Abstract
Recent evidence for the nonskeletal effects of vitamin D, coupled with recognition that vitamin D deficiency is common, has revived interest in this hormone. Vitamin D is produced by skin exposed to ultraviolet B radiation or obtained from dietary sources, including supplements. Persons commonly at risk for vitamin D deficiency include those with inadequate sun exposure, limited oral intake, or impaired intestinal absorption. Vitamin D adequacy is best determined by measurement of the 25-hydroxyvitamin D concentration in the blood. Average daily vitamin D intake in the population at large and current dietary reference intake values are often inadequate to maintain optimal vitamin D levels. Clinicians may recommend supplementation but be unsure how to choose the optimal dose and type of vitamin D and how to use testing to monitor therapy. This review outlines strategies to prevent, diagnose, and treat vitamin D deficiency in adults.
AI = adequate intake; CKD = chronic kidney disease; D2 = vitamin D2; D3 = vitamin D3; 1,25(OH)2D = 1,25-dihydroxyvitamin D; HPT = hyperparathyroidism; 25(OH)D = 25-hydroxyvitamin D; PTH = parathyroid hormone; UVB = ultraviolet B
Vitamin D has been appreciated for its role in calcium homeostasis and bone health since its identification in 1921.1 Even so, 25% to 50% or more of patients commonly encountered in clinical practice are deficient in vitamin D. Recent advances in biochemical assessment, therapeutic goals for vitamin D nutrition for optimal bone health, and the association of vitamin D deficiency with nonskeletal disease have revived interest in this hormone.
Vitamin D consists of 2 bioequivalent forms. Vitamin D2 (D2), also known as ergocalciferol, is obtained from dietary vegetable sources and oral supplements. Vitamin D3 (D3), also known as cholecalciferol, is obtained primarily from skin exposure to ultraviolet B (UVB) radiation in sunlight, ingestion of food sources such as oily fish and variably fortified foods (milk, juices, margarines, yogurts, cereals, and soy), and oral supplements. Aside from rich sources such as oily fish, the vitamin D content of most foods is between 50 and 200 IU per serving. This value varies greatly by region of the world because fortification markedly improves the availability of vitamin D through diet. Both D2 and D3 are biologically inert. Once absorbed from the intestine, they are metabolized in the liver to 25-hydroxyvitamin D [25(OH)D], composed of 25(OH)D2 and 25(OH)D3; 25(OH)D (also called calcidiol) is subsequently converted to 1,25-dihydroxyvitamin D [1,25(OH)2D], also known as calcitriol, in the kidney and select other tissues by the action of the 1α-hydroxylase enzyme. The predominant effects of vitamin D are exerted through the endocrine and autocrine actions of calcitriol via activation of the vitamin D receptor in cells.
TESTING AND INTERPRETING VITAMIN D STATUS
How Prevalent Is Vitamin D Deficiency And Who Is At Risk?
Worldwide, naturally occurring dietary sources of vitamin D are limited, and food fortification is optional, inconsistent, inadequate, or nonexistent. Therefore, for most people, vitamin D is primarily obtained by cutaneous production from sun exposure. However, many variables influence the amount of UVB from sunlight that reaches the skin and its effectiveness. These include time of day, season, latitude, altitude, clothing, sunscreen use, pigmentation, and age. In Minnesota in 2008, less than half of days provided enough solar UVB radiation at noon to effect cutaneous vitamin D production.2 Even those who normally reside in sunny climates are commonly found to be deficient in vitamin D, probably due to cultural habits and/or dress.3 Even if regularly exposed to sunlight, elderly people produce 75% less cutaneous D3 than young adults.4 Further barriers to cutaneous vitamin D production are ongoing public health campaigns promoting sunscreen use, as advocated by the American Academy of Dermatology (http://www.aad.org/forms/policies/ps.aspx, accessed December 24, 2009). Unfortunately, commonly recommended daily intakes of vitamin D are known to be insufficient if sunlight exposure is limited.5
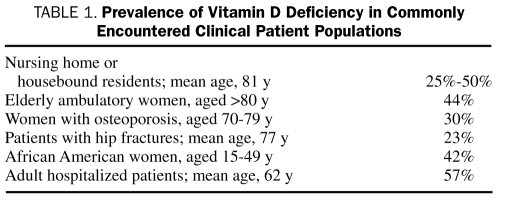
Vitamin D deficiency is more common than previously thought. The Centers for Disease Control and Prevention has reported that the percentage of adults achieving vitamin D sufficiency as defined by 25(OH)D of at least 30 ng/mL (to convert to nmol/L, multiply by 2.496) has declined from about 60% in 1988-1994 to approximately 30% in 2001-2004 in whites and from about 10% to approximately 5% in African Americans during this same time. Furthermore, more people have been found to be severely deficient in vitamin D [25(OH)D <10 ng/mL].6 Even when using a conservative definition of vitamin D deficiency, many patients routinely encountered in clinical practice will be deficient in vitamin D, as shown in Table 1.
