Abstract
In this study of the mosquito, Culex pipiens, we examined the impact of multiple bouts of dehydration and rehydration on survival, depletion of metabolic reserves and egg production in both non-diapausing and diapausing females. Mosquitoes provided with access to sugar during rehydration survived longer than those allowed to rehydrate without sugar, and their survival was similar to that of mosquitoes of the same age that were not dehydrated. Among mosquitoes not provided with sugar, each dehydration bout reduced the mosquito's dry mass – an effect likely to be due to the utilization of carbohydrates and lipid reserves. The toll on glycogen and lipid reserves is likely to be especially costly for diapausing mosquitoes that are dependent on these stored reserves for winter survival. Egg production in both non-diapausing and post-diapausing C. pipiens was also reduced in response to multiple bouts of dehydration. Although egg quality was not compromised, the number of eggs produced was reduced. Both non-diapausing and diapausing females can compensate for the nutrient loss due to dehydration by sugar feeding but the opportunity to feed on sugar is likely to be rarely available in the overwintering habitat of diapausing females, thus the impact of dehydration may be especially pronounced in overwintering populations of C. pipiens.
Keywords: dehydration bouts, nutrient reserves, egg production, Culex pipiens, diapause
INTRODUCTION
The response of insects to dehydration is defined by their ability to maintain the water pool necessary to remain functional and to prevent or recover from the stress of dehydration (Hadley, 1994). Water balance is maintained by reducing water lost through cuticular and respiratory routes, improving water re-absorption by the alimentary canal, or increasing water uptake by drinking or absorbing water vapor. Water stress is alleviated by increasing the internal concentrations of protective sugars and polyols and by up-regulating the expression of stress-related proteins that repair damaged proteins, reduce oxidative stress and maintain cellular integrity (França et al., 2007; Li et al., 2009; Lopez-Martinez et al., 2009). Previous studies have usually focused on a single bout of dehydration and rehydration to establish an insect's water balance profile (Wharton, 1985; Hadley, 1994; Benoit, 2010) but insects are likely to experience multiple bouts of dehydration, especially overwintering insects that remain dormant (in diapause) for many months.
Based on several studies of mosquito dehydration (Gray and Bradley, 2005; Rinehart et al., 2006; Benoit and Denlinger, 2007; Lee et al., 2009; Benoit et al., 2010), it is evident that mosquitoes are fairly susceptible to dehydration, i.e. they are hydrophilic. Adult female mosquitoes contain 60–70% water, and they can lose approximately 25–35% of their water content before succumbing (Benoit et al., 2010). To increase their water pool, mosquitoes rely solely on the ingestion of blood or free water (Gray and Bradley, 2005; Benoit et al., 2010). Up-regulation of heat shock proteins (Hsp70 and Hsp90) has been noted in three mosquito species during dehydration, and knockdown experiments using RNA interference indicate that these two proteins are important for the maintenance of dehydration tolerance (Benoit et al., 2010). Additionally, the diapause programme increases the resistance of adult females of Culex pipiens to dehydration (Rinehart et al., 2006; Benoit and Denlinger, 2007), and differences in dehydration resistance have also been noted between M and S forms of Anopheles gambiae (Lee et al., 2009; Gray et al., 2009), thus both developmental programmes (diapause and non-diapause) and population differences are known to impact mosquito dehydration resistance.
In this study, we examined the effect of multiple dehydration/rehydration exposures on the physiology of the northern house mosquito, C. pipiens. To do so, we analyzed the water balance characteristics and energy reserves of mosquitoes exposed to single and multiple bouts of dehydration and compared the results with individuals of the same age held under non-desiccating conditions. Additionally, we provided a sub-sample of mosquitoes with sugar and others were provided with no sugar between dehydration bouts to determine whether the negative consequences of dehydration bouts could be alleviated by the presence of a nutrient resource. Finally, we compared the effects of multiple dehydration bouts on both non-diapausing and diapausing adult females. In response to short daylength and low temperatures in late summer and autumn, adult females enter an overwintering diapause (Robich and Denlinger, 2005). Blood feeding ceases, females seek sugar resources to increase their fat reserves nearly 3-fold greater than non-diapause individuals (Robich and Denlinger, 2005; Sim and Denlinger, 2008) and reproduction is halted (Spielman and Wong, 1973; Bowen et al., 1988). Diapause represents an extended period when food is not normally available, thus the effects of dehydration bouts could be expected to be particularly stressful at this time. We demonstrate that multiple bouts of dehydration/rehydration, in the absence of sugar, significantly impact the survival of C. pipiens – most likely to be a result of depleted nutrient reserves. The consequences are also evident as a decrease in the number, but not in the quality, of eggs produced after multiple dehydration/rehydration bouts.
MATERIALS AND METHODS
Mosquitoes
The colony of Culex pipiens L. (Buckeye strain), originating in Columbus, OH, USA, was maintained as previously described (Robich and Denlinger, 2005). Larvae were fed a diet of ground fish food (Tetramin, Tetra, Mulle, Germany). Non-diapausing (ND) adult females were generated by rearing larvae and adults under long-day conditions (15 h:9 h, L:D), while diapausing (D) females were reared under short-day conditions (9 h:15 h, L:D). Both ND and D mosquitoes were reared at 18°C, 93% relative humidity (RH). Adults were fed 10% sucrose. Nearly all females (non-diapausing and diapausing) were inseminated before use in the experiments. Eggs were generated by allowing mosquitoes to feed on a live rooster (Gallus gallus L.; IACUC 2008A0206). In experiments using post-diapause females, the females were held in diapause for 50 days before diapause was broken by transfer to 15 h:9 h L:D and 25°C.
