This study aimed to evaluate the impact of this prophylactic regimen on the etiology and resistance profiles of organisms implicated in infection during the first 3 months after allogeneic HSCT. Also of interest was the incidence of Clostridium difficile colitis and vancomycin-resistant enterococci (VRE) colonization and infection in this patient population.
PATIENTS AND METHODS
Study Design
We studied a retrospective cohort of patients who underwent allogeneic HSCT from January 1, 2003, through August 31, 2008. Patients were identified through a database maintained by the Blood and Marrow Transplant Center at our institution. Patients were included in the analysis if they were aged at least 18 years and were taking 500 mg of levofloxacin orally once daily or 750 mg orally once daily before or on the day of transplant as a part of a prophylactic antibiotic regimen. Duplicate isolates, potential colonizing organisms, and organisms isolated without available susceptibility data were excluded. Swabs positive for VRE were limited to 1 occurrence per patient. Similarly, a positive C difficile assay result was considered to be a duplicate if more than 1 result was positive during the first 3 months after transplant.
Institutional Protocols
For allogeneic HSCT recipients, levofloxacin prophylaxis usually begins on day 1 of transplant and is continued for the duration of neutropenia (specifically, until the absolute neutrophil count is >0.5 × 109 /L). On day one, 500 mg of penicillin V potassium orally twice daily (or doxycycline 100 mg orally twice daily in penicillin-allergic patients) is initiated and is continued for at least a year after transplant. Active surveillance for VRE colonization occurs on inpatient admission and twice weekly thereafter while the patient remains hospitalized. A rapid polymerase chain reaction assay is used to establish VRE colonization and the presence of C difficile toxin; however, the former was detected with stool cultures before July 2004 and the latter with Enzyme ImmunoAssay before July 2007.
Definitions
Infection was defined as a test culture positive for an organism isolated from blood, urine, or cerebrospinal, bronchoalveolar lavage (BAL), pleural, or peritoneal fluid. Given that BAL samples come from a nonsterile source, we included isolated bacteria from lavage cultures that were clinically correlated with respiratory infection in the medical record. Duplicate isolates were defined as a positive culture result within a 7-day period with the same organism maintaining an identical susceptibility profile. Any change in susceptibility profile, reappearance of the same organism after a previously negative culture result from the same site, or appearance of the organism in a separate site was determined to be a new infection and counted as a separate occurrence. Suspected colonizers were defined as coagulase-negative staphylococci (CoNS), Corynebacterium spp., Propionibacterium spp., Micrococcus spp., or Stomatococcus spp. isolated from a single culture. If any of these organisms were isolated from 2 consecutive cultures, they were included as an infection. Organisms with intermediate susceptibility were considered nonsusceptible, and a GN MDR pathogen conferred resistance to 2 or more of the following: third- or fourth-generation cephalosporin, fluoroquinolone, carbapenems (eg, imipenem, meropenem), piperacillin-tazobactam, or aminoglycoside.
Statistical Analyses
The GP:GN ratio for bacterial infections was calculated for each year of the study, and the distribution between GP and GN bacterial infections was compared across calendar years using an exact Wilcoxon signed rank test for the ordered contingency table to look for a time trend. Time trends in antibiotic susceptibility were assessed for each drug or drug class using the same method. All tests were 2-sided, and P values <.05 were considered statistically significant.
Ethical Considerations
All study patients gave consent to have their records reviewed for medical studies, pursuant to Minnesota law. This retrospective cohort study was approved by the Mayo Clinic Institutional Review Board.