TABLE 1.
Prevalence of Vitamin D Deficiency in Commonly Encountered Clinical Patient Populations
Who Should Be Tested For Vitamin D Deficiency?
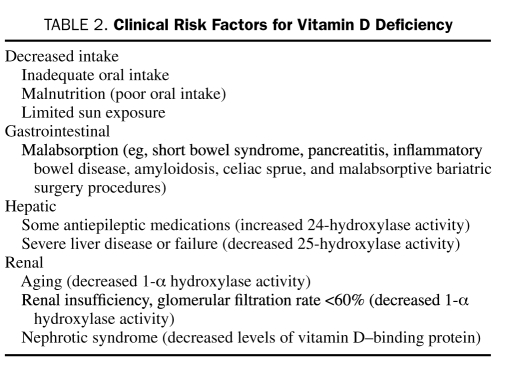
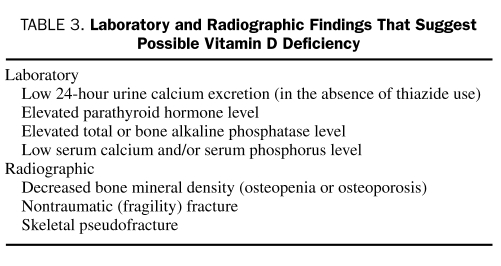
Although vitamin D deficiency is prevalent, measurement of serum 25(OH)D levels is expensive, and universal screening is not supported. However, vitamin D testing may benefit those at risk for severe deficiency (Table 2) or those with laboratory or radiographic findings commonly associated with vitamin D deficiency (Table 3). In these patients, knowledge of the 25(OH)D blood level provides an accurate assessment of vitamin D body stores, helps identify the need for vitamin D therapy, and may help to determine an effective dose. Alternatively, empiric vitamin D supplementation without testing can be justified for patients who have no overt risk factors or evidence of deficiency but are thought to have inadequate sun exposure or dietary intake.
TABLE 2.
Clinical Risk Factors for Vitamin D Deficiency
TABLE 3.
Laboratory and Radiographic Findings That suggest Possible Vitamin D Deficiency
Vitamin D deficiency can contribute to bone loss from decreased vitamin D–mediated intestinal calcium absorption and resultant secondary hyperparathyroidism (HPT). Vitamin D supplementation can improve muscle strength and reduce fall frequency by approximately 50%.7 Thus, patients who have low bone mineral density or a prior low-impact (fragility) skeletal fracture and those at risk of falling should be evaluated for vitamin D deficiency to reduce the risk of all types of skeletal fractures.8,9
Patients with chronic kidney disease (CKD) have decreased conversion of 25(OH)D to 1,25(OH)2D as a result of impaired renal 1-α hydroxylase activity. This contributes to secondary HPT and metabolic bone disease. Superimposed nutritional deficiency may aggravate secondary HPT both directly (as a result of low vitamin D levels) and indirectly (as a result of impaired vitamin D–mediated intestinal calcium absorption). Patients with stage I to III CKD should be tested and supplemented with vitamin D as needed to achieve optimal levels of 25(OH)D in addition to modifying calcium and phosphate intake. Emerging evidence is challenging our understanding of bone and vascular health in stage IV to V CKD, such that vitamin D, calcitriol, or vitamin D analogs should be used according to current CKD guidelines and under the guidance of a nephrologist.