Dehydration experiments
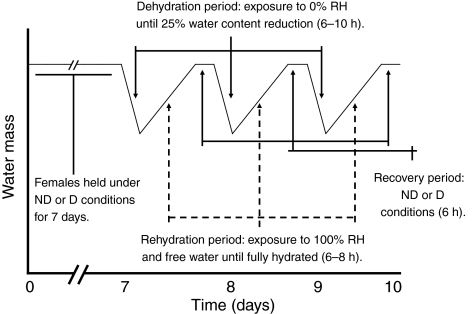
Adult females, 7 days post-emergence, were used in this study. At this point, non-diapausing and diapausing mosquitoes reach stable lipid levels (Mitchell and Briegel, 1989; Robich and Denlinger, 2005; Sim and Denlinger, 2008; Zhou and Miesfeld, 2009). During each period of dehydration, mosquitoes were held at 0% RH until individuals lost 25% of their water content, they were then moved to 100% RH in the presence of liquid water to rehydrate, and then were finally moved to colony conditions (93% RH in the presence of free water until the next period of dehydration). Each dehydration/rehydration bout lasted 20–30 h. A diagram of the dehydration bouts is presented as Fig. 1. During rehydration, one subset of mosquitoes was provided with only water (water only) and a second subset was provided with 10% sucrose and water (sugar + water), thus providing a comparison of mosquitoes exposed to dehydration/rehydration bouts with and without an energy source. Additionally, a subset of mosquitoes was held at 100% RH throughout the experiments (fully hydrated) to ensure that differences were not due to age. These mosquitoes served as a control, providing a comparison with mosquitoes that did not experience dehydration. Mosquitoes were held individually in 10 cc to prevent group effects from altering water balance characteristics (Benoit et al., 2005; Benoit et al., 2007). In this study, non-diapausing mosquitoes were compared after 0, 1, 2, 4, 6, 8 and 10 bouts of dehydration, and diapausing mosquitoes were analyzed after 0, 5, 10, 15, 20 and 25 bouts of dehydration.
Fig. 1.
Schematic representation of the experimental protocol, showing three bouts of dehydration and rehydration. The duration of each bout of dehydration/rehydration was 20–30 h. ND, non-diapause; D, diapause; RH, relative humidity.
Water balance analysis
Mass changes in the mosquitoes were monitored gravimetrically using an electrobalance (CAHN 35, Ventron Co., Cerritos, CA, USA). Each mosquito was weighed singly without enclosure after a brief CO2 knockdown (Benoit and Denlinger, 2007). Mosquitoes were transferred to the weighing pan and returned to the experimental conditions within 1 minute. Relative humidities were generated using saturated salt solutions (Winston and Bates, 1960), 0% RH was established with calcium sulfate, and double-distilled water was used to create 100% RH. All test relative humidities were validated with a hygrometer (Taylor Scientific, St Louis, MO, USA). All observations were conducted at 18°C in a controlled environment room.
The amount of water available for exchange (m, water mass) was determined according to standard methods for insects (Wharton, 1985; Hadley, 1994; Benoit and Denlinger, 2007). Mosquitoes were placed at 0% RH and 18°C until a loss of 4–6% of their mass to ensure that mass changes reflected a shift in the water pool (Wharton, 1985). Subsequently, consecutive mass determinations (0% RH, 22–24°C) were made at hourly intervals for a total of six mass readings, and then individuals were transferred to 80°C, 0% RH to determined dry mass (d, denoted by five consecutive daily mass measurements with no changes). Water mass was determined by subtracting dry mass from the initial mass. Initial, immediate and final water mass values were analyzed according by Wharton (Wharton, 1985) for determining water loss rates:
| (1) |
where mt is the water mass at any time t, m0 is the initial water mass, and k is the rate of water loss expressed as % h−1. The dehydration level at which the mosquitoes can no longer right themselves and fly when prodded was defined as the critical activity point. This denotes the dehydration tolerance – an irreversible lethal amount of water loss.
Nutritional reserve analyses
The amount of sugar, glycogen and lipids within each mosquito was determined using anthrone and vanillin assays (Van Handel, 1985a; Van Handel, 1985b; Van Handel and Day, 1988; Vaidyanathan et al., 2008). Briefly, individual mosquitoes were homogenized in 0.2 ml sodium sulfate, followed by the addition of 1.8 ml of chloroform:methanol (2:1). The supernatant was divided equally into two 16 mm×100 mm glass test tubes for the determination of lipid and sugar contents (Van Handel and Day, 1988). The precipitate was utilized to determine glycogen levels. Each glycogen sample was heated with 1 ml anthrone reagent for 10 min, and then combined with 4 ml of hot anthrone reagent for 10 min. All samples (glycogen, lipid and sugar) were cooled to room temperature, and absorbance was measured with a spectrophotometer at two wavelengths (625 nm and 560 nm for sugar and glycogen samples; 525 nm and 490 nm for lipid samples). Two samples were measured for each mosquito. Concentrations were determined using a standard curve.