RESULTS
Patient Demographics
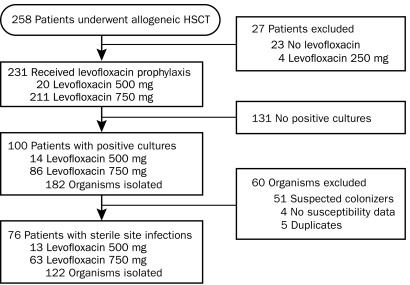
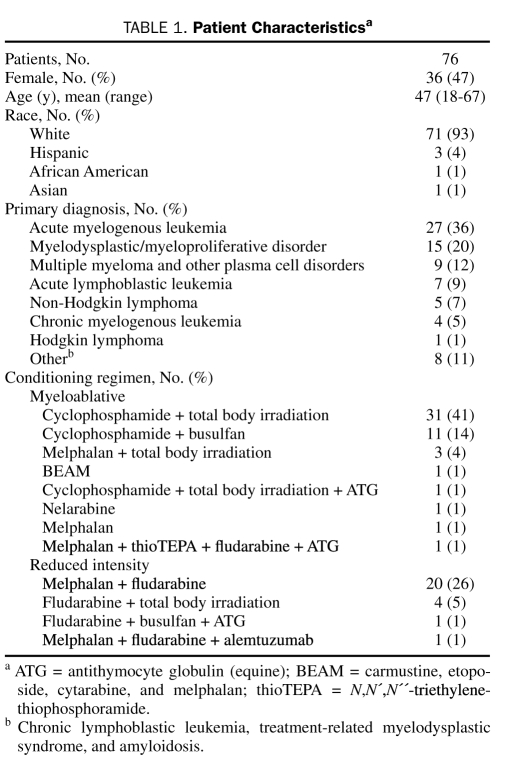
During the study period, 258 adults underwent allogeneic HCST at our institution. Of the 231 who met criteria for inclusion into this study, 76 (33%) developed a qualifying infection within 3 months after transplant (Figure 1). Baseline characteristics of these patients are listed in Table 1. Of the 76 patients, 13 (17%) received 500 mg of levofloxacin orally once daily, and 63 (83%) received 750 mg of levofloxacin orally once daily. Additional antibiotic prophylaxis included penicillin (n=55; 72%) and doxycycline (n=13; 17%). Six patients received levofloxacin monotherapy only, and 2 patients received concomitant vancomycin for GP bacteremia. These patients were pooled for analysis.
FIGURE 1.
Study design. Flow diagram of patient screening and eligibility. HSCT = hematopoietic stem cell transplant.
TABLE 1.
Patient Characteristicsa
The most common indications for allogeneic HSCT were acute myelogenous leukemia (n=27; 36%) and myelodysplastic syndrome (n=15; 20%). Most patients who developed an infection received a myeloablative conditioning regimen (n=50; 66%). The median duration of levofloxacin-based combination antimicrobial prophylaxis was 14 days (range, 3-39 days).
Epidemiology of Infections
The median time from initiation of combination levofloxacin-penicillin or levofloxacin-doxycycline prophylaxis to positive culture result was 27 days (range, 2-99 days). Among 108 positive culture results, 89 (82%) were isolated from blood, 14 (13%) from urine, 4 (4%) from BAL fluid, and 1 (1%) from the eye. A single organism was isolated in most positive cultures (n=95; 88%). Thirteen polymicrobial infections with 27 isolated organisms were identified: 16 GP and 11 GN.
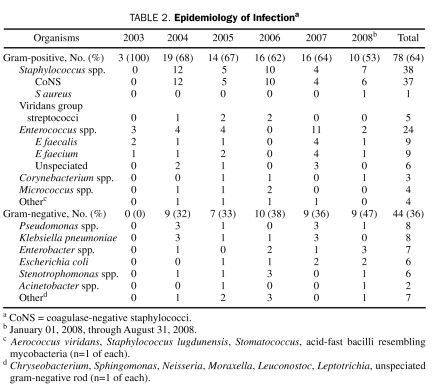
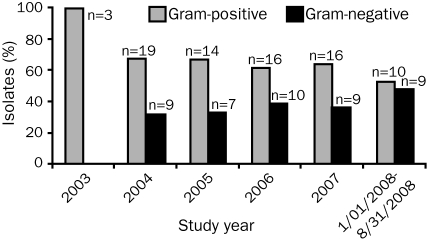
Of the 122 bacterial pathogens isolated, 78 (64%) were GP bacteria and 44 (36%) were GN bacteria. The most common GP organisms recovered were CoNS (n=37; 47%) and Enterococcus spp. (n=24; 31%), whereas the most common GN organisms were Pseudomonas aeruginosa (n=8; 18%), Klebsiella spp. (n=8; 18%), Enterobacter spp. (n=7; 16%), and Escherichia coli (n=6; 14%). A description of the organisms implicated in bacterial infections for each calendar year of the study is provided in Table 2. The ratio of GP:GN organisms decreased over time, from 2.11 in 2004 to 1.11 in 2008, but this decrease did not reach statistical significance (Figure 2; P=.20).