It has been suggested that clinicians should routinely test for hypovitaminosis D in patients with musculoskeletal symptoms, such as bone pain, myalgias, and generalized weakness, because these symptoms are often associated with hypovitaminosis D and might be misdiagnosed as fibromyalgia, chronic fatigue, age-related weakness, or even depression.10 Some studies and numerous anecdotal observations report vitamin D deficiency in 80% to 90% of children and adults with pain, myalgias, and weakness.11 However, few high-quality interventional studies support a causal relationship between vitamin D deficiency and pain. Furthermore, vitamin D status can be a surrogate marker of poor nutritional status such that the high prevalence of vitamin D deficiency in these populations may reflect suboptimal nutrition and lack of outdoor activity associated with chronic illness. Indeed, a recent randomized, blinded, placebo-controlled trial showed no benefit of vitamin D supplementation for such symptoms.12 The role of vitamin D testing in pregnant or lactating women may be refined by data from ongoing interventional trials.
Which Test Best Measures Vitamin D Status?
Ingested and cutaneously produced vitamin D is rapidly converted to 25(OH)D, but in serum only a fraction of 25(OH)D is converted to its active metabolite 1,25(OH)2D. Thus, measurement of the total 25(OH)D level is the best test to assess body stores of vitamin D. The total 25(OH)D level allows for the diagnosis and monitoring of vitamin D deficiency, whereas quantification of 25(OH)D2 and 25(OH)D3 fractions may facilitate treatment monitoring. For example, in patients without clinical improvement after D2 or D3 supplementation, lack of increase in the corresponding 25(OH)D2 or 25(OH)D3 and total 25(OH) D levels may indicate inadequate dosing, nonadherence, or malabsorption. Some laboratory assays for vitamin D cannot differentiate between 25(OH)D2 and 25(OH)D3 and will only report a total 25(OH)D level. Some laboratory assays underdetect D2 metabolites, which may give the appearance of ineffective D2 supplementation.
In people with healthy kidneys and bones, normal serum levels of calcium and phosphorus are maintained predominantly through the interaction of 2 hormones: parathyroid hormone (PTH) and calcitriol. In the setting of vitamin D deficiency, secondary HPT causes both release of calcium stored in bone and resorption of calcium by the kidney to maintain normal serum calcium and phosphorus levels. Thus, vitamin D deficiency is usually accompanied by normal blood levels for calcium and phosphorus, high-normal or elevated levels of PTH, normal to elevated levels of total alkaline phosphatase, a low 24-hour urine calcium excretion rate, and low levels of total 25(OH)D. Patients with severe and long-standing vitamin D deficiency may present with overt hypocalcemia and/or hypophosphatemia, but this is the exception. Clinicians should not measure 1,25(OH)2D levels to diagnose hypovitaminosis D. Doing so can lead to an erroneous interpretation of vitamin D status because calcitriol levels are often normal or even elevated in patients with vitamin D deficiency as a result of elevated PTH levels.
What Is an Optimal 25(OH)D Level?
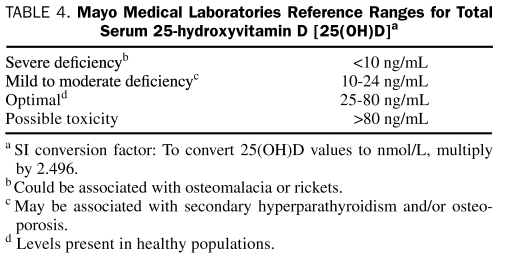
A wide “optimal” range for 25(OH)D is reported (25-80 ng/mL), and differences of opinion exist as to the definitions of vitamin D insufficiency (sometimes reported as <30 ng/mL) and deficiency (<20 ng/mL). Mild-to-modest deficiency can be associated with osteoporosis and/or secondary HPT. Severe deficiency may lead to failure to mineralize newly formed osteoid in bone, resulting in rickets in children and osteomalacia in adults. Most cells have vitamin D receptors. The consequences of vitamin D deficiency for organs other than bone are not fully known but may include impaired immunity, increased autoimmunity, myopathy, diabetes mellitus, and an increased risk of colon, breast, and prostate cancers.13 Higher vitamin D levels have also been associated with increased longevity.14,15 Thus, an optimal vitamin D level might depend on the health outcome in question. The vitamin D levels in Table 4 are those reported by Mayo Medical Laboratories and represent clinical decision-making values that apply to men and women of all ages rather than population-based reference values.