Total protein content in the mosquitoes was determined with a Bradford Assay (Bio-Rad, Hercules, CA, USA). Individual mosquitoes were placed in 500 μl of phosphate buffered saline (PBS) and sonicated. The homogenate (100 μl) was combined with 700 μl PBS and 200 μl Bradford reagent. Samples were incubated for 10 min at 25°C and absorbance at 295 nm was determined. Known amounts of bovine serum albumin were used to establish a standard curve.
Egg production
To determine egg production, mosquitoes that had been exposed to multiple bouts of dehydration were subsequently allowed to blood-feed on a rooster (G. gallus). Non-diapausing mosquitoes were exposed to their respective number of dehydration bouts, then held at 100% RH until they were 18 days post adult emergence. Diapausing mosquitoes were held under diapausing conditions for 50 days, they were then moved to non-diapausing conditions for 10 days before being offered a blood meal. After three days, a 1-liter container with 0.5 l of water was provided for oviposition. The number of eggs in each raft was counted, and carbohydrate, glycogen and lipid levels within the eggs were analyzed according to Harrington et al. (Harrington et al., 2001).
Field collections and relative humidity levels
Relative humidities within overwintering hibernacula were determined daily within culverts in the vicinity of Columbus, OH, USA, for one week in December 2006. Briefly, hygrometers (HOBO H8 Family Data Loggers, Onset Computer, Bourne, MA, USA) were secured in areas where overwintering C. pipiens were present in large numbers. All culverts were constructed of cement and the opening varied from 0.5 m to 2.5 m in diameter. From the same areas, mosquitoes were collected for eight weeks to determine if mosquito water content varied in the field. This set of experiments was designed to assess natural variation in RH and its impact on mosquito water content.
RESULTS
Basic water balance characteristics of non-diapausing females
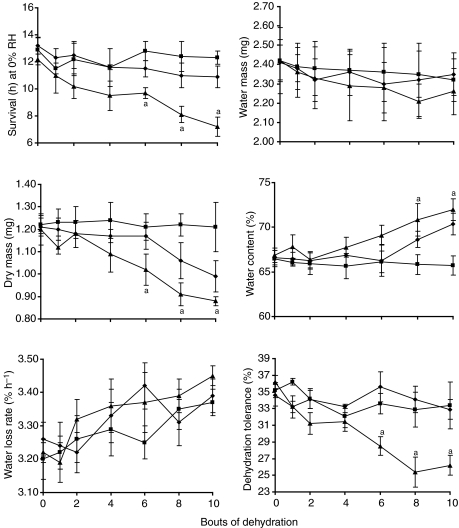
Water balance characteristics for C. pipiens exposed to no previous dehydration bouts were similar to those reported previously (Benoit and Denlinger, 2007; Benoit et al., 2010). The initial mass of the non-diapausing mosquitoes was 3.62±0.13 mg (mean ± s.e.m., N=30), and they contained 2.41±0.11 mg of water (66.6% water content). Water loss rate was 3.26±0.09% h−1, dehydration tolerance was 34.6±1.1%, and survival at 0% RH was 13.4±0.8 h. No significant differences were noted in any water balance characteristics after mosquitoes were subjected to four bouts of dehydration (Fig. 2; ANOVA; P>0.05). Following six bouts of dehydration, survival, dry mass and dehydration tolerance were significantly lower for water-only mosquitoes compared with sugar + water and fully hydrated mosquitoes (Fig. 2; ANOVA; P<0.05), and this trend continued after eight and 10 bouts of dehydration (Fig. 2). By contrast, water loss rates and water mass were not significantly different at any point in experiments using non-diapausing C. pipiens (Fig. 2; ANOVA; P>0.05). Based on these results, we conclude that survival of C. pipiens will be reduced following successive bouts of dehydration unless the mosquitoes have the opportunity to replenish their metabolic reserves.
Fig. 2.
Water balance characteristics of non-diapausing adult females of Culex pipiens after multiple bouts of dehydration. ▴, water only during recovery; ▪, 10% sucrose and water during recovery; ♦, fully hydrated controls. Each point is the mean ± s.e.m. of 24 individuals. a, denotes significance (P<0.05) between the three treatments based on ANOVA. RH, relative humidity.