TABLE 2.
Epidemiology of Infectiona
FIGURE 2.
Epidemiology of infection. Percentage of isolates caused by gram-positive and gram-negative organisms per year (P=.20; exact Wilcoxon signed rank test).
ANTIBIOTIC RESISTANCE
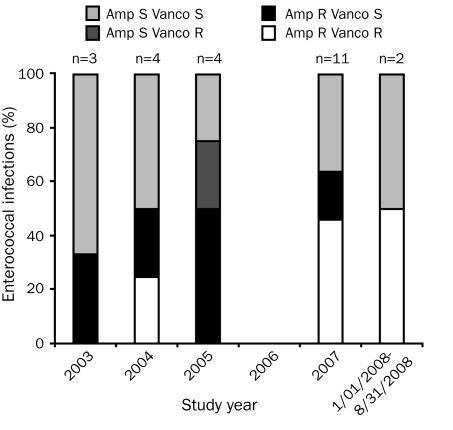
The only Staphylococcus aureus isolate was resistant to oxacillin and all tested fluoroquinolones (ie, moxifloxacin, ciprofloxacin, and levofloxacin). Among the 37 isolates of CoNS, 34 (92%) were resistant to oxacillin, and 29 (78%) were resistant to the 3 fluoroquinolones. Of the 5 viridans group streptococci isolates, 3 (60%) were resistant to penicillin; fluoroquinolone susceptibility was performed on only 1 isolate, which was resistant to levofloxacin. Our laboratory does not routinely perform fluoroquinolone susceptibility testing to the viridans group streptococci. A total of 12 (50%) ampicillin-resistant and 8 (33%) vancomycin-resistant isolates of Enterococcus spp. were identified. The changing epidemiology of infections with Enterococcus spp. is depicted in Figure 3. With the exception of 2007, when 5 VRE infections occurred, the incidence of VRE infection remained relatively low and consistent during the study period. Overall, for GP organisms with available susceptibility data, 43 (78%) of 55 isolates were resistant to levofloxacin, 31 (69%) of 45 isolates were resistant to moxifloxacin, and 42 (78%) of 54 isolates were resistant to ciprofloxacin.
FIGURE 3.
Epidemiology of infection-causing Enterococcus spp. There were no Enterococcus spp. isolates in 2006. Amp = ampicillin; R = resistant; S = susceptible; Vanco = vancomycin.
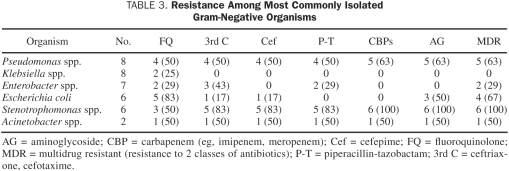
Isolates that were resistant to GN are summarized in Table 3. Of 8 P aeruginosa isolates, 5 (63%) were resistant to 2 or more antibiotic classes, and 4 (50%) were resistant to 3 or more. The 4 P aeruginosa isolates identified before 2006 retained susceptibility to fluoroquinolones; however, the latter 4 isolates were resistant to fluoroquinolones. Most of the 6 E coli isolates were resistant to fluoroquinolone (n=5; 83%). In contrast, most of the 8 Klebsiella spp. isolates retained susceptibility to this antibiotic class, with only 2 (25%) of the isolates conferring resistance. Overall, for GN organisms with reported fluoroquinolone susceptibility, 13 (31%) of 42 isolates were resistant to levofloxacin, and 18 (43%) of 42 were resistant to ciprofloxacin. Discordant fluoroquinolone susceptibility was noted in 3 (50%) of the Stenotrophomonas spp. isolates, 1 (50%) of the Acinetobacter spp. isolates, and 1 (13%) of the Klebsiella spp. isolates. One extended-spectrum β-lactamase–producing organism, E coli, was identified during the study period.