TABLE 4.
Mayo Medical Laboratories Reference Ranges for Total Serum 25-hydroxyvitamin D [25(OH)D]a
Population reference ranges for vitamin D vary widely depending on ethnic background, age, geographic location of the population, and the sampling season. In northern latitude locations in particular, up to 73% of the population may have levels of less than 20 ng/mL during winter.16 Thus, it is important to be aware that vitamin D levels are affected by both geographic and seasonal variability and that a person with an “optimal” level in the summer may well become “deficient” in the winter without any change in diet and as a result of changes in sun exposure.
HOW TO PREVENT AND TREAT VITAMIN D DEFICIENCY
Many patients and physicians think that adequate vitamin D intake can be obtained via diet alone. This assumption is erroneous. With the exception of fatty fish, the vitamin D content of most foods, including fortified dairy products, is relatively low to nonexistent. Even some dairy products in the United States are not fortified, making it important to read food labels to ensure the vitamin D content of foods.
Vitamin D supplementation is safe17 and inexpensive, but vitamin D deficiency often remains undiagnosed or is undertreated. Possible explanations for this disparity include (1) the recommended age-dependent adequate intake (AI) of vitamin D was established before publication of studies suggesting that 25(OH)D levels of greater than 30 ng/mL are needed to ensure PTH suppression into the normal range; (2) the current AI for vitamin D can easily be met by diet and/or a daily multivitamin,18 but this intake level may still be inadequate to reach optimal levels in many people, especially those at risk; and (3) physicians may be uncomfortable recommending larger doses of vitamin D. That fear is generally unmerited given the dearth of reports of vitamin D toxicity compared with the expansive literature on vitamin D deficiency. The rarity of reports of vitamin D toxicity can be explained in part by the kidney's ability to limit production of active calcitriol. Increased calcitriol levels inhibit PTH both directly (through the vitamin D response element on the PTH gene) and indirectly (by increasing intestinal calcium absorption), causing calcitriol production in the kidney to decrease. Renal 24-hydroxylase activity further limits the availability of calcitriol by creating inert metabolites of both calcitriol (1,24,25-trihydroxyvitamin D) and calcidiol (24,25-dihydroxyvitamin D). The 24-hydroxylase gene is under the transcriptional control of calcitriol, thereby providing tight negative feedback.
Vitamin D2 Vs Vitamin D3 Supplements: How Much Is Enough?
Both D2 (ergocalciferol) and D3 (cholecalciferol) are available as dietary supplements. The relative efficacy of D2 vs D3 in humans continues to be debated, although both appear to be effective for preventing or treating disease, provided that an adequate total 25(OH)D blood level is obtained. The variable efficacy of D2 vs D3 may relate primarily to differences in serum half-life and is clinically relevant for dosing and monitoring frequency. A single dose of 50,000 IU of D2 or D3 produces a similar increase in the total 25(OH)D concentration, but the apparent longer half-life of D3 suggests that less frequent dosing may be needed.19 A daily dosing study of 1000 IU of D2 vs D3 showed no difference in any resulting vitamin D level [25(OH)D2, 25(OH)D3, or total 25(OH)D].20 However, a recent study comparing 1600 IU of D2 once daily vs 1600 IU of D3 once daily vs 50,000 IU of D2 once monthly vs 50,000 IU of D3 once monthly suggested that D3 is superior in that it showed slightly higher levels of 25(OH)D3 at the end of 1 year. An important caveat of this study was that the mean total 25(OH)D level at the beginning of the study was already in the reference range (33 ng/mL), and those with hypovitaminosis D may respond differently.21 We recommend the use of D3, particularly if dosing is infrequent (ie, less than once weekly). One situation in which D2 may be preferred is a vegetarian or vegan diet. It is recommended that both D2 and D3 be taken with a meal containing fat to ensure maximum absorption.
Since 1997, the Food and Nutrition Board has advised an AI of vitamin D of 200 to 600 IU/d.18 The AI is “believed to cover the needs of all individuals” but “lack of data or uncertainty in the data” limit the ability to confidently determine a recommended daily allowance. The AI for vitamin D is based on maintenance of a total serum 25(OH)D level of at least 11 ng/mL.18 Although these recommendations are the basis for the amounts of vitamin D used to fortify foods and provided in many supplements, it is widely accepted that they are outdated.22 Revised dietary reference intakes from the Institute of Medicine are expected in 2010.