Depletion of nutritional reserves by dehydration
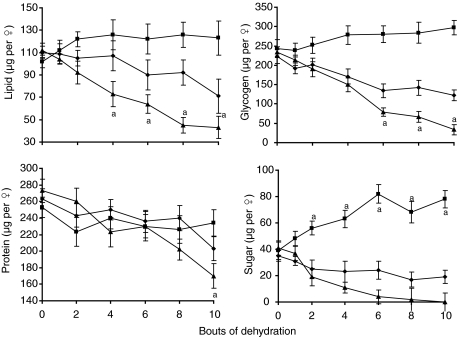
The decline in dry mass implies that metabolic reserves of C. pipiens were negatively impacted by multiple dehydration bouts. Initial lipid, carbohydrate and glycogen levels in adult females were similar to those previously reported (Vaidyanathan et al., 2008; Sim and Denlinger, 2008). Reductions in glycogen and lipid were apparent after four bouts of dehydration for water-only mosquitoes (ANOVA; P<0.05) whereas there was a significant increase in carbohydrate content for sugar + water mosquitoes after only two bouts of dehydration (Fig. 3). A significant reduction in protein content of the water-only mosquitoes was noted only after eight bouts of dehydration (Fig. 3). By comparison with females provided with sugar between dehydration bouts, water-only control females exposed to 10 bouts of dehydration had reduced lipid levels by 62%, glycogen by 85%, protein by 24% and sugar reserves were completely eliminated (Fig. 3). Lipids, glycogen and carbohydrates also significantly declined in fully hydrated controls as time progressed (Fig. 3; ANOVA, P<0.05) but these reduction levels were not as severe as the levels observed in water-only mosquitoes subjected to bouts of dehydration. Thus, nutritional reserves declined when mosquitoes were not provided with a sugar resource, and this effect was exacerbated by multiple dehydration exposures.
Fig. 3.
Nutrient reserves in non-diapausing adult females after multiple bouts of dehydration. ▴, water only during recovery; ▪, 10% sucrose and water during recovery; ♦, fully hydrated controls. Each point is the mean ± s.e.m. of 24 individuals. a, denotes significance (P<0.05) between the three treatments groups based on ANOVA.
Impact of dehydration bouts during diapause
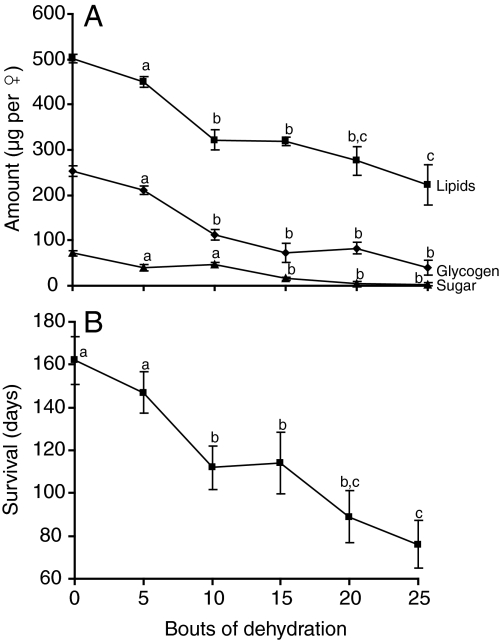
To determine if bouts of dehydration also impact diapausing mosquitoes in the same way, we monitored metabolic reserves and survival after exposures to different numbers of dehydration bouts. The first significant reduction in survival was noted after 10 dehydration bouts (Fig. 4A), at which time lipid levels had dropped by 36%, glycogen content by 56% and sugar content by 33%. After 25 dehydration bouts, lipid content was reduced by 55%, glycogen content by 84% and sugar content by 96% (Fig. 4A). Survival of diapausing C. pipiens was reduced by nearly 25% after 10 bouts and by nearly 50% when females were exposed to 25 bouts of dehydration (Fig. 4B). Thus, diapausing females of C. pipiens were affected in the same manner as observed in non-diapausing females: dehydration reduced metabolic reserves, resulting in reduced survival.
Fig. 4.
Changes in (A) nutrient reserves and (B) survival of diapausing adult females after multiple bouts of dehydration. ▴, sugar; ▪, lipids; ♦, glycogen. Each point is the mean ± s.e.m. of 15 individuals. Superscripts denote significance (P<0.05).
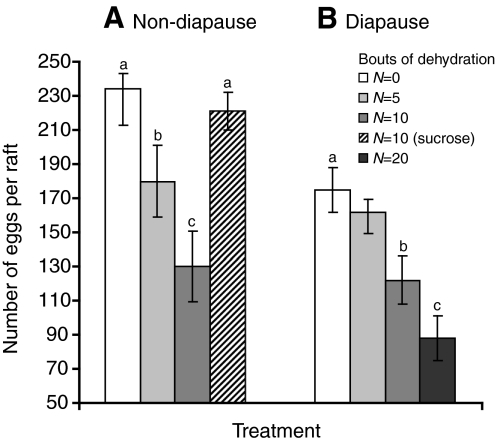
Egg production
Non-diapausing females laid significantly more eggs per raft than females that had experienced 50 days of diapause (234±19 eggs vs 175±17 eggs). Five bouts of dehydration (non-diapause females) or 10 bouts (diapause females) reduced egg production compared with controls (Fig. 5). Non-diapausing mosquitoes that were offered sugar during their recovery period produced nearly the same number of eggs per raft as those that were not dehydrated (Fig. 5, ANOVA, P>0.05). The mean carbohydrate (1.0 μg), glycogen (0.4 μg) and lipid (0.7 μg) contents within each egg did not vary between treatment groups (ANOVA, P>0.05). Thus, multiple bouts of dehydration did not influence reserves packaged within the egg but it did have a significant effect on the number of eggs per raft, and this negative effect could be countered, at least in non-diapausing females, by the provision of a sugar source.
Fig. 5.
Egg production in (A) non-diapausing and (B) post-diapause adult females following blood ingestion after multiple bouts (N=0–20) of dehydration. Each point is the mean ± s.e.m. of 15 individuals. Superscripts denote significance (P<0.05).