TABLE 3.
Resistance Among Most Commonly Isolated Gram-Negative Organisms
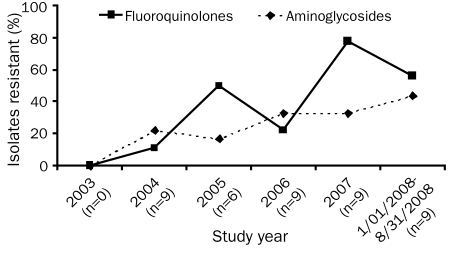
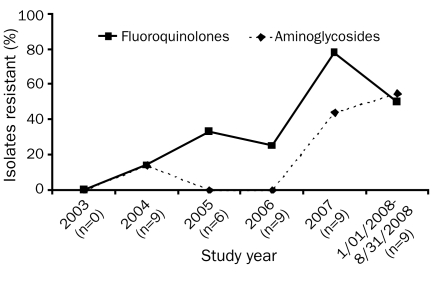
Trends of GN antimicrobial resistance over time are noted in Figures 4 and 5. Increasing resistance to fluoroquinolones is observed when all GN isolates are combined (P=.02). No change in resistance to cefepime, piperacillin-tazobactam, or the carbapenems was seen (P=.68, P=.62, and P=.82, respectively). If resistance among the 4 most common GN isolates is examined independently, the same trends are observed. There was, however, a statistically significant increase in resistance to both fluoroquinolones and aminoglycosides in these organisms over time (Figure 5, P=.05). Again, no change in resistance to cefepime, piperacillin-tazobactam, or the carbapenems was noted (P=.94, P=.82, and P=.50, respectively).
FIGURE 4.
Gram-negative resistance to fluoroquinolones (P=.02; exact Wilcoxon signed rank test) and aminoglycosides (P=.26; exact Wilcoxon signed rank test).
FIGURE 5.
Resistance of Pseudomonas, Klebsiella, and Enterobacter spp. and Escherichia coli to fluoroquinolones and aminoglycosides (P=.05 for both classes of drugs; exact Wilcoxon signed rank test).
C Difficile and VRE
The incidence of C difficile and VRE during the study period was minimal in patients who received levofloxacin and penicillin prophylaxis (n=231). Eight patients (3.5%) developed C difficile–associated disease. Colonization or infection by VRE was present in 33 patients (14%), and a statistically significant increase in combined VRE infection and colonization was discovered over time (P=.04). The 20 patients with newly discovered VRE had a documented negative VRE swab result before receipt of levofloxacin and a positive VRE swab result or VRE infection within 3 months after transplant. Notably, 5 VRE infections occurred in 2007, whereas only one or none occurred in the other study years.
DISCUSSION
During the course of this study, approximately one-third of patients developed infection within 3 months of undergoing allogeneic HSCT. The incidence of GP and GN bacterial infections did not significantly change over time at our institution. Rates of fluoroquinolone-resistant GN organisms (particularly P aeruginosa) increased, whereas resistance to extended-spectrum β-lactams remained stable. The incidence of VRE colonization and C difficile infection was negligible during the study period.
During 2003, only 19 allogeneic HSCTs were performed, compared with 45 to 55 transplants per year in all other study years. The low number of infections detected in 2003 (3 GP bacterial infections vs >20 infections observed in the other years of the study) is reflected by this observation. Not surprisingly, CoNS was the most commonly isolated organism overall. These isolates likely represented true infection in a neutropenic host given our strict inclusion criteria. Consistent with other investigations, potential colonizing organisms had to be isolated from 2 consecutive cultures to be considered an infection.12,13 A steady decrease in the ratio of GP:GN organisms has been observed in multiple studies in patients undergoing HSCT who received fluoroquinolone prophylaxis.12,14,15 The percentage of GN bacterial infections increased slightly during the study period; however, that increase did not reach statistical significance, likely because of the small sample size. We anticipate that eventually more GN bacterial infections will emerge with continued use of fluoroquinolone-based combination antimicrobial prophylaxis and subsequent selective pressure.