How much vitamin D is needed to correct severe vitamin D deficiency (<10 ng/mL)? Although not validated by clinical trials, a commonly applied strategy is to prescribe a “loading dose” (eg, 50,000 IU of vitamin D orally once weekly for 2-3 months, or 3 times weekly for 1 month). A review of multiple loading algorithms suggested that a minimum total dose of 600,000 IU best predicted an end-of-treatment 25(OH)D level greater than 30 ng/mL.23 It is important to note that none of the studied patients developed hypercalcemia. For mild to moderate deficiency (11-25 ng/mL), a shorter treatment interval or lower dose may be effective. Although many different strategies may be used in treating vitamin D deficiency, a common oversight in management is to stop treatment or provide inadequate vitamin D maintenance dosing once the 25(OH)D level reaches the optimal range. Regardless of initial vitamin D therapy, and assuming no change in lifestyle or diet, a maintenance/prevention daily dose of 800 to 2000 IU or more will be needed to avoid recurrent deficiency (Table 3).24 A maintenance dose averaging 2000 IU/d meets the current safe upper limit guidelines and is well below safe upper limits reported by others.17
Special mention is needed for patients who have malabsorption or require tube feeding or parenteral nutrition. Patients receiving tube feeding (but without malabsorption) have vitamin D dosing requirements similar to persons with oral intake. However, ergocalciferol capsules contain D2 in oil, which can clog the feeding tube and therefore should not be used. Cholecalciferol capsules and tablets contain D3 in powder form and can be used without clogging the feeding tube. Patients with malabsorption often require larger maintenance dosing of vitamin D. For example, patients with malabsorptive gastric bypass procedures may require 50,000 IU of D2 or D3 maintenance dosing from once weekly to as frequently as daily to maintain sufficiency. Standard multivitamin preparations for intravenous parenteral nutrition provide only 200 IU, a dose that helps maintain normal 25(OH)D levels in the short term but may not correct vitamin D deficiency. In extreme malabsorptive states, UVB exposure (ie, sunlight or phototherapy) can be effective for those who do not respond to large oral doses.25 Vitamin D for intramuscular administration is not commercially available in the United States; however, it can be compounded in specialty pharmacies for limited local use.26
THE IMPORTANCE OF CALCIUM AND VITAMIN D TOXICITY
What Role is Played by Calcium Nutrition?
Maintenance of normal serum calcium levels results from an array of interrelated processes, including intestinal calcium absorption, calcium uptake and release from the skeleton, and renal calcium handling. As previously noted, vitamin D plays a critical role in each of these processes. Hypovitaminosis D impairs intestinal calcium absorption and leads to secondary HPT and risk of bone loss. Heaney et al27 found that maximal calcium absorption in men occurs when 25(OH)D levels are in the range of 30 to 40 ng/mL, consistent with vitamin D levels needed to suppress PTH. However, even in the presence of vitamin D sufficiency, inadequate oral calcium intake may cause secondary HPT. The National Osteoporosis Foundation guidelines recommend that men and women younger than 50 years ingest 1000 mg/d of elemental calcium, and those older than 50 years ingest 1200 mg/d (http://www.nof.org/prevention/calcium_and_VitaminD.htm, accessed December 24, 2009).
Clinicians should be mindful of several important caveats when considering calcium supplementation.
First, up to 500 to 600 mg of elemental calcium can be efficiently absorbed in any single dose, with excess calcium passing unabsorbed through the gut.
Second, gastric acidity is necessary for calcium absorption. However, even in patients with achlorhydria, calcium absorption is reported to occur adequately if taken with meals. For patients with achlorhydria due to gastric reduction or bariatric surgery, or during gastric acid suppressive therapy (eg, protein pump inhibitor use), calcium supplementation with the more acidic calcium citrate is preferred over calcium carbonate. However, calcium citrate can clog feeding tubes and should not be administered via any feeding tube.