Field observations
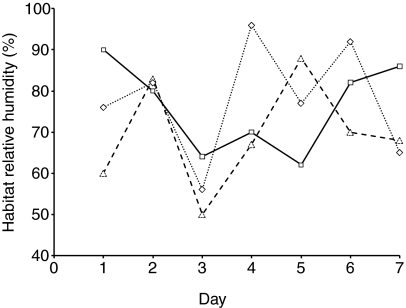
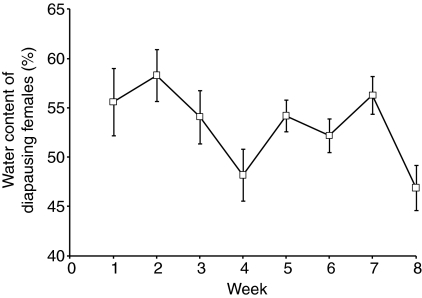
RH levels in three overwintering mosquito habitats varied considerably from 50% RH to near saturation, within a single week (Fig. 6). Mean humidity was 70–75% RH. Water content of the mosquitoes was analyzed from overwintering sites; the water content varied from 48–60% (Fig. 7) – values that were considerably lower than the 66.6% observed in our fully hydrated lab-reared females (Fig. 2). These results suggest that mosquitoes are exposed to periods of dehydration while residing in their overwintering habitats and that such fluctuations have an impact on the water content of the mosquitoes.
Fig. 6.
Relative humidity levels in three hibernacula utilized by diapausing Culex pipiens near Columbus, OH, USA, during a one-week period in December 2006.
Fig. 7.
Water content of diapausing female adults of Culex pipiens collected from their overwintering hibernacula near Columbus, OH, USA, during eight weeks in December 2006 and January 2007. Each point is the mean ± s.e.m. of 10 mosquitoes.
DISCUSSION
Drosophila melanogaster (Albers and Bradley, 2004; Folk and Bradley, 2003) and related Drosophila species (Gibbs and Matzkin, 2001; Hoffmann, 2003) have been the focus of most previous works on dipteran water balance. Studies with Drosophila demonstrate higher dehydration resistance (Gibbs and Matzkin, 2001; Matzkin et al., 2007), reduced water loss rates (Gibbs and Matzkin, 2001) and reduced metabolism in xeric-adapted species (Gibbs et al., 2003). Selection for dehydration tolerance in D. melanogaster is associated with the accumulation of glycogen and differential metabolism of carbohydrates and lipids (Djawdan et al., 1998; Bradley et al., 1999; Gibbs, 2002). Similar dehydration resistance mechanisms appear to be operating in diapausing adults of C. pipiens (Benoit and Denlinger, 2007) and in Anopheles gambiae (Gray et al., 2009). But thus far, studies on insect dehydration have nearly exclusively focused on evaluating the impact of a single exposure to dehydration. This is the first study to evaluate the impact of multiple bouts of dehydration and rehydration on the physiology of mosquitoes (or any other insect). In our experiments, each dehydration exposure was followed by a subsequent rehydration. Several recent studies suggest that dehydration and rehydration elicit distinct stress responses (Hayward et al., 2004; Lopez-Martinez et al., 2009), thus rehydration cannot be viewed as a simple reversal of dehydration stress.
Our results demonstrate a progressive decline in glycogen, lipid and sugar contents as the number of dehydration/rehydration bouts increased. This negative effect can be ameliorated by allowing the mosquito to ingest sugar between dehydration bouts. The reduction in nutritional reserves that we observed is probably the consequence of the mosquito's response to water stress. This form of stress invokes energy-depleting activities such as the mobilization of antioxidants, heat shock proteins (Sinclair et al., 2007; Lopez-Martinez et al., 2009), aquaporins, late embryogenesis abundant (LEA) proteins (França et al., 2007) and cytoskeletal proteins (Li et al., 2009). In a recent study, we showed that heat shock protein 70 (Hsp70) and Hsp90 are essential in the mosquito's response to dehydration stress (Benoit et al., 2010). Significant changes in metabolic genes have also been noted in response to dehydration resistance in Drosophila (Matzkin and Markow, 2009). In addition to changes in protein and gene levels, other molecules including trehalose and glycerol are likely to be generated to prevent protein interactions and reduce membrane changes as water levels within the mosquito fluctuate (Goyal et al., 2005; Watanabe, 2006). Unless these reserves are replenished, multiple bouts of dehydration exhaust the glycogen, lipid and sugar reserves, thus denying the mosquito the nutritional resources required to respond and subsequently recover from dehydration stress.