Our study contributes to the growing body of evidence that using prophylactic antibiotics in neutropenic patients selects for resistant organisms implicated in infections.16-19 Fluoroquinolone-resistant and MDR P aeruginosa and E coli were frequently identified in our allogeneic HSCT patients who received fluoroquinolone-based combination antimicrobial prophylaxis. These findings are consistent with a recent study describing prophylactic fluoroquinolone use as a risk factor for the isolation of MDR E coli in a broad neutropenic population.20 Other factors that play a role in the acquisition of resistance in this patient population, including previous antibiotic use, previous hospitalizations, and potential nonadherence, were not assessed in this study. Despite these limitations, our study suggests that the use of levofloxacin for prophylaxis, our current standard of practice, may be contributing to the resistance patterns observed in GN organisms. Fluoroquinolone resistance rates have increased, whereas resistance to broader-spectrum antibiotics has not changed appreciably over time. We did not measure rates of infection reduction for patients receiving prophylaxis compared with those who were not. However, given our observations, we encourage transplant centers that routinely use fluoroquinolone prophylaxis in allogeneic HSCT recipients to empirically use antipseudomonal β-lactams in the event of a febrile episode and subsequent infection.
Although our institution is a large, academic, tertiary care center, rates of bacterial resistance have remained historically low. Only 1 extended-spectrum β-lactamase–producing organism was identified in our study, whereas rates as high as 25% to 44% have been observed in allogeneic HSCT recipients at other centers.12,21 In comparing our study data with our institutional antibiogram, we note that the rate of fluoroquinolone-resistant organisms is considerably higher in our allogeneic HSCT recipients than in our general patient population, reflecting not only their higher antimicrobial exposures but perhaps also their more extensive exposure to the inpatient hospital setting. Therefore, institutional antibiograms that are compiled from all pooled patient groups may not be as useful in guiding empiric antibiotic selection in those who undergo allogeneic HSCT and other patient groups with heavy antibiotic exposures. Aminoglycosides are infrequently used at our institution. The appearance of aminoglycoside-resistant organisms over time, particularly in the most commonly isolated GN organisms, is both statistically and clinically significant. We hypothesize that a possible cross-resistance exists between fluoroquinolones and aminoglycosides, as has been described in vitro with P aeruginosa.22,23
Rates of VRE colonization are increasingly reported in those who undergo HSCT, ranging from 10% to as high as 40% at some centers24,25; however, numerous epidemiologic studies have noted a marginal (<5%) incidence of VRE infection in the HSCT patient population.12,15,19 Although the earlier time period of our study is consistent with these trends, a few concerning features were identified. First, 5 VRE infections occurred in 2007, which could represent either a contained outbreak or a more generalized trend of increased VRE prevalence in our allogeneic HSCT patient population. The latter is plausible, given the overall statistically significant increase in the occurrence of VRE (if colonization and infection are combined) during the study period. Additionally, most of the documented VRE cases in the 3 months after transplant were newly discovered cases. This increase in VRE detection may reflect an increased surveillance, our laboratory transition in 2004 from stool culture to the more sensitive polymerase chain reaction assay for identification of VRE, propagation of VRE through antimicrobial selective pressure, or an increase in imported VRE in patients referred to our institution from other medical centers. Given these confounders, we cannot confirm whether a levofloxacin-based antimicrobial prophylaxis program is contributing to the increased occurrence of VRE at our center. Newer literature suggests a 30% risk of developing VRE infection in a colonized allogeneic HSCT patient.24 Because VRE colonization is also a risk factor for 100-day mortality after allogeneic HSCT,25 we encourage transplant centers to continue to closely monitor VRE colonization.
Fluoroquinolone usage has been associated with the development of hypertoxin-producing C difficile–associated disease.26 In our study, the incidence of C difficile was surprisingly minimal in the 3 months after allogeneic HSCT; however, transplant centers should continue to monitor for C difficile infection in symptomatic patients.