Third, during vitamin D sufficiency, approximately 30% of calcium intake is normally absorbed regardless of the dietary or supplement source.27 Thus, if 1000 mg of calcium is ingested and 30% (300 mg) is absorbed, and assuming that 50 mg is required for daily bone health, the remaining 250 mg will be renally excreted (normal 24-hour urine calcium excretion approximates 100-250 mg/d). With vitamin D deficiency, as little as 10% of ingested calcium may be absorbed. Thus, calcium excretion would be low (only 50 mg for a dose of 1000 mg). Although cumbersome, 24-hour urine calcium excretion is an effective test to assess adequacy of both calcium and vitamin D intake. When assessing urine calcium values, it is important to note that thiazide diuretics, lithium, and a low-sodium diet decrease renal calcium excretion, whereas excess sodium intake increases it.
Fourth, as already noted, low 25(OH)D levels may be associated with secondary HPT and abnormal bone mineralization. Thus, increased levels of PTH, increased total or bone alkaline phosphatase levels, and low 24-hour urine calcium levels should prompt suspicion for vitamin D deficiency in some patients. For example, vitamin D deficiency should be suspected in an otherwise healthy person found to have an elevated alkaline phosphatase level, especially if findings on other liver enzyme tests are normal.
What About Vitamin D Toxicity?
Vitamin D toxicity should not be diagnosed solely on the basis of an elevated 25(OH)D level; instead, it should be recognized as a clinical syndrome of both hypervitaminosis D and hypercalcemia, in which hyperphosphatemia and hypercalciuria also commonly (although not always) occur. Patients with vitamin D toxicity could present with clinical symptoms and signs of hypercalcemia (eg, nausea, dehydration, and constipation) and hypercalciuria (eg, polyuria and kidney stones). Hypervitaminosis D in the absence of hypercalcemia may prompt further investigation to evaluate the etiology of increased vitamin D levels; however, unlike hypercalcemia, it is not a medical emergency. Although excess vitamin D supplementation can lead to hypercalcemia, vitamin D toxicity is extremely rare and generally occurs only after ingestion of large doses of vitamin D (>10,000 IU/d) for prolonged periods in patients with normal gut absorption or in patients who may be concurrently ingesting generous if not excessive amounts of calcium. A 25(OH)D level of 80 ng/mL is the lowest reported level associated with toxicity in patients without primary HPT with normal renal function. Most patients with vitamin D toxicity have levels greater than 150 ng/mL.28 Binkley et al21 have recently reported that vitamin D supplementation with 1600 IU/d or 50,000 IU monthly was not associated with any laboratory parameters of toxicity [eg, 25(OH)D, PTH, bone alkaline phosphatase, and 24-hour urine calcium] and even failed to increase total 25(OH)D levels above 30 ng/mL in 19% of participants.
CONCLUSION
Vitamin D is important for skeletal and nonskeletal health. It is now well established that many people have vitamin D levels that are less than currently recommended for optimal health. Worldwide, vitamin D is predominantly obtained through exposure to UVB radiation in the form of sunlight and cutaneous vitamin D production. Latitude, cultural dress habits, season, sun avoidance, and sunscreen protection can all limit vitamin D production. Gastrointestinal, hepatic, and renal disease may be related to low vitamin D levels, but hypovitaminosis D most commonly results from inadequate intake. Hypovitaminosis D resulting from lack of UVB exposure is not easily corrected by dietary intake alone in the absence of supplementation. Food fortification with vitamin D is based on outdated recommendations for daily AI. Supplementation with 800 to 1000 IU/d of vitamin D or 50,000 IU monthly is safe for most people and can ensure levels of vitamin D within the optimal range. This intake is within the currently recommended safe upper tolerable limit for vitamin D of 2000 IU/d for those aged 1 year and older. Revised recommended dietary intake values for vitamin D, which are needed to guide patients and physicians alike, are expected to be published in 2010.
Supplementary Material
On completion of this article, you should be able to (1) recognize patients at risk for vitamin D deficiency, (2) optimally use and interpret serum vitamin D testing, and (3) determine the optimal vitamin D therapy required to treat or prevent vitamin D deficiency in adults.
CME Questions About Vitamin D Deficiency in Adults
-
Which one of the following patients is at greatest risk for vitamin D deficiency?
A formula-fed infant
A teenaged girl eating an unrestricted diet and taking a multivitamin
A 30-year-old male nursing home resident treated with phenytoin for epilepsy
A 70-year-old woman with osteopenia taking a calcium carbonate with vitamin D supplement
A 43-year-old male farmer
-
Which one of the following biochemical tests provides the best initial assessment of a person's vitamin D status?