Non-diapausing females of C. pipiens have the potential to replenish their reserves by feeding on nectar and avian hosts that are readily available during the summer months. But, this is not the case for diapausing females. In response to short daylength of late summer and early autumn, females of C. pipiens cease feeding on blood and rely exclusively on sugar sources to generate the reserves needed to bridge the winter months (Mitchell and Briegel, 1989; Bowen, 1992; Robich and Denlinger, 2005). These additional lipids are the result of an increase in fatty acid synthesis in diapausing mosquitoes immediately after adult emergence (Robich and Denlinger, 2005; Sim and Denlinger, 2009). The diapausing females then retreat to protected habitats such as caves and culverts (Vinogradova, 2000) and thus spend the winter in locales that are unlikely to provide sugar resources. This study and that of Rinehart et al. (Rinehart et al., 2006) indicate that RH within overwintering sites can vary significantly, resulting in field-collected mosquitoes with a reduced water content that is considerably lower than in those reared in the laboratory at high humidity. We suspect that diapausing females would be the stage most likely to experience bouts of dehydration, and also due to the absence of sugar resources during the winter, it is the stage likely to be most vulnerable to this form of stress. Low temperature in the overwintering stage, however, can be expected to counter the impact of dehydration by reducing the metabolic rate and thus conserve nutrient and water resources (Robich and Denlinger, 2005; Benoit and Denlinger, 2007). Yet, C. pipiens remains in the overwintering habitat for 8–9 months, and is probably subjected to numerous bouts of low humidity during this time. High humidity, or the presence of liquid water, in the overwintering habitat is critical for survival, and our observations indicate variation in the mosquito's water content under field conditions. Responding to changes in water content can be expected to lower the energy reserves necessary to survive until diapause is terminated in the spring.
Bouts of dehydration impact not only the survival of the adult but also the number of eggs she produces. Females with reduced nutritional reserves are well known to produce fewer eggs (Foster, 1995; Ziegler and Ibrahim, 2001; Harrington et al., 2001; Zhou et al., 2004). Females allowed to feed on sugar prior to blood feeding have significantly higher lipid reserves, allowing these females to produce more eggs that contain more lipids (Ziegler and Ibrahim, 2001; Zhou et al., 2004). During the first gonotrophic cycle, carbohydrate reserves, along with lipids, are a crucial source of energy, thus a severe reduction in such reserves is likely to reduce egg production. Our experiments showed that carbohydrate, glycogen and lipid levels of adult females were negatively impacted by chronic bouts of dehydration, resulting in a lower production of eggs in both non-diapause females as well as post-diapause females exposed to multiple dehydration bouts during diapause. Interestingly, no changes in the nutritional reserves were noted within individual eggs but fewer eggs were produced; a result that is consistent with previous observations (Harrington et al., 2001) on Aedes aegypti where non-optimal blood meals reduced egg quantity rather that individual quality. Thus, our results indicate an indirect impact of water balance on egg production, presumably through the diversion of energy reserves from egg production to the combating of water stress.
In conclusion, we demonstrate that multiple bouts of dehydration and rehydration significantly impact the physiology of C. pipiens. We show that nutritional reserves are utilized in response to dehydration, resulting in the progressive erosion of dehydration resistance. This effect can be alleviated by sugar feeding between dehydration bouts. To the best of our knowledge, no previous studies have addressed the effect of numerous dehydration exposures on arthropods but several studies have tested the impact of multiple freeze/thaw cycles in insects (Bale et al., 2001; Sinclair and Chown, 2005). Our results suggest that determining how an insect responds to chronic bouts of dehydration may be important in evaluating its water balance profile. For insects undergoing dormancy, maintaining water balance is one of the most crucial factors determining survival (Danks, 2000; Benoit, 2010). Most insects in diapause have reduced water loss rates (Benoit, 2010) but in this study we show that each bout of dehydration reduces the metabolic reserves needed to survive through the dormant period, and this can impact post-diapause egg production as well. Prevention of chronic dehydration during diapause may be crucial for insects that do not feed during their dormant periods and thus rely solely on nutritional reserves obtained before the onset of dormancy.
ACKNOWLEDGEMENTS
Research was funded by a grant from the National Institutes of Health (R01 AI058279), and support for J.B.B. was provided by a Mary S. Muelhaput Endowed Presidential Fellowship from the Ohio State University. Deposited in PMC for release after 12 months.