This study has several limitations. Because we studied a retrospective cohort with a small sample size, we are unable to accurately identify a causal relationship between the use of levofloxacin and the changing epidemiology of infections in our patient population. A risk factor analysis would help determine whether a relationship exists. With the exception of reviewing the patients' prophylactic antibiotics, we did not assess other factors that could contribute to changing epidemiology and resistance profiles of the organisms isolated, such as previous antibiotic use and nonadherence to the prophylactic regimen. Additionally, we recognize that the use of penicillin and doxycycline with levofloxacin can affect the selection of GP bacterial infections; however, we think the use of doxycycline is unlikely to contribute significantly to the GN resistance trends observed.
Although many factors contribute to the acquisition of resistance, administration of fluoroquinolone prophylaxis during the period of chemotherapy-induced neutropenia may be a driving factor in the allogeneic HSCT patient population. As the rates of drug-resistant P aeruginosa, E coli, and other GN bacteria climb, the utility of fluoroquinolones to treat infections in these patients is likely to decline in the near future. In time, perhaps, the benefits of antimicrobial prophylaxis for infection rate reduction may need to be weighed against the risks of promoting resistance to select drugs. Rates of VRE colonization and infection are also increasing in HSCT recipients, making rigorous surveillance critical. Fortunately, the incidence of C difficile colitis was low in our study. We encourage HSCT centers to conduct similar studies and routinely monitor antimicrobial resistance patterns in this patient population.
CONCLUSION
A fluoroquinolone-based prophylactic regimen is a potential contributor to the emergence over time of resistant GN infections in those who undergo allogeneic HSCT. Our findings provide additional support for the current standard of practice, which is to administer empiric monotherapy with an antipseudomonal β-lactam if these patients develop fever and are thought to have an infection.
Acknowledgments
We thank Leann Batterson for her assistance with accessing patient data, Julianna Burzynski, PharmD, and Robert Wolf, PharmD, for many helpful discussions, and the Mayo Clinic Center for Translational Science Activities for statistical support.
REFERENCES
- 1.Cruciani M, Rampazzo R, Malena M, Lazzaini L, et al. Prophylaxis with fluoroquinolones for bacterial infections in neutropenic patients: a meta-analysis. Clin Infect Dis. 1996;23:795-805 [DOI] [PubMed] [Google Scholar]
- 2.Engels EA, Lau J, Barza M. Efficacy of quinolone prophylaxis in neutropenic cancer patients: a meta-analysis. J Clin Oncol. 1998;16(3):1179-1187. [DOI] [PubMed] [Google Scholar]
- 3.Cometta A, Calandta T, Bille J, et al. Escherichia coli resistant to fluoroquinolones in patients with cancer and neutropenia. N Engl J Med. 1994;330(17):1240-1241 [DOI] [PubMed] [Google Scholar]
- 4.Bucaneve G, Micozzi A, Menichetti F, et al. Levofloxacin to prevent bacterial infection in patients with cancer and neutropenia. N Engl J Med. 2005;353(10):977-987 [DOI] [PubMed] [Google Scholar]
- 5.Cullen M, Steven N, Billingham L, et al. Antibacterial prophylaxis after chemotherapy for solid tumors and lymphomas. N Engl J Med. 2005;353(10):988-998 [DOI] [PubMed] [Google Scholar]
- 6.Imran H, Tleyjeh IM, Arndt CAS, et al. Fluoroquinolone prophylaxis in patients with neutropenia: a meta-analysis of randomized placebo-controlled trials. Eur J Clin Microbiol Infect Dis. 2008;27(1):53-63 [DOI] [PubMed] [Google Scholar]
- 7.Segal BH, Freifeld AG, Baden LR, et al. Prevention and treatment of cancer-related infections. J Natl Compr Canc Netw. 2008;6(2):122-174 [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Infectious Disease Society of America. American Society of Blood and Marrow Transplantation Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients [published correction appears in MMWR Recomm Rep. 2004;14(19):396] MMWR Recomm Rep. 2000;49(RR-10):1-125 [PubMed] [Google Scholar]