Serum parathyroid hormone (PTH)
Serum 25-hydroxyvitamin D (calcidiol) [25(OH)D]
Serum 1,25-dihydroxyvitamin D (calcitriol) [1,25(OH)2 D]
Serum bone alkaline phosphatase
24-hour urine calcium excretion
-
Which one of the following sets of laboratory test findings (reference ranges provided parenthetically) is most suggestive of vitamin D toxicity?
Serum calcium, 9.7 mg/dL (8.9-10.1 mg/dL); serum phosphorus, 4.0 mg/dL (2.5-4.5 mg/dL); 24-hour urine calcium, 250 mg/spec (25-300 mg/spec); 25(OH)D, 120 ng/mL (25-80 ng/mL); and PTH, 30 pg/mL (15-50 pg/mL)
Serum calcium, 10.4 mg/dL (8.9-10.1 mg/dL); serum phosphorus, 4.8 mg/dL (2.5-4.5 mg/dL); 24-hour urine calcium, 450 mg/spec (25-300 mg/spec); 25(OH)D, 120 ng/mL (25-80 ng/mL); and PTH, 20 pg/mL (15-50 pg/mL)
Serum calcium, 11.0 mg/dL (8.9-10.1 mg/dL); serum phosphorus, 2.2 mg/dL (2.5-4.5 mg/dL); 1,25(OH)2D, 85 pg/mL (22-67 pg/mL); and PTH, 95 pg/mL (15-50 pg/mL)
Serum calcium, 10.6 mg/dL (8.9-10.1 mg/dL); serum phosphorus, 4.0 mg/dL (2.5-4.5 mg/dL); 24-hour urine calcium, 450 mg/spec (25-300 mg/spec); 25(OH)D, 26 ng/mL (25-80 ng/mL); 1,25(OH)2D, 85 pg/mL (22-67 pg/mL); and PTH, 12 pg/mL (15-50 pg/mL)
Serum calcium, 15 mg/dL (8.9-10.1 mg/dL); serum phosphorus, 4.0 mg/dL (2.5-4.5 mg/dL); 24-hour urine calcium, 450 mg/spec (25-300 mg/spec); 25(OH)D, 35 ng/mL (25-80 ng/mL); 1,25(OH)2D, <10 pg/mL (22-67 pg/mL); and PTH, <6 pg/mL (15-50 pg/mL)
-
Which one of the following treatment strategies is most likely to be safe and effective to achieve optimal vitamin D levels in a person with little sun exposure?
Daily supplementation with 400 IU of vitamin D3 (D3) via a multivitamin
Daily supplementation with 800 to 1000 IU of vitamin D2 (D2) or D3
Daily supplementation with 50,000 IU of D3
Monthly supplementation with 50,000 IU of D2
One daily serving of fortified milk
-
Which one of the following statements about measuring 25(OH)D levels is correct?
All patients should be tested for vitamin D deficiency before supplementation
A patient with a 25(OH)D level of 10 ng/mL who is beginning treatment with 800 IU/d of D3 should be rechecked after 1 month
A patient with a 25(OH)D level of 10 ng/mL who is beginning treatment with 50,000 IU of vitamin D 3 times weekly for 1 month to be followed by 50,000 IU once monthly should be rechecked after 1 month
A patient with a 25(OH)D level of 10 ng/mL who is beginning treatment with 2000 IU/d of D3 should be rechecked after 6 months
A patient with generous summertime sun exposure living at a high latitude with a low 25(OH)D level in spring should be supplemented and retested in the fall
This activity was designated for 1 AMA PRA Category 1 Credit(s).™
Because the Concise Review for Clinicians contributions are now a CME activity, the answers to the questions will no longer be published in the print journal. For CME credit and the answers, see the link on our Web site at mayoclinicproceedings.com.