REFERENCES
- Albers M., Bradley T. J. (2004). Osmotic regulation in adult Drosophila melanogaster during dehydration and rehydration. J. Exp. Biol. 207, 2313-2321 [DOI] [PubMed] [Google Scholar]
- Bale J. S., Worland M. R., Block W. (2001). Effects of summer frost exposures on cold tolerance strategy of the sub-Antarctic beetle. J. Insect Physiol. 47, 1161-1167 [DOI] [PubMed] [Google Scholar]
- Benoit J. B. (2010). Water management by dormant insects: comparisons between dehydration resistance during estivation and winter diapause. In Progress in Molecular and Subcellular Biology, Vol. Estivation (ed. Navas C., Eduardo J.), pp. 209-230 Berlin: Springer; [DOI] [PubMed] [Google Scholar]
- Benoit J. B., Denlinger D. L. (2007). Suppression of water loss during adult diapause in the northern house mosquito, Culex pipiens. J. Exp. Biol. 210, 217-226 [DOI] [PubMed] [Google Scholar]
- Benoit J. B., Yoder J. A., Rellinger E. J., Ark J. T., Keeney G. D. (2005). Prolonged maintenance of water balance by adult females of the American spider beetle, Mezium affine Boieldieu, in the absence of food and water resources. J. Insect Physiol. 51, 565-573 [DOI] [PubMed] [Google Scholar]
- Benoit J. B., Del Grosso N. A., Yoder J. A., Denlinger D. L. (2007). Resistance to dehydration between bouts of blood feeding in the bed bug, Cimex lectularius, is enhanced by water conservation, aggregation, and quiescence. Am. J. Trop. Med. Hyg. 76, 987-993 [PubMed] [Google Scholar]
- Benoit J. B., Lopez-Martinez G., Phillips Z. P., Patrick K. R., Denlinger D. L. (2010). Heat shock proteins contribute to mosquito dehydration tolerance. J. Insect Physiol. 56, 151-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen M. F. (1992). Patterns of sugar feeding in diapausing and nondiapausing Culex pipiens (Diptera:Culicidae) females. J. Med. Entomol. 29, 843-849 [DOI] [PubMed] [Google Scholar]
- Bowen M. F., Davis E. E., Haggart D. A. (1988). A behavioral and sensory analysis of host-seeking behavior in the diapausing mosquitoes Culex pipiens. J. Insect Physiol. 34, 805-813 [Google Scholar]
- Bradley T. J., Williams A. E., Rose M. R. (1999). Physiological responses to selection for desiccation resistance in Drosophila melangaster. Am. Zool. 39, 337-345 [Google Scholar]
- Danks H. V. (2000). Dehydration in dormant insects. J. Insect Physiol. 46, 837-852 [DOI] [PubMed] [Google Scholar]
- Djawdan M., Chippindale A. K., Rose M. R., Bradley T. J. (1998). Metabolic reserves and evolved stress resistance in Drosophila melanogaster. Physiol. Zool. 71, 584-594 [DOI] [PubMed] [Google Scholar]
- Folk D. G., Bradley T. J. (2003). Evolved patterns and rates of water loss and ion regulation in laboratory-selected populations of Drosophila melanogaster. J. Exp. Biol. 206, 2779-2786 [DOI] [PubMed] [Google Scholar]
- Foster W. A. (1995). Mosquito sugar feeding and reproductive energetics. Annu. Rev. Entomol. 40, 443-474 [DOI] [PubMed] [Google Scholar]
- França M. B., Panek A. D., Eleutherio E. C. A. (2007). Oxidative stress and its effect during dehydration. Comp. Biochem. Physiol. A 146, 621-631 [DOI] [PubMed] [Google Scholar]
- Gibbs A. G. (2002). Water balance in desert Drosophila: lessons from non-charismatic microfauna. Comp. Biochem. Physiol. A 133, 781-787 [DOI] [PubMed] [Google Scholar]
- Gibbs A. G., Matzkin L. M. (2001). Evolution of water balance in the genus Drosophila. J. Exp. Biol. 204, 2331-2338 [DOI] [PubMed] [Google Scholar]
- Gibbs A. G., Fukuzato F., Matzkin L. M. (2003). Evolution of water conservation mechanisms in Drosophila. J. Exp. Biol. 206, 1183-1192 [DOI] [PubMed] [Google Scholar]
- Goyal K., Walton L. J., Browne J. A., Burnell A. M., Tunnacliffe A. (2005). Molecular anhydrobiology: identifying molecules implicated in invertebrate anhydrobiosis. Integ. Comp. Biol. 45, 702-709 [DOI] [PubMed] [Google Scholar]
- Gray E. M., Bradley T. J. (2005). Physiology of desiccation resistance in Anopheles gambiae and Anopheles arabiensis. Am. J. Trop. Med. Hyg. 73, 553-559 [PubMed] [Google Scholar]
- Gray E. M., Rocca K. A. C., Costantini C., Besansky N. J. (2009). Inversion 2La is associated with enhanced desiccation resistance in Anopheles gambiae. Malaria J. 8, 215-227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley N. F. (1994). Water Relations of Terrestrial Arthropods New York: Academic Press; [Google Scholar]
- Harrington L. C., Edman J. D., Scott T. W. (2001). Why do female Aedes aegypti (Diptera: Culicidae) preferentially and frequently feed on human blood? J. Med. Entomol. 38, 422-432 [DOI] [PubMed] [Google Scholar]
- Hayward S. A. L., Rinehart J. P., Denlinger D. L. (2004). Desiccation and rehydration elicit distinct heat shock protein transcript responses in flesh fly pupae. J. Exp. Biol. 207, 963-971 [DOI] [PubMed] [Google Scholar]
- Hoffmann A. A. (2003). Low potential for desiccation adaptation in rainforest Drosophila species. Science 301, 100-102 [DOI] [PubMed] [Google Scholar]
- Lee Y., Meneses C. R., Fofana A., Lanzaro G. C. (2009). Desicccation resistance among subpopulations of Anopheles gambiae s.s. from Selinkenyi, Mali. J. Med. Entomol. 46, 316-320 [DOI] [PubMed] [Google Scholar]