- 9.Hughes WT, Armstrong D, Bodey GP, et al. 2002 Guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis. 2002;34(6):730-751 [DOI] [PubMed] [Google Scholar]
- 10.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15(10):1143-1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cosgrove S. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and healthcare costs. Clin Infect Dis. 2006;42(suppl 2):S82-S89 [DOI] [PubMed] [Google Scholar]
- 12.Mikulska M, Del Bono V, Raiola AM, et al. Blood stream infections in allogeneic hematopoietic stem cell transplant recipients: reemergence of gram-negative rods and increasing antibiotic resistance. Biol Blood Marrow Transplant. 2009;15(1):47-53 [DOI] [PubMed] [Google Scholar]
- 13.Poutsiaka DD, Price LL, Ucuzian A, Chan GW, Miller KB, Snydman DR. Blood stream infection after hematopoietic stem cell transplantation is associated with increased mortality. Bone Marrow Transplant. 2007;40(1):63-70 [DOI] [PubMed] [Google Scholar]
- 14.Collin BA, Leather HL, Wingard JR, Ramphal R. Evolution, incidence and susceptibility of bacterial bloodstream isolates from 519 bone marrow transplant patients. Clin Infect Dis. 2000;33(7):947-953 [DOI] [PubMed] [Google Scholar]
- 15.Ortega M, Rovira M, Almela M, et al. Bacterial and fungal bloodstream isolates from 796 hematopoietic stem cell transplant recipients between 1991 and 2000. Ann Hematol. 2005;84(1):40-47 [DOI] [PubMed] [Google Scholar]
- 16.Kern WV, Andriof E, Oethinger M, Kern P, Hacker J, Marre R. Emergence of fluoroquinolone-resistant Escherichia coli at a cancer center. Antimicrob Agents Chemother. 1994;38(4):681-687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Somolinos N, Arranz R, Del Rey MC, et al. Superinfections by Escherichia coli reistant to fluoroquinolones in immunocompromised patients. J Antimicrob Chemother. 1992;30(5):730-731 [DOI] [PubMed] [Google Scholar]
- 18.Kern WV, Klose K, Jellen-Ritter AS, et al. Fluoroquinolone resistance of Eschericia coli at a cancer center: epidemiologic evolution and effects of discontinuing prophylactic fluoroquinolone use in neutropenic patients with leukemia. Eur J Clin Microbiol Infect Dis. 2005;24(2):111-118 [DOI] [PubMed] [Google Scholar]
- 19.Frere P, Hermanne JP, Debouge MH, et al. Changing pattern of bacterial susceptibility to antibiotics in hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2002;29(7):589-594 [DOI] [PubMed] [Google Scholar]
- 20.Rangaraj G, Granwehr BP, Jiang Y, et al. Perils of quinolone exposure in cancer patients. Cancer 2010;116:967-973 [DOI] [PubMed] [Google Scholar]
- 21.Oliveira A, de Souza M, Carvalho-Dias VMH, et al. Epidemiology of bacteremia and factors associated with multi-drug resistant gram-negative bacteremia in hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2007;39(12):775-781 [DOI] [PubMed] [Google Scholar]
- 22.Henrichfreise B, Wiegand I, Pfister W, et al. Resistance mechanisms of multiresistant Pseudomonas aeruginosa strains from Germany and correlation with hypermutation. Antimicrob Agents Chemother. 2007;51(11):4062-4070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hocquet D, Muller A, Blanc K, et al. Relationship between antibiotic use and incidence of MexXY-OprM overproducers among clinical isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother 2008;52(3):1173-1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinstock D, Conlon M, Iovino C, et al. Colonization, bloodstream infection, and mortality caused by vancomycin-resistant Enterococcus early after allogeneic hematopoietic stem cell transplant. Biol Bone Marrow Transplant. 2007;13(5):615-621 [DOI] [PubMed] [Google Scholar]
- 25.Zirakzadeh A, Gastineau DA, Mandrekar JN, et al. Vancomycin-resistant enterococcal colonization appears associated with increased mortality among hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2008;41(4):385-392 [DOI] [PubMed] [Google Scholar]
- 26.Loo V, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353(23):2442-2449 [DOI] [PubMed] [Google Scholar]