REFERENCES
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281 [DOI] [PubMed] [Google Scholar]
- 2.Plotnikoff GA. Weather or not: the importance of vitamin D status monitoring and supplementation. Minn Med. 2009;92(11):43-46 [PubMed] [Google Scholar]
- 3.Khoja SO, Khan JA, Berry JL, Maimani AR, Woolf AD, Lanham-New SA. Nutritional influences on bone in Saudi Arabian women: widespread vitamin D deficiency Presented at the 6th International Symposium on Nutritional Aspects of Osteoporosis; Lausanne, Switzerland; May4-6, 2006 [Google Scholar]
- 4.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22:477-501 [DOI] [PubMed] [Google Scholar]
- 5.Glerup H, Mikkelsen K, Poulsen L, et al. Commonly recommended daily intake of vitamin D is not sufficient if sunlight exposure is limited. J Intern Med. 2000;247:260-268 [DOI] [PubMed] [Google Scholar]
- 6.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004. Arch Intern Med. 2009;169:626-632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bischoff HA, Stahelin HB, Dick W, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18:343-351 [DOI] [PubMed] [Google Scholar]
- 8.Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327:1637-1642 [DOI] [PubMed] [Google Scholar]
- 9.Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA 2005;293:2257-2264 [DOI] [PubMed] [Google Scholar]
- 10.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353-373 [DOI] [PubMed] [Google Scholar]
- 11.Plotnikoff GA, Quigley JM. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc. 2003;78:1463-1470 [DOI] [PubMed] [Google Scholar]
- 12.Arvold DS, Odean MJ, Dornfeld MP, et al. Correlation of symptoms with vitamin D deficiency and symptom response to cholecalciferol treatment: a randomized controlled trial. Endocr Pract. 2009;15:203-212 [DOI] [PubMed] [Google Scholar]
- 13.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S-1696S [DOI] [PubMed] [Google Scholar]
- 14.Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730-1737 [DOI] [PubMed] [Google Scholar]
- 15.Ginde AA, Scragg R, Schwartz RS, Camargo CA., Jr Prospective study of serum 25-hydroxyvitamin d level, cardiovascular disease mortality, and all-cause mortality in older U.S. Adults. J Am Geriatr Soc. 2009;57:1595-1603 [DOI] [PubMed] [Google Scholar]
- 16.Reusch J, Ackermann H, Badenhoop K. Cyclic changes of vitamin D and PTH are primarily regulated by solar radiation: 5-year analysis of a German (50 degrees N) population. Horm Metab Res. 2009;41:402-407 [DOI] [PubMed] [Google Scholar]
- 17.Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am J Clin Nutr. 2007;85:6-18 [DOI] [PubMed] [Google Scholar]
- 18.Institute of Medicine Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride Washington, DC: National Academy Press; 1999:71-145 [Google Scholar]
- 19.Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89:5387-5391 [DOI] [PubMed] [Google Scholar]
- 20.Holick MF, Biancuzzo RM, Chen TC, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab. 2008;93:677-681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Binkley N, Gemar D, Engelke J, Gangnon R, Ramamurthy R, Krueger D. Dosing with ergocalciferol or cholecalciferol, 1,600 IU daily or 50,000 IU monthly, is safe but does not assure vitamin D adequacy. J Bone Miner Res. 2009;24 (suppl 1). [Google Scholar]
- 22.Yetley EA, Brule D, Cheney MC, et al. Dietary reference intakes for vitamin D: justification for a review of the 1997 values. Am J Clin Nutr. 2009;89:719-727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pepper KJ, Judd SE, Nanes MS, Tangpricha V. Evaluation of vitamin D repletion regimens to correct vitamin D status in adults. Endocr Pract. 2009;15:95-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heaney RP. The Vitamin D requirement in health and disease. J Steroid Biochem Mol Biol. 2005;97:13-19 [DOI] [PubMed] [Google Scholar]
- 25.Chandra P, Wolfenden LL, Ziegler TR, et al. Treatment of vitamin D deficiency with UV light in patients with malabsorption syndromes: a case series. Photodermatol Photoimmunol PhotoMed. 2007;23:179-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bertino JS, Reed MD, Lambert PW, Halpin TC. Stability of an extemporaneous formulation of injectable cholecalciferol. Am J Hosp Pharm. 1981;38:1932-1933 [PubMed] [Google Scholar]
- 27.Heaney RP, Dowell MS, Bierman J, Hale CA, Bendich A. Absorbability and cost effectiveness in calcium supplementation. J Am Coll Nutr. 2001;20:239-246 [DOI] [PubMed] [Google Scholar]
- 28.Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88:582S-586S [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.