- Li A., Benoit J. B., Lopez-Martinez G., Elnitsky M. A., Lee R. E., Jr, Denlinger D. L. (2009). Distinct contractile and cytoskeletal protein patterns in the Antarctic midge are elicited by desiccation and rehydration. Proteomics 9, 2788-2797 [DOI] [PubMed] [Google Scholar]
- Lopez-Martinez G., Benoit J. B., Rinehart J. P., Elnitsky M. A., Lee R. E., Jr, Denlinger D. L. (2009). Dehydration, rehydration and overhydration alter patterns of gene expression in the Antarctic midge, Belgica antarctica. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 179, 481-491 [DOI] [PubMed] [Google Scholar]
- Matzkin L. M., Markow T. A. (2009). Transcriptional regulation of metabolism associated with increased desiccation resistance of the cactophilic Drosophila mojavensis. Genetics 182, 1279-1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzkin L. M., Watts T. D., Markow T. A. (2007). Desiccation resistance in four Drosophila species: sex and population effects. Fly 1, 268-273 [DOI] [PubMed] [Google Scholar]
- Mitchell C. J., Briegel H. (1989). In ability of diapasing Culex pipiens (Diptera: Culicidae) to use blood for producing lipid reserves for overwintering survival. J. Insect Physiol. 26, 318-326 [DOI] [PubMed] [Google Scholar]
- Rinehart J. P., Robich R. M., Denlinger D. L. (2006). Enhanced cold and desiccation tolerance in diapausing adults of Culex pipiens, and a role for Hsp70 in response to cold shock but not as a component of the diapause program. J. Med. Entomol. 43, 713-722 [DOI] [PubMed] [Google Scholar]
- Robich R. M., Denlinger D. L. (2005). Diapause in the mosquito Culex pipiens evokes a metabolic switch from blood feeding to sugar gluttony. Proc. Natl. Acad. Sci. USA 102, 15912-15917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim C., Denlinger D. L. (2008). Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc. Natl. Acad. Sci. USA 105, 6777-6781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim C., Denlinger D. L. (2009). Transcriptional profiling and regulation of fat metabolism genes in diapausing adults of the mosquito Culex pipiens. Physiol. Genomics 39, 202-209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair B. J., Chown S. L. (2005). Deleterious effects of repeated cold exposure in a freeze-tolerant sub-Antarctic caterpillar. J. Exp. Biol. 208, 869-879 [DOI] [PubMed] [Google Scholar]
- Sinclair B. J., Gibbs A. G., Roberts S. P. (2007). Gene transcription during exposure to, and recovery from, cold and desiccation stress in Drosophila melanogaster. Insect Mol. Biol. 16, 435-443 [DOI] [PubMed] [Google Scholar]
- Spielman A., Wong J. (1973). Studies on autogeny in natural populations of Culex pipiens. III. Midsummer preparation for hibernation in autogenous populations. J. Med. Entomol. 10, 319-324 [DOI] [PubMed] [Google Scholar]
- Tammariello S. P., Rinehart J. P., Denlinger D. L. (1999). Desiccation elicits heat shock protein transcription in the flesh fly, Sarcophaga crassipalpis, but does not enhance tolerance to high or low temperature. J. Insect Physiol. 45, 933-938 [DOI] [PubMed] [Google Scholar]
- Vaidyanathan R., Fleisher A. E., Minnick S. L., Simmons K. A., Scott T. W. (2008). Nutritional stress affects mosquito survival and vector competence for West Nile virus. Vector-Borne Zoonotic Dis. 8, 727-732 [DOI] [PubMed] [Google Scholar]
- Van Handel E. (1985a). Rapid determination of glycogen and sugars in mosquitoes. J. Am. Mosq. Control Assoc. 1, 299-301 [PubMed] [Google Scholar]
- Van Handel E. (1985b). Rapid determination of total lipids in mosquitoes. J. Am. Mosq. Control Assoc. 1, 302-304 [PubMed] [Google Scholar]
- Van Handel E., Day J. F. (1988). Assay of lipids, glycogen and sugars in individual mosquitoes: correlations with wing length in field-collected Aedes vexans. J. Am. Mosq. Control Assoc. 4, 549-550 [PubMed] [Google Scholar]
- Vinogradova E. B. (2000). Culex Pipiens Pipiens Mosquitoes: Taxonomy, Distribution, Ecology, Physiology, Genetics, Applied Importance and Control Sofia, Bulgaria: Pensoft Publishers; [Google Scholar]
- Watanabe M. (2006). Anhydrobiosis in invertebrates. Appl. Entomol. Zool. 41, 15-31 [Google Scholar]
- Wharton G. W. (1985). Water balance of insect. In Comprehensive Insect Physiology, Biochemistry and Pharmacology Vol.4 (ed. Kerkut G. A., Gilbert L. I.), pp. 565-603 Oxford: Pergamon Press; [Google Scholar]
- Winston P. W., Bates D. S. (1960). Saturated solutions for the control of humidity in biological research. Ecology 41, 232-237 [Google Scholar]
- Zhou G., Miesfeld R. L. (2009). Energy metabolism during diapause in Culex pipiens mosquitoes. J. Insect Physiol. 55, 40-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G., Pennington J. E., Wells M. A. (2004). Utilization of pre-exiting energy stores of female Aedes aegypti mosquitoes during the first gonotrophic cycle. Insect Biochem. Mol. Biol. 34, 919-925 [DOI] [PubMed] [Google Scholar]
- Ziegler R., Ibrahim M. M. (2001). Formation of lipid reserves in fat body and eggs of the yellow fever mosquito, Aedes aegypti. J. Insect Physiol. 47, 623-627 [DOI] [PubMed] [Google Scholar]